Abstract
Bone tissue regeneration holds the potential to solve both osteoporosis and large skeletal defects, two problems associated with significant morbidity. The differentiation of mesenchymal stem cells into the osteogenic lineage requires a specific microenvironment and certain osteogenic growth factors. Neural EGF Like-Like molecule 1 (NELL-1) is a secreted glycoprotein that has proven, both in vitro and in vivo, to be a potent osteo-inductive factor. Furthermore, it has been shown to repress adipogenic differentiation and inflammation. NELL-1 can work synergistically with other osteogenic factors such as Bone Morphogenic Protein (BMP) −2 and −9, and has shown promise for use in tissue engineering and as a systemically administered drug for the treatment of osteoporosis. Here we provide a comprehensive up-to-date review on the molecular signaling cascade of NELL-1 in mesenchymal stem cells and potential applications in bone regenerative engineering.
Keywords: Bone tissue engineering, Mesenchymal stem cells, NELL-1, NEL-like protein 1, Osteogenic differentiation
Introduction
Bone defects often pose a difficult problem for physicians. Autologous bone grafts are the gold standard of treatment in orthopedic reconstruction but often fail, and treatment for osteoporosis is difficult and unsatisfactory. Mesenchymal stem cells (MSC), progenitor stem-cells of osteoblasts, chondrocytes, adipocytes, and even myoblasts, are a resource that may be able to solve some of these problems.1, 2, 3, 4, 5 Human MSCs (hMSCs) can be derived from a variety of locations including adipose and perivascular tissue, as well as from the stromal cells of bone marrow.3, 6, 7 MSC differentiation induces changes in gene expression that lead to the phenotype of terminally differentiated cells. MSCs can be induced to differentiate by a host of factors including mechanical forces,8 electrical currents,9 and various growth factors.1 MSCs have been most extensively studied for their ability to differentiate into adipocytes and osteoblasts. The inherent potential of MSCs to become adipogenic versus osteogenic is seemingly equal; their fate is decided by their microenvironment, which includes growth factors and cytokines.10 Signaling pathways involving Bone Morphogenic Proteins (BMPs), Wnt/β-Catenin, and Hedgehog (HH) push MSCs to an osteoblastic lineage; while expression of transcription factors such as PPAR-γ and C/EBP induce adipogenesis.11, 12, 13, 14, 15, 16, 17 These pathways are implicated in many different pathological processes, most importantly in osteoporosis, where cells inappropriately become adipocytes rather than mature bone cells.18, 19, 20, 21 With over 2 million annual factures caused by osteoporosis, it is a disease that causes significant physical, emotional, and financial consequences, with few good treatments options available.22 Manipulating the Wnt and BMP pathways could be an important key in finding ways to reverse this disease process, increasing the deposition of bone in osteoporotic patients.
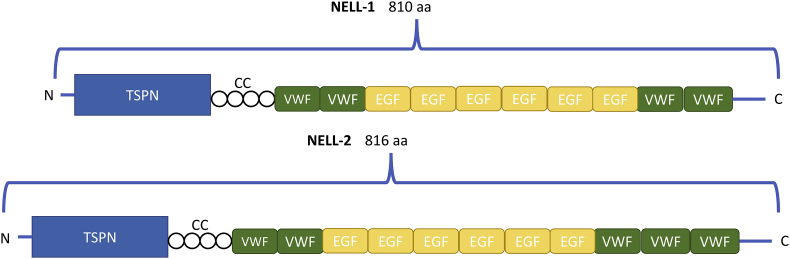
Recent studies have shown that a novel protein, Neural Epidermal Growth Factor-Like-Like 1 (NELL-1) can be a potent osteogenic stimulator, work synergistically with BMPs, and inhibit adipogenesis.23, 24, 25, 26 NELL-1 was originally discovered when Ting et al isolated the protein from, and found it to be upregulated in, surgically resected human cranial bone tissues from patients with unilateral coronal synostosis (UCS), which is a congenital cranial defect defined by premature closure of the coronal suture in the developing calvarium.27 The NELL-1 protein includes several motifs, including an N-terminal thrombospondin-1-like (TSPN) domain, a coiled-coil (CC) domain, four von Willebrand factor type C (VWC) domains, and six EGF-like domains.28 A second homologous gene NELL-2 was also identified with similar structural motifs, although NELL-1 and NELL-2 only share approximately 55% amino acid sequence homology, implying different function for the two proteins (Fig. 1).29, 30
Figure 1.
NELL family members and functional domains. This schematic shows the proposed structure of the NELL-1 and NELL-2 proteins. Both contain N-terminal TSPN domains and then a CC domain connecting to multiple VWC and EGF domains. TSPN, thrombospondin N-terminal domain; CC, Coiled Coil domain; VWC, von Willebrand factor, type C domain; EGF, EGF domain.
Functionally, NELL-1 has demonstrated its effect as a potent osteoinductive factor, i.e., its ability to recruit immature cells and stimulate them to become preosteoblasts.25, 27, 31, 32, 33 Much interest has been generated in NELL-1 as a possible novel therapeutic in osteoporosis because NELL-1 induces osteogenesis, but is distinguished from BMP2 and BMP7 by its ability to simultaneously downregulate adipogenesis.34 This review will focus on NELL-1, its known role in cellular signaling, its theorized mechanism of action in the osteogenic pathway, how it interacts with BMP and other signaling pathways in osteogenesis and its potential use in tissue engineering and the treatment of osteoporosis.
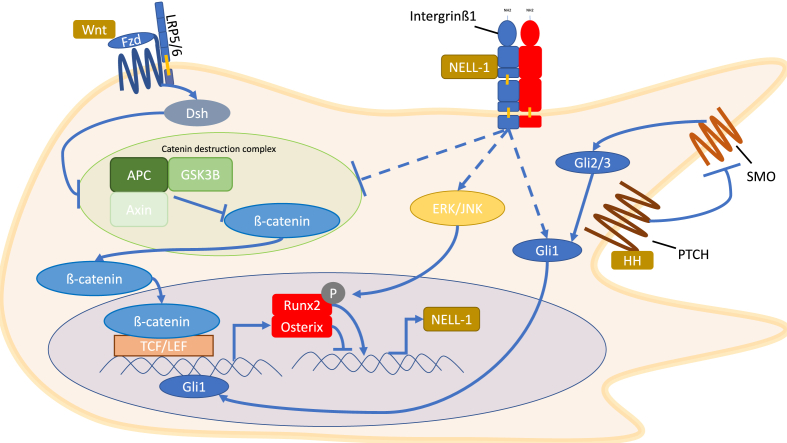
Osteogenic signaling in MSCs involves the complex interaction of multiple pathways, some of which will be discussed below. As previously mentioned, there is a fine balance between the adipogenic and osteogenic fate of MSCs; with the current consensus being that the upregulation of transcription factor PPAR-γ controls adipogenesis while upregulation of the transcription factor Runx2 controls osteogenesis.10, 11, 13, 35 There is, however, cross talk between pathways that contributes to the differentiation of MSCs, and below we will focus on some of the pathways known to interact with NELL-1 signaling, such as: Wnt, Hedgehog and BMP, and their downstream targets in osteogenic signaling, Runx2 and Osterix (Fig. 2).
Figure 2.
Cellular signaling pathways through which NELL-1 works. These pathways include the canonical Wnt pathway, HH and the MAPK pathway. Not included in the figure is the BMP pathway. NELL-1 binding to Intergrinβ1 increases β-catenin nuclear localization, which increases transcription of Runx2 and Osterix. NELL-1 can activate ERK1/2 and JNK which phosphorylate and activate Runx2. NELL-1 also increases the levels of Gli1 which increases expression of Runx2 and Osterix. Runx2 activates expression of NELL-1, while Osterix inhibits expression of NELL-1.
Wnt signaling in osteogenesis
Wingless (Wnt) proteins are secreted glycoproteins that are vital to osteoblast differentiation, as well as a variety of other cellular and developmental functions.16, 36, 37, 38 Wnt signaling is transduced into the cell by the family of Frizzled (Fzd) receptors, seven pass membrane G-protein coupled receptors, and co-receptors of the arrow/Lrp family or a Ror/Ryk transmembrane tyrosine kinase.39 Binding of a Wnt ligand to the Fzd receptor and co-receptor can lead to both canonical/β-catenin and non-canonical/β-catenin independent intracellular signaling, here we will only discuss the canonical pathway.40
Canonical signaling induces complex formation of Fzd, low density lipoprotein receptor-related protein 5/6 (LRP5/6) co-receptor, and intracellular proteins of the disheveled (DSH) family.41, 42 The formation of this complex activates DSH, and activation of DSH inhibits an intracellular complex comprised of Axin, Glycogen Synthase Kinase 3 (GSK3), and adenomatosis polyposis coli (APC) protein.43, 44 GSK3 normally phosphorylates β-catenin leading to its ubiquitination and degradation, but inhibition of the complex leads to the buildup of β-catenin in the cytosol and later the nucleus.45 β-catenin heterodimerizes with lymphoid enhancer binding factor (LEF-1) in the nucleus and elicits gene transcription activity.45, 46 Non-canonical Wnt signaling diverges after activating DSH to elicit its effects independent of β-catenin.47
Wnt signaling has been shown to both promote osteogenesis and inhibit adipogenesis. Wnt ligands inhibit PPAR-γ and C/EBP, while loss of Wnt signaling leads to inhibition of osteoblast differentiation.37, 48, 49 Wnt signaling temporally regulates Runx2 gene expression and also activates Osterix both directly and indirectly via FGF.15, 38, 50 Runx2, the master transcription factor of osteogenesis, is crucial in the commitment of MSCs to an osteogenic fate and is important in many stages of bone development.35, 51, 52 Taken together, these findings demonstrate the importance of the Wnt signaling pathway in bone development and homeostasis. Interestingly, NELL-1 was recently shown to activate the Wnt pathway, which will be discussed further in later sections.53
Hedgehog signaling in osteogenesis
Two of the three Hedgehog (HH) family proteins, Indian Hedgehog (IHH) and Sonic Hedgehog (SHH) have been shown to have vital roles in skeletal development.54, 55 A disruption of HH signaling in vivo leads to developmental skeletal defects56 and SHH is both pro-osteogenic and anti-adipogenic in various MSC cell lines.54, 57, 58 All HH morphogens follow a conserved signaling pathway: the insoluble HH peptide is cleaved, forming a soluble multimeric protein, then a cholesterol moiety is then added to the C-terminal of this protein, and a palmitate moiety added to the N-terminal. The modified protein is then exported from the cell via Dispatched, a large transmembrane protein, and binds to the Patched (PTCH) receptor on the receiving cell. This relieves the inhibition of smoothened homologue (SMO) and activates Human Glioma-Associated Oncogene Homolog 2 & 3 (Gli2/3) transcription factors, which normally act as transcriptional repressors.59, 60 Activation of Gli2/3 leads to expression of Gli1, another transcription factor which is a direct downstream target of Gli2/3.61 Gli1 is regulated by mediators of the HH pathway such as Kinesin-like protein (Kif7) and suppressor of fused homolog SuFu, which also function as nuclear translocators for Gli1.60 Gli transcription factors translocate to the nucleus where they control a variety of genes including Runx2 and Osterix.62 HH signaling in osteogenesis requires the presence of BMP signaling and the two pathways work in a positive feedback loop; Gli2 upregulates BMP2 transcription which in turn increases transcription of Gli2.63, 64
Interestingly, recent studies have shown that increased HH signaling in osteoarthritis leads to decreased bone mass, and HH inhibitors have been proposed as treatment.65 However, HH activation has been shown to increase matrix deposition in fracture healing, meaning chondrocytes are laying down the framework for bone; as well as increase the expression of Runx2 and Osterix.57, 66 Furthermore, HH agonists have shown to significantly reduce adipocyte markers in MSCs such as leptin etc., and reduces the expression of adipogenic transcription factors such as PPAR-γ and C/EBP, pushing MSCs away adipocyte formation.67, 68 Lastly, new studies have shown that NELL-1 increases the expression of HH signaling molecules, suggesting that NELL-1 may exert its anti-adipogenic effects through HH signaling.24 Specifically NELL-1 overexpression leads to increased expression of Ihh, Gli, and Ptch1.24 Also the osteogenic effects of NELL-1 were diminished when adipose derived stromal cells (ASC) were treated with a HH antagonist.58 The exact mechanism of action through which NELL-1 interacts with the HH pathway has not yet been clarified, and future research can elucidate this interaction. This evidence shows that HH signaling plays a complex role in bone metabolism, and may be a novel target for future pharmacological interventions.
BMP signaling in osteogenesis
Bone Morphogenic Proteins (BMPs) are a group of 20 extracellular cytokines that are part of the TGF-beta superfamily.69 They are thought to be indispensable in the differentiation of MSCs into the osteogenic lineage.17, 70, 71, 72, 73 The downstream targets of the BMP pathway, specifically Runx2 and Osterix, have been implicated in bone and cartilage development in several studies.52, 73, 74, 75 Many studies have demonstrated BMPs osteogenic induction, for example: mice with altered BMP receptors show decreased bone mass,76 overexpression of BMP inhibitors like Noggin impair bone formation,77 and deletion of BMP inhibitors, such as Gremlin, causes an increase in bone formation.78 BMP2 and BMP7 are FDA approved for treatment in spinal fusion surgery and BMP9 has recently shown promise as a therapeutic agent for bone growth.72, 79 The pathway starts when BMP ligands bind to serine–threonine kinase cell surface BMP type II receptors (BMPRs), and BMPR type II receptors then recruit, phosphorylate, and activate BMPR type I receptors.69 There are two type I BMP receptors that play a role in MSC differentiation: BMPR-IA and BMPR-IB.80
In addition to demonstrating pro-osteogenic function, BMPs have also been observed to be pro-adipogenic, reducing their utility as a therapeutic agent.64, 81 The tendency to induce osteogenesis over adipogenesis in BMP signaling is not well understood, and both dose-dependent and receptor mechanisms have been proposed.12 Studies have shown that activation of BMPR-IA generally leads to adipogenic effects, while BMPR-IB induces osteogenic effects,80 and that lower doses of BMP2 favor adipogenesis in contrast to higher doses favoring osteogenesis.82
Smad signaling plays a key role in BMP-induced MSC differentiation.83 When a BMP ligand activates a BMPR, the resulting intracellular signaling pathway proceeds through either Smad or mitogen activated protein kinase (MAPK) signaling.84 In the Smad signaling pathway, activation of the BMPR by the BMP ligand leads to phosphorylation of receptor-regulated Smads (R-Smads), Smads 1, 5, and 8, which then dissociate from the receptor and form a complex with a Co-Smad, Smad4.85, 86 These Smad complexes then translocate to the nucleus where they interact with transcription factors and affect gene transcription in a cell specific manner.86 In osteogenic cells, BMP-induced signaling leads to the formation of a Smad complex that physically associates with Runx2 as well as increasing its expression.73 Other studies demonstrate that BMP can upregulate Sox9 and Hox gene expression in osteogenesis as well. In adipogenic signaling the Smads form a complex with CEB/Pα and induce the expression of PPAR-γ.87
In the context of stem cell research, recent efforts have been focused on how to promote BMP-induced osteogenesis while blocking adipogenesis. Some studies have observed that NELL-1 works synergistically with BMP2 and BMP9 to promote osteogenesis while reducing their adipogenic effects.23, 53 The proposed mechanism by which this occurs is that NELL-1 increases Runx2 expression via the canonical Wnt pathway, which rescues some of the Runx2 activity lost by BMP2 activation of PPAR-γ.53 Further discussion of the interplay between BMP and NELL-1 will take place in later sections.
Crosstalk between NELL-1 and Wnt signaling
As previously discussed, numerous studies have shown that Wnt signaling contributes to osteogenesis in stem cells. For example, Wnt ligand filled liposomes accelerate bone regeneration in skeletal defects, and deletion of Wnt or ß-Catenin genes lead to significant skeletal malformations.37, 88 It is proposed that NELL-1 signaling in osteogenesis is mediated by integrin and subsequent activation of the canonical Wnt pathway. Groups have shown that NELL-1 directly binds to the cell surface receptor Integrinβ1 through its TSPN domain.89 Integrins are a group of cell surface proteins that mediate cell adhesion and integrate signals from a variety of cytokines and other growth factors. NELL-1 binding to Integrinβ1 activates an intracellular signaling cascade that promotes cell adhesion, proliferation, and osteogenic differentiation.89 The osteogenic effects are proposed to work through activation of the canonical Wnt/β-Catenin pathway mentioned above. This idea is supported by evidence that nuclear localization of β-catenin was increased in MSCs that were treated with NELL-1.53 Furthermore, the osteogenic effects of NELL-1 are disrupted when Wnt signaling is inhibited.53 For example, when LRP5/6 co-receptors were prevented from complexing with Wnt-Fzd, an important downstream effect of the Wnt pathway, the downstream effects of NELL-1 were also diminished.53 Taken together, this supports NELL-1's role in activating the Wnt pathway in the extracellular domain.53, 90 The same study showed that XAV939, a small molecule inhibitor of intracellular Wnt signaling, also blocked the downstream effects of NELL-1, providing further evidence that NELL-1 works through the Wnt pathway.53
Wnt, and therefore NELL-1 signaling stimulates osteoblast differentiation and skeletal development through stimulating the expression of Runx2, a crucial transcription factor in the control osteogenesis.15 Runx2 then acts upstream to regulate NELL-1 expression.25, 31, 91 Studies have shown that the expression of NELL-1 and Runx2 is coupled, and that Runx2 deficiency leads to low NELL-1 levels.31 These studies suggest that Runx2 is an in vivo regulator of NELL-1, this is further supported by Runx2 binding sites in the NELL-1 promoter.92 Specifically, Runx2 has been shown to upregulate NELL-1 expression by binding to OSE2 sites in its promoter region.92 In addition to these interactions, NELL-1 activated Runx2 by inducing its phosphorylation through the MAPK pathway.93 In Runx2 deficient mice, NELL-1 provided a partial rescue of the skeletal defects usually seen.31 These findings show that NELL-1 is both an upstream and downstream target of Runx2, and plays a part in the delicate balance of signaling in osteogenesis and the regulation of Runx2 and other osteogenic genes.
NELL-1 has also shown signal interaction with both the ERK1/2 and JNK MAPK pathways.93, 94 Both ERK and JNK were phosphorylated after treatment with NELL-1, leading to the phosphorylation of Runx2 and osteogenesis.93 Studies have shown that NELL-1 osteogenic effects were reduced when JNK signaling was blocked in myoblasts, but not entirely, suggesting that NELL-1 signals only partially through the JNK pathway.26 Although, in human osteosarcoma cell lines NELL-1 induced osteoblastic differentiation is accompanied by, and requires, intact JNK signaling.94 Together these demonstrate that MAPK signaling serves an important function in the NELL-1 signaling process.
NELL-1 has also been shown to directly regulate Osterix, a well characterized transcription factor that has been shown to be vital to osteoblastogenesis.95, 96, 97 Interestingly a study found that Osterix is a negative regulator of NELL-1 expression.98 It was shown that Osterix does not disrupt Runx2 binding to NELL-1 promoter regions, but instead inhibits transcription by decreasing the binding of RNA polymerase II.98 This is a surprising relationship because both Osterix and NELL-1 are pro-osteogenic, but it may be that Osterix plays a modulating role in the delicate balance of NELL-1 signaling. NELL-1 seems to be a crucial mediator in the action of Runx2; it is a transcriptional regulator of NELL-1, and NELL-1 can activate Runx2 through phosphorylation. This relationship is modulated by Osterix, which when transcribed as a result of Runx2 activation, inhibits the expression of NELL-1.
One of the most clinically promising aspects of NELL-1 signaling is its anti-adipogenic effects. Treatment of pre-adipogenic cells with rhNELL-1 or infection with NELL-1 adenovirus leads to a significant decrease in adipogenesis, showing a reduction in adipogenic gene expression and in Oil Red O staining, another marker for adipogenesis.24 The same study also showed that adenoviral mediated overexpression of NELL-1 led to an increase in Indian Hedgehog (IHH) ligand and other HH signaling proteins.24 Treatment of human adipose derived stromal cells (ASCs) with a HH agonist and NELL-1 led to an additive effect of osteogenesis and anti-adipogenesis.58 As discussed previously HH signaling is both pro-osteogenic and anti-adipogenic, and this study suggests that some of the effects of NELL-1 may be potentiated through HH signaling. The cellular mechanisms controlling MSC fate are complex, but NELL-1 has proven to be a vital mediator in this network and holds great potential for therapeutic use.
Crosstalk between NELL-1 and BMP signaling
Some of the most well characterized factors that promote bone growth, as discussed previously, are the Bone Morphogenic Proteins (BMPs). In addition to inducing osteoblastic differentiation, BMPs concurrently induce adipogenic differentiation of MSCs, although the precise mechanism by which it does so has yet to be fully elucidated. In the context of creating an optimal 3D bone scaffold, it is essential to understand what causes MSCs to commit to an osteogenic versus an adipogenic fate and to have a proper control of balance between the two. The BMPs highlighted in this review are BMP2, a FDA approved osteoinductive growth factor currently used for bone generation and repair, as well as BMP9, which is not as well characterized but considered to be one of the most potent osteogenic BMPs that induces osteoblastic differentiation of MSCs.23 Although both of these BMPs offer promising osteoinductive effects, they must be paired with the proper complementary factors that will effectively enhance bone growth, minimize inflammation, and promote the formation of a robust extracellular environment. The positive synergistic effect of NELL-1 with BMP2 and BMP9 in tissue engineering has been explored in several studies, which will be discussed below.
BMP2 is currently used for bone regeneration and repair, and has even been shown to increase spinal fusion efficacy.99 Several investigators have observed that combining BMP2 with NELL-1 causes a synergistic osteogenic effect in vitro and in vivo.24, 25, 32, 33, 100, 101, 102, 103, 104 A group studied the synergy between BMP2 and NELL-1 in myoblasts and its effect on bone formation through Alkaline Phosphatase (ALP) activity, an early marker of osteogenesis, as well as osteopontin (OPN) production, which is a well-recognized osteoblast differentiation marker. They found that although NELL-1 alone did not stimulate increase in ALP activity or OPN production, the combination of NELL-1 and BMP2 compared to BMP2 alone significantly stimulated an increase in ALP activity as well as OPN production. The same group also attempted to elucidate the mechanism by which the BMP and NELL-1 signaling pathways crosstalk. They studied the classical MAPK pathways: p28, ERK1/2, and JNK, and found that BMP2 enhanced NELL-1 induced JNK signaling. This is a pathway that is separate from BMP2-induced differentiation, suggesting that BMP2 stimulates osteoblastic differentiation via a separate mechanism when working synergistically with NELL-1. To examine the downstream effects of the synergistic activation of the JNK pathway by NELL-1 and BMP2, they blocked JNK signaling and found that OPN production was partially eliminated. Using adenoviral infection to make AdNELL-1 and AdBMP2 cells, however, allowed NELL-1 to phosphorylate and thus activate the JNK pathway, which in turn induced OPN expression and an osteoblastic phenotype in muscle stem cells. Synergistic activity was also detected in matrix mineralization. In conclusion, NELL-1 and BMP2 together increase the amount of ALP, OPN, and mineralization far beyond the effects that result from either alone, which suggests that they contribute separate, but complementary, signals to stimulate osteoblast differentiation.
Despite the potent osteoinductive effect of BMP, there have been several complications associated with the clinical use of BMP2, including bone resorption and post-operative inflammatory swelling.105, 106 Numerous studies have shown that BMP2-induced inflammation is attributable to higher doses of BMP2, above those required for bone formation, and occurs in various cell types such as endothelial cells, fibroblasts, and preosteoblasts.107, 108, 109 In endothelial cells and preosteoblasts, BMP2 induces inflammation through the generation of reactive oxygen species (ROS).109 BMP2-induced local inflammation is considered to be the clinical complication associated with the highest morbidity, as it can lead to neck-swelling resulting in dysphagia, possible respiratory failure, seroma, or radiculopathies.105, 110, 111, 112, 113 While Shen et al was attempting to study the synergy between BMP2 and NELL-1 in bone regeneration, they discovered that NELL-1 not only promotes BMP2-induced bone growth, but also that it suppressed BMP-induced ROS-dependent inflammation in vivo and in vitro. This group also demonstrated that NELL-1 is able to mitigate the adipogenic phenotype of preadipocytes by directly reducing adipogenic gene expression.
In a more recent study, we investigated the effect of NELL-1 on BMP9-induced osteogenic versus adipogenic differentiation of MSCs using BMP9. We first showed that BMP9 was able to induce NELL-1 expression in MSCs at as early as 24 h post Ad-BMP9 infection.23 In addition, we found that NELL-1 potentiated BMP9-induced late stage osteogenic differentiation while inhibiting early osteogenic marker ALP. Taken together, these results indicate that NELL-1 and BMP have a synergistic effect in bone formation and that crosstalk exists between the two pathways. More specifically, NELL-1 overexpression potentiates BMP9-induced expression of important osteogenic and chondrogenic markers, including Runx2, Osterix, and OPN. We further analyzed how the interaction between NELL-1 and BMP9 in MSCs leads to a cell-specific fate, and found that forced expression of NELL-1 both enhances the mineralization and maturity of BMP9-induced ectopic bone formation while suppressing BMP9-induced adipogenic differentiation of MSCs. Overall, these findings suggest that it may be beneficial in regenerative medicine to use NELL-1 as a co-osteogenic factor to promote BMP9-induced bone formation while also suppressing adipogenesis.
Association of NELL-1 with osteoporosis
Osteoporosis is marked by pathological bone loss caused by an imbalance between bone formation by osteoblastic cells and resorption by osteoclastic cells.34 Osteoporosis is responsible for greatly increasing the probability of severe fractures, ultimately debilitating patients who suffer the over 2 million fractures due to osteoporosis annually.114 By 2025, the economic burden of osteoporotic fractures is projected to grow by almost 50%, incurring $25.3 billion in costs per year.114 The substantial morbidity, mortality, and economic damages caused by osteoporotic fractures make the potential for NELL-1 treatment of osteoporosis an extremely attractive option. While the osteogenic effects of NELL-1 have been well established, more recent evidence has demonstrated an association between NELL-1 and osteoporosis. A 2010 genome-wide study of single-nucleotide polymorphisms (SNPs) found a link between NELL-1 and osteoporosis in women,115 and since then numerous other studies have showed promising links for therapeutic potential of NELL-1 and osteoporosis.
A major setback that routinely negates the therapeutic value of bone anabolic agents (such as BMP2 and retinoic acid) is the tightly coupled osteoclastic response that is secondarily activated as a result of the increased osteoblastic response.116, 117 As a result, the success in using anabolic bone factors in treating osteoporosis will depend on the uncoupling of osteoblastic and osteoclastic activity, likely by focusing on the Wnt/β-catenin signaling pathway.34 As evidenced through the use of Nell-1 haploinsufficient mice, one study found that Nell-1 protein expression showed a significant decline with age, resulting in osteoporosis.34 In addition to the osteoporotic phenotype, the study was able to show that the Nell-1 haploinsufficient mice had increased bone fragility and decreased bone stiffness, with a decrease in proliferation and differentiation of osteoblastic precursors, while increasing the activity and bone resorption of osteoclastic cell.34
The same study further built the evidence around NELL-1 as a potential therapeutic to osteoporosis by testing the effects of recombinant human NELL-1 (rhNELL-1) on osteoporotic sheep, and by examining the effects of rhNELL-1 through a systemic administration. Osteoporotic vertebrae of sheep showed a significant increase in bone mineral density, bone volume, cortical bone thickness, and increased trabecular bone density, in addition to an increased osteoblast:osteoclast ratio when treated with rhNELL-1.34 Systemic rhNELL-1 administration showed significant bone formation in osteoporotic mice, without observations of adverse effects in animal morbidity or mortality across the study period.34
NELL-1 as a therapeutic agent for bone tissue engineering
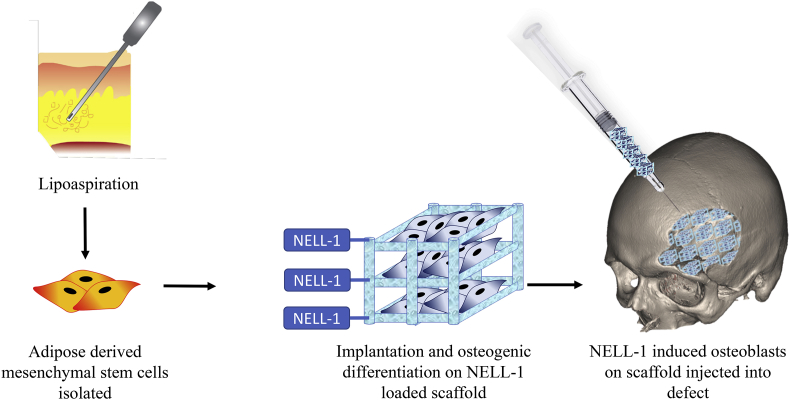
One of the challenges in treating osteoporosis is that it renders bone unsatisfactory for procedures requiring autologous bone graft.118 This may be due to an age-related loss of osteogenic progenitor cells, and loss of their function.119 It's conceivable that NELL-1 can be exploited as a bone-forming factor to enhance the bone repair process (Fig. 3). The use of high dose human perivascular stem cells (hPSCs) combined with NELL-1 has been demonstrated to increase the spinal fusion rate in osteoporotic rats, with a fusion rate at 83.3%, compared to a fusion rate of 20–28.6% with hPSCs alone. The hPSC + NELL-1 rats showed robust bone formation between the transverse processes in addition to significant increases in bone volume compared to the control, which had clear clefts between transverse processes with minimal bone formation.118 The study ultimately concluded that the administration of hPSCs + NELL-1 was able to restore both the diminished osteoprogenitor cells, as well as the osteoinductive microenvironment normally lost in osteoporotic bone.
Figure 3.
Potential applications of NELL-1 in bone tissue engineering. First MSCs are isolated from adipose tissue, then they are seeded into a biodegradable scaffold (PPCN) modified with NELL-1, and then injected into a skeletal defect where new bone will form.
Another study has demonstrated the ability of NELL-1 to enhance in situ osteogenesis in bone marrow, which is one of the fundamental underlying causes of osteoporosis.120 Because osteoblasts and adipocytes are derived from the same bone marrow stem cells, age related increases in adipogenesis in bone marrow infringe upon the potential for osteoblastogenesis.121 This decreases the osteoblast population in addition to causing a decline in their function and survival.122 NELL-1 was able to increase local bone formation by increasing osteoblast activity, without a concomitant osteoclast response, which is an extremely hopeful discovery for future therapeutic uses. The aforementioned study used an in vivo model with ovariectomy (OVX)-induced osteoporotic mice, and demonstrated that NELL-1 injections were effective in maintaining comparable bone volume, bone mineral density, and trabecular thickness in the femurs of osteoporotic mice as compared to the non-osteoporotic control group. Additionally, the study demonstrated that the intramarrow NELL-1 injected femurs of osteoporotic mice maintained their trabecular bone over time, while the osteoporotic control femurs showed continuous loss from the distal femur throughout the time points of the study. The in vivo data corroborated the findings in vitro, where it was found that NELL-1 increased all markers of osteodifferentiation after having been lost in the OVX mice.120
In addition to its osteogenic properties, NELL-1 is an attractive candidate for the treatment of osteoporotic bone because of its demonstrated ability to inhibit the complications that result from high doses of BMP-2, such as inflammation and cystic bone formation,106, 123 in addition to repressing adipogenic differentiation.34 It's lack of toxicity in mice, promotion of osteodifferentiation of bone marrow stem cells, as well as its ability to increase multiple measures of bone quality without provoking a secondary osteoclastic response make it a promising candidate for therapeutic use in humans.34, 120
Concluding remarks and future directions
NELL-1 has the immense potential to be an effective agent for the treatment osteoporosis; as shown in studies that have been done in vitro and in vivo using both rat and sheep models.34, 118, 120, 124 NELL-1 is particularly promising because of its ability to suppress adipogenesis24 while promoting bone growth,91 and its demonstrated efficacy in treatment of osteoporosis.120 Further research needs to be done on using NELL-1 as a systemic drug. Researchers have previously commented on the rapid systemic elimination of NELL-1, making it difficult to use as a treatment,34 but new promising research from Kwak et al has examined the pharmacokinetics of PEGylated NELL-1 as one possible solution.125 PEGylation was developed as a way of addressing the common issue of rapid clearance and immunogenicity in therapeutic proteins. PEGylation involves the conjugation of the protein to polyethylene glycol, which is a polymer that improves solubility, increases longevity, and increases the safety and effectiveness of peptide and protein therapeutics.126, 127, 128 Researchers at UCLA tested the PEGylated NELL-1 in mice, and found that compared to unconjugated NELL-1, it had a higher maximum concentration and a longer half-life.125 Furthermore, they found the PEGylated protein had significantly increased uptake in bone tissue.125 Assays looking at the systemic osteogenic capacity for the PEGylated NELL-1 showed increase bone density, and increased new bone formation.125 Most recently the same group studied the efficacy of intraperitoneal (IP) injection of the PEGylated NELL-1, showing that IP administration is a safe and effective way to increase bone mineral density and osteoblastic activity.129 These studies are leading the way to find the optimal method for the therapeutic delivery of NELL-1.
In addition to PEGylation of NELL-1, there are other technologies under investigation to improve NELL-1 delivery, such as the conjugation of NELL-1 to biodegradable scaffolds, which then promote differentiation of MSCs into bone. New technology has emerged in the biomaterials field with the creation of biodegradable scaffolds to grow stem cells. Recently researchers described a citric acid based polymer called poly(polyethylene glycol citrate-co-N-isopropylacrylamide) (PPCN) that has the potential to be used as an injectable biomaterial scaffold for cells.130 PPCN is thermoresponsive, has anti-oxidant properties, and supports the viability and proliferation of cells.130 Our group showed that PPCN could be used to effectively create new bone in vivo. When BMP9 expressing MSCs were seeded into the PPCN scaffold and injected into critical sized bone defects in mouse calvarias, new bone was effectively formed.131 Injection of the scaffold seeded with the BMP9 MSCs showed a reduction of the size of the defect and mature bone formation.131 This recent evidence shows that PPCN holds great promise for tissue engineering. Our lab is currently investigating the efficacy of chemically conjugating the NELL-1 protein to the PPCN scaffold. Previous groups have attached NELL-1 to a biologic scaffold and found that the addition of the protein augmented the constructive remodeling of the tissue.132 The modification of PPCN with NELL-1 may provide an extremely effective method for the growth of new bone in an injectable form. Such combinations of scaffold materials and NELL-1 should be particularly useful for craniofacial defect repair (Fig. 3).
In conclusion, NELL-1 signals through a complex network of cellular players including the Wnt, HH, and BMP pathways. NELL-1 has proven to have a strong effect in activating osteogenic signaling while repressing adipogenic signaling, and holds promise as a therapy for osteoporosis and bone regeneration.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
Research in the authors' laboratories was supported in part by research grants from the National Institutes of Health (AT004418, DE020140 to TCH and RRR), the US Department of Defense (OR130096 to JMW), the Scoliosis Research Society (TCH and MJL), and the 973 Program of the Ministry of Science and Technology (MOST) of China (# 2011CB707906 to TCH). MP and SM were recipients of the Pritzker Summer Research Fellowship funded through the National Institute of Health (NIH) T-35 training grant (NIDDK) #T35DK062719-30. The reported work was also supported in part by The University of Chicago Cancer Center Support Grant (P30CA014599) and the National Center for Advancing Translational Sciences of the National Institutes of Health through Grant Number UL1 TR000430. Funding sources were not involved in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Chamberlain G., Fox J., Ashton B., Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells Dayt Ohio. 2007;25(11):2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 2.Nakahara H., Dennis J.E., Bruder S.P., Haynesworth S.E., Lennon D.P., Caplan A.I. In vitro differentiation of bone and hypertrophic cartilage from periosteal-derived cells. Exp Cell Res. 1991;195(2):492–503. doi: 10.1016/0014-4827(91)90401-f. [DOI] [PubMed] [Google Scholar]
- 3.Castro-Malaspina H., Gay R.E., Resnick G. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood. 1980;56(2):289–301. [PubMed] [Google Scholar]
- 4.Ashton B.A., Allen T.D., Howlett C.R., Eaglesom C.C., Hattori A., Owen M. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin Orthop. 1980;151:294–307. [PubMed] [Google Scholar]
- 5.Pittenger M.F., Mackay A.M., Beck S.C. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 6.James A.W., Zara J.N., Zhang X. Perivascular stem cells: a prospectively purified mesenchymal stem cell population for bone tissue engineering. Stem Cells Transl Med. 2012;1(6):510–519. doi: 10.5966/sctm.2012-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levi B., Longaker M.T. Concise review: adipose-derived stromal cells for skeletal regenerative medicine. Stem Cells Dayt Ohio. 2011;29(4):576–582. doi: 10.1002/stem.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olivares-Navarrete R., Lee E.M., Smith K. Substrate stiffness controls osteoblastic and chondrocytic differentiation of mesenchymal stem cells without exogenous stimuli. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0170312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen L., Zhang C., Nong Y., Yao Q., Song Z. Mild electrical pulse current stimulation upregulates S100A4 and promotes cardiogenesis in MSC and cardiac myocytes coculture monolayer. Cell Biochem Biophys. 2013;65(1):43–55. doi: 10.1007/s12013-012-9402-x. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q., Shou P., Zheng C. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 2016;23(7):1128–1139. doi: 10.1038/cdd.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Z., Rosen E.D., Brun R. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell. 1999;3(2):151–158. doi: 10.1016/s1097-2765(00)80306-8. [DOI] [PubMed] [Google Scholar]
- 12.James A.W. Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Scientifica. 2013;2013 doi: 10.1155/2013/684736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tontonoz P., Hu E., Spiegelman B.M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79(7):1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 14.Freytag S.O., Geddes T.J. Reciprocal regulation of adipogenesis by Myc and C/EBP alpha. Science. 1992;256(5055):379–382. doi: 10.1126/science.256.5055.379. [DOI] [PubMed] [Google Scholar]
- 15.Gaur T., Lengner C.J., Hovhannisyan H. Canonical Wnt signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280(39):33132–33140. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- 16.Baron R., Kneissel M. Wnt signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19(2):179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 17.Kang Q., Song W.-X., Luo Q. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev. 2009;18(4):545–558. doi: 10.1089/scd.2008.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pino A.M., Rosen C.J., Rodríguez J.P. In osteoporosis, differentiation of mesenchymal stem cells (MSCs) improves bone marrow adipogenesis. Biol Res. 2012;45(3):279–287. doi: 10.4067/S0716-97602012000300009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pei L., Tontonoz P. Fat's loss is bone's gain. J Clin Invest. 2004;113(6):805–806. doi: 10.1172/JCI21311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L., Li D., Wu J., Wu W., Chen H., Mao Y. [A potential role for the bone marrow mesenchymal stem cell in the pathogenesis of osteoporosis by ovariectomy in rat] Sheng Wu Yi Xue Gong Cheng Xue Za Zhi J Biomed Eng Shengwu Yixue Gongchengxue Zazhi. 2006;23(1):129–135. [PubMed] [Google Scholar]
- 21.Foo C., Frey S., Yang H.H., Zellweger R., Filgueira L. Downregulation of beta-catenin and transdifferentiation of human osteoblasts to adipocytes under estrogen deficiency. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2007;23(9):535–540. doi: 10.1080/09513590701556483. [DOI] [PubMed] [Google Scholar]
- 22.Dempster D.W. Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care. 2011;17(Suppl. 6):S164–S169. [PubMed] [Google Scholar]
- 23.Wang J., Liao J., Zhang F. NEL-like molecule-1 (Nell1) is regulated by bone morphogenetic protein 9 (BMP9) and potentiates BMP9-induced osteogenic differentiation at the expense of adipogenesis in mesenchymal stem cells. Cell Physiol Biochem. 2017;41(2):484–500. doi: 10.1159/000456885. [DOI] [PubMed] [Google Scholar]
- 24.James A.W., Pan A., Chiang M. A new function of Nell-1 protein in repressing adipogenic differentiation. Biochem Biophys Res Commun. 2011;411(1):126–131. doi: 10.1016/j.bbrc.2011.06.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu S.S., Zhang X., Soo C. The osteoinductive properties of Nell-1 in a rat spinal fusion model. Spine J Off J North Am Spine Soc. 2007;7(1):50–60. doi: 10.1016/j.spinee.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 26.Cowan C.M., Jiang X., Hsu T. Synergistic effects of Nell-1 and BMP-2 on the osteogenic differentiation of myoblasts. J Bone Min Res Off J Am Soc Bone Min Res. 2007;22(6):918–930. doi: 10.1359/jbmr.070312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ting K., Vastardis H., Mulliken J.B. Human NELL-1 expressed in unilateral coronal synostosis. J Bone Min Res Off J Am Soc Bone Min Res. 1999;14(1):80–89. doi: 10.1359/jbmr.1999.14.1.80. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura Y., Hasebe A., Takahashi K. Oligomerization-induced conformational change in the C-terminal region of Nel-like molecule 1 (NELL1) protein is necessary for the efficient mediation of murine MC3T3-E1 cell adhesion and spreading. J Biol Chem. 2014;289(14):9781–9794. doi: 10.1074/jbc.M113.507020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luce M.J., Burrows P.D. The neuronal EGF-related genes NELL1 and NELL2 are expressed in hemopoietic cells and developmentally regulated in the B lineage. Gene. 1999;231(1–2):121–126. doi: 10.1016/s0378-1119(99)00093-1. [DOI] [PubMed] [Google Scholar]
- 30.Kuroda S., Oyasu M., Kawakami M. Biochemical characterization and expression analysis of neural thrombospondin-1-like proteins NELL1 and NELL2. Biochem Biophys Res Commun. 1999;265(1):79–86. doi: 10.1006/bbrc.1999.1638. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X., Ting K., Bessette C.M. Nell-1, a key functional mediator of Runx2, partially rescues calvarial defects in Runx2+/− mice. J Bone Min Res. 2011;26(4):777–791. doi: 10.1002/jbmr.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siu R.K., Lu S.S., Li W. Nell-1 protein promotes bone formation in a sheep spinal fusion model. Tissue Eng Part A. 2011;17(7-8):1123–1135. doi: 10.1089/ten.tea.2010.0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aghaloo T., Cowan C.M., Chou Y.-F. Nell-1-induced bone regeneration in calvarial defects. Am J Pathol. 2006;169(3):903–915. doi: 10.2353/ajpath.2006.051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.James A.W., Shen J., Zhang X. NELL-1 in the treatment of osteoporotic bone loss. Nat Commun. 2015;6:7362. doi: 10.1038/ncomms8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu J., Li Z., Hou Y., Fang W. Potential mechanisms underlying the Runx2 induced osteogenesis of bone marrow mesenchymal stem cells. Am J Transl Res. 2015;7(12):2527–2535. [PMC free article] [PubMed] [Google Scholar]
- 36.Bennett C.N., Longo K.A., Wright W.S. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA. 2005;102(9):3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brault V., Moore R., Kutsch S. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Dev Camb Engl. 2001;128(8):1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 38.Cai T., Sun D., Duan Y. Wnt/β-catenin signaling promotes VSMCs to osteogenic transdifferentiation and calcification through directly modulating Runx2 gene expression. Exp Cell Res. 2016;345(2):206–217. doi: 10.1016/j.yexcr.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Karner C.M., Long F. Wnt signaling and cellular metabolism in osteoblasts. Cell Mol Life Sci CMLS. 2017;74(9):1649–1657. doi: 10.1007/s00018-016-2425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Logan C.Y., Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 41.Holmen S.L., Salic A., Zylstra C.R., Kirschner M.W., Williams B.O. A novel set of Wnt-frizzled fusion proteins identifies receptor components that activate beta-catenin-dependent signaling. J Biol Chem. 2002;277(38):34727–34735. doi: 10.1074/jbc.M204989200. [DOI] [PubMed] [Google Scholar]
- 42.Schweizer L., Varmus H. Wnt/Wingless signaling through beta-catenin requires the function of both LRP/arrow and frizzled classes of receptors. BMC Cell Biol. 2003;4:4. doi: 10.1186/1471-2121-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Itoh K., Antipova A., Ratcliffe M.J., Sokol S. Interaction of dishevelled and Xenopus axin-related protein is required for Wnt signal transduction. Mol Cell Biol. 2000;20(6):2228–2238. doi: 10.1128/mcb.20.6.2228-2238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willert K., Shibamoto S., Nusse R. Wnt-induced dephosphorylation of axin releases beta-catenin from the axin complex. Genes Dev. 1999;13(14):1768–1773. doi: 10.1101/gad.13.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huber O., Korn R., McLaughlin J., Ohsugi M., Herrmann B.G., Kemler R. Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mech Dev. 1996;59(1):3–10. doi: 10.1016/0925-4773(96)00597-7. [DOI] [PubMed] [Google Scholar]
- 46.Pandur P., Maurus D., Kühl M. Increasingly complex: new players enter the Wnt signaling network. BioEssays News Rev Mol Cell Dev Biol. 2002;24(10):881–884. doi: 10.1002/bies.10164. [DOI] [PubMed] [Google Scholar]
- 47.Kühl M. Non-canonical Wnt signaling in Xenopus: regulation of axis formation and gastrulation. Semin Cell Dev Biol. 2002;13(3):243–249. doi: 10.1016/s1084-9521(02)00050-2. [DOI] [PubMed] [Google Scholar]
- 48.Li C., Zhou L. Inhibitory effect 6-gingerol on adipogenesis through activation of the Wnt/β-catenin signaling pathway in 3T3-L1 adipocytes. Toxicol Vitro Int J Publ Assoc BIBRA. 2015;30(1 Pt B):394–401. doi: 10.1016/j.tiv.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 49.Jeon M., Rahman N., Kim Y.-S. Wnt/β-catenin signaling plays a distinct role in methyl gallate-mediated inhibition of adipogenesis. Biochem Biophys Res Commun. 2016;479(1):22–27. doi: 10.1016/j.bbrc.2016.08.178. [DOI] [PubMed] [Google Scholar]
- 50.Felber K., Elks P.M., Lecca M., Roehl H.H. Expression of osterix is regulated by FGF and Wnt/β-catenin signalling during osteoblast differentiation. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0144982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Komori T., Yagi H., Nomura S. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89(5):755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 52.Lee K.-S., Kim H.-J., Li Q.-L. Runx2 is a common target of transforming growth factor β1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol Cell Biol. 2000;20(23):8783–8792. doi: 10.1128/mcb.20.23.8783-8792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen J., James A.W., Zhang X. Novel Wnt regulator NEL-like molecule-1 antagonizes adipogenesis and augments osteogenesis induced by bone morphogenetic protein 2. Am J Pathol. 2016;186(2):419–434. doi: 10.1016/j.ajpath.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spinella-Jaegle S., Rawadi G., Kawai S. Sonic Hedgehog increases the commitment of pluripotent mesenchymal cells into the osteoblastic lineage and abolishes adipocytic differentiation. J Cell Sci. 2001;114(Pt 11):2085–2094. doi: 10.1242/jcs.114.11.2085. [DOI] [PubMed] [Google Scholar]
- 55.St-Jacques B., Hammerschmidt M., McMahon A.P. Indian Hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13(16):2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nanni L., Ming J.E., Bocian M. The mutational spectrum of the Sonic Hedgehog gene in holoprosencephaly: SHH mutations cause a significant proportion of autosomal dominant holoprosencephaly. Hum Mol Genet. 1999;8(13):2479–2488. doi: 10.1093/hmg/8.13.2479. [DOI] [PubMed] [Google Scholar]
- 57.Tian Y., Xu Y., Fu Q., Dong Y. Osterix is required for Sonic Hedgehog-induced osteoblastic MC3T3-E1 cell differentiation. Cell Biochem Biophys. 2012;64(3):169–176. doi: 10.1007/s12013-012-9369-7. [DOI] [PubMed] [Google Scholar]
- 58.James A.W., Pang S., Askarinam A. Additive effects of Sonic Hedgehog and Nell-1 signaling in osteogenic versus adipogenic differentiation of human adipose-derived stromal cells. Stem Cells Dev. 2012;21(12):2170–2178. doi: 10.1089/scd.2011.0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murone M., Luoh S.-M., Stone D. Gli regulation by the opposing activities of fused and suppressor of fused. Nat Cell Biol. 2000;2(5):310–312. doi: 10.1038/35010610. [DOI] [PubMed] [Google Scholar]
- 60.Simpson F., Kerr M.C., Wicking C. Trafficking, development and hedgehog. Mech Dev. 2009;126(5–6):279–288. doi: 10.1016/j.mod.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 61.Ikram M.S., Neill G.W., Regl G. GLI2 is expressed in normal human epidermis and BCC and induces GLI1 expression by binding to its promoter. J Invest Dermatol. 2004;122(6):1503–1509. doi: 10.1111/j.0022-202X.2004.22612.x. [DOI] [PubMed] [Google Scholar]
- 62.Nakamura T., Naruse M., Chiba Y. Novel hedgehog agonists promote osteoblast differentiation in mesenchymal stem cells. J Cell Physiol. 2015;230(4):922–929. doi: 10.1002/jcp.24823. [DOI] [PubMed] [Google Scholar]
- 63.Zhao M., Qiao M., Harris S.E., Chen D., Oyajobi B.O., Mundy G.R. The zinc finger transcription factor Gli2 mediates bone morphogenetic protein 2 expression in osteoblasts in response to Hedgehog signaling. Mol Cell Biol. 2006;26(16):6197–6208. doi: 10.1128/MCB.02214-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sottile V., Seuwen K. Bone morphogenetic protein-2 stimulates adipogenic differentiation of mesenchymal precursor cells in synergy with BRL 49653 (rosiglitazone) FEBS Lett. 2000;475(3):201–204. doi: 10.1016/s0014-5793(00)01655-0. [DOI] [PubMed] [Google Scholar]
- 65.Lin A.C., Seeto B.L., Bartoszko J.M. Modulating hedgehog signaling can attenuate the severity of osteoarthritis. Nat Med. 2009;15(12):1421–1425. doi: 10.1038/nm.2055. [DOI] [PubMed] [Google Scholar]
- 66.Baht G.S., Silkstone D., Nadesan P., Whetstone H., Alman B.A. Activation of hedgehog signaling during fracture repair enhances osteoblastic-dependent matrix formation. J Orthop Res Off Publ Orthop Res Soc. 2014;32(4):581–586. doi: 10.1002/jor.22562. [DOI] [PubMed] [Google Scholar]
- 67.Fontaine C., Cousin W., Plaisant M., Dani C., Peraldi P. Hedgehog signaling alters adipocyte maturation of human mesenchymal stem cells. Stem Cells Dayt Ohio. 2008;26(4):1037–1046. doi: 10.1634/stemcells.2007-0974. [DOI] [PubMed] [Google Scholar]
- 68.Sinha S., Chen J.K. Purmorphamine activates the Hedgehog pathway by targeting Smoothened. Nat Chem Biol. 2006;2(1):29–30. doi: 10.1038/nchembio753. [DOI] [PubMed] [Google Scholar]
- 69.Miyazono K., Maeda S., Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16(3):251–263. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 70.Wang R.N., Green J., Wang Z. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014;1(1):87–105. doi: 10.1016/j.gendis.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng H., Jiang W., Phillips F.M. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Jt Surg Am. 2003;85-A(8):1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 72.Li X., Cao X. BMP signaling and skeletogenesis. Ann N Y Acad Sci. 2006;1068:26–40. doi: 10.1196/annals.1346.006. [DOI] [PubMed] [Google Scholar]
- 73.Nishimura R., Hata K., Matsubara T., Wakabayashi M., Yoneda T. Regulation of bone and cartilage development by network between BMP signalling and transcription factors. J Biochem (Tokyo) 2012;151(3):247–254. doi: 10.1093/jb/mvs004. [DOI] [PubMed] [Google Scholar]
- 74.Nishimura R., Hata K., Ikeda F. Signal transduction and transcriptional regulation during mesenchymal cell differentiation. J Bone Min Metab. 2008;26(3):203. doi: 10.1007/s00774-007-0824-2. [DOI] [PubMed] [Google Scholar]
- 75.Lee M.-H., Kwon T.-G., Park H.-S., Wozney J.M., Ryoo H.-M. BMP-2-induced Osterix expression is mediated by Dlx5 but is independent of Runx2. Biochem Biophys Res Commun. 2003;309(3):689–694. doi: 10.1016/j.bbrc.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 76.Mishina Y., Starbuck M.W., Gentile M.A. Bone morphogenetic protein type IA receptor signaling regulates postnatal osteoblast function and bone remodeling. J Biol Chem. 2004;279(26):27560–27566. doi: 10.1074/jbc.M404222200. [DOI] [PubMed] [Google Scholar]
- 77.Zhu W., Kim J., Cheng C. Noggin regulation of bone morphogenetic protein (BMP) 2/7 heterodimer activity in vitro. Bone. 2006;39(1):61–71. doi: 10.1016/j.bone.2005.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gazzerro E., Smerdel-Ramoya A., Zanotti S. Conditional deletion of gremlin causes a transient increase in bone formation and bone mass. J Biol Chem. 2007;282(43):31549–31557. doi: 10.1074/jbc.M701317200. [DOI] [PubMed] [Google Scholar]
- 79.Song D., Zhang F., Reid R.R. BMP9 induces osteogenesis and adipogenesis in the immortalized human cranial suture progenitors from the patent sutures of craniosynostosis patients. J Cell Mol Med. May 2017:1–14. doi: 10.1111/jcmm.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen D., Ji X., Harris M.A. Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J Cell Biol. 1998;142(1):295–305. doi: 10.1083/jcb.142.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.James A.W., LaChaud G., Shen J. A review of the clinical side effects of bone morphogenetic Protein-2. Tissue Eng Part B Rev. 2016;22(4):284–297. doi: 10.1089/ten.teb.2015.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang E.A., Israel D.I., Kelly S., Luxenberg D.P. Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors Chur Switz. 1993;9(1):57–71. doi: 10.3109/08977199308991582. [DOI] [PubMed] [Google Scholar]
- 83.Miyazono K., Kusanagi K., Inoue H. Divergence and convergence of TGF-beta/BMP signaling. J Cell Physiol. 2001;187(3):265–276. doi: 10.1002/jcp.1080. [DOI] [PubMed] [Google Scholar]
- 84.von Bubnoff A., Cho K.W. Intracellular BMP signaling regulation in vertebrates: pathway or network? Dev Biol. 2001;239(1):1–14. doi: 10.1006/dbio.2001.0388. [DOI] [PubMed] [Google Scholar]
- 85.Chen Y., Bhushan A., Vale W. Smad8 mediates the signaling of the receptor serine kinase. Proc Natl Acad Sci USA. 1997;94(24):12938–12943. doi: 10.1073/pnas.94.24.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Massagué J., Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19(8):1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jin W., Takagi T., Kanesashi S. Schnurri-2 controls BMP-dependent adipogenesis via interaction with Smad proteins. Dev Cell. 2006;10(4):461–471. doi: 10.1016/j.devcel.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 88.Minear S., Leucht P., Jiang J. Wnt proteins promote bone regeneration. Sci Transl Med. 2010;2(29):29ra30. doi: 10.1126/scitranslmed.3000231. [DOI] [PubMed] [Google Scholar]
- 89.Shen J., James A.W., Chung J. NELL-1 promotes cell adhesion and differentiation via Integrinβ1. J Cell Biochem. 2012;113(12):3620–3628. doi: 10.1002/jcb.24253. [DOI] [PubMed] [Google Scholar]
- 90.Mao B., Wu W., Li Y. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411(6835):321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 91.Zhang X., Zara J., Siu R.K., Ting K., Soo C. The role of NELL-1, a growth factor associated with craniosynostosis, in promoting bone regeneration. J Dent Res. 2010;89(9):865–878. doi: 10.1177/0022034510376401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Truong T., Zhang X., Pathmanathan D., Soo C., Ting K. Craniosynostosis-associated gene nell-1 is regulated by runx2. J Bone Min Res Off J Am Soc Bone Min Res. 2007;22(1):7–18. doi: 10.1359/jbmr.061012. [DOI] [PubMed] [Google Scholar]
- 93.Bokui N., Otani T., Igarashi K. Involvement of MAPK signaling molecules and Runx2 in the NELL1-induced osteoblastic differentiation. FEBS Lett. 2008;582(2):365–371. doi: 10.1016/j.febslet.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen F., Walder B., James A.W. NELL-1-dependent mineralisation of Saos-2 human osteosarcoma cells is mediated via c-Jun N-terminal kinase pathway activation. Int Orthop. 2012;36(10):2181–2187. doi: 10.1007/s00264-012-1590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Karsenty G. Transcriptional control of skeletogenesis. Annu Rev Genomics Hum Genet. 2008;9:183–196. doi: 10.1146/annurev.genom.9.081307.164437. [DOI] [PubMed] [Google Scholar]
- 96.Koga T., Matsui Y., Asagiri M. NFAT and Osterix cooperatively regulate bone formation. Nat Med. 2005;11(8):880–885. doi: 10.1038/nm1270. [DOI] [PubMed] [Google Scholar]
- 97.Nakashima K., Zhou X., Kunkel G. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108(1):17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 98.Chen F., Zhang X., Sun S. NELL-1, an osteoinductive factor, is a direct transcriptional target of osterix. PLoS One. 2011;6(9) doi: 10.1371/journal.pone.0024638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lissenberg-Thunnissen S.N., de Gorter D.J.J., Sier C.F.M., Schipper I.B. Use and efficacy of bone morphogenetic proteins in fracture healing. Int Orthop. 2011;35(9):1271–1280. doi: 10.1007/s00264-011-1301-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aghaloo T., Jiang X., Soo C. A study of the role of Nell-1 gene modified goat bone marrow stromal cells in promoting new bone formation. Mol Ther J Am Soc Gene Ther. 2007;15(10):1872–1880. doi: 10.1038/sj.mt.6300270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang X., Péault B., Chen W. The Nell-1 growth factor stimulates bone formation by purified human perivascular cells. Tissue Eng Part A. 2011;17(19–20):2497–2509. doi: 10.1089/ten.tea.2010.0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li W., Zara J.N., Siu R.K. Nell-1 enhances bone regeneration in a rat critical-sized femoral segmental defect model. Plast Reconstr Surg. 2011;127(2):580–587. doi: 10.1097/PRS.0b013e3181fed5ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li W., Lee M., Whang J. Delivery of lyophilized Nell-1 in a rat spinal fusion model. Tissue Eng Part A. 2010;16(9):2861–2870. doi: 10.1089/ten.tea.2009.0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Siu R.K., Zara J.N., Hou Y. NELL-1 promotes cartilage regeneration in an in vivo rabbit model. Tissue Eng Part A. 2012;18(3–4):252–261. doi: 10.1089/ten.tea.2011.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mindea S.A., Shih P., Song J.K. Recombinant human bone morphogenetic protein-2-induced radiculitis in elective minimally invasive transforaminal lumbar interbody fusions: a series review. Spine. 2009;34(14):1480–1484. doi: 10.1097/BRS.0b013e3181a396a1. discussion 1485. [DOI] [PubMed] [Google Scholar]
- 106.Zara J.N., Siu R.K., Zhang X. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng Part A. 2011;17(9–10):1389–1399. doi: 10.1089/ten.tea.2010.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shields L.B.E., Raque G.H., Glassman S.D. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine. 2006;31(5):542–547. doi: 10.1097/01.brs.0000201424.27509.72. [DOI] [PubMed] [Google Scholar]
- 108.Robin B.N., Chaput C.D., Zeitouni S., Rahm M.D., Zerris V.A., Sampson H.W. Cytokine-mediated inflammatory reaction following posterior cervical decompression and fusion associated with recombinant human bone morphogenetic protein-2: a case study. Spine. 2010;35(23):E1350–E1354. doi: 10.1097/BRS.0b013e3181e85756. [DOI] [PubMed] [Google Scholar]
- 109.Akeel S., El-awady A., Hussein K. Recombinant bone morphogenetic protein-2 induces up-regulation of vascular endothelial growth factor and interleukin 6 in human pre-osteoblasts: role of reactive oxygen species. Arch Oral Biol. 2012;57(5):445–452. doi: 10.1016/j.archoralbio.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 110.Garrett M.P., Kakarla U.K., Porter R.W., Sonntag V.K.H. Formation of painful seroma and edema after the use of recombinant human bone morphogenetic protein-2 in posterolateral lumbar spine fusions. Neurosurgery. 2010;66(6):1044–1049. doi: 10.1227/01.NEU.0000369517.21018.F2. discussion 1049. [DOI] [PubMed] [Google Scholar]
- 111.Smucker J.D., Rhee J.M., Singh K., Yoon S.T., Heller J.G. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine. 2006;31(24):2813–2819. doi: 10.1097/01.brs.0000245863.52371.c2. [DOI] [PubMed] [Google Scholar]
- 112.Yaremchuk K., Toma M., Somers M. Acute airway obstruction associated with the use of bone-morphogenetic protein in cervical spinal fusion. The Laryngoscope. 2010;120(Suppl. 4):S140. doi: 10.1002/lary.21604. [DOI] [PubMed] [Google Scholar]
- 113.Vaidya R., Carp J., Sethi A., Bartol S., Craig J., Les C.M. Complications of anterior cervical discectomy and fusion using recombinant human bone morphogenetic protein-2. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2007;16(8):1257–1265. doi: 10.1007/s00586-007-0351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Burge R., Dawson-Hughes B., Solomon D.H., Wong J.B., King A., Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Min Res Off J Am Soc Bone Min Res. 2007;22(3):465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 115.Karasik D., Hsu Y.-H., Zhou Y., Cupples L.A., Kiel D.P., Demissie S. Genome-wide pleiotropy of osteoporosis-related phenotypes: the Framingham study. J Bone Min Res. 2010;25(7):1555–1563. doi: 10.1002/jbmr.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cowan C.M., Aalami O.O., Shi Y.-Y. Bone morphogenetic protein 2 and retinoic acid accelerate in vivo bone formation, osteoclast recruitment, and bone turnover. Tissue Eng. 2005;11(3–4):645–658. doi: 10.1089/ten.2005.11.645. [DOI] [PubMed] [Google Scholar]
- 117.Jensen E.D., Pham L., Billington C.J. Bone morphogenic protein 2 directly enhances differentiation of murine osteoclast precursors. J Cell Biochem. 2010;109(4):672–682. doi: 10.1002/jcb.22462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee S., Zhang X., Shen J. Brief report: human perivascular stem cells and Nel-like Protein-1 synergistically enhance spinal fusion in osteoporotic rats. Stem Cells Dayt Ohio. 2015;33(10):3158–3163. doi: 10.1002/stem.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.D'Ippolito G., Schiller P.C., Ricordi C., Roos B.A., Howard G.A. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Min Res Off J Am Soc Bone Min Res. 1999;14(7):1115–1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 120.Kwak Jinny, Zara J.N., Chiang M. NELL-1 injection maintains long-bone quantity and quality in an ovariectomy-induced osteoporotic senile rat model. Tissue Eng Part A. 2013;19(3–4):426–436. doi: 10.1089/ten.tea.2012.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gimble J.M., Zvonic S., Floyd Z.E., Kassem M., Nuttall M.E. Playing with bone and fat. J Cell Biochem. 2006;98(2):251–266. doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]
- 122.Dominguez L.J., Di Bella G., Belvedere M., Barbagallo M. Physiology of the aging bone and mechanisms of action of bisphosphonates. Biogerontology. 2011;12(5):397–408. doi: 10.1007/s10522-011-9344-5. [DOI] [PubMed] [Google Scholar]
- 123.Shen J., James A.W., Zara J.N. BMP2-induced inflammation can Be suppressed by the osteoinductive growth factor NELL-1. Tissue Eng Part A. 2013;19(21–22):2390–2401. doi: 10.1089/ten.tea.2012.0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.James A.W., Chiang M., Asatrian G. Vertebral implantation of NELL-1 enhances bone formation in an osteoporotic sheep model. Tissue Eng Part A. 2016;22(11–12):840–849. doi: 10.1089/ten.tea.2015.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kwak J.H., Zhang Y., Park J. Pharmacokinetics and osteogenic potential of PEGylated NELL-1 in vivo after systemic administration. Biomaterials. 2015;57:73–83. doi: 10.1016/j.biomaterials.2015.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Harris J.M., Chess R.B. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2(3):214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 127.Pasut G., Veronese F.M. PEGylation for improving the effectiveness of therapeutic biomolecules. Drugs Today Barc Spain 1998. 2009;45(9):687–695. doi: 10.1358/dot.2009.45.9.1416421. [DOI] [PubMed] [Google Scholar]
- 128.Veronese F.M., Mero A. The impact of PEGylation on biological therapies. BioDrugs Clin Immunother Biopharm Gene Ther. 2008;22(5):315–329. doi: 10.2165/00063030-200822050-00004. [DOI] [PubMed] [Google Scholar]
- 129.Tanjaya J., Zhang Y., Lee S. Efficacy of intraperitoneal administration of PEGylated NELL-1 for bone formation. BioResearch Open Access. 2016;5(1):159–170. doi: 10.1089/biores.2016.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang J., van Lith R., Baler K., Hoshi R.A., Ameer G.A. A thermoresponsive biodegradable polymer with intrinsic antioxidant properties. Biomacromolecules. 2014;15(11):3942–3952. doi: 10.1021/bm5010004. [DOI] [PubMed] [Google Scholar]
- 131.Dumanian Z.P., Tollemar V., Ye J. Repair of critical sized cranial defects with BMP9-transduced calvarial cells delivered in a thermoresponsive scaffold. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0172327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Turner N.J., Londono R., Dearth C.L., Culiat C.T., Badylak S.F. Human NELL1 protein augments constructive tissue remodeling with biologic scaffolds. Cells Tissues Organs. 2013;198(4):249–265. doi: 10.1159/000356491. [DOI] [PubMed] [Google Scholar]