Abstract
Background and objectives
This controlled single-blind trial compared the efficacy of a lip balm with propolis special extract GH 2002 at a concentration of 0.5% in the treatment of episodes of herpes labialis with that of 5% aciclovir cream.
Methods
Patients in the erythematous/papular stage were randomized: 189 patients were treated with propolis cream, 190 patients were treated with aciclovir cream (intention-to-treat population). Application was 5 times daily. The primary parameter was the difference in median time to complete encrustation or epithelialization of lesions. Secondary parameters were the development of typical herpes symptoms (eg, pain, burning and itching, tension, and swelling), the global assessment of efficacy, and the safety of application.
Results
The predefined clinical situation was reached after a median of 4 days with propolis and after 5 days with aciclovir (P < 0.0001). Significant differences in favor of the study preparation were found with all secondary parameters and symptoms. No allergic reactions, local irritations, or other adverse events were observed.
Conclusions
A formulation of 0.5% propolis GH 2002 extract lip balm was found to be superior in the treatment of episodes of herpes labialis over 5% aciclovir cream in patients in the papular/erythematous phase upon inclusion. EudraCT Registration No. 2006-001971-38.
Key words: aciclovir, clinical study, herpes labialis, propolis, superiority
Introduction
Episodes of herpes labialis typically start with a prodromal phase with local pain, tingling, and burning followed by erythematous and papular phases with inflamed and reddened papules, followed by a vesicular phase with fluid-filled blisters. Via the ulceration phase or the bursting of the vesicle with wound formation, it finally leads to the incrustation and healing phase.1 The typical duration of the natural course of untreated herpes labialis is 7 to 10 days and sometimes up to 15 days.1, 2 Accepted local and systemic standard treatments for herpes labialis consist of nucleoside analogues of the aciclovir type.3, 4, 5
Recently, a number of studies demonstrated antiviral effects of propolis special extract GH 2002 against herpes simplex virus type I and II in vitro.6, 7 In addition, antimicrobial effects of GH 2002 were demonstrated in vitro against methicillin-resistant Staphylococcus aureus, and vancomycin-resistant Enterococcus faecium, Candida, and Streptococcus pyogenes.8 A clinical dose-finding study compared concentrations of 0.1%, 0.5%, and 1% of propolis extract GH 2002 in a lip balm for the treatment of herpes labialis.9 The study resulted in the recommendation of 0.5% as a concentration with a sufficiently strong effect and an acceptable level of safety. The latter point was also examined and confirmed in an open observational study.10 The intention of the present study was the evaluation of the clinical usefulness and formal demonstration of efficacy of 0.5% GH 2002 in a lip balm through a reference-controlled trial, using a cream containing 5% aciclovir as an accepted topical comparator medication with marketing authorization for the treatment of herpes labialis.
Materials and Methods
The study was designed as a randomized single-blind, parallel group, reference-controlled multicenter trial (clinical Phase III), with a comparison of the effects of topically applied 0.5% propolis GH 2002 extract lip balm and 5% aciclovir cream in the treatment of herpes labialis. It was performed at the dermatology department of the Medicinal Faculty of the Charles University of Prague and at 3 dermatology ambulatory care centers with participants recruited from regular outpatients presenting for herpes episodes. No financial incentive was given for study participation.
Patients were assigned a consecutive random number according to their entry into the trial. A random list in blocks of 10 ensuring a balanced distribution of patients to the 2 study arms was prepared in advance by the study sponsor using a random number generator (RandList version 1.2; DatInf GmbH, Tübingen, Germany). The random list was closed after labeling and only reopened after official closing of the study and the database during analysis. The physicians had no access to the random code.
The study duration was up to 10 days or up to the visit when the lesions were fully epithelialized or encrusted. Examinations and documentations by the physician were made at Days 0, 2, 3, 4, and 5. Additional examinations on Days 8 (±1 day) and 10 were foreseen for patients still requiring therapy at the previous visit.
Study medication
One study arm received a lip balm containing 0.5% propolis special extract GH 2002 (Herpetino batch No. 20/0913; Gehrlicher Pharmazeutische Extrakte, Eurasburg, Germany). The active constituent of the study preparation was the purified propolis semiliquid extract GH 2002 (extract batch No. 9494, drug extract ratio 2:1, extraction solvent ethanol; excipients in the lip balm: water, xylitol, Butyrospermum parkii, hydrogenated polyisobutene, Simmondsia chinensis seed oil, Prunus amygdalis dulcis (sweet almond) oil, panthenol, polyglyceryl-4-isostearate, cetyl PEG/PPG-10/1, dimethicone, hexyl laurate, sodium chloride, tocopheryl acetate, and bisabolol). The extract GH 2002 is purified from potentially allergenic pollen, waxes, and resins, and is standardized to a defined content of flavonoids, polyphenols, and phenylcarboxylic acids.11
The second study arm applied a 5% aciclovir cream (Herpesin batch No. 10/1013; Teva Pharmaceuticals CR, Prague, Czech Republic) with ingredients including carbomer 934, sodium hydroxide, dimethicone, cetyl alcohol, sodium lauryl sulphate, methyl parabene, and purified water.
The 2 creams were manufactured to be similar in appearance and consistency; both were prepared as externally undistinguishable tubes with 10 g cream (sufficient for a study period of 10 days) and were delivered to the study center with a prenumbered code according to the blinded random number list. The content of the tubes differed slightly with respect to color and odor, which is why the study was considered single-blinded because as the physicians might have known the color and odor difference. The patients were not expected to know the difference between the study preparations; thus, no undue bias was expected.
Each preparation was applied 5 times daily (every 3–5 hours) to the entire upper and lower lip, corresponding to a daily dose of approximately 1 g cream.
Primary and secondary parameters
The first aim of the study was the comparison of median times to full encrustation or epithelialization between groups. According to previous experience with aciclovir and propolis extract the median time to this clinical point was estimated to be reached within approximately 5 days.
Treatment was planned for up to 10 days to cover the typical duration of an untreated episode of herpes labialis. After 10 days the healing process should be in its final stages even in patients not responding to the study medication.
Secondary parameters were the assessments of typical symptoms of lip sores (pain on a visual analogue scale, itching/burning, and tension/swelling on a 4-point verbal rating scale), and an evaluation of global efficacy by the physician on a 4-point verbal rating scale. A descriptive analysis of the development of the individual symptoms of the episode was foreseen as a secondary outcome parameter, including the percentage of patients skipping the vesicular or erosive phase and the calculation of the number needed to treat. Subgroup analyses according to age and gender were also planned.
Safety of application was addressed through an analysis of adverse events, including allergic reactions and skin irritation actively discussed with the patients at each visit. In addition, patients were asked to bring the medication tubes back for inspection for the assessment of compliance.
Inclusion and exclusion criteria
Patients of both genders aged 18 to 70 years could only be included if they had visible eruptions (erythematous or papular) for no more than 30 hours before the first examination, and if their history showed at least 4 previous episodes of herpes labialis. Patients in the prodromal stage; that is, with burning or tension of the lips only, and patients with progressed stages (ie, vesicular, erosive, or encrusted) were not eligible. The inclusion and exclusion criteria with respect to the stage of the episode had to be strictly adhered to because they were the foundation of a comparability of results with published data on aciclovir.
Further exclusion criteria were hypersensitivity to any component of the test preparation or the reference, concomitant viral infections, acquired or malignant immunodeficiency, including HIV or leukemia, the severity of the herpes labialis requiring systemic treatment, or the concurrent use of other topical preparations or systemic antiviral medication. The use of such preparations was also not permitted for the duration of the trial.
Case number calculation
A case number calculation was made based on the results of a previously performed dose-finding study.9 The study preparation had reduced the time to complete encrusting of herpes lesions by approximately 1 day compared to a 0.1% formulation, which still showed activity. It was therefore anticipated that 0.5% propolis would reduce time to complete encrusting or epithelialization by approximately 1.5 days compared with the untreated course of the episode. Aciclovir shortens the duration of herpes labialis episodes by approximately 1 day versus placebo. The case number calculation was therefore based on the assumption that the study preparation should be superior over aciclovir by approximately 0.5 days, as judged by the median time to full encrusting or epithelialization. The case number calculation was based on an expected dropout rate of 20% and a power of at least 80% in the superiority testing, which led to the conclusion of 190 patients to be included per group.
Vote of the ethics committee
The study was planned and carried out in accordance with the criteria of Good Clinical Practice and the ethical standards defined in the Declaration of Helsinki. An approval of the ethics committees of the study centers (Etická komise fakultní nemocnice Královské Vinohrady, Srobárova 50, 10035 Praha 10; and Etická komie pro multicentrické klinické hodnoceni fakultní nemocnice v Motole, V uvalu 84, 15005 Praha 5) and the Czech drug authorization authority, as well as a signed informed consent form for all participants was obtained. Initiation of the trial was on February 24, 2012. The final visit of the last participant took place on January 14, 2014.
Statistical analysis
IBM-SPSS Statistics version 21.0.0 (IBM-SPSS Inc, Armonk, NY) was used as the statistical software to perform the analyses.
The intention-to-treat (ITT) group included all patients who received study drugs and returned for at least 1 visit after the admission examination. The per-protocol group (PP) included all patients who followed the protocol. The safety population comprised all patients exposed to study medication.
Superiority calculation was made primarily in the ITT population through a 1-sided Mann-Whitney U test by the SPSS Exact module giving exact P values, with a level of significance set to 0.025. The threshold for superiority was defined as a difference between medians of the groups for full encrustation or epithelialization with minimum of 0.5 days in favor of propolis.
Missing values were to be replaced by the worst-case imputation method, replacing missing values in the propolis group by the worst value of the propolis group, and missing values in the aciclovir group by the best value of the aciclovir group. The worst-case imputation is favorable for the comparator and makes superiority testing more robust.
Individual improvement of pain as a secondary parameter was the difference of the average pain at Visits 3 and 4 minus pain at Visit 1. Between-group comparisons were made with the group averages using the Mann-Whitney U test at a 2-sided significance level of 0.05.
Presence and intensity of itching/burning and tension/swelling, and the physician’s global assessment of efficacy were compared between groups using the Fisher exact test and Mann-Whitney U test at a 2-sided significance level of 0.05. All other parameters were analyzed descriptively, with significance between groups examined using the Mann-Whitney U test or the Fisher exact test, as appropriate. All P values for secondary end points do not possess confirmatory value.
Results
Demographic data
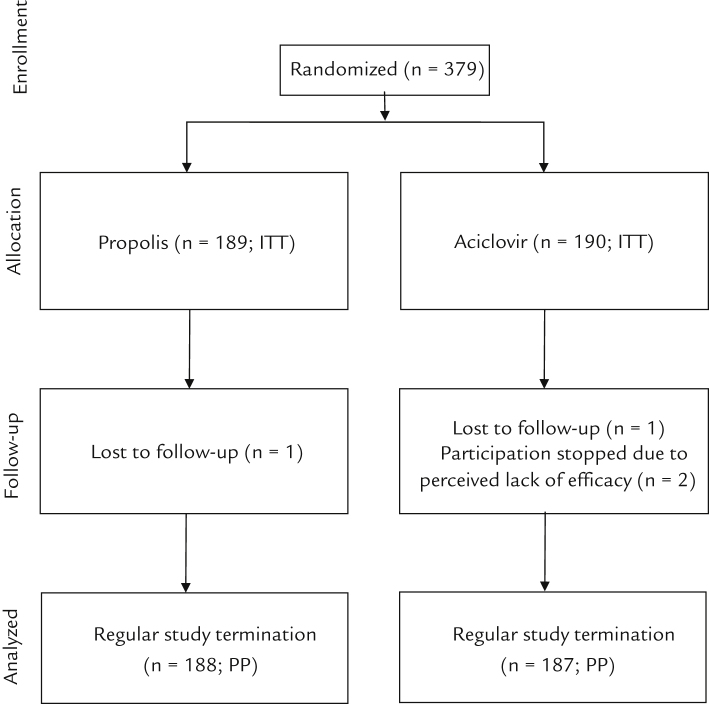
Three hundred seventy-nine outpatients with the diagnosis of herpes labialis were included (ITT population) (Figure 1). The inclusion and exclusion criteria, including those specifically referring to excluded comedication, were met in all cases. Two hundred thirty-seven patients were women and 142 were men. One hundred eighty-nine patients were assigned to the study preparation (mean (SD) age 40.7 [13.0] years and 58.2% female), 190 to reference (age 41.1 [13.8] years and 66.8% female). There were no statistically significant differences between groups with respect to age and expression of herpes lesions.
Figure 1.
Flow chart of patient distribution to treatment groups. ITT = intention-to-treat population; PP = per protocol population.
Four patients prematurely discontinued participation in the study: 1 patient with propolis (lost to follow-up) and 3 patients with aciclovir (1 patient lost to follow-up and 2 patients for lack of efficacy). The PP population therefore consisted of a total of 375 participants (188 treated with the study preparation and 187 treated with the reference preparation).
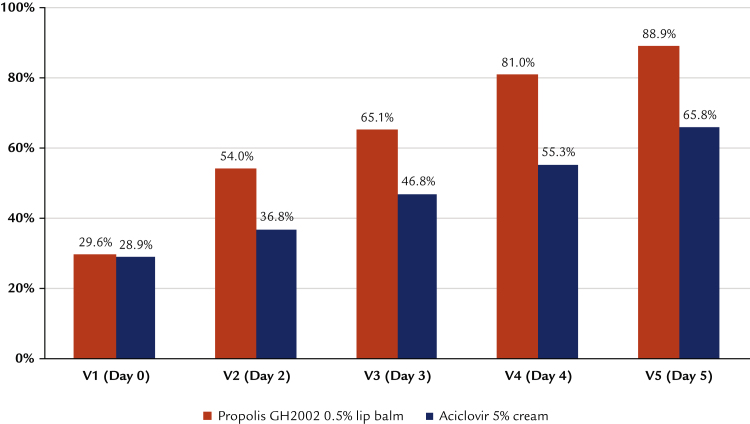
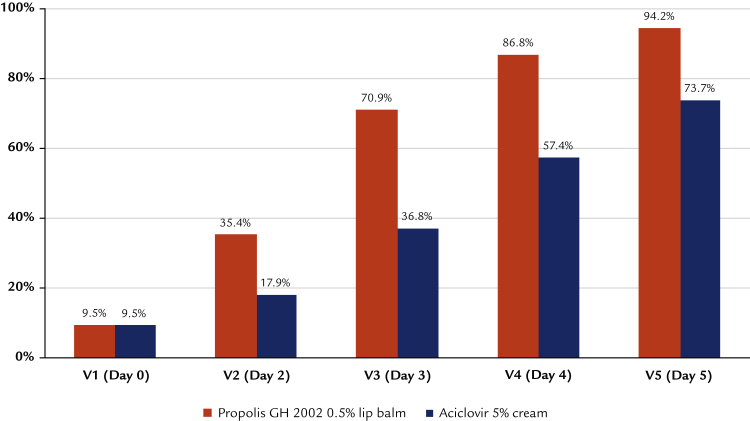
Development of lesions over time
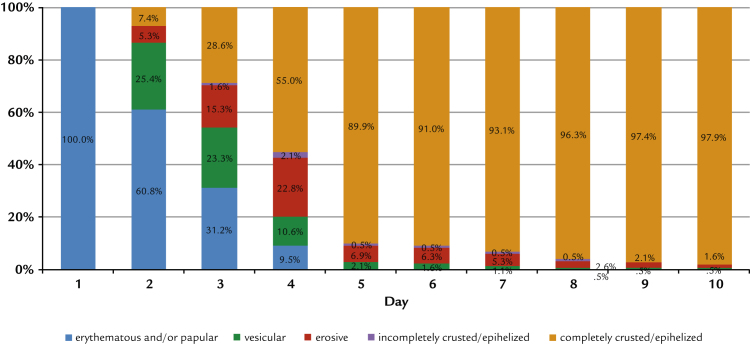
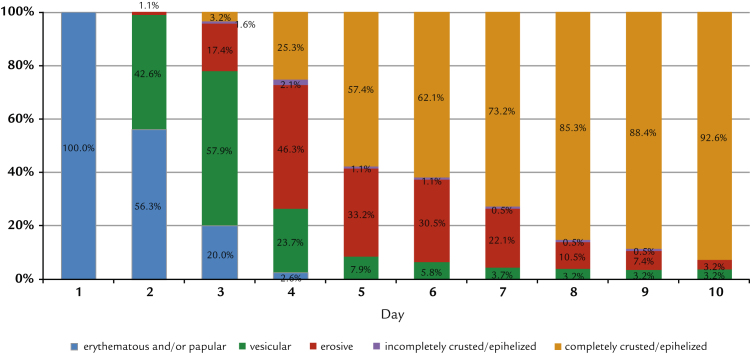
As demanded by the inclusion criteria, all patients presented only erythematous or papular lesions at inclusion. The further development shows distinct and statistically significant differences between groups: at Day 4, 55.0% of the participants treated with propolis had no more erythema, papules, vesicles, or erosions (Figure 2). This increased to 89.9% on Day 5. With aciclovir, the same clinical end point was reached for only 25.3% of patients, whereas on Day 5, 57.5% of patients were fully encrusted or epithelialized (Figure 3). In addition, significantly fewer study participants treated with the study preparation than patients treated with reference preparation developed vesicles or erosions (42% vs 85% vesicles and 39% vs 72% erosions; in both cases P < 0.0001 by Fisher exact test). Converting these figures into numbers needed to treat, the comparison results in 2.3 and 3.0 patients treated with reference to avoid the formation of vesicles and erosions to the same extent as for 1 patient treated with the study preparation.
Figure 2.
Development of herpes lesions treated by propolis extract. (Intention-to-treat population n = 189).
Figure 3.
Development of herpes lesions treated by aciclovir (intention-to-treat population; n = 190).
Superiority testing
The course of the herpes episodes, as shown in Figure 2, Figure 3, gives a first impression on the clinical effects of propolis treatment versus aciclovir. For statistical comparison in the context of superiority testing, the course of the herpes episodes had to be transformed into statistically comparable figures. The mathematical approach was based on the median time to reach full encrustation or epithelialization in the population of each study arm.
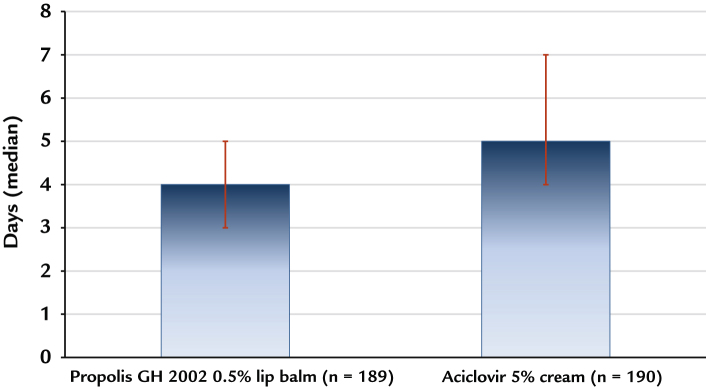
The superiority test was performed as planned in the ITT population, with a threshold of a difference between groups of 0.5 days in favor of propolis. The median time to complete encrustation/epithelialization was 4.0 days with propolis (average 4.41 [1.63] days), and 5.0 days with aciclovir (average 5.54 [1.87]). The superiority of propolis was highly significant (P < 0.0001; Mann-Whitney U test modified for superiority testing with a threshold 0.5 days; Figure 4).
Figure 4.
Superiority testing: Time in days to complete encrustation or epithelialization of herpes lesions (intention-to-treat population n = 189 for propolis and 190 for aciclovir). Bars represent the 25th to 75th percentile.
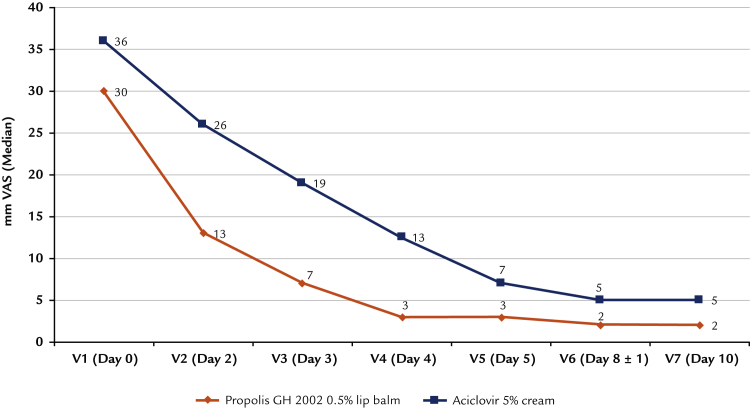
Development of pain
Mean (SD) pain at baseline, assessed on a 100-mm visual analogue scale, was 34 (25) with propolis and 39 (26) with aciclovir (ITT). Pain decreased continuously in both groups during the study, but the difference between means was 10 mm on a visual analogue scale and more at the visits on Days 2, 3, and 4 (Figure 5). The predefined calculation of the difference of the average of pain at days 3 and 4 to pain at baseline (V1) resulted in a pain reduction of 27 mm visual analogue scale with the propolis and 19 mm with aciclovir. The difference of 8 mm on a visual analogue scale in favor of propolis extract GH 2002 was statistically significant (P < 0.001 based on Mann-Whitney U test).
Figure 5.
Development of pain assessment by visual analogue scale (VAS) (intention-to-treat population).
Itching/burning and tension/swelling
Itching/burning and tension/swelling were evaluated through a 4-step verbal rating scale (absent, mild, moderate, and severe). At baseline, approximately 30% of patients indicated the absence of itching or burning and approximately 10% the absence of tension or swelling, with no statistically significant difference between groups (ITT population). Overall, patients in the aciclovir group had more severe tension and swelling than patients in the group using propolis (P = 0.042 based on Mann-Whitney U test; data not shown).
For the further visits on Days 2 to 5 there was a significant difference in favor of propolis, reaching 89% versus 66% free of itching or burning (Figure 6), and 94% versus 74% free of tension and swelling (Figure 7) at Day 5 for propolis versus aciclovir (P < 0.001 based on Mann-Whitney U test). Results obtained with the PP population were practically identical.
Figure 6.
Percentage of patients free of burning and itching during the course of the study (intention-to-treat population).
Figure 7.
Percentage of patients free of tension and swelling during the course of the study (intention-to-treat population).
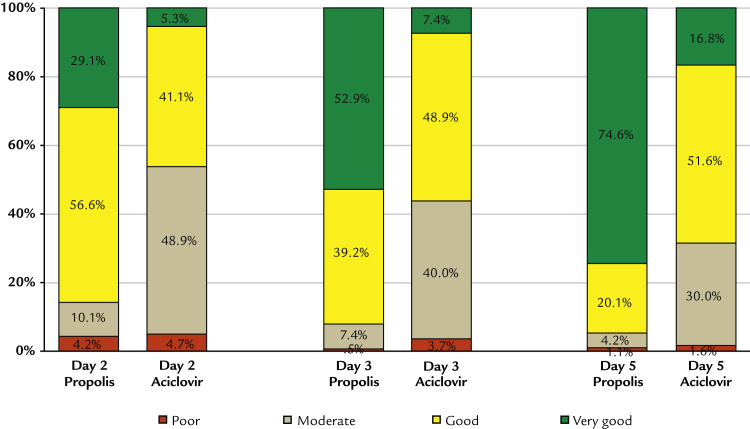
Global assessment of efficacy by a physician
Differences between groups in favor of propolis were also confirmed in the assessment of global efficacy by the physicians (Figure 8). At the visit on Day 5, 94.7% of patients treated with propolis and 68.4% of patients treated with aciclovir received efficacy assessments of “good” to “very good.” Differences were statistically significant at all visits on Days 2, 3, 4, and 5 (ITT group P < 0.0001 based on Mann-Whitney U test). Results for the PP group were practically identical, with 94.7% and 69.0% of ratings as “good” and “very good” on Day 5 for propolis and aciclovir, respectively.
Figure 8.
Global assessment of efficacy by the physician (intention-to-treat population).
Subgroup analyses
Subgroup analyses according to age and gender indicate that young female patients may have profited to a slightly larger extent. The difference of means with respect to the difference in time to full encrustation/epithelialization was 1.2 days for women, and 0.95 days for men. Similarly, younger patients profited slightly more than older patients (1.4 vs 0.8 days). The strength of the effect of propolis and aciclovir was not influenced by the severity of symptoms at baseline.
Safety and compliance
In both treatment groups there were no adverse events observed, which includes the absence of local reactions or superinfections. This finding is in line with the experience with the application of both study preparations. There was no hint of poor compliance through the safety assessment and the inspection of the returned medication for consumption.
Discussion
The preparation 0.5% propolis GH 2002 lip balm tested in this study has previously shown positive results in the treatment of herpes labialis,9, 10 but has not yet been tested against an active reference in a controlled study. For a better comparability of outcomes, the design and end points of this study were selected to match those of placebo-controlled studies with aciclovir, and to justify the use of aciclovir as a reference treatment for the verification of the therapeutic applicability of the propolis preparation.
GH 2002 cream with 0.5% propolis special extract was found to be superior to 5% aciclovir cream under the clinical conditions defined in this study; that is, a start of treatment in the early, erythematous or papular phase. The advantage of propolis lip balm over aciclovir was especially visible within the first 3 to 5 days after the beginning of treatment. It is no contradiction that after 10 days there was no longer a significant difference between propolis and aciclovir lip balm: episodes of herpes labialis are self-limiting conditions and would normally be expected to heal within 10 to 14 days, if left untreated. The aim of a medication is to shorten this period, and a shortening of the herpes episode was demonstrated for both aciclovir and propolis extract.
The lack of a placebo control might be considered a downside of the study protocol. However, the efficacy of aciclovir in the tested concentration is generally accepted as proven, and can also be derived from the outcome of this study. In the Czech Republic, where this study was performed, the use of placebo is considered ethically unacceptable when there is a reference treatment with accepted efficacy. The dosing of aciclovir corresponded to a dose accepted as efficacious. The worst-case imputation method for missing values would be in favor of aciclovir. The median and average time to complete encrustation or epithelialization was approximately 1 day shorter in the propolis group than in the aciclovir group, which underlines the clinical importance of the findings beyond statistical significance. In addition, a higher proportion of patients in the propolis group skipped the vesicular and the erosive stage.
The measured advantage of the study preparation over the reference preparation with respect to pain reduction, reaching 8 mm on the VAS at mid study, was considered of borderline clinical importance. Such a difference is still noticeable to the patients,12, 13 especially because the patients treated with propolis reached a defined amount of pain improvement approximately 1 to 2 days earlier than patients treated with the reference balm.
Conclusions
An important aspect of studies in the treatment of herpes labialis is the selection of a clinically exact defined starting point for the assurance of comparability between groups. In this study, all patients were in the papular or erythematous stage. The findings are therefore applicable to the early stages of a herpes episode. We are already examining the question of whether the advantages of propolis over aciclovir are still visible if lip balm is first applied when patients are already in the vesicular stage.
Conflicts of Interest
This clinical study was supported by funds from the companies Harras Pharma Curarina GmbH (Munich, Germany; applicant for the marketing authorization of the study preparation) and Gehrlicher Pharmazeutische Extrakte GmbH (Eurasburg, Germany; manufacturer of the study preparation). The study sponsors proposed the trial and provided funding and honoraria for the data collection, the statistical evaluation, and manuscript preparation. The sponsors were not involved in the planning, execution, data analysis and evaluation, and the publication of the clinical trial results. These tasks were carried out under the responsibility of the principal investigator.
Acknowledgments
P.A. was the principal investigator, M.A. and S.H. were treating physicians. M.H. was responsible for statistical evaluations. B.O. designed the study and performed the monitoring.
References
- 1.Platz G., Gross G. Aciclovir Creme bei Lippenherpes. DtApothZtg. 2006;145:1653–1658. [Google Scholar]
- 2.Spruance S.L., Rea T.L., Thoming C., Tucker R., Saltzman R., Boon R. Penciclovir cream for the treatment of herpes simplex labialis. A randomized, multicenter, double-blind, placebo-controlled trial. Topical Penciclovir Collaborative Study Group. JAMA. 1997;277:1374–1379. [PubMed] [Google Scholar]
- 3.Raborn G.W., McGaw W.T., Grace M., Percy J., Samuels S. Herpes labialis treatment with acyclovir 5% modified aqueous cream: a double-blind randomized trial. Oral Surg Oral Med Oral Pathol. 1989;67:676–679. doi: 10.1016/0030-4220(89)90007-8. [DOI] [PubMed] [Google Scholar]
- 4.Spruance S.L., Schnipper L.E., Overall J.C., Jr, Kern E.R., Wester B., Modlin J. Treatment of herpes simplex labialis with topical acyclovir in polyethylene glycol. J Infect Dis. 1982;146:85–90. doi: 10.1093/infdis/146.1.85. [DOI] [PubMed] [Google Scholar]
- 5.Spruance S.L., Nett R., Marbury T., Wolff R., Johnson J., Spaulding T. Acyclovir cream for treatment of herpes simplex labialis: results of two randomized, double-blind, vehicle-controlled, multicenter clinical trials. Antimicrob Agents Chemother. 2002;46:2238–2243. doi: 10.1128/AAC.46.7.2238-2243.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schnitzler P., Neuner A., Nolkemper S., Zundel C., Nowack H., Sensch K.H. Antiviral activity and mode of action of propolis extracts and selected compounds. Phytother Res. 2010;24:S20–S28. doi: 10.1002/ptr.2868. [DOI] [PubMed] [Google Scholar]
- 7.Nolkemper S., Reichling J., Sensch K.H., Schnitzler P. Mechanism of herpes simplex virus type 2 suppression by propolis extracts. Phytomedicine. 2010;17:132–138. doi: 10.1016/j.phymed.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Astani A., Zimmermann S., Hassan E., Reichling J., Sensch K.H., Schnitzler P. Antimicrobial activity of propolis special extract GH 2002 against multidrug-resistant clinical isolates. Pharmazie. 2013;68:695–701. [PubMed] [Google Scholar]
- 9.Holcová S., Hladiková M. Efficacy and tolerability of propolis special extract GH 2002 as a lip balm against herpes labialis: a randomized, double-blind three-arm dose finding study. Health. 2011;3:49–56. [Google Scholar]
- 10.Holcová S., Hladiková M. Inhibition of the development of cold sores through early use of a nursing lip balm containing the active constituent propolis special extract GH 2002 in comparison with aciclovir cream 5% (in German) Kosmetische Med. 2012;33:100–104. [Google Scholar]
- 11.Tomanova D., Holcova S., Hladikova M. Clinical study: Lotion containing propolis special extract GH 2002 0.5% vs. placebo as on-top treatment of Herpes zoster. Health. 2017;9:1337–1347. [Google Scholar]
- 12.Eberle E., Ottillinger B. Clinically relevant change and clinically relevant difference in knee osteoarthritis. Osteoarthritis Cartilage. 1999;7:502–503. doi: 10.1053/joca.1999.0246. [DOI] [PubMed] [Google Scholar]
- 13.Ehrich E.W., Davies G.M., Watson D.J., Bolognese J.A., Seidenberg B.C., Bellamy N. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol. 2000;27:2635–2641. [PubMed] [Google Scholar]