Abstract
Partial seasonal migration is ubiquitous in many species. We documented this phenomenon in plains zebra (Equus burchelli) in Etosha National Park, Namibia (ENP), and provided a cost-benefit analysis as it relates to the spatial distribution of water, vegetation and endemic anthrax. This analysis draws upon two years of ENP zebra movement data that reveal two sub-populations: migrators and non-migrators. Migrators are shown to be behaviorally dominant in the way they utilize space and use water holes. We raise the possibility that the co-existence of these two groups reflects an evolutionary process, and the size of each group maintains evolutionary equilibrium.
Keywords: partial migration, GPS telemetry, movement ecology, Bacillus anthracis, tick load, water holes
Introduction
Partial seasonal migration of plains zebra (Equus burchelli) in Etosha National Park (ENP), Namibia, whereby some individuals migrate and some do no,t remains a conundrum. Better than a strict migrant-resident dichotomy is a continuum point of view in which extreme migrating and total non-migratory individuals are the end-points (LeResche 1974, Ball et al. 2011). In Etosha, however, distinct migrant and resident groups exist. In addition, distinct early and late migrant groups exist. The migrating distance is large and the areas of the wet season and the dry or semi-dry seasons do not overlap.
The phenomenon of partial migration, widely studied in birds and somewhat less in mammals (Dingle 1996), is taxonomically widespread (Chapman et al. 2011). It is found in ungulates (Ball et al. 2001), including 15,000 zebra living in the central sector of ENP. These zebra inhabit the western side of the ENP central sector during the wet season (Fig. 1) and during the dry and semi-dry seasons, the majority (around 70% with some annual variation) migrates to the eastern part of this sector, which contains many water holes (Fig. 1), while the rest stay in the western part.
Figure 1.
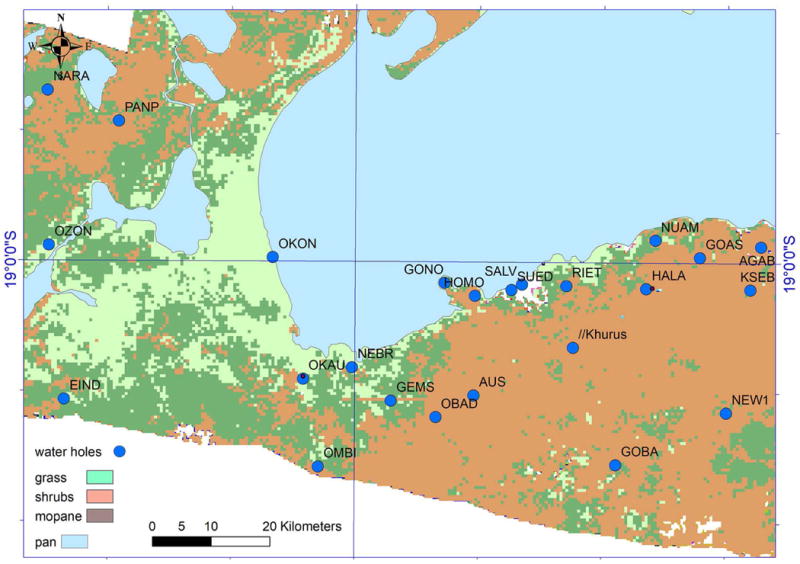
Study area and water sources within the central region of Etosha national park, Namibia. The zebra live in the west of this region, where water sources are scarce throughout the wet season (left polygon). Okon in the north-east part of the polygon is salty and with an abundance of predators. The majority of the zebra migrate in the dry season to the east, and all of them return to the west at the beginning of the wet season.
Water availability is likely a key driver for eastward dry and semi-dry season migrations. In the wet season many ephemeral sources of water occur on the western plains of the central sector. All these water sources typically dry up by the beginning of August (W. Kilian, personal communication), so in the dry and semi-dry seasons proximity to water becomes an issue. Based on a tradeoff concept driving the evolution of partial migration processes (Berthold 1996), we use our data to explore an anthrax/water-availability tradeoff explanation for partial migration of zebra in ENP. Annually, anthrax, reaches epidemic proportions in the western side of the central sector during the wet season. Anthrax, an acute and often fatal disease caused by Bacillus anthracis, is distributed globally and occurs in wild and domestic vertebrates (Turnbull et al. 1998, Turnbull 2002). In wildlife, anthrax is typified by seasonal outbreaks, mostly affecting ungulates and often has considerable impact on their dynamics (de Voss 1990, Lindeque and Turnbull 1994, Dragon and Rennie 1995, Dixon et al. 1999).
The seasonal patterns of the disease differ among regions. For example, in the central part of ENP, Namibia, outbreaks in zebra and other plain ungulates occur annually, mainly in the wet season (Lindeque and Turnbull 1994, Hampson et al. 2011), while in northern Canada, anthrax epidemics in bison are episodic, sometimes decades apart, and occur during the hot, dry season between late June and early September (Broughton 1987). In Kruger National Park, South Africa, outbreaks always occur at the end of the dry season (Braack and de Vos 1990). This diversity in the temporal dynamics of anthrax suggests that local factors, such as the ecology of the host, influence the disease dynamics. In ENP, the notion that animals are infected by inhalation of airborne spores was rejected (Turnbull et al. 1998). Rather, anthrax in herbivores in ENP is contracted by ingesting soil laden with infectious B. anthracis spores while grazing (Turner et al. 2016).
Our data pertain to the movement of radio-tagged zebra in ENP. These data, along with information on herd size, habitat structure, anthrax prevalence, and tick load, allow us to identify migrating and non-migrating individuals and associated movement statistics, habitat preference, visits to water, and location relative to other individuals and groups. We use our data to test the hypothesis that migrating zebra dominate non-migrating zebra with regard habitat availability and use. The support we find for this hypothesis, together with other data, suggests that the migration represents, in part, an evolutionary response to a risk of anthrax, mediated by interspecific competition that differentially impacts the tradeoff for dominant and less dominant individuals. In particular, our analysis suggests that migration is an appropriate anthrax mitigation strategy for dominant but not subordinate individuals.
Methods
Study Area
Etosha National Park is an approximately 23,000 km2 wildlife reserve located in northern Namibia between 18°30′ S–19°30′ S and 14°15′ E–17°10′ E. Our study focused on the zebra population occupying the central part of ENP between 18°80′ S–19°23′ S and 15°70′ E–16°5′ E (approximately 5500 km2). The average elevation is 1,050 m, and annual precipitation is about 430 mm, with large annual fluctuations.
ENP has three characteristic seasons (Berry 1980): the wet (rainy) season (January–April), cool-dry season (May–August) and hot-semidry season (September–December). The vegetation is classified as arid savanna including open grasslands and groves. The common grass species are Cynodondactylon, Eragrostis micrantha, E. rotifer, Diplachnefusca, and Chlorisvirgata. Colophospermum mopane is the dominant tree species (Le Roux et al. 1987). ENP supports a high density of mammal populations with many herbivores that are anthrax susceptible (Turnbull et al. 1998). Zebra and springboks are the two most abundant plains ungulate species, with estimated populations of 13,000 zebra and 15,600 springboks. Elephants have an estimated population of ∼2000 (de Beer and van Aarde 2008).
During the wet season, all 13,000 zebra reside in the west area of the central region. During the dry season most zebra (around 70%) migrate to the east. The west and east are similar in terms of vegetation structure, but the total grassland area in the east is approximately 20% of that found in the west. However, there are far fewer permanent water sources in the west (Fig. 1) some of which are salty. The reproductive pattern is not seasonal, and both conception and parturition are year-round (Turner and Getz 2010).
Anthrax
By way of a very brief summary, Bacillus anthracis is a spore-forming bacterium, thought to be an obligate pathogen with limited ability to propagate in soil. Anthrax occurs after spores enter the host following inhalation or ingestion or through abrasions in the skin (Dixon et al. 1999). The incubation period of Anthrax is relatively short, and in one to seven days the animal dies (Turnbull et al. 2008). Anthrax cannot spread directly from one animal to another because infected animals have only the actively growing bacteria (vegetative cells) and do not have the infectious spores (Dragon and Rennie 1995), which are found primarily in the soil (Turner et al. 2016). After an infected animal dies of anthrax, the vegetative cells sporulate on being exposed to oxygen as the animal bleeds out and is ripped open by scavengers, or consumed by necrophagous insects (Bellan et al. 2013). The spores are shed to the ground where they attach strongly to soil particles. This strong attachment of the spores to the soil causes the soil in carcass sites to stay contaminated with many spores for an unknown period of time, and creates local infectious zones (LIZs). When released into the environment, Bacillus anthracis spores can survive for decades (Dragon and Rennie 1995). However, the spore concentration within the LIZ is diluted by wind, rain and germination of some of the spores (Turnbull et al. 1998).
Anthrax outbreaks in ENP occur in plain zebra (Equus burchelli), African elephants (Loxodonta africana), wildebeest (Connochaetes taurinus), springboks (Antidorcas marsupialis), and to a lesser extent in several other species of antelope. The peak outbreak among zebra and other plain ungulates (not in all of them) occurs in March/April towards the end of the wet season. Specifically in zebra, ∼50% of the annual mortality from anthrax occurs in these two months (Cizauskas et al. 2014; Turner et al. 2013). Detailed numbers are described in the results below. The increment is gradual, with peaks around February and May (Turner et al. 2013).
Most anthrax incidence in ENP appears to be concentrated around the Okaukuejo area (Fig. 1, labeled as Okau in the west polygon) (Lindeque and Turnbull 1994, Kilian, personal communication; Berkeley Anthrax Team carcass surveys). By contrast, in the eastern part of the central section, to which part of the zebra migrate, there are much fewer recorded cases of anthrax.
Monitoring
Between March 2008 and August 2010, 62 zebra (55 females and 7 males) were radio-collared with either GPS platforms (manufactured by LoxoTrack, Aeroeskoebing, Denmark and Africa Wildlife Tracking, Pretoria, South Africa) or VHF devices.
Forty-five zebra were collared in March 2008 (30 females with VHF collars, 8 females and 7 males with GPS collars). The collared individuals were randomly chosen within the estimated wet-season home range of the zebra population. During October 2008 7 more females were GPS collared and the collars of 8 females that were previously collared were replaced with new GPS collars. An additional 15 collars were replaced during March 2009 (10 females with GPS-GSM collars) and August 2009 (5 females with GPS collars). All animals were handled as described in animal use protocol R217-0509B (University of California, Berkeley, California, USA). In summary, the total number of collared zebra is 52: 30 individuals collared with VHF and 22 with GPS devices.
The GPS collars were programmed to obtain a location every 20 minutes. In addition to remote relocations, the collared individuals (GPS-UHF, GPS-GSM and VHF) were also located visually for direct observations twice a week nearly every week from October 2008 to August 2010 (see below).
Among the collared zebra, several patterns of migration were observed. In addition to the large-scale migration patterns, we also focused on seven key factors we considered potentially relevant:
1. Herd size
Herd size may be an important correlate of migrating in zebra (Hack and Rubenstein 2004) because it is linked to levels of intra-specific competition. We distinguish the wet season from the two dry seasons, because in the wet season all the zebra live in the same place (i.e., no zebra remain in the east) and the partial migration occurs during the dry and semi-dry seasons. We recorded herd size whenever a collared individual (GPS or VHF) was observed. For each observation we estimated the number of zebra within a 100 m radius of the collared individual. Only individuals with at least six separate recordings of herd size were included in this analysis. All sightings were done by a single observer. The position of the observer was usually less than 30 m from the group, and always less than 100 m. Small groups were counted accurately, and large groups were divided into categories of 50, 100, 150 etc.
2. Movement
In herbivores, movement is a major component of the cost of foraging. Using GPS devices, we focused on the 12 individuals from 12 different herds—7 migratory and 5 non-migratory—for which we had a nearly full year's (2009) data set. Locations were taken every 20 minutes, for a maximum (taking failed readings into account) of 72 locations each day. We omitted days for which fewer than 65 locations were recorded. We then calculated the daily distance traveled as the sum of the Pythagorean distances between consecutive GPS points, and calculated the mean daily distance for each individual for each of the two seasons (daily movement during migration included in the dry seasons).
3. Vegetation preference
We classified the vegetation following Le Roux et al. (1987). The flora was divided into three categories (Fig. 1): grasslands, shrub lands and mopane woodlands. Grasslands include grass savanna and steppe in the phraseology of Le Roux et al. (1987). We merged the GPS data of migrating and sedentary zebra with the vegetation map. For each individual we recorded the vegetation associated with each location, and then calculated the proportion of use for each day. Finally, we calculated the average of all days for which we had data.
4. Use of water holes
We compared proximity of migrating and non-migrating zebra to water holes. We defined a visit to a water hole when one or more consecutive GPS locations fell within a 500 m radius around the center of the water hole. We used a relatively large radius to make sure that visits to water holes are not missed due to the one location per 20 minutes recording interval (i.e., if animals spent less than 20 minutes at the water hole a reading may not necessarily be obtained when the animal is at the water hole). We assumed that an animal within a range of 500 m of a water hole is in the vicinity for the purpose of drinking. The duration of time spent at the water hole was calculated as 20 minutes for each location of a given sequence of locations within the 500 m radius.
We calculated the distance from nearest water hole as follows. For every GPS position obtained for each zebra we found the nearest water hole and recorded the distance. We then calculated the average of all distances for each given day. Finally, we produced the average value of all the days together for each of the zebras. We then used this measure to compare between the migrating and non-migrating zebra. We did it only for positions in the dry and semi-dry seasons (when drinking is a key component of the time budget). In the wet season the zebra drink from small puddles, hence the distance from water holes becomes insignificant.
5. Distance from population center
Assuming that the dominant groups graze on the best grounds and these are expected to be towards the center of the patch, the distance from population center reflects dominance. Thus, we used this parameter as a proxy for dominance. The organization of harems and herds in zebra is very dynamic (Hack and Rubenstein 2004) and the center of the population changes all the time (Bar-David et al. 2009). The average distance from the center, however, may help us to distinguish between dominant and subordinate individuals, especially if those on the fringes appear to occupy less favorable habitat.
Twice a week, always at the same hour, the positions of all tagged zebra were sampled and a virtual center point was taken to be the average of these positions. This provides the best estimate we have of the center of the population as a whole. We measured the distance of the zebra from this virtual population center during the wet season when all the zebra are in the west. The distance of each individual was then calculated, from its GPS signal to the virtual center. We focused on the 12 zebra that had a continuous and stable GPS data.
6. Prevalence of anthrax
We relied on an existing anthrax mortality data in zebra in our study area for the years 2005–2011 to determine the spatial and temporal patterns of the disease (Bellan et al. 2013).
7. Tick load
Ticks are a general indicator of condition, nutrition and stress (Gladney et al. 1973, Gallivan et al. 1995). The number of ticks on the body of an individual may serve as evidence of the physical disposition of the animal (Taddese and Mustefa 2013), and may also reflect the density of individuals in its nearby surrounding.
We estimated the number of ticks on the body of zebra immobilized for tagging between March 2008 and August 2010. We externally examined the entire body of immobilized individuals. For individuals immobilized twice (or thrice), the number of ticks was averaged over all the measurements.
Results
1. Herd size
The average herd size of 6 radio-tagged migrating zebra in the wet season was 84.3(SE ± 13.8), and was larger than the herd size of the 2 radio-tagged non-migrating zebra in the same season: 29.3 (SE ± 19.0), p=0.0024 and t6=5.02 (two-tailed t test). The average herd size for 11 migrating zebra in the dry and semi-dry seasons was 77.0 (SE ± 11.0), while for 2 non-migrating zebra it was 29.9 (SE ± 6.7), p=0.0048 and t11=3.52.
2. Movement
During our study no zebra were observed in the eastern part throughout the wet season. At the end of the wet season (i.e., the end of April) zebra began to migrate eastward. The migration process was both partial and gradual (Fig. 2). The first and main wave of zebra that migrated to the east did so at the beginning of May. Many zebra, however, were still present in the west throughout the dry season. In the semi-dry season, as water resources continued to decline, three processes occurred: a second migration wave occurred in July; the range of the migration (towards the east) increased; and the non-migrating zebra remaining in the west began seeking water sources further afield (Fig. 2). In our data, all zebra moved back to the west at the end of December, including those individuals collared in the east.
Figure 2.
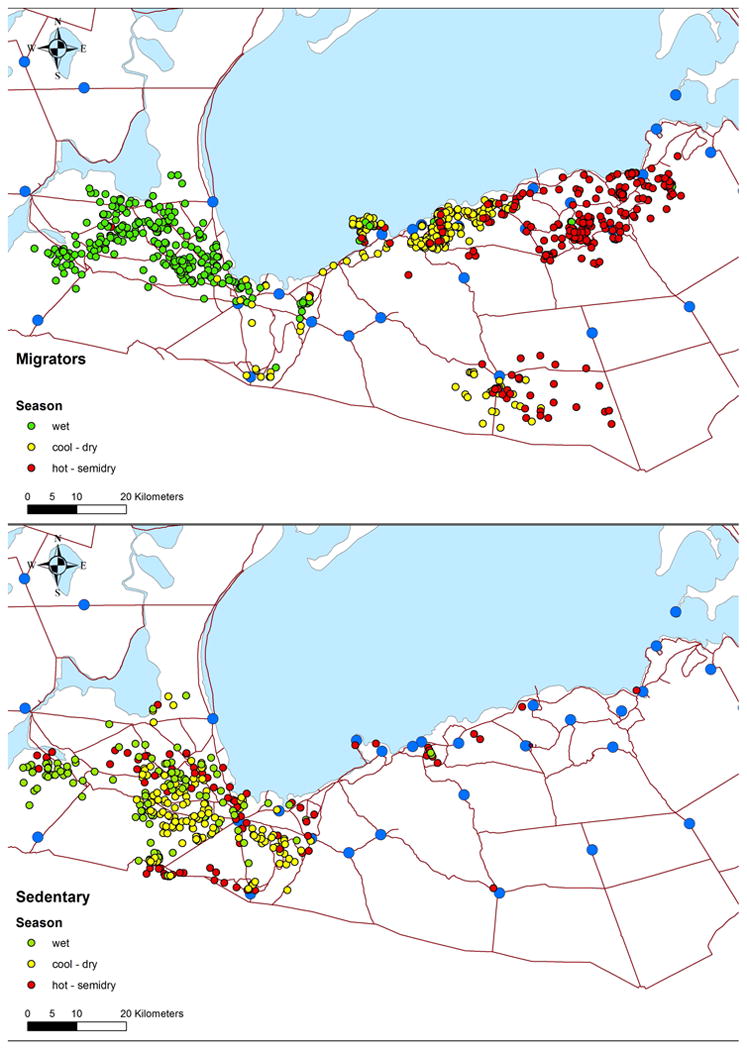
Migrating group (upper part) and non-migrating group (lower part). Blue circles are water holes. The zebra position is described in three colors: green for the wet season, yellow for the dry season and red for the semi-dry season. In the upper map, one can see the individual that moves to the south.
Three individuals (out of 45 collared zebra) were anomalous and moved with a very small group to a water hole in the south (Gobaub; marked as Goba in Fig. 1, see also the locations in the upper panel of Fig. 2). These zebra seem to be quite different from other individuals. In particular, the water hole in the south is located in the middle of a mopane forest, which is not the typical zebra habitat in ENP. It is hard to classify these individuals as migrators—they do not migrate like all the others, nor can they be classified as non-migratory—they do not stay in the west. It seems that they form a unique group that relies on s single water hole in lesser quality habitat. Hence we did not take these individuals into account in our analyses.
Due to a shortage of water sources in the west during the dry and semi-dry seasons, non-migratory zebra must walk further to water holes. Daily movement was obtained from the 12 GPS collared zebra belonging to different herds—7 from migratory herds and 5 from non-migratory herds. The salient finding is that non-migratory zebra walked throughout the dry and semi-dry season significantly more than migrating zebra (Fig. 3), 16. 5 km/day (non-migratory), SE ± 0.6 vs. 13.2 km/day (migrating) with SE ± 0.13 (p=0.0035 and t10=3.7994). In the wet season there is no apparent difference between the two groups with respect to distance traveled, 15.1 km/day(SE ± 0.90) vs.16.1 km/day (SE ± 0.14), respectively (p=0.404, t10=−0.87). Thus, in terms of movement costs it is advantageous to be migratory.
Figure 3.
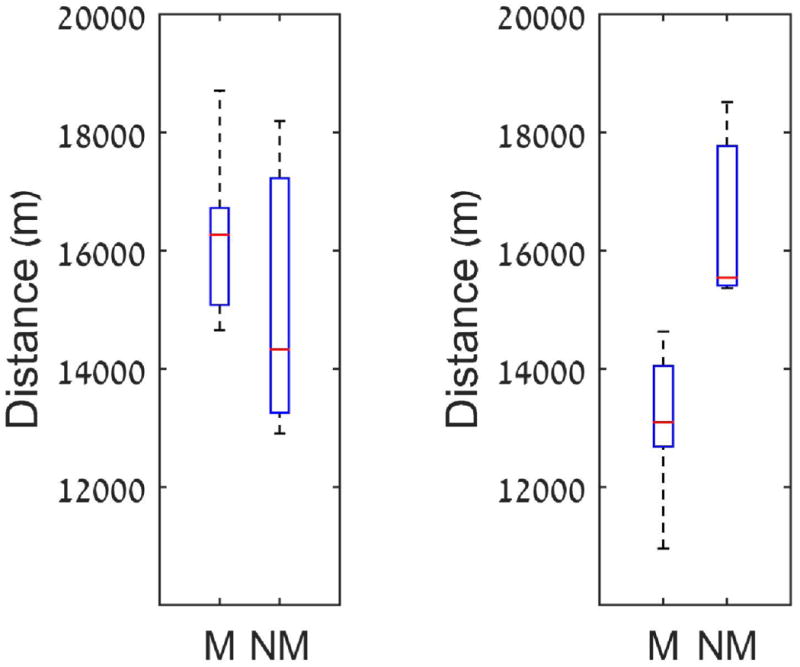
Daily movement along the year is presented. The left panel exhibits the daily movement in the wet season; the right panel in the dry and semi-dry seasons (together). The distance is calculated as an average over the distinct individuals of each group. The red line is the sample median. The top and bottom of each box are 25th and 75th percentiles of the samples. The whiskers are the minimal and maximal values.
3. Vegetation preference
We compared the migrating zebra with non-migratory zebra with respect to their vegetation/habitat preference (Fig. 4). In the wet season, migrating zebra spent 65.2% of the time in the grass habitat (SE ± 2.1), 31.8% in shrubs (SE ± 1.7), and 2.7% in the mopane (SE ± 0.6 habitat). The rest are locations with no vegetation classification (e.g., water hole). By contrast, non-migratory zebra spent 54.5% in the grass habitat (SE ± 10.3), 33.8% in shrubs (SE ± 5.1) and 10.2% in mopane trees (SE ± 5.5).
Figure 4.
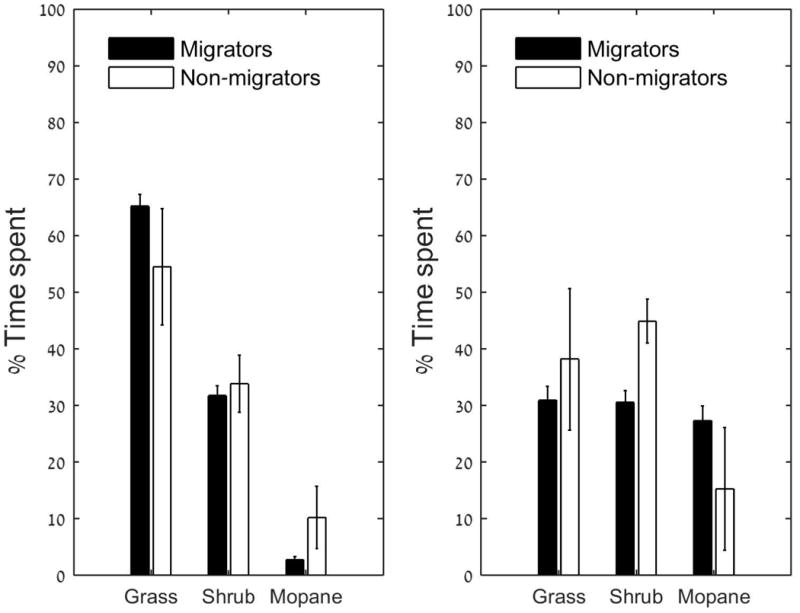
Percent time spent at each habitat according to season. The left panel describes the wet season. The right panel describes the dry seasons.
In the dry seasons, migrating zebra spent 30.9% in grass habitat (SE ± 2.5), 30.5% in shrubs (SE ± 2.1) and 27.3% in mopane trees (SE ± 2.6).Non-migratory zebra spent 38.2% in grass (SE ± 12.5), 44.9% in shrubs (SE ± 3.9) and 15.3% in mopane (SE ± 10.9).
4. Use of water holes
During the combined dry and semi-dry seasons migratory zebra spent significantly more time near water holes than non-migratory zebra. The average duration of a water hole visit for the migratory group was 73.8 minutes (SE ± 2.1). The average of duration for the non-migratory group was 28.5(SE ± 2.83),p=0.0023, t10=-3.9493.
Another difference in the behavior of the two groups of zebra at water holes is mirrored in their daily arrival time. Migratory groups arrived earlier in the morning (on average) to water sources, at 8:38 (UTC; SE ± 3 minutes). Non-migratory zebra arrived later, at 9:29 (UTC, on average; SE ± 6 minutes) p=0.018, t10=2.82. In addition, while migratory zebra arrived at the water holes during the night (albeit at lower frequencies), non-migratory zebra avoided water holes during the night (Fig. 5).
Figure 5.
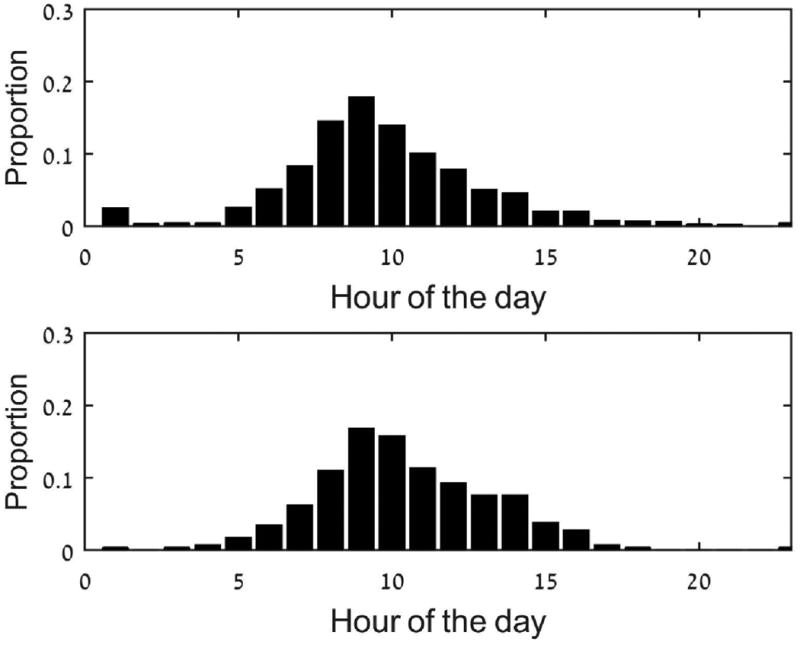
Arrival times into water holes. The data includes the dry and semi-dry seasons. The upper panel is the migrating zebra and the lower one is the non-migratory zebra.
Non-migratory zebra spent most of their time further from water sources than migratory zebra. The total distance traveled during the dry and semi-dry seasons (in which the zebra use water holes) was calculated for each tagged individual. On average the migratory zebra travelled a total of 4,803.1 m (SE ± 164.3). By contrast, the overall traveled distance of the non-migratory zebra was 9,422.4 m (SE ± 164.29) p=0.00042 and t10=5.1663. Finally, the percentage of days in which a visit to a water hole was recorded for migratory zebra was 56.463% (SE ± 0.0078) vs. 42.34% (SE ± 0.0243) for non-migratory zebra (p=0.0003 and t10=5.2991).
The general picture of behavior around water holes is coherent: non-migratory zebra arrive later, and stay shorter amount of times at water holes. They visit water holes fewer times (per month), avoid night visits and the average distance from water holes is larger.
5. Distance from population center
The distance between the collared zebra and the center of population during the wet season differed between the two groups. The average distance of the migratory group was 5,865 (SE ± 122). The average distance of the non-migratory group was 12,428 (SE ± 1,219), p=0.0004 and t10=5.15. This reflects the habitat usage by the two groups (see item 3, Vegetation preference above) with the migrators occupying the superior grassland habitat (Figs. 1 and 2). During the dry season after the migrants leave, non-migratory individuals move to the center into the grassland and close to water, and remain there during the semi-dry season, albeit more dispersed (Fig. 2). Upon the return of the migratory herds in the following wet season, non-migratory zebra once again move into the periphery of the habitats and exploit lower quality ranges than those at the population center.
6. Anthrax mortality
Most anthrax mortality occurred in the wet season. Zebra in the west die from anthrax during the dry season as well, but in lower numbers (Turner et al. 2013). Referring to a data set along the years 2005–2011, the average number of cases that were considered anthrax-related mortality between July and January is 5–10 per month. In February and May the number is around 30 cases, while in March and April it steps up to 60–80 cases. All the instances of anthrax in this period (i.e., the wet period) are in the west, i.e. within the group of non-migratory zebra. There were no documented cases of anthrax in the eastern part, during the research period.
7. Tick load
We examined 33 zebra, 26 belonged to the migratory group and 7 to the non-migratory group. We found no difference in tick load between the migratory zebra (the average is 5.02, SE ± 0.65) and non-migratory ones (the average is 3.29, SE ± 0.89), p=0.21, t31=−1.28.
Discussion
Cost-benefit dynamics mold the behavior of animals through natural selection and are considered the driving force of migration. Dominance relationships play a key role in such systems as migrating individuals scramble to settle into better seasonal habitat at the end of migration. Thus natural selection is involved in producing migratory patterns, including behavioral polymorphisms, which are evolutionarily advantageous. This line of thinking is central with respect to partial migration (Dingle 1996). Trying to evaluate the costs and benefits to zebra in the ecological system of ENP, we should consider the key limiting factors, especially with regard to the dry seasons in which the migration occurs and the two distinct life-history strategies of the zebra - migrators and non-migrators. These include:
1. Drinking
During the wet season drinking has little cost for zebra as rain-puddles are ubiquitous. During the dry and semi-dry seasons, however, the fewer holes in the west dictate more movement as resources closer to the holes are rapidly depleted (Redfern et al. 2003). This results in the cost of movement (as determined by total seasonal distance traveled) during the dry and semi-dry seasons being two-fold higher for the non-migratory zebra, even with the migration distance factored in. Thus, while migrating zebra must travel long distances during migration, after the migration has been completed (it takes only a few weeks) the migrating zebra can walk much less on a daily basis so their overall mean daily movement for the entire season (including the migrations) is half as much as for the non-migrators. Increased movement not only carries an energetic cost, it also increases the risk of predation: the western side of ENP has fewer water holes so the arrival of prey at these holes during the dry season is more predictable for predators. This, in turn, implies that waterhole visits should preferably be done during the day, resulting in the non-migratory animals arriving later in the day at the water holes. Migrating animals are also subject to this risk, but since these are not regularly used paths, predators may have limited ability to take advantage of these movements. The fact that none of the tagged migrating individuals died during migration suggests this risk is, in fact, relatively minor.
2. Foraging and habitat
Zebra typically prefer open grasslands (Fischhoff et al. 2007, Turner and Getz 2010), and this preference was evident in our study during the wet season. During the dry season the value of grasslands declines as they wilt and there is no re-growth and the zebra must rely on other sources (e.g., the mopane). Thus, although grasslands in the east are scarcer (20% of their total in the west), the migration eastward during the dry and semi-dry season bares lower costs because of the lowered value of the grasslands.
3. Anthrax
Anthrax in ENP accounts for a significant part of zebra mortality (Getz 2011, Appendix D). However, the disease is unique in its dynamics, typified by a short incubation period (one week) which is lethal, and the formation of LIZs which reportedly deteriorate over 20–40 weeks (Lindeque and Turnbull 1994, Bellan et al. 2013). A directional migration over several weeks would, consequently, weed out infected individuals, and the LIZs formed by their death in route as well as LIZs formed in the pre-migration habitat would be far less effective upon the return of the animals. Thus, in the absence of a non-migrating population to sustain the disease, anthrax would die-out. This explains why anthrax has not been recorded in the east section, as no zebra remain there to sustain the disease during the wet season.
4. Dominance
Partial migration is often typified by non-migratory dominant individuals while the weaker individuals are forced to migrate due to seasonally intensified intra-specific competition (e.g., Ketterson and Nolan 1976). The reasoning is two-fold: Stronger individuals can survive the poorer conditions without migrating (Gauthreaux 1982), and they can win the struggle for the fewer resources (Kalela 1954). However, in some instances the migrating individuals have better survival abilities (Boyle 2008, Chapman et al. 2011), as in salmonids (Hendry et al. 2004) and kingbirds (Jahn et al. 2010). Our data suggests that a dominance hierarchy of some form exists among the zebra, and that it is the dominant groups that migrate. We have no direct evidence as to how this dominance is manifested, but some form of dominance hierarchy does exist. Whether this constitutes active intraspecific interactions or is a manifest of past generations interactions followed by maternal effects is difficult to tell. Indeed, during the wet season non-migrating groups occupy the poorer habitat along the boundaries of the western area. The non-migrating groups are also smaller reflecting the lower quality habitat (but see Dennehy 2001).
The benefit during the dry season to the non-migrators is, therefore, reduced competition after most of the population leaves. But this comes at a cost including continued exposure to anthrax at nearly similar rates as the wet season, and increased energy expenditure on movement and elevated predation risk resulting from this movement. It appears that these costs would greatly outweigh the benefits of not migrating (especially the risk of anthrax), although we do not have the necessary data to conclusively show this.
Unlike many instances of migration, it seems that differences in weather have only a proximate role in the partial migration of zebra in ENP, as there is no noteworthy difference between the eastern and the western parts in this respect (Berry 1980). Specifically, temporal changes in the weather influences the timing of migration, because rainfall determines the availability of water puddles. Disease, however, is considered a key driver of migration (Altizer et al. 2011) and we argue that in ENP, anthrax is a fundamental cause of migration. Below we provide a scenario that could well have led to such different strategies in individuals sharing the same area for at least part of the year.
Possible scenario: The dynamics of anthrax and its prevalence in ENP suggests that migratory behavior would be strongly selected for by the presence of disease. We cannot ascertain whether migration would occur in the absence of anthrax, but since both the east and the west could apparently support some zebra year round (in terms of water and habitat) it is probable that, in the absence of the disease, selection would have favored two resident populations (a large population in the west and a small population in the east) and some lower-ranking migratory individuals moving seasonally as the relative value of the two areas changes with season and occupying peripheral habitat. An invasion of anthrax into this system would then result in very high mortality rates and far smaller resident populations. The invasion of the disease would thus give the migrants a clear selective advantage over all others because en route they would not be subject to the disease and on arrival after migration would occupy areas where intra-specific competition has been reduced by anthrax mortality. Eventually, the migratory behavior would be favored over the non-migratory one.
The remaining question then is: why does a resident population continue to exist in the west? Based on our data, it is evident that migrating zebra consist of dominant groups while sedentary zebra are subordinate. We speculate that the habitat in the east is unable to support the entire population and a dominance hierarchy between the different herds forces submissive groups to become non-migratory in the larger habitat (the west). These subordinate herds continue to be subject to high mortality rates from anthrax in addition to increased cost of movement and risk of predation during the dry season. In fact, given the documented mortality rates from anthrax, the non-migratory population may be a sink population sustained by an overflow from the migrants. This resident population sustains the disease and continues to promote selection for the migratory behavior of the more dominant groups.
We conclude that anthrax is an important component in the ecology of zebra in ENP. The system is maintained at an equilibrium consisting of a large migrating population and a small subordinate non-migrating population that sustains the disease. By and large, natural selection at the individual level has been recognized as meaningful with respect to partial migration (Chapman et al. 2011). It has also been claimed that partial migration is an intermediate stage which leads to full migration (Dingle 1996). In the case of zebra in ENP, the pattern observed reflects an evolutionary steady-state in which the dominant groups migrate and the submissive ones are forced to remain sedentary by intensified intra-specific competition pressure that is enhanced during the dry season. Possibly, the sedentary group experiences a negative growth rate due to the continuous exposure to anthrax and is sustained by an overflow from the migrating population, making the sedentary population a sink. It is conceivable, therefore, that the relative sizes of these two groups (migrating and non-migrating) form the optimal equilibrium in which all the ecological features are taken into account.
Acknowledgments
We thank the Namibian Ministry of Environment and Tourism for permission to do this research, the Directorate of Parks, Wildlife and Management for permission to work in Etosha, and the staff of the Directorate of Scientific Services at the Etosha Ecological Institute for logistical support and assistance. We would like to give special thanks to veterinarians Mark Jago, Conrad Brain, Peter Morkel, and Ortwin Aschenborn for their assistance with animal captures, as well as to S. Bellan, Carrie Cizauskas, Martina Küsters, Shayne Kötting, Gabriel Shatumbu, Wendy Turner, Wilferd Versfeld, Marthin Kasaona and Werner Kilian, among others, for their tremendous help in the field. We acknowledge the helpful comments of two anonymous reviewer. The first two authors were supported by Ronen Kadmon's lab, and they wish to thank him. Royi Zidon carried out the field work and wrote the paper as part of his Ph.D., David Saltz and Wayne, M. Getz were advisors to Royi Zidon and assisted in writing and editing the paper, Shimon Garti raised the idea of integrating the evolution process with the observed migration behavior. This project was approved by the UC Berkeley Animal Care and Use Committee (protocol no. R217-0510B) and supported in part by grant NIH grant GM83863 to W.M.G.This is publication number 940 of the Mitrani Department for Desert Ecology.
Literature Cited
- Altizer S, Bartel R, Han BA. Animal migration and infectious disease risk. Science. 2011;331:296–302. doi: 10.1126/science.1194694. [DOI] [PubMed] [Google Scholar]
- Ball JP, Nordengren C, Wallin K. Partial migration by large ungulates: characteristics of seasonal moose Alces alces ranges in northern Sweden. Wildlife Biology. 2001;7:39–47. [Google Scholar]
- Bar-David S, Bar-David I, Cross PC, Ryan SJ, Knechtel CU, Getz WM. Methods for assessing movement path recursion with application to African buffalo in South Africa. Ecology. 2009;90:2467–2479. doi: 10.1890/08-1532.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beer Y, van Aarde RJ. Do landscape heterogeneity and water distribution explain aspects of elephant home range in southern Africa's arid savannas? Journal of Arid Environments. 2008;72:2017–2025. [Google Scholar]
- Bellan SE, Turnbull PCB, Beyer W, Getz WM. Effects of experimental exclusion of scavengers from carcasses of anthrax-infected herbivores on Bacillus anthracis sporulation, survival, and distribution. Applied and Environmental Microbiology. 2013;79:3756–3761. doi: 10.1128/AEM.00181-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry HH. Dissertation. Department of Biological Sciences, University of Capetown; Capetown, South Africa: 1980. Behavioural and eco-physiological studies on blue wildebeest Connochaetes taurinus at the Etosha National Park. [Google Scholar]
- Berthold P. Control of bird migration. First edition. Chapman & Hall; London, UK: 1996. [Google Scholar]
- Boyle WA. Partial migration in birds: tests of three hypotheses in a tropical lekking frugivore. Journal of Animal Ecology. 2008;77:1122–1128. doi: 10.1111/j.1365-2656.2008.01451.x. [DOI] [PubMed] [Google Scholar]
- Chapman BB, Brönmark C, Nilsson JÅ, Hansson LA. The ecology and evolution of partial migration. Oikos. 2011;120:1764–1775. [Google Scholar]
- Cizauskas CA, Turner WC, Wagner B, Küstersrs M, Vance RE, Getz WM. Gastrointestinal helminths may affect host susceptibility to anthrax through seasonal immune trade-offs. BMC Ecology. 2014;14:1. doi: 10.1186/s12898-014-0027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennehy JJ. Influence of social dominance rank on diet quality of pronghorn females. Behavioral Ecology. 2001;12:177–181. [Google Scholar]
- Dingle H. Migration: the biology of life on the move. First edition. Oxford University Press, New York; New York, USA: 1996. [Google Scholar]
- Dixon TC, Meselson M, Guillemin J, Hanna PC. Medical progress: anthrax. New England Journal of Medicine. 1999;341:815–826. doi: 10.1056/NEJM199909093411107. [DOI] [PubMed] [Google Scholar]
- Dragon DC, Rennie RP. The ecology of anthrax spores: tough but not invincible. Canadian Veterinary Journal. 1995;36:295–301. [PMC free article] [PubMed] [Google Scholar]
- Hack M, Rubenstein DI. Natural and sexual selection and the evolution of multi-level societies: insights from zebras with comparisons to primates. In: Kappeler P, van Schaik CP, editors. Sexual selection in primates: new and comparative perspectives. Cambridge University Press, New York; New York, USA: 2004. pp. 266–279. [Google Scholar]
- Gallivan GJ, Culverwell J, Girdwood R, Surgeoner GA. Ixodid ticks of impala (Aepyceros melampus) in Swaziland: effect of age class, sex, body condition and management. South African Journal of Zoology. 1995;30:178–186. [Google Scholar]
- Gasaway WC, Gasaway KT, Berry HH. Persistent low densities of plains ungulates in Etosha National Park, Namibia: testing the food-regulating hypothesis. Canadian Journal of Zoology. 1996;74:1556–1572. [Google Scholar]
- Gladney WJ, Graham OH, Trevino JL, Ernst SE. Boophilus annulatus: effect of host nutrition on development of female ticks. Journal of Medical Entomology. 1973;10:123–130. doi: 10.1093/jmedent/10.2.123. [DOI] [PubMed] [Google Scholar]
- Gauthreaux SA., Jr . The ecology and evolution of avian migration systems. In: Farner DS, King JR, Parkes KC, editors. Avian biology. Academic Press; Oxford, UK: 1982. pp. 93–168. [Google Scholar]
- Getz WM. Biomass transformation webs provide a unified approach to consumer–resource modeling. Ecology Letters. 2011;14:113–124. doi: 10.1111/j.1461-0248.2010.01566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn AE, Levey DJ, Hostetler JA, Mamani AM. Determinants of partial bird migration in the Amazon Basin. Journal of Animal Ecology. 2010;79:983–992. doi: 10.1111/j.1365-2656.2010.01713.x. [DOI] [PubMed] [Google Scholar]
- Kalela O. Populationsökologische gesichtspunkte zur entstehung des Vogelzugs. Über den Revierbesitz bei Vögeln und Säugetieren als populationsökologischer Faktor II. Annales Botanici Societatis Zoologicae-Botanicae Fennicae “Vanamo”. 1954;16(4):1–30. [Google Scholar]
- Ketterson ED, Nolan V. Geographic variation and its climatic correlates in the sex ratio of eastern-wintering Dark-Eyed Juncos (Junco hyemalis hyemalis) Ecology. 1976;57:679–693. [Google Scholar]
- LeResche RE. Moose migrations in North America. Naturaliste Canadien. 1974;101:393–415. [Google Scholar]
- Le Roux CJG, Gunow JO, Morris JW, Bredenkamp GJ, Scheepers JCA. Classification of the vegetation of the Etosha National Park. South African Journal of Botany. 1987;54:1–10. [Google Scholar]
- Lindeque PM, Turnbull PCB. Ecology and epidemiology of anthrax in the Etosha National Park, Namibia. Onderstepoort Journal of Veterinary Research. 1994;61:71–84. [PubMed] [Google Scholar]
- Redfern JV, Grant R, Biggs H, Getz WM. Surface-water constraints on herbivore foraging in the Kruger National Park, South Africa. Ecology. 2003;84:2092–2107. [Google Scholar]
- Rubenstein DI. The ecology of female social behavior in horses, zebras, and asses. In: Jarman P, Rossiter A, editors. Animal societies: Individuals, interactions, and organization. Kyoto University Press; Kyoto, Japan: 1994. pp. 13–28. [Google Scholar]
- Smith KL, DeVos V, Bryden H, Price LB, Hugh-Jones ME, Keim P. Bacillus anthracis diversity in Kruger National Park. Journal of Clinical Microbiology. 2000;38:3780–3784. doi: 10.1128/jcm.38.10.3780-3784.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddese A, Mustefa MA. Study on camel ticks in and around Dire Dawa, eastern Ethiopia. Acta Parasitologica Globalis. 2013;4:64–70. [Google Scholar]
- Turnbull PCB. Introduction: anthrax history, disease and ecology. Current Topics in Microbiology and Immunology. 2002;271:1–19. doi: 10.1007/978-3-662-05767-4_1. [DOI] [PubMed] [Google Scholar]
- Turnbull PC, Lindeque PM, Le Roux J, Bennett AM, Parks SR. Airborne movement of anthrax spores from carcass sites in the Etosha National Park, Namibia. Journal of Applied Microbiology. 1998;84:667–676. doi: 10.1046/j.1365-2672.1998.00394.x. [DOI] [PubMed] [Google Scholar]
- Turner WC, Getz WM. Seasonal and demographic factors influencing gastrointestinal parasitism in ungulates of Etosha National Park. Journal of Wildlife Diseases. 2010;46:1108–1119. doi: 10.7589/0090-3558-46.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner WC, Imologhome P, Havarua Z, Kaaya GP, Mfune JKE, Mpofu IDT, Getz WM. Soil ingestion, nutrition and the seasonality of anthrax in herbivores of Etosha National Park. Ecosphere. 2013;4:1–19. [Google Scholar]
- Turner WC, et al. Lethal exposure: an integrated approach to pathogen transmission via environmental reservoirs. Scientific Reports. 2016;6:27311. doi: 10.1038/srep27311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittemyer G, Getz WM, Vollrath F, Douglas-Hamilton I. Social dominance, seasonal movements, and spatial segregation in African elephants: a contribution to conservation behavior. Behavioral Ecology and Sociobiology. 2007;61:1919–1931. [Google Scholar]


