Abstract
Background
Protein energy malnutrition (PEM) increases susceptibility to infectious diseases, including influenza infection, but no studies have addressed the potential influences of PEM on the immunogenicity and protective efficacy of avian influenza A(H5N1) vaccine.
Methods
We investigated the role of PEM on vaccine-mediated protection after a lethal challenge with recombinant A(H5N1) virus using isocaloric diets providing either adequate protein (AP; 18% protein) or very low protein (VLP; 2% protein) in an established murine model of influenza vaccination.
Results
We demonstrated that mice maintained on a VLP diet succumb to lethal challenge at greater rates than mice maintained on an AP diet, despite comparable immunization regimens. Importantly, there was no virus-induced mortality in both VLP and AP groups of mice when either group was immunized with adjuvanted low-dose A(H5N1) subvirion vaccine.
Conclusions
Our results suggest that adjuvanted vaccination in populations where PEM is endemic may be one strategy to boost vaccination-promoted immunity and improve outcomes associated with highly pathogenic A(H5N1).
Keywords: malnutrition, protein energy malnutrition, virus, influenza, vaccination, adjuvant, avian influenza
Malnutrition is a significant public health concern, with protein energy malnutrition (PEM) being the most lethal form of malnutrition [1]. Defined as an imbalance between food intake (protein and energy) and the levels necessary for a host to achieve optimal growth [2, 3], PEM affects every fourth child worldwide and is implicated in at least half of the 10.4 million child deaths annually [4]. Although 95.9% of the world’s undernourished population of 826 million are in developing countries [5], the elderly, specifically those hospitalized or living in long-term care facilities, are significantly affected by malnutrition [6, 7].
The ongoing circulation of highly pathogenic avian influenza A (H5N1) virus in poultry and its intermittent ability to infect humans, particularly in endemic parts of the globe, poses an imminent threat to global health (http://www.cdc.gov/flu/avianflu/h5n1-people.htm). Since 2003, a total of 846 human cases of confirmed avian influenza A (H5N1) have been reported to the World Health Organization, with >53% (449 cases) resulting in death [8]. Serious illness, high fatality rates, and the ability for influenza viruses to rapidly undergo mutation as a result of antigenic shift underscore concerns about a potential A(H5N1) pandemic. Moreover, lack of immunity to A(H5N1) in humans makes vaccination the most effective prophylactic strategy for counteracting transmission during an A(H5N1) pandemic.
PEM has been associated with increased incidence of infections, including influenza [1, 3, 9]. Given the global burden of PEM, and the current epidemiological demographic of A(H5N1), it is important to understand the implications of nutritional inadequacies on the immunogenicity and efficacy of A(H5N1) vaccine. A(H5N1) vaccine is inherently less immunogenic than antigens routinely included in the seasonal influenza vaccines [10]. Consequently, higher doses of antigen and/or other vaccine strategies are necessary to elicit effective protection against an emerging avian influenza virus such as A(H5N1), which may constitute the use of an adjuvanted system to limit the amount of antigen and improve the host immune response. More recently, safety and immunogenicity studies have underscored the feasibility of using adjuvants for improving immunogenicity of A(H5N1) vaccines [11, 12].
In the present study, the murine model was used to ascertain the impact of PEM on adjuvanted A(H5N1) (A/Vietnam/1203/2004/H5N1; clade 1 monovalent vaccine) vaccination and subsequent lethal challenge. Weanling mice were fed isocaloric diets supplemented with either adequate (18%) or very low (2%) levels of protein [9, 13], followed by different vaccination regimens and viral challenge with a lethal dose of rgH5N1, a reverse genetics–derived A(H5N1) (A/Vietnam/1203/2004) virus on A/PR/8/1934 background. Our findings illustrate enhanced protection after challenge and suggest a role for adjuvanted vaccines in decreasing the susceptibility and improving influenza (H5N1)–induced outcomes, specifically in malnourished settings.
MATERIALS AND METHODS
Mice and Diets
Four-week-old female C57BL/6 mice were purchased from the Jackson Laboratory, quarantined for 3 days, and randomly assigned to isocaloric diets (Harlan Laboratories) containing either 18% or 2% protein, defined as adequate protein (AP) and very low protein (VLP), respectively. The composition and caloric content of the diets used in the study have been described elsewhere [9]. Also as described elsewhere [9], mice were randomly assigned, and the amount of food provided to the control group of mice (AP group) was matched to that consumed by the experimental group (VLP group) to confirm comparable feed consumption. Based on this, we replenished the same amount of feed every 3 days and weighed the amount of feed consumed (per week) by the AP and VLP groups of mice. All studies contained herein were approved by the Centers for Disease Control and Prevention (CDC) Institutional Animal Care and Use Committee and carried out in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Immunizations
Five weeks after onset of the postisocaloric diet onset, mice were vaccinated intramuscularly with influenza A virus monovalent vaccine (A/Vietnam/1203/2004/H5N1, clade 1; prepared by Sanofi Pasteur for use in humans, obtained through the Biomedical Advanced Research and Development Authority), hereafter referred to as H5 (A/Vietnam/1203/2004/H5N1)-HA (hemagglutinin), containing either 3 or 10 μg of H5-HA in a total volume of 100 μL per mouse. Adjuvanted groups were vaccinated with 3 μg of H5-HA formulated with an oil-in-water nanoemulsion adjuvant system [14] at a concentration of 1:,1 in a total volume of 100 μL per mouse. The nanoemulsion adjuvant consisted of squalene and α-tocopherol, Tween 20, and phosphate-buffered saline (PBS). The detailed protocol for preparation of an oil-in-water emulsion- based adjuvant system developed in our laboratory is described elsewhere [14]. Each mouse in the adjuvant control group was administered 100 μL of adjuvant formulated with PBS in a 1:1 concentration. Two weeks later, mice received a booster immunization dose, according to the same procedure used for primary immunization.
Hemagglutination Inhibition Assay
Mouse serum samples were was treated overnight with receptor- destroying enzyme (Denka Seiken) and tested for reactivity to rgH5N1 virus using the standard hemagglutination inhibition (HI) assay with 0.5% horse red blood cells (RBCs), as described elsewhere [15]. In short, 25 μL of cold ×1 PBS was added to the wells of a 96-well V-bottom plate (Corning). Receptor-destroying enzyme–treated serum samples (25 μL) was then added to each column and serially diluted. Eight hemagglutination units of rgH5N1 virus were added to each well and incubated at room temperature for 60 minutes. Finally, 50 μL of 0.5% horse RBCs in ×1 PBS was added to each well and incubated at room temperature for 60 minutes. The HI titer was recorded as the serial dilution of serum showing complete HI.
Virus Infection
For virus challenge studies, we used rgH5N1 (A/Vietnam/1203/2004), a virus strain containing the H5-HA gene (with a modified basic amino acid cleavage site) and the N1 neuraminidase gene from A(H5N1) virus (A/Vietnam/1203/2004) on an A/PR/8/34 background. Details of generation and propagation of rgH5N1 have been described elsewhere [16]. Briefly, rgH5N1 virus was propagated in 10-day-old embryonated chicken eggs and stored at –80°C. The median lethal dose of rgH5N1 (equivalent to 6 × 105 plaque-forming units/50 μL) was determined in mice fed an AP diet. For virus challenge studies, mice were deeply anesthetized with 2,2,2-tribromoethanol in tert-amyl alcohol (Avertin; Sigma-Aldrich) before intranasal inoculation with 4 times the median lethal dose of rgH5N1. All mice that lost ≥25% preinfection body weight were humanely euthanized, according to CDC Institutional Animal Care and Use Committee guidelines.
Lung Viral Titer Quantitation
Lungs were aseptically removed on day 4 after infection and stored at –80° C. Samples were thawed and homogenized in antibiotic-supplemented PBS on the day of assessment. As described elsewhere [9], serial dilutions were titrated in eggs to determine virus infectivity (limit of detection, 101.5 egg infectious dose 50 [EID50]/mL). Allantoic fluid from the inoculated eggs was added to wells containing 0.5% turkey RBCs in PBS. Virus titers were calculated using the method described by Reed and Muench [17] and are expressed as the mean log10 EID50/mL (with standard error of the mean).
Lung Cytokine Assay
Lung homogenates (see methods for lung viral titer quantitation, above) were added in duplicate to a Bioplex plate (Bio-Rad Laboratrories) previously coated with anti-mouse antibody– conjugated beads specific for interleukin 1β, 6, and 12p40 (IL- 1β, IL-6, and IL-12p40), interferon (IFN) γ, and tumor necrosis factor (TNF) α. Bioplex plate was processed and read according to the manufacturer’s protocol on a Bioplex 200 Array Reader (Bio-Rad Laboratrories).
Statistical Analysis
All statistical analyses were performed using GraphPad Prism version 5.0 software (GraphPad Software). Analysis of variance was used when >2 groups were being compared, with post hoc Tukey multiple-comparison testing to delineate significance among experimental groups at a 95% confidence interval. Lung viral titer, lung cytokine concentration, morbidity rate, and serum HI titer were presented as means with standard deviations. All differences were considered statistically significant when at P< .05.
RESULTS
Serum HI Responses in AP and VLP Diet Groups of Mice
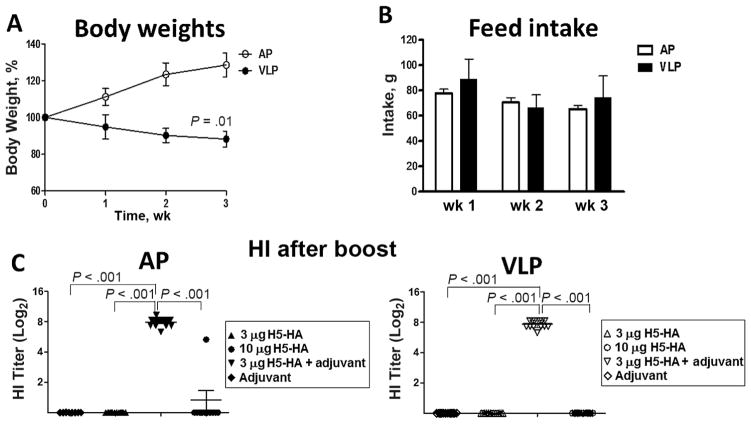
Earlier studies using 2 isocaloric diets showed that, compared with a diet containing AP, the VLP diet increased susceptibility to influenza infection [9]. In the current study, to determine the effects of PEM on influenza vaccination, we used AP and VLP diets on weanling mice, followed by immunizations with different doses of H5-HA or adjuvanted-H5-HA. First, as shown in Figure 1, weanling mice maintained on an AP or VLP diet were examined weekly for feed consumption and body weight gain over a period of 3 weeks. As shown in Figure 1, whereas mice maintained on an AP diet regimen gained body weight, the VLP group showed significant growth retardation (percentage body weight gain) (Figure 1A), despite comparable levels of feed consumption in the 2 groups (Figure 1B). The difference in percentage body weight between the groups was evident as early as 1 week after initiation of custom diets and markedly different by 3 weeks. In earlier studies using AP and VLP diets, we found that mice fed a VLP diet show a significant decrease in serum leptin levels, a key determinant of PEM [18, 19]. Next, as shown in Supplementary Figure 1, both groups of mice (AP and VLP) received different doses of H5-HA or adjuvanted H5-HA at week 9, followed by booster immunization at week 11.
Figure 1.
Weekly body weight gain, feed intake, and hemagglutination inhibition (HI) titer in mice fed diets with adequate protein (AP) or very low protein (VLP). A, Change in percentage body weight gain over 3 weeks. B, Weekly feed intake. C, D, Serum HI titers in AP and VLP groups treated with H5-HA, adjuvanted H5-HA, or adjuvant. Data in all 4 panels represent results from 2 experiments (20 mice per group). Values in A and B represent means with standard deviations; in C, each symbol represents an individual mouse from 2 experiments with 10–14 mice per group, and horizontal lines represent mean group values.
Serum samples were collected from all groups of mice before and 2 weeks after booster immunization to assess rg-H5N1– specific HI response. Serum samples collected after primary immunization did not show detectable HI titer in any experimental groups (data not shown). Similarly, serum samples from mice receiving booster immunization with low (3-μg) dose of H5-HA did not show appreciable levels of HI titer (Figure 1C; left and right). Moreover, with the exception of 1 mouse in the AP group, serum samples from both groups of mice immunized with a high (10-μg) dose of H5-HA did not show detectable HI titer. Interestingly, both AP and VLP groups immunized with adjuvanted H5-HA showed significantly higher HI titer than groups immunized with H5-HA alone (Figure 1C; left and right). Moreover, the levels of HI titer induced after booster immunization were comparable between the AP and VLP groups (Figure 1C; left and right). Mice receiving adjuvant alone did not show any detectable HI titer.
Immunization With an Oil-In-Water Nanoemulsion Adjuvant Leads to Enhanced Survival of VLP Diet Fed Mice Challenged With rgH5N1
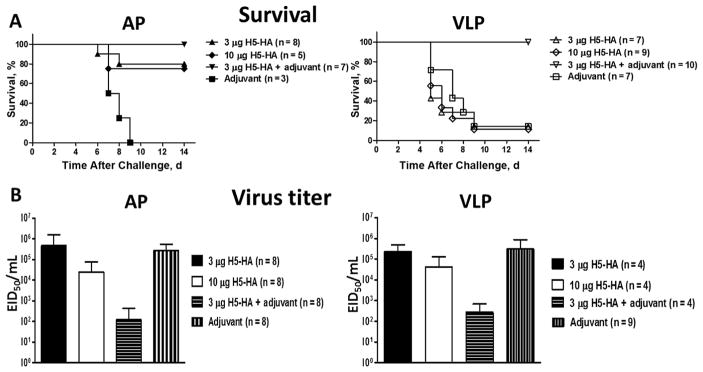
The immunization regimen used in this study demonstrated the potential for an adjuvanted vaccine regimen to induce HI antibody response in mice maintained on either an AP or a VLP diet. To determine whether the adjuvant-induced effect on antibody response could help protect against lethal virus challenge, both AP and VLP groups were challenged with rgH5N1 virus 2 weeks after booster immunization. Interestingly, in both AP and VLP groups, all the mice that received adjuvanted H5-HA survived the lethal viral challenge (Figure 2A, left and right). Furthermore, groups of mice on the AP diet that received a low (3-μg) or high (10-μg) dose of H5-HA also showed significant survival (Figure 2A, left). However, mice fed a VLP diet that received either low- or high-dose H5-HA showed significantly lower survival levels than mice receiving adjuvanted H5-HA (Figure 2A, right). Mice receiving adjuvant alone showed poor survival in both AP and VLP groups (Figure 2A, left and right).
Figure 2.
Survival rate and lung virus titer in response to immunization and lethal viral challenge in mice fed diets with adequate protein (AP) or very low protein (VLP). A, AP (left) and VLP (right) groups of mice receiving HA, adjuvanted HA, or adjuvant alone were assessed for protection from lethal (rgH5N1) viral challenge. Survival rates for AP and VLP groups are shown; data represent results from 2 independent experiments. B, Lung tissues harvested from mice on the AP or VLP diets at day 4 after viral challenge were homogenized and assayed for virus titer, as described in Materials and Methods. Data represent means and standard deviations from 2 independent experiments. The differences in survival percentage by treatment were statistically significant, as follows: AP groups: 3 μg of H5 (A/Vietnam/1203/2004/H5N1)-HA (hemagglutinin) vs adjuvant, P < .01; 10 μg of H5-HA vs adjuvant, P < .05; 3 μg of H5-HA plus adjuvant vs adjuvant alone, P < .001. VLP groups: 3 μg of H5-HA plus adjuvant vs adjuvant alone, 3 μg of H5-HA vs 3 μg H5-HA plus adjuvant, and 10 μg of H5-HA vs 3 μg of H5-HA plus adjuvant, all P < .001. Abbreviation: EID50, egg infectious dose50.
Because the immunization regimen used in this study could influence protection by modulating virus clearance, we determined the virus titer and key inflammatory mediators in the lung tissue on day 4 after viral challenge. As shown in Figure 2B, in both groups (AP and VLP), mice that received adjuvanted H5-HA showed 2–3-log lower levels of virus titer than mice treated with H5-HA or adjuvant alone. An increase in the survival rate of mice immunized with H5-HA in the AP group prompted us to investigate differences in antiviral and inflammatory mediators. Analysis of antiviral cytokine (IFN-γ) and inflammatory (IL-1β, IL-6, IL-12p40, and TNF- α) mediators showed that, compared with the AP group, the VLP group showed lower levels of all the inflammatory markers (Supplementary Figure 2). However, with the exception of IFN-γ in the AP group, for which mice receiving adjuvanted vaccine had significantly lower amounts of cytokine than those receiving H5-HA, there was no difference in any inflammatory marker among the 4 treatment groups for either AP or VLP diet–fed mice (Supplementary Figure 2).
DISCUSSION
The reciprocal relationship between nutrition, specifically as it relates to protein deficiency, and vaccination has been appreciated for some time [20, 21]. In this study, using the mouse model of influenza vaccination, we examined the impact of PEM, a major form of malnutrition in developing countries, on protection against A(H5N1) infection. Because A(H5N1) vaccine (H5-HA) is poorly immunogenic [10], we used 2 different doses of vaccine and an oil-in-water nanoemulsion as adjuvant for immunizations. We found that while both doses of H5-HA failed to elicit HI response in the AP and VLP groups of mice, adjuvanted H5-HA induced comparable HI responses in AP and VLP groups of mice. Furthermore, adjuvanted H5-HA provided complete protection against lethal viral challenge even in the VLP group, which previously has been shown to be highly susceptible to influenza (H1N1) infection [9]. Adjuvanted H5-HA– mediated protection was also reflected by >2-log decrease in virus titer in both AP and VLP groups. Taken together, these results demonstrate that malnourished hosts may not be able to respond effectively to H5-HA–based vaccines and further highlights the need for an adjuvanted vaccine to overcome the PEM-induced immune deficits, and achieve comparable protection in healthy and malnourished hosts.
Earlier studies using the mouse model of PEM have shown the utility of the model for studying the impact of PEM on viral infections [9, 13, 18]. Although in the current study we used body weight as a marker to assess malnutrition in the VLP group, we and others have found low levels of serum leptin in VLP-fed mice to be yet another marker for diet-induced PEM [9, 18]. However, despite our efforts to use the same source of experimental mice and isocaloric custom diets as in earlier [9] and more current studies, we observed in the present study a relatively higher gain in body weight in the AP groups and a higher loss in the VLP groups within 3 weeks after study initiation. Nonetheless, change in percentage body weight gain over time in the VLP group was not due to any difference in consumption, because feed intake was comparable between the AP and VLP groups. Both diet groups were monitored routinely and were in adequate health until the time of viral challenge.
Considering the poor immunogenicity of HA, several experimental approaches have been tested for improving the immunogenicity and efficacy of A(H5N1) vaccines [12, 22]. Some of these approaches include high-dose vaccination, dose-sparing regimens, different formulations of adjuvanted vaccine, and different routes of vaccination [23–26]. In this study, we used 3 or 10 μg of H5-HA for the immunization protocol because our preliminary studies with 10-, 3-, 1-, and 0.3-μg doses of H5-HA administered intra-muscularly in 6-week-old mice showed poor immunogenicity in the absence of an adjuvant (S.S., W.C., S.G., unpublished observations). Furthermore, in the current study, even after boosting, neither dose elicited any significant HI response, a key parameter for assessing protection from viral challenge in mice. Interestingly, adjuvanted H5-HA was able to induce comparable HI response in both AP and VLP groups of mice. Furthermore, comparable HI titers between AP and VLP groups were also reflected in complete protection of mice from lethal viral challenge and >2-log reduction of virus titer in the lung tissue. However, the AP group that received either high- or low-dose H5-HA alone also showed significant survival from viral challenge, compared with a poor survival rate for a similar immunization regimen in the VLP group, a difference probably due to differences in weight gain over the course of experiment and increased susceptibility of mice fed a VLP diet to the virus dose used in the study.
Notably, no HI titer was detectable in the AP groups of mice immunized with either high- or low-dose H5-HA. The reverse genetics–derived H5N1 virus (rg-H5N1stock) used in the current study showed a low concentration of hemagglutination units (64–128 hemagglutination units) and low virus stock titer (106 plaque-forming units/mL), which restricted its use in our study for carrying out microneutralization assays [27], another sensitive neutralization assay for characterizing virus-specific antibody responses. It is possible that other H5-HA–mediated protective mechanisms may be responsible for enhanced survival of AP group of mice from lethal challenge. Alternatively, because a similar effect was not seen in the VLP group, a higher protein (AP) diet could be inducing additional immune stimulatory pathways, contributing to survival mechanisms. Aptly, lung homogenates from the AP groups of mice showed higher levels of IL-1β, IL-6, IL-12p40, IFN-γ, and TNF-α (Supplementary Figure 2) cytokines than the VLP groups. Further studies involving antibody-mediated protection by passive transfer of serum as well as characterization of inflammatory responses of cell types could aid in defining the immune mechanisms involved in these immunization protocols in the face of AP and VLP diets.
Some ongoing clinical trials have aimed at understanding immune responses to adjuvanted A(H5N1)-specific influenza vaccine in healthy populations [28]. Adjuvanted influenza vaccines including an adjuvant containing oil-in-water emulsion of squalene oil are approved for use among persons aged ≥65 years [29]. Notably, results from meta-analysis of several clinical trials found no safety concerns associated with use of adjuvanted vaccines [30]. However, both experimental and preclinical studies addressing the safety and efficacy of adjuvanted vaccines in children and older population at different geographic locations are needed before the application of such strategies to PEM settings can be considered. Some studies have also investigated the relationship between influenza vaccine responses and individuals’ nutritional status [31–34].
However, vaccination studies involving PEM, yet another major risk factor for increased susceptibility to influenza infection and mortality [35, 36], are necessary to elucidate PEM-induced and vaccine-specific immune deficits. Such studies will help in devising novel vaccine strategies to achieve optimal responses against seasonal and potentially pandemic A(H5N1) infections. Given the high prevalence of PEM in underdeveloped countries, and different forms of PEM such as kwashiorkar and marasmus [37], identification of populations manifesting different forms and degree of PEM (eg, mild vs moderate or moderate vs severe) is critical for initiation of such studies and work toward developing safe and efficacious vaccination strategies. In the present study, we used custom diets representing either “adequate” or “severe” deficiency in protein-derived energy to address the effects of PEM in a mouse model of influenza vaccination. Because PEM in humans can also involve mild-to-moderate protein deficiencies, studies using additional protein diets as well as immunization strategies that elicit both antibody and cell-mediated immune responses in mice and other relevant animal models are interesting areas for further investigation.
Supplementary Material
Acknowledgments
We thank members of the Immunology and Pathogenesis Branch in the CDC’s Influenza Division for providing constructive comments on this research.
Financial support. E. N. J. was supported by an Association of Public Health Laboratories/CDC Emerging and Infectious Diseases fellowship.
Footnotes
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplement sponsorship. This work is part of a supplement sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Schaible UE, Kaufmann SH. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med. 2007;4:e115. doi: 10.1371/journal.pmed.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck MA, Handy J, Levander OA. Host nutritional status: the neglected virulence factor. Trends Microbiol. 2004;12:417–23. doi: 10.1016/j.tim.2004.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scrimshaw NS, SanGiovanni JP. Synergism of nutrition, infection, and immunity: an overview. Am J Clin Nutr. 1997;66:464S–477S. doi: 10.1093/ajcn/66.2.464S. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. [Accessed 25 November 2016];The world health report 2002—reducing risks, promoting healthy life. Chapter 4 http://www.who.int/whr/2002/en/ [Google Scholar]
- 5.Katona P, Katona-Apte J. The interaction between nutrition and infection. Clin Infect Dis. 2008;46:1582–88. doi: 10.1086/587658. [DOI] [PubMed] [Google Scholar]
- 6.Guyonnet S, Rolland Y. Screening for malnutrition in older people. Clin Geriatr Med. 2015;31:429–37. doi: 10.1016/j.cger.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Boyd KP, Andea A, Hughey LC. Acute inpatient presentation of kwashiorkor: not just a diagnosis of the developing world. Pediatr Dermatol. 2013;30:e240–1. doi: 10.1111/j.1525-1470.2012.01747.x. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. [Accessed 25 November 2016];Cumulative number of confirmed human cases of avian influenza A(H5N1) reported to WHO. http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en.
- 9.Taylor AK, Cao W, Vora KP, et al. Protein energy malnutrition decreases immunity and increases susceptibility to influenza infection in mice. J Infect Dis. 2013;207:501–10. doi: 10.1093/infdis/jis527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luke CJ, Subbarao K. Improving pandemic H5N1 influenza vaccines by combining different vaccine platforms. Expert Rev Vaccines. 2014;13:873–83. doi: 10.1586/14760584.2014.922416. [DOI] [PubMed] [Google Scholar]
- 11.Nolan TM, Richmond PC, Skeljo MV, et al. Phase I and II randomised trials of the safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in healthy adults. Vaccine. 2008;26:4160–67. doi: 10.1016/j.vaccine.2008.05.077. [DOI] [PubMed] [Google Scholar]
- 12.Belshe RB, Frey SE, Graham IL, et al. Immunogenicity of avian influenza A/Anhui/01/2005(H5N1) vaccine with MF59 adjuvant: a randomized clinical trial. J Am Med Assoc. 2014;312:1420–8. doi: 10.1001/jama.2014.12609. [DOI] [PubMed] [Google Scholar]
- 13.Peña-Cruz V, Reiss C, McIntosh K. Effect of respiratory syncytial virus infection on mice with protein malnutrition. J Med Virol. 1991;33:219–23. doi: 10.1002/jmv.1890330402. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Davis WG, Sambhara S, Jacob J. Strategies to alleviate original antigenic sin responses to influenza viruses. Proc Natl Acad Sci U S A. 2012;109:13751–6. doi: 10.1073/pnas.0912458109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephenson I, Wood JM, Nicholson KG, Zambon MC. Sialic acid receptor specificity on erythrocytes affects detection of antibody to avian influenza haemagglutinin. J Med Virol. 2003;70:391–8. doi: 10.1002/jmv.10408. [DOI] [PubMed] [Google Scholar]
- 16.Hoelscher MA, Singh N, Garg S, et al. A broadly protective vaccine against globally dispersed clade 1 and clade 2 H5N1 influenza viruses. J Infect Dis. 2008;197:1185–8. doi: 10.1086/529522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–7. [Google Scholar]
- 18.Chatraw JH, Wherry EJ, Ahmed R, Kapasi ZF. Diminished primary CD8 T cell response to viral infection during protein energy malnutrition in mice is due to changes in microenvironment and low numbers of viral-specific CD8 T cell precursors. J Nutr. 2008;138:806–12. doi: 10.1093/jn/138.4.806. [DOI] [PubMed] [Google Scholar]
- 19.Kilic M, Taskin E, Ustundag B, Aygun AD. The evaluation of serum leptin level and other hormonal parameters in children with severe malnutrition. Clin Biochem. 2004;37:382–7. doi: 10.1016/j.clinbiochem.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Prendergast AJ. Malnutrition and vaccination in developing countries. Philos Trans R Soc Lond B Biol Sci. 2015:370. doi: 10.1098/rstb.2014.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savy M, Edmond K, Fine PE, et al. Landscape analysis of interactions between nutrition and vaccine responses in children. J Nutr. 2009;139:2154S–2218S. doi: 10.3945/jn.109.105312. [DOI] [PubMed] [Google Scholar]
- 22.Hoelscher M, Gangappa S, Zhong W, Jayashankar L, Sambhara S. Vaccines against epidemic and pandemic influenza. Expert Opin Drug Deliv. 2008;5:1139–57. doi: 10.1517/17425247.5.10.1139. [DOI] [PubMed] [Google Scholar]
- 23.Winokur PL, Patel SM, Brady R, et al. Safety and immunogenicity of a single low dose or high dose of clade 2 influenza A(H5N1) inactivated vaccine in adults previously primed with clade 1 influenza A(H5N1) vaccine. J Infect Dis. 2015;212:525–30. doi: 10.1093/infdis/jiv087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter NJ, Plosker GL. Prepandemic influenza vaccine H5N1 (split virion, inactivated, adjuvanted) [Prepandrix]: a review of its use as an active immunization against influenza A subtype H5N1 virus. BioDrugs. 2008;22:279–92. doi: 10.2165/00063030-200822050-00001. [DOI] [PubMed] [Google Scholar]
- 25.Ledgerwood JE, Hu Z, Gordon IJ, et al. Influenza virus H5 DNA vaccination is immunogenic by intramuscular and intradermal routes in humans. Clin Vaccine Immunol. 2012;19:1792–7. doi: 10.1128/CVI.05663-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price GE, Soboleski MR, Lo CY, et al. Single-dose mucosal immunization with a candidate universal influenza vaccine provides rapid protection from virulent H5N1, H3N2 and H1N1 viruses. PLoS One. 2010;5:e13162. doi: 10.1371/journal.pone.0013162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. [Accessed 25 November 2016];Serological diagnosis of influenza by microneutralization assay. 2010 http://www.who.int/influenza/gisrs_laboratory/2010_12_06.
- 28.National Institutes of Health. [Accessed 25 November 2016];Clinical research studies. http://clinicalstudies.info.nih.gov/cgi/cs/processqry2.pl?search1=influenza&searchtype=e&SearchButton99a=Submit+Query.
- 29.Centers for Disease Control and Prevention. [Accessed 25 November 2016];FLUAD—flu vaccine with adjuvant. 2016 https://www.cdc.gov/flu/protect/vaccine/adjuvant.htm.
- 30.Stassijns J, Bollaerts K, Baay M, Verstraeten T. A systematic review and meta-analysis on the safety of newly adjuvanted vaccines among children. Vaccine. 2016;34:714–22. doi: 10.1016/j.vaccine.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 31.Sundaram ME, Meydani SN, Vandermause M, Shay DK, Coleman LA. Vitamin E, vitamin A, and zinc status are not related to serologic response to influenza vaccine in older adults: an observational prospective cohort study. Nutr Res. 2014;34:149–54. doi: 10.1016/j.nutres.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Crogan NL, Velasquez D, Gagan MJ. Testing the feasibility and initial effects of iron and vitamin C to enhance nursing home residents’ immune status following an influenza vaccine. Geriatr Nurs. 2005;26:188–94. doi: 10.1016/j.gerinurse.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Sagawa M, Kojimahara N, Otsuka N, Kimura M, Yamaguchi N. Immune response to influenza vaccine in the elderly: association with nutritional and physical status. Geriatr Gerontol Int. 2011;11:63–8. doi: 10.1111/j.1447-0594.2010.00641.x. [DOI] [PubMed] [Google Scholar]
- 34.Langkamp-Henken B, Wood SM, Herlinger-Garcia KA, et al. Nutritional formula improved immune profiles of seniors living in nursing homes. J Am Geriatr Soc. 2006;54:1861–70. doi: 10.1111/j.1532-5415.2006.00982.x. [DOI] [PubMed] [Google Scholar]
- 35.Kikafunda JK, Walker AF, Collett D, Tumwine JK. Risk factors for early childhood malnutrition in Uganda. Pediatrics. 1998;102:E45. doi: 10.1542/peds.102.4.e45. [DOI] [PubMed] [Google Scholar]
- 36.Garenne M, Kahn K, Tollman S, Gear J. Causes of death in a rural area of South Africa: an international perspective. J Trop Pediatr. 2000;46:183–90. doi: 10.1093/tropej/46.3.183. [DOI] [PubMed] [Google Scholar]
- 37.Castiglia PT. Protein-energy malnutrition (kwashiorkor and marasmus) J Pediatr Health Care. 1996;10:28–30. doi: 10.1016/S0891-5245(96)90071-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.