Abstract
This review describes recent progress in tolerance-inducing strategies across xenogeneic immunological barriers as well as the potential benefit of a tolerance strategy for islets and kidney xenotransplantation.
Recent findings
Using advanced gene editing technologies, xenotransplantation from multi-transgenic alpha-1,3-galactosyltransferase knockout pigs has demonstrated marked prolongation of renal xenograft survival, ranging from days to greater than several months for life-supporting kidneys, and >2 years in a heterotopic non-life-supporting cardiac xenograft model. Continuous administration of multiple immunosuppressive drugs has been required and attempts to taper immunosuppression have been unsuccessful. It appears likely that low levels of T cell-dependent antibodies and activation of innate responses are responsible for xenograft loss. Mixed chimerism and thymic transplantation approaches have achieved xenogeneic tolerance in pig-to-mouse models and both have recently been extended to pig-to-baboon models. Encouraging results have been reported, including persistence of macrochimerism, prolonged pig skin graft survival, donor-specific unresponsiveness in vitro and detection of recent T cell emigrants in vivo.
Summary
Although tolerance induction in vivo has not yet been achieved in pig-to-baboon models, recent results are encouraging that this goal will be attainable through genetic engineering of porcine donors.
Keywords: Immune tolerance, xenotransplantation, mixed chimerism, vascularized thymic transplantation, thymus transplantation
I. Introduction
CRISPR/CAS9 and other modern gene editing technologies have markedly improved the efficiency of gene manipulations in porcine xenograft source animals (1, 2). Utilizing these technologies, xenotransplantation from multi-transgenic alpha-1,3-galactosyltransferase knockout (GalT-KO) pigs has demonstrated marked prolongation of renal xenograft survival, ranging from days to greater than six months in a life-supporting model (3, 4), >6 months in islets in induced diabetes in non-human primates (5) (6) (7–9), and >2 years in a heterotopic non-life-supporting cardiac xenograft model (10). Xenotransplantation is thus becoming a more realistic strategy for solving the organ shortage crisis. However, in order to prevent rejection, continuous administration of high dose anti-CD40 or anti-CD154 mAb-based immunosuppression, combined with multiple immunosuppressive drugs, has been required. The recipients eventually died from infection associated with chronic immunosuppression or from graft rejection (3, 4, 10, 11). Attempts to taper immunosuppression have been unsuccessful (12). These data are consistent with previous reports (13, 14) indicating that the human-anti-porcine T-cell response is similar or stronger than that across allogeneic barriers. Because of the strength of both innate and adaptive immunity in xenotransplantation, the level of continuous immunosuppression needed to control these immune responses and prolong xenograft survival has been associated with prohibitive morbidity and mortality (3, 4, 6, 7). These results provide compelling rationale to pursue a clinically applicable strategy for the induction of tolerance. In this review we summarize attempts to date toward successful tolerance induction in xenotransplantation.
II. Tolerance-inducing strategies across xenogeneic immunological barriers
Three successful tolerance induction approaches have been explored in large animals models: the use of mixed hematopoietic chimerism (15–17), T regulatory cells (18) and thymic transplantation (19–22). It has been demonstrated that tolerance is possible in humans by successful clinical application of the mixed chimerism approach to renal transplantation (22, 23) and by the T regulatory cell approach to liver allografts (24). Despite the greater immunologic differences between species than within species, both mixed chimerism and thymic transplantation approaches have been shown to be capable of tolerizing human T cells to porcine xenografts in humanized mouse models(20, 25, 26), suggesting the feasibility of pig-to-primate xenograft tolerance.
a) Induction of tolerance in xenogeneic T cells – Thymic transplantation
Prior to overcoming HAR, there was early speculation that xenogeneic T-cell responses would be less severe than allogeneic responses (27). Early in vitro studies of mouse anti-primate responses revealed (i) primary xenogeneic helper responses were absent, whereas primary allogeneic responses were brisk, and (ii) secondary xenogeneic helper responses were dependent on CD4+ T cells and responder antigen-presenting cells (APCs) (27). However, studies done in the early 1990’s at the authors’ (13) and other (14) laboratories, demonstrated that the direct pathway of activation exists in the pig-to-human model. Our laboratory has demonstrated that human T-cells respond to xeno-MHC antigens as they do to allo-MHC antigens in mixed lymphocyte reaction (MLR) assays. Additionally, human-anti-pig T-cell responses appear to share similar antigen presenting cell (APC) requirements for stimulators (direct pathway) or responders (indirect pathway). The majority of the primary human-anti-pig xeno-responses are directed toward porcine MHC class II antigens. These involve interactions with human CD4 accessory molecules (13). Activation of CD4 T cells provides help to B cells and NK cells as well as CD8 cytotoxic T cell progenitors (28). These data are consistent with reports by Korsgren and colleagues which indicated that even a small number of T cells is sufficient to initiate rejection of porcine islets by macrophages in T-cell-deficient rodents (29). More recently, Shin et al. reported that pig islets, engrafted >500 days in non-human primates, were fully rejected by activated immune cells, particularly CD4+ and CD8+ T cells, when immunosuppressive maintenance drugs were discontinued (12). Therefore, strategies directed at inhibition of the direct and indirect pathways must be included for successful xenotransplantation between pigs and primates.
Thus far, thymic transplantation has proven to be a powerful strategy to induce T cell tolerance across both allogeneic and xenogeneic barriers in pig-to-pig, pig-to-mouse, pig-to-humanized mouse and pig-to-baboon models (19, 21, 22, 30–33). T-cell education occurs in the thymus and the thymus is known to play an integral role in self-tolerance. Thymic transplantation is thought to lead to transplantation tolerance via central mechanisms.
Early studies in Dr. Sykes’s laboratory demonstrated that transplantation of fetal or neonatal pig thymic tissue to thymectomized mice produced tolerance to pig skin grafts (19, 34). Further studies showed that polyclonal, functional human T cells can develop in swine thymic tissue in humanized mice, and these cells exhibit donor-specific unresponsiveness and accept donor skin grafts (20, 25, 26), providing important proofs of principle in the pig-human combination. However, they also found that the homeostasis and function of peripheral human T cells derived from a pig thymus was suboptimal compared to those derived from a human thymus in the humanized mouse model (25). Moreover, in the pig to mouse model, sixty percent of grafted nude mice and 10% of grafted thymectomized B6 mice developed an illness resembling chronic graft-versus-host disease (35). This was shown to be due to the inability of pig thymus-derived Tregs to inhibit autoimmunity and to an increased propensity of pig thymus-derived effector cells to react to the mouse tissues. We hypothesized that both defects likely reflected a lack of host mouse tissue-specific antigens in the thymus, due to the absence of murine thymic epithelial cells, and hence a failure to positively select Tregs and negatively select effector T cells specific for murine tissue-specific antigens, whose thymic expression is limited to TECS. Indeed, both defects were corrected by the addition of murine thymic stromal cells, including TECs, to the porcine thymic graft (33, 36). In order to prevent development of autoimmunity and improve the peripheral function of human T cells developing in a porcine thymus graft, a similar strategy, involving incorporation of human thymic epithelial cells (TEC) in the grafted pig thymus, is being tested in the humanized mouse model as well as pig to a nonhuman primate model in the authors’ laboratories.
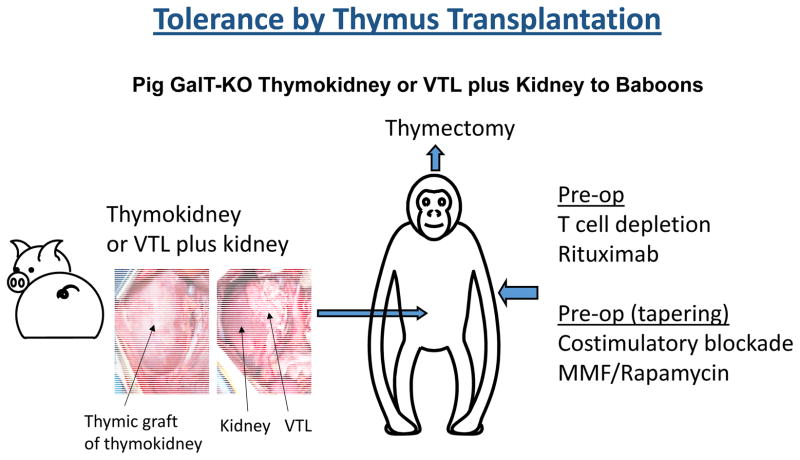
However, initial attempts to implant minced swine thymic tissue into primates in a similar manner failed, with evidence of graft rejection by days 15–30 (37). It was reasoned that since complete T cell depletion at similar levels to mouse models is not practical in non-human primates or clinically, the ischemic period of revascularization required for thymic tissue to become vascularized might lead to rejection of transplanted allogeneic or xenogeneic thymic tissue in large animals. Moreover, evidence that regulatory T cells developing in pig thymus grafts played a role in suppressing xenoreactivity of pre-existing T cells that were not depleted by conditioning(38) suggested that earlier engraftment and function of a porcine thymus graft would be beneficial. In order to eliminate the damage that occurs during revascularization and achieve more rapid function of the grafted thymus, we have developed two methods for vascularized thymic transplantation (39, 40); First is preparation of a composite “thymokidney” in which autologous thymic tissue is allowed to engraft for 1–2 months under the donor’s own kidney capsule before allogeneic or xenogeneic transplantation (39). The other is the “VTL procedure”, which is the transplantation of an isolated vascularized thymic lobe (VTL) (40). Using these two techniques, we have demonstrated that vascularized thymic tissue can successfully induce tolerance and support thymopoiesis across full allogeneic barriers in MGH miniature swine (21, 22, 32)(Figure 1a). Based on these encouraging results, this vascularized thymic transplant strategy has been extended to pig-to-baboon models of renal xenotransplantation. In initial studies, recipients of GalT-KO kidneys and vascularized thymic grafts from the same donors maintained normal creatinine levels up to 83 days, while GalT-KO kidneys without thymic grafts were rejected by 34-days, even with potent immunosuppression (41) (31). Recently, further modification of the induction regimen facilitated survival of life-supporting thymokidneys for over 6 months (30). Notably, multiple recipients bearing functional thymokidneys for over 3 months developed T cells with the phenotype of new thymic emigrants (CD4+/CD31+/CD45+) as well as donor-specific unresponsiveness at the T and B cell levels. Because recipients were completely thymectomized prior to transplantation, these thymic emigrants most likely developed in the vascularized thymic grafts of the composite thymokidneys (30).
Figure 1.
Schematic diagram of vascularized thymus plus kidney transplantation in pig-to-baboon models.
b) Mixed chimerism
Mixed chimerism approaches with transient chimerism by infusion of donor BM has been successful for induction of tolerance in clinical renal transplantation (42, 43). However, because of the activation of T cells, which in turn activate B cells and NK cells sequentially (44–46), durable chimerism is likely required to induce stable T/B/NK cell tolerance across xenogeneic barriers. Using mouse models, we found that mouse anti-rat natural antibodies, which had undefined specificities, disappeared when non-myeloablative mixed chimerism was induced (47). We later used GalTKO mice as recipients and were able to show that successful engraftment of GalT+/+ mouse or rat bone marrow leads not only to disappearance of anti-Gal antibodies, but also to clear-cut tolerization of the B cells producing these antibodies (48) through mechanisms that involved early anergy and later deletion (49, 50) and appeared to depend on stromal CR1/Cr2-expressing cells (51). These animals successfully accepted donor-matched heart grafts for > 100 days, indicating the presence of T cell and B cell tolerance to GAL-expressing heart xenografts (52). Because humans may carry a titer of anti-pig antibodies much higher than that of the mouse model, a second set of experiments using mice sensitized with Gal antigens was performed(53). Although the standard model of non-myeloablative conditioning and bone marrow transplantation did not lead to engraftment, we found that larger doses of bone marrow did result in engraftment and lasting B cell tolerance. These mice subsequently accepted cardiac xenografts without signs of rejection (60). The data from these rodent models suggest that a non-myeloablative induction regimen with bone marrow transplantation may be capable of tolerizing porcine antigen-reactive B/plasma cells. More recently, we have performed similar studies in humanized mice and preliminary results suggest that human B cells may likewise be tolerized to porcine xenoantigens by the induction of mixed xenogeneic chimerism (H.W. Li and M. Sykes, unpublished data).
Mixed chimerism also has the capacity to tolerize natural killer (NK) cells (54). In the case of allogeneic chimerism, the tolerance is specific for the donor, with otherwise preserved NK cell function. However, in the rat-to-mouse xenogeneic chimerism model, mouse NK cells were rendered globally unresponsive by the presence of even low levels of rat chimerism (55). In the pig-human mixed chimerism model in humanized mice, we recently showed that human NK cells are rendered tolerant by porcine mixed chimerism, as reflected in some cases by specific unresponsiveness with otherwise preserved NK cell function and, in others, global hyporesponsiveness (54). Collectively, our data argue for a mechanism of NK cell tolerance involving anergy induced by repeated, unopposed activating signals, as opposed to a requirement for licensing by the presence of inhibitory ligands, as we have discussed previously (56). Based on our findings in humanized mice, we believe that expression by porcine hematopoietic cells of a human NK cell inhibitory ligand is likely to be the best way to avoid NK cell-mediated rejection while preserving NK cell function overall.
Despite promising results in rodent models, macrochimerism in non-human primates has not been achieved (57, 58). Most porcine cells were cleared within 24–48 hours post-bone marrow infusion, although specific humoral unresponsiveness was induced in some recipients (57), indicating that additional strategies are required to achieve persistence of donor chimerism.
Recent work to circumvent rapid clearance of BM cells has focused on innate immunity and macrophage-associated mechanisms. Specifically, attention is on SIRP-alpha, a transmembrane protein with intracellular tyrosine kinase activity that is present on macrophages, dendritic cells, and neutrophils. The ligand of this receptor is CD47, and recognition of CD47 by SIRP-alpha down-regulates phagocytosis by macrophages (59, 60). In a small animal study in which human hematopoietic stem cells were transplanted into severely immunocompromised mice to allow for human hematopoiesis, the authors found significant improvement in hematopoiesis and engraftment in mice transgenic for human SIRP-alpha (61).
In order to test the ability of human CD47 (hCD47) to similarly improve engraftment of porcine cells in baboons, transgenic swine were produced on the GalT-KO inbred miniature swine background (62). Two swine expressing markedly different levels of the hCD47 transgene on peripheral blood mononuclear cells were produced, one expressing high levels (18286) and one expressing very low levels (18289). Mobilized peripheral blood stem cells (PBSC) from each of these animals were transplanted into conditioned baboons. Both baboons receiving PBSC from the 18286 demonstrated macro-chimerism in peripheral blood for several days following cell infusions, while chimerism was rapidly lost in baboons receiving PBSC from 18289. These results thus demonstrated a profound protective effect of hCD47 expression on the survival of porcine hematopoietic cells following transplantation into primates.
There was no evidence for sensitization of the recipient baboons at either the antibody or T-cell levels (62). To test for effects of the HSC transplants on recipient responses, split-thickness skin grafts (STSG) from the transgenic PBSC donors were placed onto full-thickness graft beds at 14 weeks after the cell transplants, by which time all exogenous immunosuppression had been discontinued for over four weeks. A control baboon (B360) received identical conditioning and immunosuppression regimens, but no cell transplants. Skin grafts on both baboons that received HSC from 18286 showed markedly prolonged survivals (70 and >23 days, respectively), while skin grafts on both baboons that received HSC from 18289 showed no difference in survival from the control baboon (14 days). These results therefore indicate a strong correlation between hCD47 expression, prolonged peripheral porcine chimerism, and marked prolongation of porcine skin grafts in the absence of concurrent immunosuppression(62)..
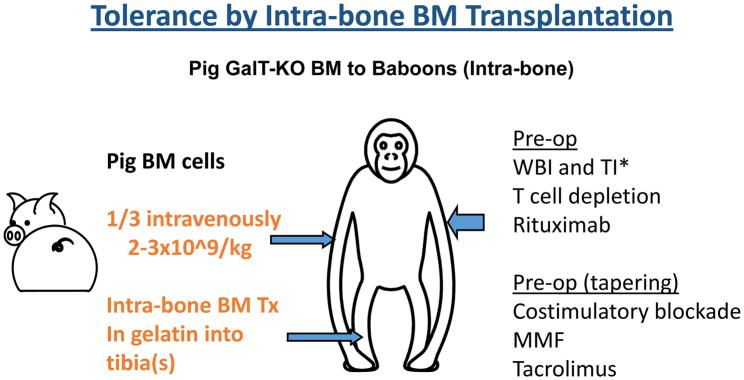
Another strategy for improving pig-to-baboon chimerism is intra-bone bone marrow transplantation (IBBMTx) (Figure 1b). Previous studies using porcine BM cells infused intravenously following ex vivo immune-adsorption of nAb have only demonstrated transient macro-chimerism, with most infused cells undetectable within 24 hours (57, 58). Recent data in allogeneic models demonstrated that direct injection of donor BM cells into recipient BM spaces (IBBM-Tx) produced rapid reconstitution and higher survival rates compared to IV injection (63). Therefore, a modified IBBM-Tx procedure was developed for a preclinical pig-to-baboon model (64) to assess whether this would achieve improved, persistent macro-chimerism and engraftment of BM across a xenogeneic barrier. A modified IBBM-Tx procedure using a 3D collagen gel matrix in a preclinical pig-to-baboon model has led to markedly higher percentages of chimerism (average of 8.6%, max 29% at day 2) and prolonged detectable peripheral macro-chimerism over 3 weeks, along with higher incidence (4 of 6 recipients) of BM engraftment both at injection site (local engraftment) and systemically (64). Projects that combine the strategy of IBBM-Tx with vascularized thymic grafts (see below) using hCD47 Tg MHC-inbred donors are in progress.
III. The potential benefit of a tolerance strategy for islets and kidney xenotransplantation
Thus far, the longest survival using xenogeneic transplants in life-supporting models have been shown with islets and kidneys. However, the outstanding outcomes seen with the use of allogeneic kidney transplants as well as insulin treatment regimens may argue against the adoption of xenotransplantation as an alternative. Therefore, stronger justification besides organ shortage is needed for implementation of xenogenic transplantation.
Since porcine insulin is very similar to human insulin, it is very likely that the pig pancreas (or islets) will support the insulin needs of diabetic patients. Several groups have achieved >6 months insulin free period after pig islet transplantation in non-human primates (5–7, 9). With the advent of transgenic pigs and extended survival, it may be assumed that this will be the next attempt in clinical xenograft. However, one problem is the requirement of indefinite daily administration of immunosuppressive drugs. Even if patients become insulin independent, they must take life-long immunosuppressive drugs. The New Zealand group recently reported 8 clinical cases of islet xenotransplantation using encapsulated neonatal porcine islets without immunosuppressive drugs which resulted in clinical benefit for unstable type 1 diabetic patients (65). This result is encouraging, however, although there was improvement of HbA1c, no patients became insulin-free. One major issue was insufficient vascularization for oxygen supply and yield of islets in the encaspulized strategy. Therefore, strategies aimed at induction of tolerance to islet grafts might be of great advantage.
Likewise, in favor of the kidney is that failure of transplant is not fatal, as the patient could be put back on dialysis. On the other hand, the availability of living donor transplants, and markedly improved patient survival of allogeneic kidney at 3 years (66, 67). Thus, stronger justification besides organ shortage may be needed for implementation of xenogenic transplantation. There are several justifications for the use of xenotransplantation. First is that using pig donors may provide kidneys to patients who have a small chance to receive organs from human donors. Numerous patients on waiting lists for kidneys are highly sensitized to potential human donors (i.e. high PRA) (68). We have previously demonstrated that allo-sensitization does not increase the risk of xeno-reactivity to GalT-KO miniature swine in patients on transplantation waiting lists (69), suggesting that highly allo-sensitized patients may be at no increased risk of xeno-sensitization over their non-sensitized cohorts. Although a recent report demonstrated that some, not all of, HLA antibodies in sensitized patients cross-react with class I SLA.SLA class I is a target for genome editing in pig donors which may resolve the issue (70). Second is tolerance induction. Although clinical applicability of vascularized thymic transplantation in clinical allotransplantation is limited due to involution of thymus (71), this limitation does not apply for xenotransplantation using MHC inbred transgenic GalT-KO pigs. MHC-inbred Tg GalTKO allows recipients to receive size matched kidney from a pig donor and thymus from a juvenile donor that is MHC matched to the kidney donors.
III. Conclusions
For xenografts, the level of immunosuppressive agents needed to fully suppress immune responses is greater than for allografts and would likely lead to greater complications. For this reason, adoption of tolerance strategies is imperative. Additionally, antibody-mediated responses appear to be more prevalent and robust in xenograft rejection than in allograft rejection, even when major responses to Gal have been eliminated by use of Tg/GalT-KO donors. Even though current immunosuppression seems to be controlling T cell responses in long-term acceptors (3, 4, 9, 10), it appears likely that low levels of T cell-dependent antibodies (44, 58) and activation of innate responses (72) still develop, potentially leading to xenograft loss. Tolerance induction has the potential to avoid such persistent immune reactivity and therefore overcome the antibody-mediated response as well.
Figure 2.
Schematic diagram of intra-bone bone marrow transplantation in pig-to-baboon models. * WBI: whole body irradiation. TI: thymic irradiation.
Key points.
Although continuous administration of high dose anti-CD40 or anti-CD154 mAb-based immunosuppression combined with multiple immunosuppressive drugs seems to be controlling T cell responses in a non-specific manner, attempts to taper immunosuppression without tolerance strategies have been unsuccessful.
Both mixed chimerism and thymic transplantation have achieved xenogeneic tolerance in pig-to-mouse/humanized mice models.
More recently, there has been demonstration of persistence of macrochimerism or prolonged pig skin graft acceptance following pig bone marrow transplantation, as well as donor-specific unresponsiveness in vitro and recent T cell emigrants in vivo following vascularized thymic transplantation in pig to baboon models.
Recent results are encouraging and inducing tolerance across pig-to-primates may be attainable.
Acknowledgments
The authors would like to thank Dr. Thomas Pomposelli for his helpful advice and review of this manuscript.
Financial support and sponsorship
This research was supported by NIH grant P01AI45897.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
* of specific interest
** of outstanding interest
- 1*.Cowan PJ. The use of CRISPR/Cas associated technologies for cell transplant applications. Current opinion in organ transplantation. 2016;21(5):461–6. doi: 10.1097/MOT.0000000000000347. This article summarized recent developments in the use of the clustered, regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated 9 (Cas9) genome editing system for cell transplant applications, ranging from transplantation of corrected autologous patient stem cells, to the treatment of inherited diseases, as well as the tailoring of donor pigs for cell xenotransplantation. [DOI] [PubMed] [Google Scholar]
- 2.Hai T, Teng F, Guo R, Li W, Zhou Q. One-step generation of knockout pigs by zygote injection of CRISPR/Cas system. Cell research. 2014;24(3):372–5. doi: 10.1038/cr.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3**.Higginbotham L, Mathews D, Breeden CA, Song M, Farris AB, 3rd, Larsen CP, et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation. 2015;22(3):221–30. doi: 10.1111/xen.12166. This article reported long-term survival (>133 and >126 days) of rhesus macaques that had low-titer levels of preformed natural antibodies that were treated with an anti-CD154-based regimen and then transplanted with galactose-α1,3-galactose knockout/CD55 transgenic pig kidneys. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4**.Iwase H, Hara H, Ezzelarab M, Li T, Zhang Z, Gao B, et al. Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts. Xenotransplantation. 2017;24(2) doi: 10.1111/xen.12293. The authors used porcine renal grafts from six-gene specific genetically modified swine that survied for >7 months in baboons. Although the number of experiments is very limited, the authors concluded that expression of human endothelial protein C receptor (±CD55) in the graft might be important to avoid coagulation dysregulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hering BJ, Wijkstrom M, Graham ML, Hardstedt M, Aasheim TC, Jie T, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nature medicine. 2006;12(3):301–3. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 6.Cardona K, Korbutt GS, Milas Z, Lyon J, Cano J, Jiang W, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nature medicine. 2006;12(3):304–6. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 7.van der Windt DJ, Bottino R, Casu A, Campanile N, Smetanka C, He J, et al. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(12):2716–26. doi: 10.1111/j.1600-6143.2009.02850.x. [DOI] [PubMed] [Google Scholar]
- 8.Bottino R, Wijkstrom M, van der Windt DJ, Hara H, Ezzelarab M, Murase N, et al. Pig-to-monkey islet xenotransplantation using multi-transgenic pigs. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(10):2275–87. doi: 10.1111/ajt.12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin JS, Kim JM, Kim JS, Min BH, Kim YH, Kim HJ, et al. Long-term control of diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015;15(11):2837–50. doi: 10.1111/ajt.13345. [DOI] [PubMed] [Google Scholar]
- 10**.Mohiuddin MM, Singh AK, Corcoran PC, Thomas ML, 3rd, Clark T, Lewis BG, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nature communications. 2016;7:11138. doi: 10.1038/ncomms11138. This article reported the median (298 days) and longest (945 days) graft survival of heterotopically transplanted GTKO.hCD46.hTBM pig hearts into baboons. To date, it represents the longest report of a cardiac xenograft survival in a non-human primate and it is a major step towards a life-supporting orthotopic cardiac xenotransplantation model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwase H, Liu H, Wijkstrom M, Zhou H, Singh J, Hara H, et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation. 2015;22(4):302–9. doi: 10.1111/xen.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Shin JS, Min BH, Kim JM, Kim JS, Yoon IH, Kim HJ, et al. Failure of transplantation tolerance induction by autologous regulatory T cells in the pig-to-non-human primate islet xenotransplantation model. Xenotransplantation. 2016;23(4):300–9. doi: 10.1111/xen.12246. The authors tested if long-term survivors (n=2) that maintained normoglycemia over 6 months while on immunosuppressive maintenance with a CD40-CD154 blockade as well as sirolimus with autologous Tregs could be weaned off immunosuppression. They found that the engrafted pig islets were fully rejected by activated immune cells, particularly T cells, when immunosuppressant’s were stopped, showing a failure of transplantation tolerance. [DOI] [PubMed] [Google Scholar]
- 13.Yamada K, Sachs DH, DerSimonian H. Human anti-porcine xenogeneic T cell response. Evidence for allelic specificity of mixed leukocyte reaction and for both direct and indirect pathways of recognition. Journal of immunology. 1995;155(11):5249–56. [PubMed] [Google Scholar]
- 14.Murray AG, Khodadoust MM, Pober JS, Bothwell AL. Porcine aortic endothelial cells activate human T cells: direct presentation of MHC antigens and costimulation by ligands for human CD2 and CD28. Immunity. 1994;1(1):57–63. doi: 10.1016/1074-7613(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 15.Fuchimoto Y, Huang CA, Yamada K, Shimizu A, Kitamura H, Colvin RB, et al. Mixed chimerism and tolerance without whole body irradiation in a large animal model. The Journal of clinical investigation. 2000;105(12):1779–89. doi: 10.1172/JCI8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawai T, Cosimi AB, Colvin RB, Powelson J, Eason J, Kozlowski T, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59(2):256–62. [PubMed] [Google Scholar]
- 17.Yamada Y, Boskovic S, Aoyama A, Murakami T, Putheti P, Smith RN, et al. Overcoming memory T-cell responses for induction of delayed tolerance in nonhuman primates. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(2):330–40. doi: 10.1111/j.1600-6143.2011.03795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bashuda H, Kimikawa M, Seino K, Kato Y, Ono F, Shimizu A, et al. Renal allograft rejection is prevented by adoptive transfer of anergic T cells in nonhuman primates. The Journal of clinical investigation. 2005;115(7):1896–902. doi: 10.1172/JCI23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y, Rodriguez-Barbosa JI, Swenson K, Zhao G, Arn JS, Sykes M. Highly disparate xenogeneic skin graft tolerance induction by fetal pig thymus in thymectomized mice: Conditioning requirements and the role of coimplantation of fetal pig liver. Transplantation. 2001;72(10):1608–15. doi: 10.1097/00007890-200111270-00006. [DOI] [PubMed] [Google Scholar]
- 20.Nikolic B, Gardner JP, Scadden DT, Arn JS, Sachs DH, Sykes M. Normal development in porcine thymus grafts and specific tolerance of human T cells to porcine donor MHC. Journal of immunology. 1999;162(6):3402–7. [PubMed] [Google Scholar]
- 21.Yamada K, Shimizu A, Utsugi R, Ierino FL, Gargollo P, Haller GW, et al. Thymic transplantation in miniature swine. II. Induction of tolerance by transplantation of composite thymokidneys to thymectomized recipients. Journal of immunology. 2000;164(6):3079–86. doi: 10.4049/jimmunol.164.6.3079. [DOI] [PubMed] [Google Scholar]
- 22.Kamano C, Vagefi PA, Kumagai N, Yamamoto S, Barth RN, LaMattina JC, et al. Vascularized thymic lobe transplantation in miniature swine: thymopoiesis and tolerance induction across fully MHC-mismatched barriers. Proc Natl Acad Sci U S A. 2004;101(11):3827–32. doi: 10.1073/pnas.0306666101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strober S, Spitzer TR, Lowsky R, Sykes M. Translational studies in hematopoietic cell transplantation: treatment of hematologic malignancies as a stepping stone to tolerance induction. Seminars in immunology. 2011;23(4):273–81. doi: 10.1016/j.smim.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24*.Todo S, Yamashita K, Goto R, Zaitsu M, Nagatsu A, Oura T, et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology. 2016;64(2):632–43. doi: 10.1002/hep.28459. The authors reported operational tolerance with an ex vivo-generated regulatory T-cell-based cell therapy in clinical living donor liver transplantation. 4 patients have been drug free for more than 24 months. The other 3 recipients with autoimmune liver diseases developed mild rejection during weaning and then resumed conventional low-dose immunotherapy. [DOI] [PubMed] [Google Scholar]
- 25.Kalscheuer HOT, Dahmani A, Li H, Holzl M, Yamada K, Sykes M. Xenograft tolerance and immune function of human T cell developing in pig thymus xenografts. Journal of immunology. 2014;192(7):3442–50. doi: 10.4049/jimmunol.1302886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimizu I, Fudaba Y, Shimizu A, Yang YG, Sykes M. Comparison of human T cell repertoire generated in xenogeneic porcine and human thymus grafts. Transplantation. 2008;86(4):601–10. doi: 10.1097/TP.0b013e318182d47a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moses RD, Pierson RN, 3rd, Winn HJ, Auchincloss H., Jr Xenogeneic proliferation and lymphokine production are dependent on CD4+ helper T cells and self antigen-presenting cells in the mouse. The Journal of experimental medicine. 1990;172(2):567–75. doi: 10.1084/jem.172.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seebach JD, Yamada K, McMorrow IM, Sachs DH, DerSimonian H. Xenogeneic human anti-pig cytotoxicity mediated by activated natural killer cells. Xenotransplantation. 1996;3:188–97. [Google Scholar]
- 29.Karlsson-Parra A, Ridderstad A, Wallgren AC, Moller E, Ljunggren HG, Korsgren O. Xenograft rejection of porcine islet-like cell clusters in normal and natural killer cell-depleted mice. Transplantation. 1996;61(9):1313–20. doi: 10.1097/00007890-199605150-00005. [DOI] [PubMed] [Google Scholar]
- 30**.Tanabe T, Watanabe H, Shah JA, Sahara H, Shimizu A, Nomura S, et al. Role of Intrinsic (Graft) Versus Extrinsic (Host) Factors in the Growth of Transplanted Organs Following Allogeneic and Xenogeneic Transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2017 doi: 10.1111/ajt.14210. The authors demonstrated that baboon recipients of GalT-KO kidney co-transplanted with vascularized thymic grafts (thymokidney) without additional gene modification had markedly prolonged renal graft survival without any immunologic sensitization up to 193 days. In addition, the authors examined renal function of growth-rate mismatched combinations in both xenogeneic and allogeneic transplant models. They found that intrinsic factors are responsible, at least in part, for the growth of donor organs and that this property should be taken into consideration for growth-curve-mismatched transplants, especially for life-supporting growth mismatched organ transplants (pig-to-non hunman primates/primate xenotransplantation) and organs transplanted into a limited recipient space (intrathoracic space) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griesemer AD, Hirakata A, Shimizu A, Moran S, Tena A, Iwaki H, et al. Results of gal-knockout porcine thymokidney xenografts. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(12):2669–78. doi: 10.1111/j.1600-6143.2009.02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nobori S, Samelson-Jones E, Shimizu A, Hisashi Y, Yamamoto S, Kamano C, et al. Long-term acceptance of fully allogeneic cardiac grafts by cotransplantation of vascularized thymus in miniature swine. Transplantation. 2006;81(1):26–35. doi: 10.1097/01.tp.0000200368.03991.e0. [DOI] [PubMed] [Google Scholar]
- 33.Fudaba Y, Onoe T, Chittenden M, Shimizu A, Shaffer JM, Bronson R, et al. Abnormal regulatory and effector T cell function predispose to autoimmunity following xenogeneic thymic transplantation. Journal of immunology. 2008;181(11):7649–59. doi: 10.4049/jimmunol.181.11.7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee LA, Gritsch HA, Sergio JJ, Arn JS, Glaser RM, Sablinski T, et al. Specific tolerance across a discordant xenogeneic transplantation barrier. ProcNatlAcadSciUSA. 1994;91:10864–7. doi: 10.1073/pnas.91.23.10864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Y, Rodriguez-Barbosa JI, Shimizu A, Sachs DH, Sykes M. Despite efficient intrathymic negative selection of host-reactive T cells, autoimmune disease may develop in porcine thymus-grafted athymic mice: evidence for failure of regulatory mechanisms suppressing autoimmunity. Transplantation. 2003;75(11):1832–40. doi: 10.1097/01.TP.0000065292.20062.F0. [DOI] [PubMed] [Google Scholar]
- 36.Kalscheuer H, Onoe T, Dahmani A, Li HW, Holzl M, Yamada K, et al. Xenograft tolerance and immune function of human T cells developing in pig thymus xenografts. Journal of immunology. 2014;192(7):3442–50. doi: 10.4049/jimmunol.1302886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haller GW, Esnaola N, Yamada K, Wu A, Shimizu A, Hansen A, et al. Thymic transplantation across an MHC class I barrier in swine. Journal of immunology. 1999;163(7):3785–92. [PubMed] [Google Scholar]
- 38.Rodriguez-Barbosa JI, Zhao Y, Barth R, Zhao G, Arn JS, Sachs DH, et al. Enhanced CD4 reconstitution by grafting neonatal porcine tissue in alternative locations is associated with donor-specific tolerance and suppression of pre-existing xenoreactive T cells. Transplantation. 2001;72:1223–31. doi: 10.1097/00007890-200110150-00007. [DOI] [PubMed] [Google Scholar]
- 39.Yamada K, Shimizu A, Ierino FL, Utsugi R, Barth RN, Esnaola N, et al. Thymic transplantation in miniature swine. I. Development and function of the “thymokidney”. Transplantation. 1999;68(11):1684–92. doi: 10.1097/00007890-199912150-00011. [DOI] [PubMed] [Google Scholar]
- 40.LaMattina JC, Kumagai N, Barth RN, Yamamoto S, Kitamura H, Moran SG, et al. Vascularized thymic lobe transplantation in miniature swine: I. Vascularized thymic lobe allografts support thymopoiesis. Transplantation. 2002;73(5):826–31. doi: 10.1097/00007890-200203150-00032. [DOI] [PubMed] [Google Scholar]
- 41.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nature medicine. 2005;11(1):32–4. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 42.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. The New England journal of medicine. 2008;358(4):353–61. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Millan MT, Shizuru JA, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. The New England journal of medicine. 2008;358(4):362–8. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 44.Cretin N, Bracy J, Hanson K, Iacomini J. The role of T cell help in the production of antibodies specific for Gal alpha 1-3Gal. Journal of immunology. 2002;168(3):1479–83. doi: 10.4049/jimmunol.168.3.1479. [DOI] [PubMed] [Google Scholar]
- 45.Tonomura N, Shimizu A, Wang S, Yamada K, Tchipashvili V, Weir GC, et al. Pig islet xenograft rejection in a mouse model with an established human immune system. Xenotransplantation. 2008;15(2):129–35. doi: 10.1111/j.1399-3089.2008.00450.x. [DOI] [PubMed] [Google Scholar]
- 46.Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108(2):487–92. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- 47.Aksentijevich I, Sachs DH, Sykes M. Humoral tolerance in xenogeneic BMT recipients conditioned with a non-myeloablative regimen. Transplantation. 1992;53:1108–14. doi: 10.1097/00007890-199205000-00025. [DOI] [PubMed] [Google Scholar]
- 48.Yang Y-G, deGoma E, Ohdan H, Bracy JL, Xu X, Iacomini J, et al. Tolerization of anti-gala1-3gal natural antibody-forming B cells by induction of mixed chimerism. JExpMed. 1998;187(8):1335–42. doi: 10.1084/jem.187.8.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohdan H, Yang Y-G, Shimizu A, Swenson KG, Sykes M. Mixed bone marrow chimerism induced without lethal conditioning prevents T cell and anti-Gala1,3Gal-mediated graft rejection. JClinInvest. 1999;104:281–90. doi: 10.1172/JCI6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawahara T, Shimizu I, Ohdan H, Zhao G, Sykes M. Differing mechanisms of early and late B cell tolerance induced by mixed chimerism. AmJTransplant. 2005;5(12):2821–9. doi: 10.1111/j.1600-6143.2005.01121.x. [DOI] [PubMed] [Google Scholar]
- 51.Shimizu I, Kawahara T, Haspot F, Bardwell PD, Carroll MC, Sykes M. B-cell extrinsic CR1/CR2 promotes natural antibody production and tolerance induction of anti-alpha-Gal-producing B-1 cells. Blood. 2006;109(4):1773–81. doi: 10.1182/blood-2006-02-002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohdan H, Yang YG, Swenson KG, Kitamura H, Sykes M. T cell and B cell tolerance to GALalpha1,3GAL-expressing heart xenografts is achieved in alpha1,3-galactosyltransferase-deficient mice by nonmyeloablative induction of mixed chimerism. Transplantation. 2001;71(11):1532–42. doi: 10.1097/00007890-200106150-00009. [DOI] [PubMed] [Google Scholar]
- 53.Ohdan H, Swenson KG, Kitamura H, Yang YG, Sykes M. Tolerization of Gal alpha 1,3Gal-reactive B cells in pre-sensitized alpha 1,3-galactosyltransferase-deficient mice by nonmyeloablative induction of mixed chimerism. Xenotransplantation. 2001;8(4):227–38. doi: 10.1034/j.1399-3089.2001.00006.x. [DOI] [PubMed] [Google Scholar]
- 54**.Li HW, Vishwasrao P, Holzl MA, Chen S, Choi G, Zhao G, et al. Impact of Mixed Xenogeneic Porcine Hematopoietic Chimerism on Human NK Cell Recognition in a Humanized Mouse Model. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2017;17(2):353–64. doi: 10.1111/ajt.13957. The authors demonstrated that mixed xenogeneic chimerism induces human NK cell hypo responsiveness to pig cells in a humanized mouse model. Their results supported the use of this approach towards inducing xenogeneic tolerance in the clinical setting. Additional approaches are required however to improve the efficacy of tolerance induction while ensuring adequate NK cell function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawahara T, Rodriguez-Barbosa JI, Zhao Y, Zhao G, Sykes M. Global Unresponsiveness as a Mechanism of Natural Killer Cell Tolerance in Mixed Xenogeneic Chimeras. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7(9):2090–7. doi: 10.1111/j.1600-6143.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- 56.Griesemer A, Yamada K, Sykes M. Xenotransplantation: immunological hurdles and progress toward tolerance. Imm Revs. 2014;258(1):241–58. doi: 10.1111/imr.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Griesemer A, Liang F, Hirakata A, Hirsh E, Lo D, Okumi M, et al. Occurrence of specific humoral non-responsiveness to swine antigens following administration of GalT-KO bone marrow to baboons. Xenotransplantation. 2010;17(4):300–12. doi: 10.1111/j.1399-3089.2010.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liang F, Wamala I, Scalea J, Tena A, Cormack T, Pratts S, et al. Increased levels of anti-non-Gal IgG following pig-to-baboon bone marrow transplantation correlate with failure of engraftment. Xenotransplantation. 2013;20(6):458–68. doi: 10.1111/xen.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ide K, Wang H, Tahara H, Liu J, Wang X, Asahara T, et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(12):5062–6. doi: 10.1073/pnas.0609661104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang H, VerHalen J, Madariaga ML, Xiang S, Wang S, Lan P, et al. Attenuation of phagocytosis of xenogeneic cells by manipulating CD47. Blood. 2007;109(2):836–42. doi: 10.1182/blood-2006-04-019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang C, Wang H, Ide K, Wang Y, Van Rooijen N, Ohdan H, et al. Human CD47 expression permits survival of porcine cells in immunodeficient mice that express SIRPalpha capable of binding to human CD47. Cell transplantation. 2011;20(11–12):1915–20. doi: 10.3727/096368911X566253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tena AA, Sachs DH, Mallard C, Yang YG, Tasaki M, Farkash E, et al. Prolonged Survival of Pig Skin on Baboons After Administration of Pig Cells Expressing Human CD47. Transplantation. 2017;101(2):316–21. doi: 10.1097/TP.0000000000001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okada M, Yoshihara S, Taniguchi K, Kaida K, Ikegame K, Kato R, et al. Intrabone marrow transplantation of unwashed cord blood using reduced-intensity conditioning treatment: a phase I study. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18(4):633–9. doi: 10.1016/j.bbmt.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 64.Tasaki M, Wamala I, Tena A, Villani V, Sekijima M, Pathiraja V, et al. High incidence of xenogenic bone marrow engraftment in pig-to-baboon intra-bone bone marrow transplantation. Am J Transplant. 2015;15(4):974–83. doi: 10.1111/ajt.13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65*.Matsumoto S, Abalovich A, Wechsler C, Wynyard S, Elliott RB. Clinical Benefit of Islet Xenotransplantation for the Treatment of Type 1 Diabetes. EBioMedicine. 2016;12:255–62. doi: 10.1016/j.ebiom.2016.08.034. The authors tested two different doses of encapsulated neonatal porcine islets that were transplanted twice (totaling approximately 10,000IEQ/kg and 20,000IEQ/kg) into eight type-1 diabetic patients. Although the four patients in the higher dose group had slightly improved HbA1 as well as reduction in the frequency of unknown hypoglycemic events, no patient became completely insulin independent. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66*.Okumi M, Toki D, Nozaki T, Shimizu T, Shirakawa H, Omoto K, et al. ABO-Incompatible Living Kidney Transplants: Evolution of Outcomes and Immunosuppressive Management. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016;16(3):886–96. doi: 10.1111/ajt.13502. The authors examined the longitudinal changes in the outcomes from ABO-compatible living kidney transplantation (ABO-ILKT) compared with those from (ABO-CLKT) over the last 25 years. They reported that ABO-ILKT recipients showed substantial improvements in graft survival rate over time. Graft survival was almost identical over the past decade, regardless of ABO-incompatibility. [DOI] [PubMed] [Google Scholar]
- 67.UNOS National Data. 2017 https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#.
- 68.Duquesnoy RJ, Witvliet M, Doxiadis II, de Fijter H, Claas FH. HLAMatchmaker-based strategy to identify acceptable HLA class I mismatches for highly sensitized kidney transplant candidates. Transplant international : official journal of the European Society for Organ Transplantation. 2004;17(1):22–30. doi: 10.1007/s00147-003-0641-z. [DOI] [PubMed] [Google Scholar]
- 69.Wong BS, Yamada K, Okumi M, Weiner J, O’Malley PE, Tseng YL, et al. Allosensitization does not increase the risk of xenoreactivity to alpha1,3-galactosyltransferase gene-knockout miniature swine in patients on transplantation waiting lists. Transplantation. 2006;82(3):314–9. doi: 10.1097/01.tp.0000228907.12073.0b. [DOI] [PubMed] [Google Scholar]
- 70*.Martens GR, Reyes LM, Butler JR, Ladowski JM, Estrada JL, Sidner RA, et al. Humoral Reactivity of Renal Transplant-Waitlisted Patients to Cells From GGTA1/CMAH/B4GalNT2, and SLA Class I Knockout Pigs. Transplantation. 2017;101(4):e86–e92. doi: 10.1097/TP.0000000000001646. Sera from 820 patients were screened on GGTA1/CMAH/B4GalNT2 KO cells and a subset with elevated binding was evaluated. The authors found that many waitlisted patients have minimal xenoreactive antibody binding to the triple KO pig, but some HLA antibodies in sensitized patients cross-react with class I SLA. SLA class I is a target for genome editing in xenotransplantation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nobori S, Shimizu A, Okumi M, Samelson-Jones E, Griesemer A, Hirakata A, et al. Thymic rejuvenation and the induction of tolerance by adult thymic grafts. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(50):19081–6. doi: 10.1073/pnas.0605159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang YG. CD47 in xenograft rejection and tolerance induction. Xenotransplantation. 2010;17(4):267–73. doi: 10.1111/j.1399-3089.2010.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]