Abstract
Introduction
We present a case of membranous nephropathy associated with a secondary syphilis infection in a patient with HIV.
Case Presentation
A 37-year-old white man with HIV who was receiving highly active antiretroviral therapy presented to the Emergency Department with 6 weeks of rectal pain. He had a CD3–CD4 count of 656 cells/mm3 and an undetectable viral load. On admission, he was found to have an anal ulcer, a serum creatinine of 1.4 mg/dL (baseline 0.7 to 1.0 mg/dL), elevated transaminases, positive rapid plasmin reagin, and a urine protein/creatinine ratio revealing nephrotic-range proteinuria. Renal biopsy demonstrated membranous nephropathy with features suggestive of a secondary cause. Our patient was treated with penicillin for secondary syphilis, with normalization of renal function, resolution of the nephrotic syndrome, and improvement of his elevated transaminases.
Discussion
This case is a reminder that patients with HIV are not infrequently coinfected with Treponema pallidum and that secondary syphilis can have systemic manifestations, including elevated transaminases and nephrotic syndrome. Prompt diagnosis and treatment will result in resolution of these problems.
INTRODUCTION
Syphilis, as a disease entity, became widely known in the Western world during the Renaissance, when large outbreaks occurred that rapidly spread throughout Europe and Asia.1 The etiologic agent, Treponema pallidum, was identified in 1905,2 and with the development of medications, particularly of penicillin, and of serologic testing, syphilis became a relatively small public health issue. Since 2000, however, when the historically lowest incidence rates of syphilis in the US were observed, rates have doubled from 2.1 cases/100,000 population in 2000 to 5.3 cases/100,000 in 2013.3 The largest increase in incidence is seen among men who have sex with men in all age groups and among all ethnicities.3
Because of its shared mode of transmission, coinfection of syphilis with HIV is frequent.4 The presence of mucosal syphilitic ulcers facilitates the transmission of HIV. Moreover, the presence of immunodeficiency caused by HIV may result in syphilis presenting atypically, with a more rapid clinical course and aggressive manifestations, including neurologic and ophthalmologic involvement.5 Serologic diagnosis of syphilis using nontreponemal testing followed by confirmation using more specific treponemal testing is applicable regardless of HIV status.6 In 2010, Horberg et al7 examined the Kaiser Permanente Northern California patient population and reported that the adjusted incidence rate ratio on syphilis infection in HIV vs non-HIV-infected individuals was 86.0, and that this ratio increased with time.
Nephrotic syndrome is well known to be associated with HIV infection. Although the disease entity termed HIV-associated nephropathy (HIVAN), which is characterized by collapsing focal and segmental glomerulosclerosis and acute interstitial nephritis with microcystic tubular dilatation, is predominantly seen among African American patients with high viral loads and low CD4 counts,8 nephrotic syndrome in patients with HIV may be caused by any number of glomerular pathologies, including immune complex glomerulonephritis, minimal change disease, and immunoglobulin A (IgA) nephropathy, among others.9,10 Thus, it would be a mistake to assume that all nephrotic syndromes presenting in an HIV-positive patient is necessarily caused by HIVAN. This is particularly true if the patient is coinfected with either syphilis, hepatitis B, or hepatitis C, each of which may cause other distinct glomerular diseases.
The following case serves as a reminder that nephrotic syndrome in a patient with HIV may not necessarily be caused by the HIV.
CASE PRESENTATION
Presenting Concerns
A 37-year-old white man with a medical history of HIV who was receiving highly active antiretroviral therapy presented to the Emergency Department with 6 weeks of rectal pain. He had been evaluated both in the Emergency Department and the urgent care clinic several times before his admission. Initially, his symptoms were thought to be caused by unprotected anal intercourse exacerbated by hard bowel movements. Seven days before his admission, the patient presented to the urgent care clinic with persistent rectal pain and subjective fevers. Examination revealed a 1-cm indurated anal lesion for which he received oral cephalexin. A biopsy of the lesion was performed. He received intravenous metronidazole before the biopsy and subsequently received trimethoprim/sulfamethoxazole when an intraoperative wound culture returned enterococcus, Pseudomonas aeruginosa, and Bacteroides fragilis. On the day of admission, he returned to the Emergency Department with reports of nausea, vomiting, dark urine, and nonbloody diarrhea for 3 days. His subjective fevers had resolved since cephalexin had been started.
His medical history was significant for depression and HIV diagnosed in 2004, which was treated with highly active antiretroviral therapy. His last CD3–CD4 count was 656 cells/mm3, and his HIV viral load was undetectable 3 months before admission. He had previously been treated for gonorrhea and syphilis. He had a history of surgical removal of anal condylomata. He was allergic to abacavir, which caused rash. He drank 2 to 3 glasses of wine and 1 to 2 beers weekly and occasionally used recreational marijuana. He never smoked tobacco. His medications before admission included efavirenz-emtricitabine-tenofovir (600-200-300 mg combination) 1 tablet daily, cephalexin (500 mg) 4 times daily, sertraline (50 mg) daily, diltiazem 2% in petrolatum ointment, dibucaine 1% ointment, and docusate sodium (250 mg) daily. He took no over-the-counter or herbal medications.
On presentation, his temperature was 37.3°C, blood pressure was 115/68 mmHg without orthostatic changes, pulse was 56 beats/min, respiratory rate was 19 breaths/min, and oxygen saturation was 99% on room air. In general, he was a well-appearing man in no acute distress. He had no scleral icterus, and cardiopulmonary and abdominal exams were unremarkable. No abdominal tenderness was demonstrated. He had no peripheral edema. He had significant bilateral inguinal but no cervical or axillary lymphadenopathy. He had no skin rash. His rectal examination revealed ulceration at the posterolateral external anal sphincter, which was tender to palpation. Initial laboratory analysis revealed white blood cell count of 7.2 × 103/mL, hemoglobin 12.9 g/dL, mean corpuscular volume 91.5 fL, platelet 451 × 103/L, sodium 132 mEq/L, potassium 3.8 mEq/L, chloride 99 mEq/L, bicarbonate 27 mEq/L, blood urea nitrogen 17 mg/dL, and creatinine of 1.4 mg/dL. His baseline creatinine the previous year was 0.7 mg/dL to 1.0 mg/dL. Other laboratory test results included aspartate aminotransferase 221 U/L, alanine aminotransferase 255 U/L, total bilirubin 1.1 mg/dL, alkaline phosphatase 881 U/L, gamma-glutamyl transpeptidase 920 U/L, and lipase 26 U/L. Serum total protein was 4.6 g/dL and albumin was 1.1 g/dL. Prothrombin time international normalized ratio was 1.0. Both C3 and C4 complement factors were normal, as was rheumatoid factor and antinuclear antibody. Urinalysis revealed a specific gravity of > 1.050 and no leukocyte esterase or nitrite; urine protein was > 600 mg/dL, urine hemoglobin 0.20 mg/dL, urobilinogen 4.0 mg/dL, and urine bilirubin 6.0 mg/dL. Urine microscopy demonstrated urine white blood cell count > 25/high-power field, urine red blood cell 4 to 10/high-power field, and 5 to 10 hyaline casts, with 0 to 2 granular casts/low-power field. Urine culture was negative. Hansel’s stain of the urine demonstrated no urinary eosinophils. Urine electrolytes yielded a urine sodium 37 mEq/L, urine potassium 92 mEq/L, urine chloride 45 mEq/L, and urine creatinine > 400 mg/dL. The urinary protein/creatinine ratio was 8.2 g/g. Urine was negative for detectable acetaminophen or salicylates.
Therapeutic Intervention and Treatment
Our patient presented with several medical issues that included elevated transaminases, acute kidney injury, nephrotic range proteinuria, and rectal ulceration. He received intravenous fluid hydration for presumed prerenal azotemia. Trimethoprim/sulfomethoxazole was continued. Abdominal ultrasound did not find signs of biliary obstruction or cholecystitis but did find bilateral echogenic kidneys without hydronephrosis. Antimitochondrial and antismooth-muscle antibodies were negative; ceruloplasmin and iron saturation were normal. Treponemal enzyme immuoassay antibody was positive, and rapid plasmin reagin was 1:16. His rapid plasmin reagin 3 months before admission was negative although his treponemal antibody was positive, confirming past infection. Hepatitis B surface antigen, core antibody, and surface antibody, and hepatitis C antibody were negative; however, hepatitis A immunoglobulin M (IgM) was positive. Herpes simplex viral culture of the anal lesion was negative. The anal ulcer biopsy revealed lymphoplasmacytoid cell proliferation without evidence of clonality or lymphoma. On hospital day 2, the patient’s serum creatinine rose to 1.58 mg/dL. The patient underwent a percutaneous renal biopsy for acute kidney injury and nephrotic syndrome. The kidney biopsy demonstrated mild acute tubular necrosis, mild arterial and arteriolar nephrosclerosis, and findings consistent with early secondary membranous nephropathy.
Pathology
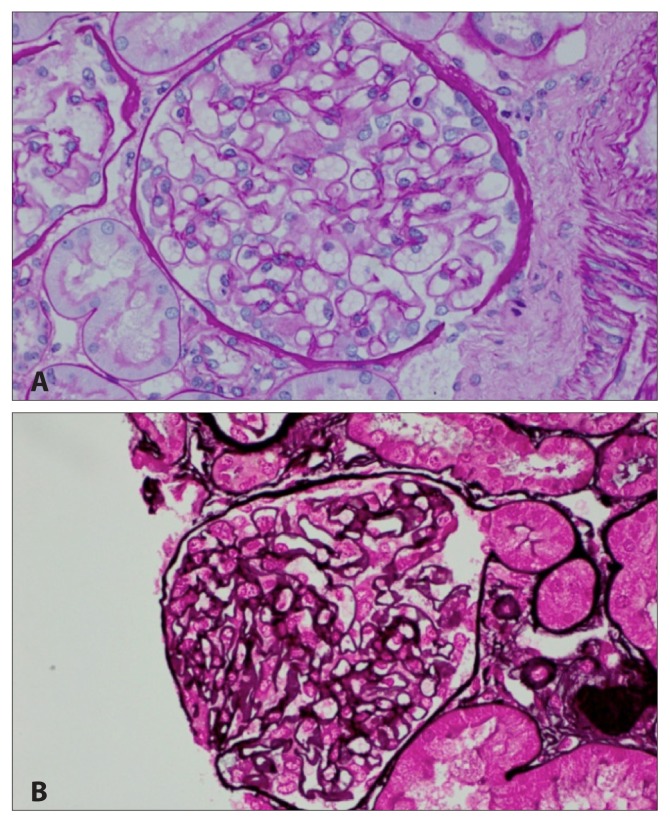
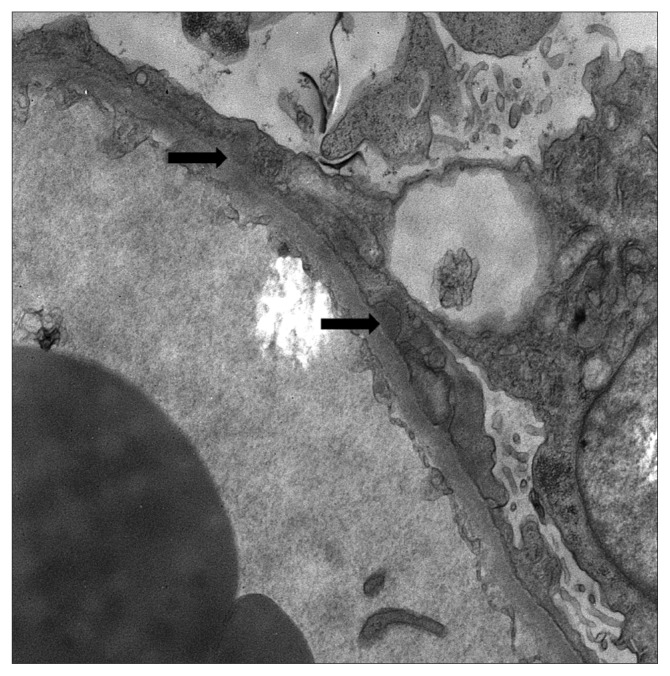
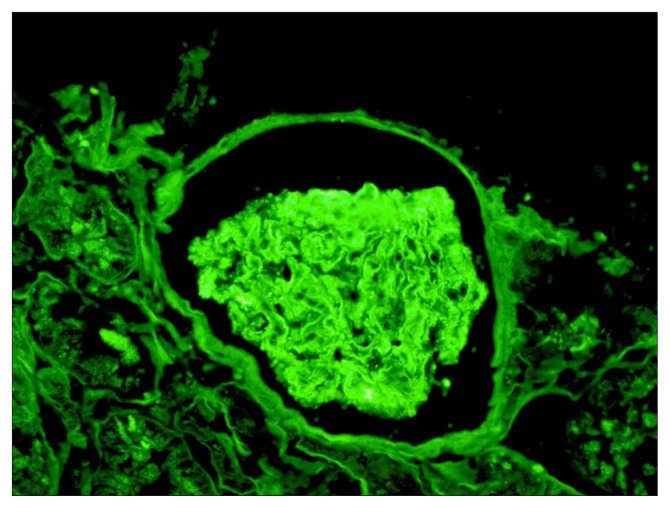
Figures 1 to 3 demonstrate the results of the renal biopsy. By light microscopy (Figures 1A and 1B), there were 14 glomeruli in the biopsy specimen. All were normal in appearance. There were no crescents or segments of sclerosis. Mild acute tubular necrosis and mild arterial and arteriolar nephrosclerosis were observed. Immunofluorescence microscopy, however, demonstrated positive staining for immunoglobulin G (IgG) (4+), IgM (1–2+), C3 (3–4+), C1q (3+), and both kappa and lambda light chains (2–3+) in the capillary walls in a granular pattern and in mesangial regions. Strong granular capillary wall and mesangial staining for IgG is illustrated in Figure 2. By electron microscopy, small electron-dense deposits were identified in subepithelial and paramesangial regions (Figure 3). Podocyte foot processes were extensively effaced, and tubuloreticular inclusions were not identified.
Figure 1.
Renal biopsy by light microscopy, A) periodic acid-Schiff stain and B) Jones silver stain, showing mild acute tubular necrosis and mild arterial and arteriolar nephrosclerosis.
Figure 3.
Renal biopsy by electron microscopy. Arrows indicate subepithelial electron-dense deposits in the subepithelial and paramesangial regions.
Figure 2.
Renal biopsy by immunofluorescence microscopy, showing positive staining for immunoglobulin G, immunoglobulin M, C3, C1q, and kappa and lambda light chains in the capillary walls and mesangial regions.
Follow-up and Outcomes
Benzathine penicillin 2,400,000 IU once per week for 3 weeks was started for treatment of secondary syphilis. Nephrotic syndrome resolved. Follow-up laboratory tests 6 weeks after starting penicillin therapy demonstrated a serum creatinine of 0.88 mg/dL, serum albumin 3.8 mg/dL, alanine aminotransferase 50 U/L, alkaline phosphatase 96 U/L, urine protein/creatinine ratio of < 0.15 mg/mg, and normal urinalysis. See Table 1 for a timeline of the patient’s case.
Table 1.
Case timeline
| Date | Venue | Summary | Diagnostic test results | Intervention |
|---|---|---|---|---|
| 19 December 2013 | Emergency Department | 37-y-old man with HIV presents with 3–4 w of anal pain | Examination: anal ulcer | Psyllium; zinc oxide; dibucaine cream |
| 6 January 2014 | Emergency Department | Anal pain and subjective fevers | Examination: anal ulcer | Surgical consult; cephalexin |
| 9 January 2014 | Surgery center | Anal pain but fevers resolved | Examination: under anesthesia, anal ulcer | Cultures; punch biopsies |
| 11 January 2014 | Emergency Department | Nausea and vomiting | Examination: anal ulcer; inguinal adenopathy; creatinine 1.4; +3 protein; alanine transaminase 255; alkaline phosphatase 881; bilirubin 1.1; abdominal ultrasound | Hospitalization; hydration; switch to trimethoprim/sulfamethoxazole on basis of culture results |
| 13 January 2014 | Hospital | Nausea and vomiting resolved | Creatinine 1.58; urine protein/creatinine 8.2; rapid plasma reagin 1:16 | Nephrology, Gastroenterology, Infectious Disease consults; switch to penicillin |
| 16 January 2014 | Hospital | Asymptomatic | Renal biopsy | None |
| 2 February 2014 | Nephrology clinic | Asymptomatic | Creatinine 0.99; urine protein/creatinine < 0.13; alanine transaminase 62; alkaline phosphatase 265 | Prescribed to complete total of 3 w of penicillin therapy |
| 6 March 2014 | Nephrology clinic | Asymptomatic | Creatinine 0.88; urine protein/creatinine < 0.15; alanine transaminase 50; alkaline phosphatase 96 | None |
Renal Manifestations of Syphilis.
| Glomerular | Tubular |
| Minimal change disease/focal sclerosis1–3 | Acute tubular necrosis18,19 |
| Membranous nephropathy4–14 | Interstitial nephritis20,21 |
| Crescentic glomerulonephritis15 | Vascular |
| Postinfectious glomerulonephritis14,16 | Renal artery stenosis22–24 |
| Amyloidosis17 | Endarteritis13 |
| Mass lesion | |
| Renal gumma25 |
Hartley AJ, Rajakariar R, Sheaff M, Buckland M, Goh B, O’Connell R. Syphilis masquerading as focal segmental glomerulosclerosis. Int J STD AIDS 2014 Jun;25(7):529–31. DOI: https://doi.org/10.1177/0956462413516940.
Stubanus M, Göbel H, Rieg S, Walz G, Gerke P. Quiz page. Minimal change glomerulonephritis associated with secondary syphilis. Am J Kidney Dis 2007 Jun;49(6):A49–50.
Krane NK, Espenan P, Walker PD, Bergman SM, Wallin JD. Renal disease and syphilis: A report of nephrotic syndrome with minimal change disease. Am J Kidney Dis 1987 Feb;9(2):176–9. DOI: https://doi.org/10.1016/s0272-6386(87)80096-3.
Hunte W, al-Ghraoui F, Cohen RJ. Secondary syphilis and the nephrotic syndrome. J Am Soc Nephrol 1993 Jan;3(7):1351–5.
Sølling J, Sølling K, Jacobsen KU, Olsen S, From E. Circulating immune complexes in syphilitic nephropathy. A case report. Br J Vener Dis 1978 Feb;54(1):53–6. DOI: https://doi.org/10.1136/sti.54.1.53.
Gamble CN, Reardon JP. Immunopathogenesis of syphilitic glomerulonephritis. Elution of antitreponemal antibody from glomerular immune-complex deposits. New Engl J Med 1975 Feb 27;292(9):449–54. DOI: https://doi.org/10.1056/nejm197502272920903.
O’Regan S, Fong JS, de Chadarévian JP, Rishikof JR, Drummond KN. Treponemal antigens in congenital and acquired syphilitic nephritis: Demonstration by immunofluorescence studies. Ann Intern Med 1976 Sep;85(3):325–7. DOI: https://doi.org/10.7326/0003-4819-85-3-325.
Handoko ML, Duijvestein M, Scheepstra CG, de Fijter CW. Syphilis: A reversible cause of nephrotic syndrome. BMJ Case Rep 2013 Feb 8;2013. DOI: https://doi.org/10.1136/bcr-2012-008279.
Chen YM, Marcos LA, Liapis H, Steinberg TH, Morrison AR. An unusual cause of membranous glomerulonephritis in a patient with HIV. Int Urol Nephrol 2012 Jun;44(3):983–6. DOI: https://doi.org/10.1007/s11255-011-9945-6.
Mora Mora MT, Gallego Domínguez MS, Castellano Cerviño MI, Novillo Santana R, Gómez-Martino Arroyo JR. Membranous glomerulonephritis in a patient with syphilis. [Article in English, Spanish]. Nefrologia 2011;31(3):372–3. DOI: https://doi.org/10.3265/Nefrologia.pre2011.Mar.10819.
Bani-Hani S, Patel V, Larsen CP, Walker PD, Cooke CR, Showkat A. Renal disease in AIDS: It is not always HIVAN. Clin Exp Nephrol 2010 Jun;14(3):263–7. DOI: https://doi.org/10.1007/s10157-009-0253-8.
Satoskar AA, Kovach P, O’Reilly K, Nadasdy T. An uncommon cause of membranous glomerulonephritis. Am J Kidney Dis 2010 Feb;55(2):386–90. DOI: https://doi.org/10.1053/j.ajkd.2009.06.015.
Hruby Z, Kuźniar J, Rabczyński J, Bogucki J, Steciwko A, Weyde W. The variety of clinical and histopathologic presentations of glomerulonephritis associated with latent syphilis. Int Urol Nephrol 1992;24(5):541–7. DOI: https://doi.org/10.1007/bf02550123.
Bhorade MS, Carag HB, Lee HJ, Potter EV, Dunea G. Nephropathy of secondary syphilis. A clinical and pathological spectrum. JAMA 1971 May 17;216(7):1159–66. DOI: https://doi.org/10.1001/jama.216.7.1159.
Walker PD, Deeves EC, Sahba G, Wallin JD, O’Neill WM Jr. Rapidly progressive glomerulonephritis in a patient with syphilis. Identification of antitreponemal antibody and treponemal antigen in renal tissue. Am J Med 1984 Jun;76(6):1106–12. DOI: https://doi.org/10.1016/s0022-5347(17)49052-5.
Yoshikawa K, Aida Y, Seki N, et al. Early syphilitic hepatitis concomitant with nephrotic syndrome followed by acute kidney injury. Clin J Gastroenterol 2014 Aug;7(4):349–54. DOI: https://doi.org/10.1007/s12328-014-0499-x.
Dowling GB. Syphilitic nephritis. Br Med J 1925 Sep 26;2(3378):551–2. DOI: https://doi.org/10.1136/bmj.2.3378.551.
Nelson TV, Blaceri S, Biederman JI. Rhabdomyolysis and acute renal failure with syphilitic myositis. Kidney Int 2016 May;89(5):1169. DOI: https://doi.org/10.1016/j.kint.2015.09.004.
Estévez RF. Neurosyphilis presenting as rhabdomyolysis and acute renal failure with subsequent irreversible psychosis and dementia. Psychosomatics 2006 Nov–Dec;47(6):538–9. DOI: https://doi.org/10.1176/appi.psy.47.6.538.
Zhou J, Hu C, Zheng F, Cheng H, Xuan J, Li H. Nephrogenic diabetes insipidus secondary to syphilis infection. J Clin Endocrinol Metab 2013 Jul;98(7):2663–6. DOI: https://doi.org/10.1210/jc.2013-1074.
Chen YC, Lee N, Chang CT, Wu MS. Salt loss and hyponatraemia in a patient with syphilitic nephritis. Nephrol Dial Transplant 2005 Jun;20(6):1248–50. DOI: https://doi.org/10.1093/ndt/gfh778.
Kharge J, Bharatha A, Raghu TR, Manjunath CN. Bilateral coronary ostial stenosis with bilateral renal ostial stenosis in cardiovascular syphilis: De novo percutaneous coronary intervention and in-stent restenosis. Eur Heart J 2013 Sep;34(34):2682. DOI: https://doi.org/10.1093/eurheartj/eht036.
Joshi SV, Desai R, Agarwal N, et al. Successful management of ruptured suprarenal aortic aneurysm with left artery stenosis (a case report). J Postgrad Med 1988 Apr;34(2):105–7.
Price RK, Skelton R. Hypertension due to syphilitic occlusion of the main renal arteries. Br Heart J 1948 Jan;10(1):29–33. DOI: https://doi.org/10.1136/hrt.10.1.29.
de Carvalho JG, Slongo EL, Sobral AC. Kidney mass and osteolytic lesion: Is it always malignancy? Nephrol Dial Transplant 2007 Feb;22(2):645–8.
DISCUSSION
Membranous nephropathy is among the most common diagnoses for adults with nephrotic syndrome in the general population and accounts for approximately 20% of cases of nephrotic syndrome.11 Histologically, membranous nephropathy is characterized by diffuse thickening of the glomerular basement membrane with immune complex deposits located in the subepithelial space, and the absence of significant inflammatory or proliferative changes. Membranous nephropathy can be either idiopathic or secondary to an underlying cause, such as solid malignant tumors, medications (eg, penicillamine, gold, nonsteroidal anti-inflammatory drugs), autoimmune diseases (eg, lupus, rheumatoid arthritis, mixed connective tissue disease), or infections (eg, hepatitis B, hepatitis C). In our case, the renal biopsy confirmed a diagnosis of early membranous nephropathy. Although the glomeruli in early membranous nephropathy may look completely normal under light microscopy, as it did in our case, the diagnosis of membranous nephropathy was confirmed by further examination of the specimen using both immunofluorescence and electron microscopy. Moreover, in our case, the immunofluorescence pattern and the presence of both subepithelial and mesangial immune complex deposits suggested that membranous nephropathy was secondary to an underlying condition, which we presumed to be syphilis.
Renal manifestations of syphilis are varied and include not only membranous nephropathy, but also a host of other glomerular and nonglomerular conditions (see the Sidebar: Renal Manifestations of Syphilis). Membranous nephropathy is the most common glomerular lesion seen with syphilis patients.12 Circulating immune complexes have been seen among patients with secondary syphilis,13 and immune complexes containing antitreponemal antibodies have been eluted from renal biopsy specimens.14,15 As opposed to idiopathic membranous nephropathy, where IgG and C3 staining are typically seen by immunofluorescence studies on renal biopsies, membranous nephropathy secondary to syphilis typically has staining not only for IgG and C3, but also occasionally with IgA, IgM, and C1q (“full house pattern”),10,14 which, with the exception of IgA staining, were seen in our case. At the time of this case presentation, immunostaining for phospholipase A2 receptor, frequently positive in primary, as opposed to secondary, membranous nephropathy,16 was not yet available in our facility. Our patient’s rapid clinical response to antitreponemal treatment also speaks in favor of syphilis as being the underlying cause of his membranous nephropathy.
Membranous nephropathy has also been seen in patients infected with HIV. Although the predominant glomerular lesion seen in nephrotic HIV-infected patients is collapsing focal and segmental glomerulosclerosis with interstitial inflammation and tubular microcystic changes, also known as HIVAN, other immune complex glomerulonephritides have been reported in HIV-infected patients, including membranous nephropathy.9,10 In a study of patients with HIV who underwent renal biopsies, those patients who had non-HIVAN renal disease had a slower rate of progression to renal replacement therapy than did those patients with HIVAN. Moreover, in contrast to those with HIVAN, patients with non-HIVAN renal disease had a clinical course that was not significantly affected by intensification of antiretroviral therapy or the absence of a detectable viral load.10 The treatment of HIV-associated membranous nephropathy remains uncertain. There is one case report of a patient with HIV-associated membranous nephropathy who responded dramatically to prednisone17 and another report of a patient who responded to intensification of antiviral therapy.18 In our case, although we cannot rule out HIV-associated membranous nephropathy, our patient’s rapid response to antibiotic therapy and the absence of detectable glomerular endothelial tubuloreticular structures, which is frequently seen in HIV-associated renal disease, speak against HIV as playing a major role in our patient’s nephrotic syndrome.
Syphilis has also been associated with acute hepatitis particularly among HIV-infected patients.19,20 Although our patient had only moderately elevated transaminases without jaundice or clinical signs or symptoms of acute hepatitis, florid acute hepatitis with jaundice caused by syphilis has been reported, which resolved completely with antimicrobial therapy.21 Liver biopsies have demonstrated periportal hepatocyte necrosis and pericholangiolar inflammation.22 As such, serum alkaline phosphatase is typically elevated out of proportion to either bilirubin or transaminase levels,19–21 as it was in our patient. Interestingly, primary syphilitic proctitis, as was presumably seen in our patient, has been associated with liver involvement, perhaps because of their common venous drainage.23 Our patient also had a detectable IgM against hepatitis A virus. The prolonged presence of antihepatitis A virus IgM following infection has been reported and indeed, false positive antihepatitis A virus IgM, confirmed by the absence of detectable viral RNA, has also been seen.24 Our patient’s clinical course with rapid resolution after treatment was not consistent with an acute hepatitis A infection.
Our patient was also taking medications that could have affected his renal function. Tenofovir is well known to have nephrotoxic effects and may present with evidence of proximal tubular dysfunction (ie, glycosuria in the absence of hyperglycemia, phosphaturia, amino aciduria, renal tubular acidosis—termed Fanconi syndrome) and proteinuria.25 Sulfa antibiotics are frequently implicated in cases of acute interstitial nephritis.26 Sulfadiazine, a sulfa antibiotic used in toxoplasmosis, is sparingly soluble and has been associated with crystal-induced acute renal failure.27 Moreover, sulfamethoxisole, similar to cimetidine, inhibits the tubular secretion of creatinine, resulting in an elevated serum level without an effect on the actual glomerular filtration rate.28 Finally, synthetic marijuana use has been associated with cases of acute kidney injury, presumably caused by acute tubular necrosis.29
CONCLUSION
HIV-infected patients are frequently coinfected with other pathogens, such as syphilis, hepatitis B, and/or hepatitis C. Although similar cases have been reported,30–34 our recent case serves as a reminder that syphilis, the “great mimicker,” is commonly seen in patients with HIV and can lead to disease states such as nephrotic syndrome and hepatitis. Prompt diagnosis and treatment is essential to prevent morbidity and mortality.
A Disease of an Unusual Nature
A disease of an unusual nature has invaded Italy and many other regions. In the beginning pustules are on the private parts, soon on the whole body … Moreover to this disease the physicians of our time do not yet give a name, but is called by the common name of French disease, as if this contagion were imported from France into Italy or because Italy was invaded at the same time both by the disease itself and the armies of the French.
— Niccolò Leoniceno, 1428–1524, Italian physician and humanist
Acknowledgment
Mary Corrado, ELS, provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
References
- 1.Frith J. Syphilis—its early history and treatment until penicillin and the debate on its origins. Journal of Military and Veterans’ Health. 2012;20(4):40–58. [Google Scholar]
- 2.de Souza EM. A hundred years ago, the discovery of Treponema pallidum. An Bras Dermatol. 2005 Sep-Oct;80(5):547–8. DOI: https://doi.org/10.1590/S0365-05962005000600017. [Google Scholar]
- 3.Patton ME, Su JR, Nelson R, Weinstock H Centers for Disease Control and Prevention (CDC) Primary and secondary syphilis—United States, 2005–2013. MMWR Morb Mortal Wkly Rep. 2014 May 9;63(18):402–6. [PMC free article] [PubMed] [Google Scholar]
- 4.Wasserheit JN. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 1992 Mar-Apr;19(2):61–77. DOI: https://doi.org/10.1097/00007435-199219020-00001. [PubMed] [Google Scholar]
- 5.Lynn WA, Lightman S. Syphilis and HIV: A dangerous combination. Lancet Infect Dis. 2004 Jul;4(7):456–66. doi: 10.1016/S1473-3099(04)01061-8. DOI: https://doi.org/10.1016/s1473-3099(04)01061-8. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006 Aug 4;55(RR-11):1–94. Erratum in: MMWR Recomm Rep 2006 Sep 15;55(36):997. [PubMed] [Google Scholar]
- 7.Horberg MA, Ranatunga DK, Quesenberry CP, Klein DB, Silverberg MJ. Syphilis epidemiology and clinical outcomes in HIV-infected and HIV-uninfected patients in Kaiser Permanente Northern California. Sex Transm Dis. 2010 Jan;37(1):53–8. doi: 10.1097/OLQ.0b013e3181b6f0cc. DOI: https://doi.org/10.1097/olq.0b013e3181b6f0cc. [DOI] [PubMed] [Google Scholar]
- 8.Kimmel PL, Barisoni L, Kopp JB. Pathogenesis and treatment of HIV-associated renal diseases: Lessons from clinical and animal studies, molecular pathologic correlations, and genetic investigations. Ann Intern Med. 2003 Aug 5;139(3):214–26. DOI: https://doi.org/10.7326/0003-4819-139-3-200308050-00011. [PubMed] [Google Scholar]
- 9.Szczech LA, Gupta SK, Habash R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004 Sep;66(3):1145–52. doi: 10.1111/j.1523-1755.2004.00865.x. DOI: https://doi.org/10.1111/j.1523-1755.2004.00865.x. [DOI] [PubMed] [Google Scholar]
- 10.D’Agati V, Appel GB. Renal pathology of human immunodeficiency virus infection. Semin Nephrol. 1998;18(4):406–21. [PubMed] [Google Scholar]
- 11.Braden GL, Mulhern JG, O’Shea MH, Nash SV, Ucci AA, Jr, Germain MJ. Changing incidence of glomerular diseases in adults. Am J Kidney Dis. 2000 May;35(5):878–83. doi: 10.1016/s0272-6386(00)70258-7. DOI: https://doi.org/10.1016/s0272-6386(00)70258-7. [DOI] [PubMed] [Google Scholar]
- 12.Hunte W, al-Ghraoui F, Cohen RJ. Secondary syphilis and the nephrotic syndrome. J Am Soc Nephrol. 1993 Jan;3(7):1351–5. doi: 10.1681/ASN.V371351. [DOI] [PubMed] [Google Scholar]
- 13.Sølling J, Sølling K, Jacobsen KU, Olsen S, From E. Circulating immune complexes in syphilitic nephropathy. A case report. Br J Vener Dis. 1978 Feb;54(1):53–6. doi: 10.1136/sti.54.1.53. DOI: https://doi.org/10.1136/sti.54.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gamble CN, Reardon JP. Immunopathogenesis of syphilitic glomerulonephritis. Elution of antitreponemal antibody from glomerular immune-complex deposits. New Engl J Med. 1975 Feb 27;292(9):449–54. doi: 10.1056/NEJM197502272920903. DOI: https://doi.org/10.1056/nejm197502272920903. [DOI] [PubMed] [Google Scholar]
- 15.O’Regan S, Fong JS, de Chadarévian JP, Rishikof JR, Drummond KN. Treponemal antigens in congenital and acquired syphilitic nephritis: Demonstration by immunofluorescence studies. Ann Intern Med. 1976 Sep;85(3):325–7. doi: 10.7326/0003-4819-85-3-325. DOI: https://doi.org/10.7326/0003-4819-85-3-325. [DOI] [PubMed] [Google Scholar]
- 16.Larsen CP, Messias NC, Silva FG, Messias E, Walker PD. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Mod Pathol. 2013 May;26(5):709–15. doi: 10.1038/modpathol.2012.207. DOI: https://doi.org/10.1038/modpathol.2012.207. [DOI] [PubMed] [Google Scholar]
- 17.Mattana J, Siegal FP, Schwarzwald E, et al. AIDS-associated membranous nephropathy with advanced renal failure: Response to prednisone. Am J Kidney Dis. 1997 Jul;30(1):116–9. doi: 10.1016/s0272-6386(97)90573-4. DOI: https://doi.org/10.1016/s0272-6386(97)90573-4. [DOI] [PubMed] [Google Scholar]
- 18.Alarcón-Zurita A, Salas A, Antón E, et al. Membranous glomerulonephritis with nephrotic syndrome in a HIV positive patient—remarkable remission with triple therapy. Nephrol Dial Transplant. 2000 Jul;15(7):1097–8. doi: 10.1093/ndt/15.7.1097. DOI: https://doi.org/10.1093/ndt/15.7.1097. [DOI] [PubMed] [Google Scholar]
- 19.Crum-Cianflone N, Weekes J, Bavaro M. Syphilitic hepatitis among HIV-infected patients. Int J STD AIDS. 2009 Apr;20(4):278–84. doi: 10.1258/ijsa.2008.008324. DOI: https://doi.org/10.1258/ijsa.2008.008324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mullick CJ, Liappis AP, Benator DA, Roberts AD, Parenti DM, Simon GL. Syphilitic hepatitis in HIV-infected patients: A report of 7 cases and review of literature. Clin Infect Dis. 2004 Nov 15;39(10):e100–5. doi: 10.1086/425501. DOI: https://doi.org/10.1086/425501. [DOI] [PubMed] [Google Scholar]
- 21.Sabbatani S, Manfredi R, Attard L, Salfi N, Chiodo F. Secondary syphilis presenting with acute severe hepatic involvement in a patient with undiagnosed HIV disease. AIDS Patient Care STDS. 2005 Sep;19(9):545–9. doi: 10.1089/apc.2005.19.545. DOI: https://doi.org/10.1089/apc.2005.19.545. [DOI] [PubMed] [Google Scholar]
- 22.Sobel HJ, Wolf EH. Liver involvement in early syphilis. Arch Pathol. 1972 Jun;93(6):565–8. [PubMed] [Google Scholar]
- 23.Le Marchant JM, Ferrand B. [Primary syphilitic proctitis associated with liver involvement (author’s transl)]. Sem Hop. 1981 Sep 18–25;57(33–36):1434–8. [Article in French] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention (CDC) Positive test results for acute hepatitis A virus infection among persons with no recent history of acute hepatitis—United States, 2002–2004. MMWR Morb Mortal Wkly Rep. 2005 May 13;54(18):453–6. [PubMed] [Google Scholar]
- 25.Scherzer R, Estrella M, Li Y, et al. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012 Apr 24;26(7):867–75. doi: 10.1097/QAD.0b013e328351f68f. DOI: https://doi.org/10.1097/qad.0b013e328351f68f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garvey JP, Brown DM, Chotirmall SH, Dorma AM, Conlon PJ, Walshe JJ. Trimethoprim-sulfamethoxazole induced acute interstitial nephritis in renal allografts: Clinical course and outcome. Clin Nephrol. 2009 Nov;72(5):331–6. doi: 10.5414/cnp72331. DOI: https://doi.org/10.5414/cnp72331. [DOI] [PubMed] [Google Scholar]
- 27.Díaz F, Collazos J, Mayo J, Martínez E. Sulfadiazine-induced multiple urolithiasis and acute renal failure in a patient with AIDS and toxoplasma encephalitis. Ann Pharmacother. 1996 Jan;30(1):41–2. doi: 10.1177/106002809603000108. DOI: https://doi.org/10.1177/106002809603000108. [DOI] [PubMed] [Google Scholar]
- 28.Berglund F, Killander J, Pompeius R. Effect of trimethoprim-sulfamethoxazole on the renal excretion of creatinine in man. J Urol. 1975 Dec;114(6):802–6. doi: 10.1016/s0022-5347(17)67149-0. DOI: https://doi.org/10.1016/s0022-5347(17)67149-0. [DOI] [PubMed] [Google Scholar]
- 29.Kazory A, Aiyer R. Synthetic marijuana and acute kidney injury: An unforeseen association. Clin Kidney J. 2013 Jun;6(3):330–3. doi: 10.1093/ckj/sft047. DOI: https://doi.org/10.1093/ckj/sft047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tatu AL, Popescu M, Rotarescu R, Popescu SD. A rare case of syphilis associated with renal and hepatic involvement. Dermatol Pract Concept. 2013 Oct 31;3(4):53–4. doi: 10.5826/dpc.0304a13. DOI: https://doi.org/10.5826/dpc.0304a13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrison EB, Normal DA, Wingo CS, Henrich WL. Simultaneous hepatic and renal involvement in acute syphilis. Case report and review of the literature. Dig Dis Sci. 1980 Nov;25(11):875–8. doi: 10.1007/BF01338531. DOI: https://doi.org/10.1007/bf01338531. [DOI] [PubMed] [Google Scholar]
- 32.Tsai YC, Chen LI, Chen HC. Simultaneous acute nephritis and hepatitis in secondary syphilis. Clin Nephrol. 2008 Dec;70(6):532–6. doi: 10.5414/cnp70532. DOI: https://doi.org/10.5414/cnp70532. [DOI] [PubMed] [Google Scholar]
- 33.Tang AL, Thin RN, Croft DN. Nephrotic syndrome and hepatitis in early syphilis. Postgrad Med J. 1989 Jan;65(759):14–5. doi: 10.1136/pgmj.65.759.14. DOI: https://doi.org/10.1136/pgmj.65.759.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Makker J, Bajantri B, Nayudu SK. Secondary syphilis with hepatitis and nephrotic syndrome: A rare concurrence. J Clin Med Res. 2016 Jul;8(7):550–4. doi: 10.14740/jocmr2595w. [DOI] [PMC free article] [PubMed] [Google Scholar]