Abstract
BACKGROUND
Few long-term or controlled studies of bariatric surgery have been conducted to date. We report the 12-year follow-up results of an observational, prospective study of Roux-en-Y gastric bypass that was conducted in the United States.
METHODS
A total of 1156 patients with severe obesity comprised three groups: 418 patients who sought and underwent Roux-en-Y gastric bypass (surgery group), 417 patients who sought but did not undergo surgery (primarily for insurance reasons) (non-surgery group 1), and 321 patients who did not seek surgery (nonsurgery group 2). We performed clinical examinations at baseline and at 2 years, 6 years, and 12 years to ascertain the presence of type 2 diabetes, hypertension, and dyslipidemia.
RESULTS
The follow-up rate exceeded 90% at 12 years. The adjusted mean change from baseline in body weight in the surgery group was −45.0 kg (95% confidence interval [CI], −47.2 to −42.9; mean percent change, −35.0) at 2 years, −36.3 kg (95% CI, −39.0 to −33.5; mean percent change, −28.0) at 6 years, and −35.0 kg (95% CI, −38.4 to −31.7; mean percent change, −26.9) at 12 years; the mean change at 12 years in nonsurgery group 1 was −2.9 kg (95% CI, −6.9 to 1.0; mean percent change, −2.0), and the mean change at 12 years in nonsurgery group 2 was 0 kg (95% CI, −3.5 to 3.5; mean percent change, −0.9). Among the patients in the surgery group who had type 2 diabetes at baseline, type 2 diabetes remitted in 66 of 88 patients (75%) at 2 years, in 54 of 87 patients (62%) at 6 years, and in 43 of 84 patients (51%) at 12 years. The odds ratio for the incidence of type 2 diabetes at 12 years was 0.08 (95% CI, 0.03 to 0.24) for the surgery group versus nonsurgery group 1 and 0.09 (95% CI, 0.03 to 0.29) for the surgery group versus nonsurgery group 2 (P<0.001 for both comparisons). The surgery group had higher remission rates and lower incidence rates of hypertension and dyslipidemia than did nonsurgery group 1 (P<0.05 for all comparisons).
CONCLUSIONS
This study showed long-term durability of weight loss and effective remission and prevention of type 2 diabetes, hypertension, and dyslipidemia after Roux-en-Y gastric bypass. (Funded by the National Institute of Diabetes and Digestive and Kidney Diseases and others.)
The first surgical procedure performed specifically for weight loss took place in 1954.1 Since then, bariatric procedures have become less invasive and safer, and insights regarding the beneficial metabolic effects of such procedures have led to additional indications for these procedures. Relatively short-term, randomized, controlled trials have investigated clinical outcomes in obese patients who had type 2 diabetes and had undergone a bariatric surgical procedure or had received intensive, nonsurgical therapies, such as lifestyle and pharmacologic interventions.2–8 Although such trials have made important clinical contributions, large gaps remain in the understanding of the long-term benefits and risks of bariatric surgery.
This article addresses the durability of health benefits related to Roux-en-Y gastric bypass. The current study represents a long-term, observational, prospective study of Roux-en-Y gastric bypass in the United States with high follow-up rates. We compared changes in weight and the incidence and remission rates of type 2 diabetes, hypertension, and dyslipidemia in patients with severe obesity who underwent Roux-en-Y gastric bypass with respective findings in two groups of patients with severe obesity who did not undergo bariatric surgery.
METHODS
STUDY DESIGN
This observational, prospective study was initiated in July 2000, and patients were followed through March 2016. Of the 1156 patients enrolled, 835 patients with severe obesity had visited a single bariatric surgical center (Rocky Mountain Associated Physicians, Salt Lake City) seeking Roux-en-Y gastric bypass. Of these patients, half (418 patients) proceeded with surgery (surgery group) and the remaining 417 patients did not undergo surgery, primarily because their insurance did not cover the procedure (nonsurgery group 1). In addition, a population-based sample of 321 adults with severe obesity who had not previously undergone bariatric surgery was recruited (nonsurgery group 2).9 Participants were 18 to 72 years of age, had no history of alcohol or narcotics abuse, had not undergone bariatric surgery, and had not had gastric or duodenal ulcers, a myocardial infarction (in the previous 6 months), or active cancer (in the previous 5 years). Other selection criteria are noted in the Supplementary Appendix, available with the full text of this article at NEJM.org, or have been published previously.10,11 The study protocol was approved by the institutional review boards at the University of Utah and at Intermountain Healthcare, and written informed consent was obtained from each patient.
Follow-up results from the 2-year and 6-year clinical examinations have been reported previously.11,12 At each examination, data on medical history, lifestyle interventions, and medications were recorded, and clinical measurements were performed. After the baseline examination, patients in the surgery group underwent Roux-en-Y gastric bypass.13 Participants in the two nonsurgery groups received no study-based intervention for weight loss, although they were free to seek such therapy.
STUDY END POINTS
The primary end points were the percentage of original weight lost and the incidence and remission rates of type 2 diabetes, hypertension, and dyslipidemia among the survivors at 12 years. Patients were considered to have type 2 diabetes if they met one or more of the following conditions: a fasting blood glucose level of at least 126 mg per deciliter (7.0 mmol per liter), a glycated hemoglobin level of at least 6.5%, or current use of any antidiabetic medication. Patients were considered to have hypertension if they had a blood pressure of at least 140/90 mm Hg while seated, if they reported current use of antihypertensive medication, or both. Patients were considered to have dyslipidemia if they met one or more of the following conditions: a fasting low-density lipoprotein cholesterol level of at least 160 mg per deciliter (4.1 mmol per liter), a high-density lipoprotein cholesterol level of less than 40 mg per deciliter (1.0 mmol per liter), a triglyceride level of at least 200 mg per deciliter (2.3 mmol per liter), or current use of lipid-lowering medication. Remission of the prevalent-disease end points at the follow-up examination was defined as the absence of disease according to the criteria above. Quality of life and mortality rate were assessed as secondary study end points (see the Supplementary Appendix).
FOLLOW-UP
All the patients were invited to return for a 12-year examination. Clinical information on the patients who did not return for the 12-year examination was obtained from primary care providers, searches of electronic medical records from large health care databases, records from hospitals in Utah, and telephone interviews (Fig. 1). The National Death Index was used to determine vital status and causes of death through 2014.14
Figure 1.
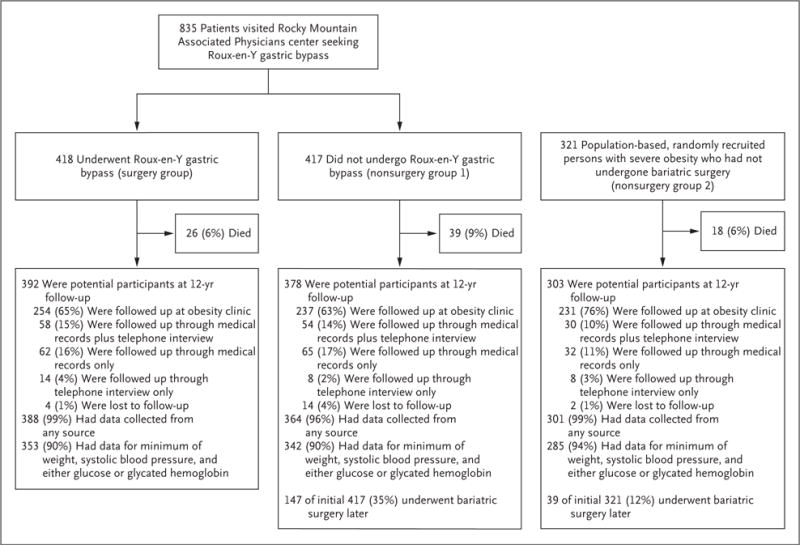
Study Design and 12-Year Follow-up Rates.
STATISTICAL ANALYSIS
Biochemical and blood pressure variables that were known to be affected by certain medications were adjusted to their estimated premedication levels for patients who were receiving such medication during the course of the study, as described previously.11 Covariates used for this adjustment included sex, age, baseline body-mass index, marital status, income, and educational level. Log transformations were applied to glucose levels, insulin levels, glycated hemoglobin levels, levels of insulin resistance as measured with the use of homeostatic model assessment, and triglyceride levels. Changes in each outcome variable were compared between the surgery group and each of the two nonsurgery groups after adjustment for the baseline level of the outcome variable and the six covariates. Logistic regression was used to analyze the between-group differences in the incidence and remission rates of type 2 diabetes, dyslipidemia, and hypertension. Data from the patients who had a prevalent disease at baseline were excluded from the analyses of incidence, and data only from the patients who had a prevalent disease at baseline were used for the analyses of remission rates. Adjustments for multiple comparisons were performed as described in the Supplementary Appendix. Because this was an observational study, the possibility of unmeasured confounding cannot be excluded. However, as detailed in the Supplementary Appendix, multiple issues related to study validity have been addressed.
RESULTS
FOLLOW-UP PARTICIPATION
After excluding deceased patients, we obtained at least some clinical follow-up data at 12 years for 388 of 392 patients (99%) in the surgery group, 364 of 378 patients (96%) in nonsurgery group 1, and 301 of 303 patients (99%) in nonsurgery group 2 (Fig. 1). Weight, blood pressure, and either a glucose level or a glycated hemoglobin level were measured for 353 of 392 patients (90%) in the surgery group, 342 of 378 patients (90%) in nonsurgery group 1, and 285 of 303 patients (94%) in nonsurgery group 2, and for patients whose clinical measurements were not available, medical end points were obtained by telephone interview or by medical record review. During the course of the 12-year follow-up period, a total of 147 of the 417 patients (35%) in nonsurgery group 1 and 39 of the 321 patients (12%) in nonsurgery group 2 subsequently underwent bariatric surgery.
CLINICAL DATA
Unadjusted mean baseline and 12-year values for the clinical variables are shown in Table 1. For each of the two nonsurgery groups, results of analyses performed with and without data from surviving patients who later had bariatric surgery are reported. Values for the clinical variables at 2 years and at 6 years were reported previously, although additional 2-year and 6-year data from some patients were obtained at 12 years and were included in the current analysis.11,12 The mean unadjusted change from baseline in body weight in the surgery group was −46.8 kg (95% confidence interval [CI], −48.0 to −45.5; mean percent change, −35.0) at 2 years, as compared with −37.3 kg (95% CI, −38.8 to −35.8; mean percent change, −28.0) at 6 years and −35.5 kg (95% CI, −37.2 to −33.7; mean percent change, −26.9) at 12 years. The mean unadjusted change in body weight from baseline to year 12 in nonsurgery groups 1 and 2 was −2.9 kg (95% CI, −5.2 to −0.5; mean percent change, −2.0) and −1.0 kg (95% CI, −3.2 to 1.1; mean percent change, −0.9), respectively, among patients who did not later undergo bariatric surgery.
Table 1.
Unadjusted Mean Baseline and 12-Year Follow-up Values for Clinical Variables, According to Study Group.*
| Variable | Surgery Group | Nonsurgery Group 1 | Nonsurgery Group 2 | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Years | Baseline | 12 Years | Baseline | 12 Years | |||
| Excluding Patients Who Underwent Subsequent Bariatric Surgery | Including Patients Who Underwent Subsequent Bariatric Surgery | Excluding Patients Who Underwent Subsequent Bariatric Surgery | Including Patients Who Underwent Subsequent Bariatric Surgery | |||||
| Age — yr | ||||||||
| No. with data | 418 | 388 | 417 | 217 | 364 | 321 | 262 | 301 |
| Mean (95% CI) | 42.5 (41.5 to 43.6) |
54.1 (53.0 to 55.2) |
42.9 (41.9 to 44.0) |
54.8 (53.3 to 56.2) |
53.9 (52.8 to 54.9) |
49.4 (48.1 to 50.6)† |
60.6 (59.3 to 62.0)† |
60.3 (59.1 to 61.6)† |
| Female sex — % | 84 | 85 | 84 | 88 | 85 | 76 | 76 | 77 |
| Weight — kg | ||||||||
| No. with data | 418 | 387 | 417 | 217 | 363 | 321 | 262 | 301 |
| Mean (95% CI) | 133.9 (131.5 to 136.3) |
97.5 (94.7 to 100.2) |
129.8 (127.4 to 132.2)‡ |
122.9 (119.2 to 126.6)† |
115.1 (112.1 to 118.0)† |
124.0 (121.2 to 126.7)† |
122.2 (118.8 to 125.6)† |
119.5 (116.3 to 122.7)† |
| Change from baseline weight — % | ||||||||
| No. with data | – | 387 | – | 217 | 363 | – | 262 | 301 |
| Mean (95% CI) | – | −26.9 (−28.2 to −25.6) |
– | −2.0 (−3.8 to−0.3)† |
−10.0 (−11.6 to −8.5)† |
– | −0.9 (−2.5 to −0.6)† |
−3.5 (−5.2 to —1.8)† |
| Systolic blood pressure — mm Hg | ||||||||
| No. with data | 418 | 369 | 417 | 211 | 353 | 321 | 253 | 291 |
| Mean (95% CI) | 126.3 (124.6 to 128.1) |
120.9 (119.0 to 122.7) |
125.6 (123.8 to 127.4) |
126.7 (124.3 to 129. l)† |
124.0 (122.1 to 125.9)‡ |
128.8 (126.8 to 130.8) |
127.2 (125.0 to 129.4)† |
126.8 (124.7 to 128.8)† |
| Diastolic blood pressure — mm Hg | ||||||||
| No. with data | 418 | 369 | 417 | 211 | 353 | 321 | 253 | 291 |
| Mean (95% CI) | 71.9 (70.9 to 73.0) |
72.1 (71.0 to 73.2) |
72.0 (70.9 to 73.0) |
73.6 (72.2 to 75.1) |
73.0 (71.9 to 74.1) |
72.3 (71.1 to 73.5) |
71.1 (69.8 to 72.4) |
71.0 (69.8 to 72.2) |
| Glucose — mg/dl | ||||||||
| No. with data | 415 | 356 | 417 | 201 | 336 | 321 | 245 | 281 |
| Mean (95% CI) | 101.4 (98.1 to 104.8) |
91.7 (87.4 to 96.0) |
106.8 (103.4 to 110.1)‡ |
113.8 (108.1 to 119.5)† |
106.2 (101.7 to 110.8)† |
107.5 (103.7 to 111.3)† |
111.9 (106.7 to 117.1)† |
110.9 (106.0 to 115.9)† |
| Glycated hemoglobin — % | ||||||||
| No. with data | 416 | 296 | 412 | 179 | 296 | 319 | 232 | 267 |
| Mean (95% CI) | 5.8 (5.7 to 5.9) |
5.7 (5.5 to 5.9) |
6.0 (5.9 to 6.1)‡ |
6.5 (6.3 to 6.7)† |
6.2 (6.0 to 6.3)† |
6.0 (5.8 to 6.1)‡ |
6.5 (6.3 to 6.7)† |
6.5 (6.3 to 6.6)† |
| LDL cholesterol — mg/dl | ||||||||
| No. with data | 417 | 310 | 416 | 175 | 301 | 321 | 228 | 264 |
| Mean (95% CI) | 108.8 (106.2 to 111.4) |
95.8 (92.2 to 99.3) |
106.7 (104.1 to 109.3) |
104.5 (99.7 to 109.2)§ |
102.3 (98.8 to 105.9)‡ |
109.3 (106.3 to 112.3) |
100.7 (96.5 to 104.8) |
100.5 (96.7 to 104.3) |
| HDL cholesterol — mg/dl | ||||||||
| No. with data | 417 | 311 | 416 | 182 | 308 | 321 | 235 | 271 |
| Mean (95% CI) | 46.6 (45.5 to 47.7) |
61.5 (59.7 to 63.3) |
44.8 (43.7 to 45.8)‡ |
48.1 (45.7 to 50.5)† |
51.5 (49.7 to 53.4)† |
47.0 (45.8 to 48.2) |
47.4 (45.3 to 49.5)† |
48.2 (46.3to50.2)† |
| Triglycerides — mg/dl | ||||||||
| No. with data | 417 | 310 | 416 | 181 | 308 | 321 | 231 | 267 |
| Mean (95% CI) | 185.7 (172.7 to 198.7) |
103.3 (95.8 to 110.9) |
192.5 (179.5 to 205.5) |
156.9 (147.0 to 166.8)† |
140.4 (133.0 to 147.9)† |
186.0 (171.2 to 200.8) |
146.1 (137.3 to 154.8)† |
142.9 (134.9 to 150.8)† |
The surgery group comprised patients with severe obesity who sought and underwent gastric bypass. Nonsurgery group 1 comprised patients with severe obesity who sought but did not undergo gastric bypass (primarily for insurance reasons). Nonsurgery group 2 comprised a population-based sample of adults with severe obesity who were randomly recruited in the study. At baseline, the sample sizes of the surgery group and nonsurgery groups 1 and 2 include data from all the patients examined. At the 12-year follow-up, the sample size for all the groups does not include data from the patients who died or were lost to follow-up. No adjustment of the confidence intervals or significance levels was performed for multiple comparisons. To convert the values for glucose to millimoles per liter, multiply by 0.05551. To convert the values for cholesterol to millimoles per liter, multiply by 0.02586. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129. CI denotes confidence interval, LDL low-density lipoprotein, and HDL high-density lipoprotein.
P<0.001 for the comparison with the surgery group.
P<0.05 for the comparison with the surgery group.
P<0.01 for the comparison with the surgery group.
Table 2 shows the adjusted mean changes from baseline in the clinical variables at 12 years (12-year value minus baseline value). As with the unadjusted values, the adjusted values at 2 years and at 6 years were reported previously.11,12 The mean change from baseline in body weight in the surgery group was −45.0 kg (95% CI, −47.2 to −42.9; mean percent change, −35.0) at 2 years, −36.3 kg (95% CI, −39.0 to −33.5; mean percent change, −28.0) at 6 years, and −35.0 kg (95% CI, −38.4 to −31.7; mean percent change, −26.9) at 12 years; the mean change at 12 years in nonsurgery group 1 was −2.9 kg (95% CI, −6.9 to 1.0; mean percent change, −2.0), and the mean change at 12 years in nonsurgery group 2 was 0 kg (95% CI, −3.5 to 3.5; mean percent change, −0.9).
Table 2.
Adjusted Mean Change from Baseline at 12 Years, According to Study Group.*
| Variable | Surgery Group Mean Change (95% CI) | Nonsurgery Group 1 Mean Change (95% CI) | Nonsurgery Group 2 Mean Change (95% CI) | ||
|---|---|---|---|---|---|
| Excluding Patients Who Underwent Subsequent Bariatric Surgery | Including Patients Who Underwent Subsequent Bariatric Surgery | Excluding Patients Who Underwent Subsequent Bariatric Surgery | Including Patients Who Underwent Subsequent Bariatric Surgery | ||
| Weight — kg | −35.0 (−38.4 to −31.7) |
−2.9 (−6.9 to 1.0)† |
−12.7 (−16.4 to −9.0)† |
0 (−3.5 to 3.5)† |
−3.4 (−7.2 to 0.4)† |
| Body-mass index $ | −11.5 (−12.7 to−10.3) |
0.1 (−1.3 to 1.5)† |
−3.4 (−4.7 to −2.0)† |
1.2 (−0.1 to 2.4)† |
−0.1 (−1.5 to 1.3)† |
| Systolic blood pressure — mm Hg | 0.1 (−3.7 to 3.8) |
10.1 (5.5 to 14.8)† |
6.5 (2.8 to 10.2)† |
8.3 (4.2 to 12.5)† |
7.2 (3.3 to 11.1) † |
| Diastolic blood pressure — mm Hg | 3.1 (0.5 to 5.7) |
10.0 (6.9 to 13.2)† |
7.7 (5.2 to 10.2)† |
7.5 (4.7 to 10.4)§ |
6.8 (4.1 to 9.4)¶ |
| Glucose — mg/dl | −8.0 (−15.5 to−0.5) |
14.4 (5.1 to 23.7)† |
4.7 (−2.8 to 12.1)† |
10.5 (2.3 to 18.7)† |
7.4 (−0.4 to 15.2)† |
| Glycated hemoglobin — % | 0 (−0.3 to 0.2) |
0.4 (0.2 to 0.7)† |
0.2 (0 to 0.4) |
0.5 (0.3 to 0.8)†− |
0.4 (0.2 to 0.6)† |
| LDL cholesterol — mg/dl | −11.0 (−18.2 to −3.8) |
19.3 (10.5 to 28.2)† |
13.8 (6.7 to 20.9)† |
16.5 (8.9 to 24.2)† |
14.3 (6.9 to 21.6)† |
| HDL cholesterol — mg/dl | 12.9 (9.9 to 16.0) |
−2.3 (−6.0 to 1.4)† |
0.8 (−2.4 to 3.9)† |
−3.3 (−6.5 to 1.4)† |
−2.6 (−5.7 to 0.6)† |
| Triglycerides — mg/dl | −62.8 (−79.0 to 46.6) |
11.2 (−8.6 to 31.0)† |
−6.5 (−32.0 to 19.1)† |
11.7 (−5.5 to 28.8)† |
−7.1 (−33.3 to 19.2)† |
Confidence intervals and significance levels were adjusted for multiple comparisons (see the Supplementary Appendix).
P<0.001 for the comparison with the surgery group.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
P<0.01 for the comparison with the surgery group.
P<0.05 for the comparison with the surgery group.
Figure 2 further illustrates weight change after Roux-en-Y gastric bypass in individual patients from baseline to years 2, 6, and 12. Despite a wide variation in change in body weight across the sample, 360 of 387 patients (93%) in the surgery group maintained at least a 10% weight loss from baseline to year 12, 271 (70%) maintained at least a 20% weight loss, and 155 (40%) maintained at least a 30% weight loss. Only 4 of 387 patients (1%) in the surgery group had regained all their postsurgical weight loss. Patients in nonsurgery groups 1 and 2 who did not later undergo bariatric surgery had no significant changes from baseline to year 12 in weight, body-mass index, or waist circumference.
Figure 2. Mean Percent Changes in Body Weight from Baseline to Years 2, 6, and 12.
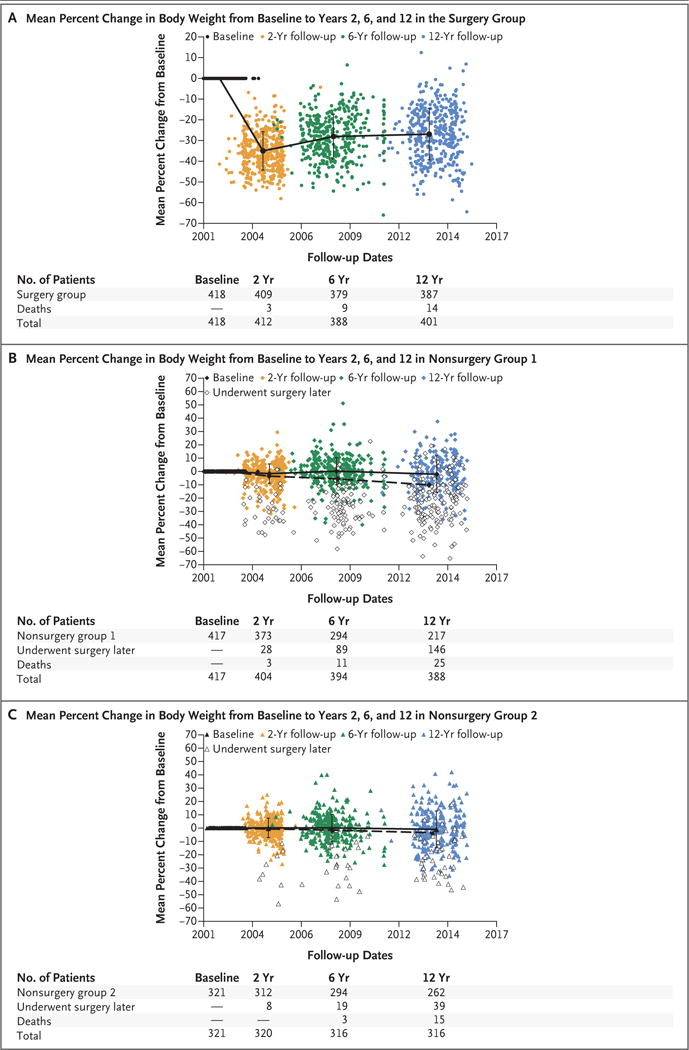
Mean percent changes in body weight from baseline to years 2, 6, and 12 are shown for the surgery group (Panel A), nonsurgery group 1 (Panel B), and nonsurgery group 2 (Panel C). The patients in the nonsurgery groups (Panels B and C) who later underwent any type of bariatric surgery (including adjustable lap band) are represented as open symbols; in addition, the solid line represents patients in the nonsurgery groups who did not later undergo bariatric surgery, and the dashed line represents all the patients in the nonsurgery groups (i.e., patients who did not later undergo bariatric surgery and patients who later chose to undergo bariatric surgery combined). Among the 147 patients in non-surgery group 1 who later underwent bariatric surgery, body weight was not available for 1 patient at the 12-year follow-up examination.
INCIDENCE, REMISSION RATE, AND MORTALITY RATE
Table 3 shows that the 12-year incidence of type 2 diabetes was 3% (8 of 303 patients) in the surgery group, as compared with 26% (42 of 164 patients) in nonsurgery group 1 and 26% (47 of 184 patients) in nonsurgery group 2. The adjusted odds ratio for the incidence of type 2 diabetes in the surgery group versus nonsurgery group 1 was 0.08 (95% CI, 0.03 to 0.24; P<0.001), and the adjusted odds ratio in the surgery group versus nonsurgery group 2 was 0.09 (95% CI, 0.03 to 0.29; P<0.001). The incidence rates of hypertension and dyslipidemia were also significantly lower in the surgery group than in each of the two nonsurgery groups (Table 3).
Table 3.
Incidence and Remission Rates at 12 Years for Type 2 Diabetes, Hypertension, and Dyslipidemia, According to Study Group.*
| End Point | Surgery Group | Nonsurgery Group 1 | Nonsurgery Group 2 | Surgery Group vs. Nonsurgery Group 1 | Surgery Group vs. Nonsurgery Group 2 | |||
|---|---|---|---|---|---|---|---|---|
| No./Total No. | % (95% CI) | No./Total No. | % (95% CI) | No./Total No. | % (95% CI) | Adjusted Odds Ratio (95% CI)† | Adjusted Odds Ratio (95% CI)† | |
| Incidence at 12 years | ||||||||
| Type 2 diabetes | 8/303 | 3 (0 to 5) | 42/164 | 26 (16 to 35) | 47/184 | 26 (17 to 35) | 0.08 (0.03 to 0.24)‡ |
0.09 (0.03 to 0.29)‡ |
| Hypertension | 37/226 | 16 (9 to 23) | 51/123 | 41 (29 to 54) | 61/131 | 47 (34 to 59) | 0.23 (0.11 to 0.49)‡ |
0.23 (0.11 to 0.51)‡ |
| Low H DL cholesterol | 7/234 | 3 (0 to 6) | 22/130 | 17 (8 to 26) | 28/170 | 16 (8 to 24) | 0.12 (0.03 to 0.46)‡ |
0.16 (0.04 to 0.6)‡ |
| High LDL cholesterol | 53/312 | 17 (11 to 23) | 93/185 | 50 (40 to 61) | 119/213 | 56 (46 to 65) | 0.17 (0.09 to 0.31)‡ |
0.19 (0.1 to 0.36)‡ |
| High triglycerides | 3/225 | 1 (−1 to 3) | 11/137 | 8 (2 to 15) | 12/153 | 8 (2 to 14) | 0.15 (0.02 to 0.97)§ |
0.17 (0.02 to 1.15) |
| Remission at 12 years | ||||||||
| Type 2 diabetes | 43/84 | 51 (36 to 67) | 5/52 | 10 (−2 to 21) | 4/76 | 5 (−2 to 12) | 8.9 (2.0 to 40.0)‡ |
14.8 (2.9 to 75.5)‡ |
| Hypertension | 59/162 | 36 (26 to 47) | 9/93 | 10 (1 to 18) | 18/130 | 14 (5 to 22) | 5.1 (1.7 to 15.6)‡ |
2.4 (0.9 to 5.9) |
| Low HDLcholesterol | 127/154 | 82 (74 to 91) | 48/87 | 55 (40 to 70) | 49/92 | 53 (39 to 68) | 3.8 (1.6 to 9.3)‡ |
3.3 (1.3 to 8.1)¶ |
| High LDL cholesterol | 45/76 | 59 (43 to 75) | 6/32 | 19 (−1 to 38) | 3/49 | 6 (−4 to 16) | 7.1 (1.6 to 31.7)¶ |
18.6 (2.8 to 124.2)‡ |
| High triglycerides | 154/163 | 94 (89 to 100) | 44/80 | 55 (39 to 71) | 78/109 | 72 (59 to 84) | 14.7 (4.5 to 48.4)‡ |
7.0 (2.1 to 23.4)‡ |
The table excludes data from patients who died, patients who were lost to follow-up, and patients who later chose to undergo any type of bariatric surgical procedure.
The odds ratios are adjusted for age, sex, baseline body-mass index, income, education level, and marital status. Confidence intervals and significance levels were adjusted for multiple comparisons (see the Supplementary Appendix).
P<0.001 for the comparison with the surgery group.
P<0.05 for the comparison with the surgery group.
P<0.01 for the comparison with the surgery group.
In the surgery group, remission of type 2 diabetes was observed in 66 of 88 patients (75%) at 2 years, in 54 of 87 patients (62%) at 6 years,11,12 and in 43 of 84 patients (51%) at 12 years (Table 3). Of the 62 patients in the surgery group who had initial remission at 2 years and had 12-year follow-up data, 69% remained free of type 2 diabetes at 12 years. When the remission rate of type 2 diabetes at 12 years in the surgery group was compared with that in the nonsurgery groups, the adjusted odds ratio for remission was 8.9 (95% CI, 2.0 to 40.0) for the surgery group versus nonsurgery group 1 and 14.8 (95% CI, 2.9 to 75.5) for the surgery group versus nonsurgery group 2 (P<0.001 for both comparisons) (Table 3).
Successful remission of type 2 diabetes was strongly predicted by baseline medication status. Remission of type 2 diabetes at 12 years was observed in 16 of 22 patients in the surgery group (73%; 95% CI, 46 to 99) who had type 2 diabetes but had not been receiving antidiabetic medications at baseline, as compared with 24 of 43 patients with diabetes (56%; 95% CI, 35 to 77) who had been receiving only oral medications at baseline and 3 of 19 patients with diabetes (16%; 95% CI, −8 to 39) who had been receiving insulin (with or without additional oral antidiabetic medication) at baseline. The odds ratios for patients who had received oral medications only versus those who had received no medication and for patients who had received insulin versus those who had received no medication were both significant (P<0.001, and P = 0.007 for trend across the three medication-status groups). Among the patients who had type 2 diabetes both at baseline and at the 12-year follow-up, improvement was still evident, with a decreased mean (±SD) number of antidiabetic medications from baseline to 12 years in the surgery group (−0.3±1.4), as compared with increases in the mean numbers of antidiabetic medications from baseline to 12 years in nonsurgery group 1 (0.8±1.4, P = 0.002 by the Kruskal–Wallis test) and in nonsurgery group 2 (1.1±1.3, P<0.001 by the Kruskal–Wallis test).
The remission rate of hypertension in the surgery group was significantly higher than the rate in nonsurgery group 1 (adjusted odds ratio, 5.1; 95% CI, 1.7 to 15.6; P<0.001) but was not significantly higher than the rate in nonsurgery group 2 (adjusted odds ratio, 2.4; 95% CI, 0.9 to 5.9) at 12 years. Furthermore, the remission rates of the three variables contributing to dyslipidemia (high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides) were significantly higher in the surgery group than in each of the nonsurgery groups, with adjusted odds ratios varying from 3.3 (95% CI, 1.3 to 8.1) to 18.6 (95% CI, 2.8 to 124.2) (Table 3).
Details on all-cause and cause-specific mortality rates at the 12-year follow-up are provided in the Supplementary Appendix. There were 7 deaths by suicide (5 in the surgery group and 2 in nonsurgery group 1); both suicide deaths in nonsurgery group 1 occurred after the patients had undergone bariatric surgery.
DISCUSSION
The 12-year results of this controlled, prospective study show that Roux-en-Y gastric bypass offered long-term durability of weight loss and was associated with fewer obesity-related coexisting conditions than among patients who did not undergo gastric bypass. The mean percent weight loss in the surgery group remained stable between 6 years (28.0% weight loss) and 12 years (26.9%). Furthermore, at 12 years, incident type 2 diabetes was still uncommon among patients who underwent Roux-en-Y gastric bypass, and the remission rate of type 2 diabetes also remained high. Remission of type 2 diabetes was much more likely if the Roux-en-Y gastric bypass occurred before the onset of treatment with insulin, presumably owing to the ability of partially viable beta cells to improve their function. Clinical variables related to metabolic health (glucose levels, glycated hemoglobin levels, systolic blood pressure, and lipid levels) as well as remission and incidence rates of both hypertension and dyslipidemia were significantly more favorable in the surgery group than in the nonsurgery groups.
Another published long-term, prospective, controlled study of bariatric surgery, the Swedish Obese Subjects (SOS) study, had a 13.4-year recruitment period.15 Beginning in 1987, participants in the study underwent primarily vertical banded gastroplasty (which is no longer performed), although later in the recruitment process, Roux-en-Y gastric bypass was performed more often than vertical banded gastroplasty. By the end of the recruitment process, patients in the surgery group who underwent Roux-en-Y gastric bypass (265 patients) represented only 13.2% of the patients in the surgery group.16,17 In the SOS study, the Roux-en-Y gastric bypass group at 10 years (34 patients) had a weight change of −25%,18 which is similar to the −26.9% weight change at 12 years in the current study (387 patients). The leveling off of weight regain between 6 years (intermediate-term) and 12 years (long-term) in the current study was also seen in the SOS study between 10 years and 15 years.15
In a retrospective cohort of 1787 veterans (of whom 73.1% were men) who had undergone Roux-en-Y gastric bypass and were matched with 5305 participants who had not undergone such surgery, the 564 veterans who had undergone gastric bypass and were seen at the 10-year follow-up had a mean weight change of −28.6%, which, again, was very similar to the weight change in our study.19 Furthermore, at 10 years, 72% of the patients who had undergone Roux-en-Y gastric bypass had maintained at least a 20% weight loss from baseline, and 40% had maintained at least a 30% weight loss,19 which was also nearly identical to the results at year 12 in the current study. We note the emerging use of sleeve gastrectomy as an alternative to Roux-en-Y gastric bypass and a decreasing use of the adjustable gastric band procedure. However, few data are available on the long-term benefit and risk of sleeve gastrectomy.
Multiple short-term studies2,5–8,11,15,18,20–27 have shown significant remission rates, lower incidence rates, or both, of type 2 diabetes after bariatric surgery. Given the value of longer-term follow-up, our U.S. study, the SOS study, and a study with a 10-year follow-up involving 22 patients who underwent biliopancreatic diversion26 are of interest, since these studies have followed patients for more than 5 years with respect to end points associated with type 2 diabetes. Although the remission rate of type 2 diabetes in the SOS study was 72% after 2 years, the rate fell to 36% at 10 years.18 In comparison, the remission rates of type 2 diabetes in our study were 75% at 2 years, 62% at 6 years, and 51% at 12 years. These longer-term differences in remission between the two studies may be attributable to the exclusive use of the Roux-en-Y gastric bypass procedure in our study as compared with the primary use of vertical banded gastroplasty and the limited use of Roux-en-Y gastric bypass in the SOS study.17,18 In the current study, the remission rate of type 2 diabetes after Roux-en-Y gastric bypass was much higher among the patients with diabetes who had not received anti-diabetic treatment at baseline than among the patients with diabetes for whom insulin had already been prescribed and was still significantly higher among the patients with diabetes who had received only oral medications at baseline than among the patients with diabetes for whom insulin had already been prescribed. Thus, it is intuitive to suggest that the more advanced the type 2 diabetes, the less the glycemic benefit from Roux-en-Y gastric bypass.
In the current study, Roux-en-Y gastric bypass resulted in a 91 to 92% lower incidence of new-onset type 2 diabetes at 12 years than that among patients in the nonsurgery groups. The low incidence of type 2 diabetes may be a result of the combined effects of a reduction in insulin resistance and appropriate increases in insulin secretion after surgery. After a median follow-up of 10 years in the SOS study, the incidence of type 2 diabetes was 83% lower among all the patients who underwent surgery and 88% lower among patients who underwent Roux-en-Y gastric bypass than among patients in the control group.22
Deaths by suicide occurred only among patients in the surgery group or among patients in nonsurgery group 1 after they underwent bariatric surgery, a finding consistent with the 2-year and 6-year follow-up results of our study.11,12 The possible association of suicide and bariatric surgery was reviewed across 28 studies.28 The review showed that suicides, self-harm emergencies, or both were higher among patients who had undergone bariatric surgery than among persons in the general population, persons in control groups, and presurgical patients.29–31 Potential risk factors for suicide after bariatric surgery included age younger than 35 years32; hormonal changes; persistence of coexisting conditions; preexisting depression and other mood disorders; worsening or lack of improvement in health-related quality of life; social, sexual, and relationship issues; poor body image; and a history of maltreatment during childhood.33 Furthermore, the reduced bioavailability of some serotonin reuptake inhibitors 1 month after gastric bypass34 and an association between binge-eating disorder before bariatric surgery and the use of psychiatric-related medications35 have been reported. Whether the increase in suicides is attributable solely to bariatric surgery itself or whether any large, sustained weight loss would also be associated with an increased risk of suicide is unknown. On the basis of the results of the current study and of other reports of increased self-harm after bariatric surgery,28,33,36–40 there is an apparent pressing, unmet need to better predict and prevent this uncommon but very serious sequela of bariatric surgery.36,40
In conclusion, the results from the current 12-year follow-up of a U.S.-based, long-term, prospective study of bariatric surgery indicate long-term durability of weight loss after Roux-en-Y gastric bypass. The weight increase between the 6-year and 12-year follow-up was minimal, near-complete prevention of new-onset type 2 diabetes was observed, and the remission rate of type 2 diabetes 12 years after surgery was 51%. Substantial improvement was also seen in systolic hypertension and lipid levels.
Supplementary Material
Acknowledgments
Supported by a grant (DK-55006) from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health, a U.S. Public Health Service research grant (MO1-RR00064) from the National Center for Research Resources, Biomedical Research Program funds from Weill Cornell Medicine, and Intermountain Healthcare.
Dr. Kolotkin reports receiving royalties for licensing of “Impact of Weight on Quality of Life-Lite questionnaire (IWQOL-Lite)” from Duke University; and Dr. Ibele, receiving fees for services rendered during the Oblation Intragastric Balloon System trial from Obalon Therapeutics.
We thank the staff of the Division of Cardiovascular Genetics for their assistance with this study, including Loni Gardner, Sara A. Wilkins, Sally I. Bradstreet, and Sawsan Ibrahim; and Ray Wilde, Director of Rocky Mountain Associated Physicians. We gratefully acknowledge the strong contributions to this study and to the field of bariatric surgery in general of Drs. Sherman Smith and R. Chad Halversen (two of the five bariatric surgeons who operated on patients who participated in this study), who died during the 12-year follow-up period.
Footnotes
No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Kremen AJ, Linner JH, Nelson CH. An experimental evaluation of the nutritional importance of proximal and distal small intestine. Ann Surg. 1954;140:439–48. doi: 10.1097/00000658-195409000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes — 5-year outcomes. N Engl J Med. 2017;376:641–51. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–23. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 4.Liang Z, Wu Q, Chen B, Yu P, Zhao H, Ouyang X. Effect of laparoscopic Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus with hypertension: a randomized controlled trial. Diabetes Res Clin Pract. 2013;101:50–6. doi: 10.1016/j.diabres.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386:964–73. doi: 10.1016/S0140-6736(15)00075-6. [DOI] [PubMed] [Google Scholar]
- 6.Halperin F, Ding SA, Simonson DC, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg. 2014;149:716–26. doi: 10.1001/jamasurg.2014.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Courcoulas AP, Belle SH, Neiberg RH, et al. Three-year outcomes of bariatric surgery vs lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg. 2015;150:931–40. doi: 10.1001/jamasurg.2015.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikramuddin S, Billington CJ, Lee WJ, et al. Roux-en-Y gastric bypass for diabetes (the Diabetes Surgery Study): 2-year outcomes of a 5-year, randomised, controlled trial. Lancet Diabetes Endocrinol. 2015;3:413–22. doi: 10.1016/S2213-8587(15)00089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams RR, Hunt SC, Barlow GK, et al. Health family trees: a tool for finding and helping young family members of coronary and cancer prone pedigrees in Texas and Utah. Am J Public Health. 1988;78:1283–6. doi: 10.2105/ajph.78.10.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams TD, Avelar E, Cloward T, et al. Design and rationale of the Utah obesity study: a study to assess morbidity following gastric bypass surgery. Contemp Clin Trials. 2005;26:534–51. doi: 10.1016/j.cct.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Adams TD, Davidson LE, Litwin SE, et al. Health benefits of gastric bypass surgery after 6 years. JAMA. 2012;308:1122–31. doi: 10.1001/2012.jama.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams TD, Pendleton RC, Strong MB, et al. Health outcomes of gastric bypass patients compared to nonsurgical, non-intervened severely obese. Obesity (Silver Spring) 2010;18:121–30. doi: 10.1038/oby.2009.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith SC, Edwards CB, Goodman GN, Halversen RC, Simper SC. Open vs laparoscopic Roux-en-Y gastric bypass: comparison of operative morbidity and mortality. Obes Surg. 2004;14:73–6. doi: 10.1381/096089204772787329. [DOI] [PubMed] [Google Scholar]
- 14.Horm J. Assignment of probabilistic scores to National Death Index record matches. In: Bildgrad R, editor. National Death Index Plus: coded causes of death. Hyattsville, MD: Division of Vital Statistics, National Center for Health Statistics; 1996. pp. A5–A12. [Google Scholar]
- 15.Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial — a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219–34. doi: 10.1111/joim.12012. [DOI] [PubMed] [Google Scholar]
- 16.Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 17.Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311:2297–304. doi: 10.1001/jama.2014.5988. [DOI] [PubMed] [Google Scholar]
- 18.Sjöström L, Lindroos A-K, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–93. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 19.Maciejewski ML, Arterburn DE, Van Scoyoc L, et al. Bariatric surgery and long-term durability of weight loss. JAMA Surg. 2016;151:1046–55. doi: 10.1001/jamasurg.2016.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–256.e5. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 21.Aminian A, Brethauer SA, Andalib A, et al. Can sleeve gastrectomy “cure” diabetes? Long-term metabolic effects of sleeve gastrectomy in patients with type 2 diabetes. Ann Surg. 2016;264:674–81. doi: 10.1097/SLA.0000000000001857. [DOI] [PubMed] [Google Scholar]
- 22.Carlsson LMS, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med. 2012;367:695–704. doi: 10.1056/NEJMoa1112082. [DOI] [PubMed] [Google Scholar]
- 23.Arterburn DE, Bogart A, Sherwood NE, et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg. 2013;23:93–102. doi: 10.1007/s11695-012-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart. 2012;98:1763–77. doi: 10.1136/heartjnl-2012-301778. [DOI] [PubMed] [Google Scholar]
- 25.Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus nonsurgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. doi: 10.1136/bmj.f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iaconelli A, Panunzi S, De Gaetano A, et al. Effects of bilio-pancreatic diversion on diabetic complications: a 10-year follow-up. Diabetes Care. 2011;34:561–7. doi: 10.2337/dc10-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Purnell JQ, Selzer F, Wahed AS, et al. Type 2 diabetes remission rates after laparoscopic gastric bypass and gastric banding: results of the Longitudinal Assessment of Bariatric Surgery Study. Diabetes Care. 2016;39:1101–7. doi: 10.2337/dc15-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterhänsel C, Petroff D, Klinitzke G, Kersting A, Wagner B. Risk of completed suicide after bariatric surgery: a systematic review. Obes Rev. 2013;14:369–82. doi: 10.1111/obr.12014. [DOI] [PubMed] [Google Scholar]
- 29.Tindle HA, Omalu B, Courcoulas A, Marcus M, Hammers J, Kuller LH. Risk of suicide after long-term follow-up from bariatric surgery. Am J Med. 2010;123:1036–42. doi: 10.1016/j.amjmed.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhatti JA, Nathens AB, Thiruchelvam D, Grantcharov T, Goldstein BI, Redelmeier DA. Self-harm emergencies after bariatric surgery: a population-based cohort study. JAMA Surg. 2016;151:226–32. doi: 10.1001/jamasurg.2015.3414. [DOI] [PubMed] [Google Scholar]
- 31.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–61. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 32.Davidson LE, Adams TD, Kim J, et al. Association of patient age at gastric bypass surgery with long-term all-cause and cause-specific mortality. JAMA Surg. 2016;151:631–7. doi: 10.1001/jamasurg.2015.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell JE, Crosby R, de Zwaan M, et al. Possible risk factors for increased suicide following bariatric surgery. Obesity (Silver Spring) 2013;21:665–72. doi: 10.1002/oby.20066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamad GG, Helsel JC, Perel JM, et al. The effect of gastric bypass on the pharmacokinetics of serotonin reuptake inhibitors. Am J Psychiatry. 2012;169:256–63. doi: 10.1176/appi.ajp.2011.11050719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell JE, King WC, Courcoulas A, et al. Eating behavior and eating disorders in adults before bariatric surgery. Int J Eat Disord. 2015;48:215–22. doi: 10.1002/eat.22275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dixon JB. Self-harm and suicide after bariatric surgery: time for action. Lancet Diabetes Endocrinol. 2016;4:199–200. doi: 10.1016/S2213-8587(16)00013-9. [DOI] [PubMed] [Google Scholar]
- 37.Backman O, Stockeld D, Rasmussen F, Näslund E, Marsk R. Alcohol and substance abuse, depression and suicide attempts after Roux-en-Y gastric bypass surgery. Br J Surg. 2016;103:1336–42. doi: 10.1002/bjs.10258. [DOI] [PubMed] [Google Scholar]
- 38.Lagerros YT, Brandt L, Hedberg J, Sundbom M, Bodén R. Suicide, self-harm, and depression after gastric bypass surgery: a nationwide cohort study. Ann Surg. 2017;265:235–43. doi: 10.1097/SLA.0000000000001884. [DOI] [PubMed] [Google Scholar]
- 39.Morgan DJ, Ho KM. Incidence and risk factors for deliberate self-harm, mental illness, and suicide following bariatric surgery: a state-wide population-based linked-data cohort study. Ann Surg. 2017;265:244–52. doi: 10.1097/SLA.0000000000001891. [DOI] [PubMed] [Google Scholar]
- 40.Courcoulas A. Who, why, and how? Suicide and harmful behaviors after bariatric surgery. Ann Surg. 2017;265:253–4. doi: 10.1097/SLA.0000000000002037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


