Abstract
Purpose
To test the genetic association between Japanese patients with primary open-angle glaucoma (POAG) and the previously reported POAG susceptibility loci and to perform genotype–phenotype analysis.
Methods
Genetic associations for 27 SNPs from 16 loci previously linked to POAG were assessed using genome-wide SNP data of the primary cohort (565 Japanese POAG patients and 1,104 controls). Reproducibility of the assessment was tested in 607 POAG cases and 455 controls (second cohort) with a targeted genotyping approach. For POAG-associated variants, a genotype–phenotype correlation study (additive, dominant, recessive model) was performed using the objective clinical data derived from 598 eyes of 598 POAG patients.
Results
Among 27 SNPs from 16 loci previously linked to POAG, genotypes for total of 20 SNPs in 13 loci were available for targeted association study. Among 8 SNPs in 3 loci that showed at least nominal association (P < 5.00E-02) in the primary cohort, a representative SNP for each loci (rs2157719 for CDKN2B-AS1, rs33912345 for SIX6, and rs9913911 for GAS7) were selected. For these SNPs the association was found significant in both the second cohort analysis and meta-analysis. The genotype–phenotype analysis revealed significant correlations between CDKN2B-AS1 (rs2157719) and decreased intraocular pressure (β = -6.89 mmHg, P = 1.70E-04; dominant model) after multiple corrections. In addition, nominal correlation was observed between CDKN2B-AS1 (rs2157719) and optic nerve head blood flow (β = -0.54 and -0.67 arbitrary units (AU), P = 2.00E-02 and 1.39E-02), between SIX6 (rs33912345) and decreased total peripapillary retinal nerve fiber layer thickness (β = -2.16 and -2.82 μm, P = 4.68E-02 and 2.40E-02, additive and recessive model, respectively) and increased optic nerve head blood flow (β = 0.44 AU, P = 2.20E-02; additive model) and between GAS7 (rs9913911) and increased cup volume (β = 0.03 mm3, P = 4.60E-02) and mean cup depth (β = 0.03 mm3, P = 4.11E-02; additive model) and decreased pattern standard deviation (β = -0.87 dB, P = 2.44E-02; dominant model).
Conclusion
The association between SNPs near GAS7 and POAG was found in Japanese patients for the first time. Clinical characterization of the risk variants is an important step toward understanding the pathology of the disease and optimizing treatment of patients with POAG.
Introduction
Glaucoma, one of the leading causes of blindness worldwide, is a neurodegenerative optic neuropathy that leads to irreversible visual field damage.[1] It is characterized by morphological changes in the optic nerve head caused by progressive loss of retinal ganglion cells (RGCs) and their axonal projections, which results in thinning of the retinal nerve fiber layer (RNFL) and enlargement of the optic nerve cup, the concave depression at the optic nerve head. As a consequence, patients have a progressive decrease in visual field sensitivity. Primary open angle glaucoma (POAG), the most common form of the disease, is considered to have multifactorial etiologies, which include elevated intraocular pressure (IOP), systemic or ocular blood flow abnormalities, older age, myopia and oxidative stress.[2–5]
Family history is also a well-known risk factor for POAG. First-degree relatives of patients have a three- to nine-fold higher risk of disease development compared with the general population.[6,7] This indicates that genetic components play important roles in the pathogenesis of POAG. Recent progress in genome-wide association studies (GWASs) has uncovered at least 16 susceptibility loci for POAG, although most investigations were performed in cohorts of Caucasian ancestry.[8–12] Several GWASs targeting POAG in Asians have been reported.[13–19] These studies have uncovered a few disease-associated loci, including those near ABCA1, PMM2 and CDC7/TGFBR3.[18,19] In Japan, a few GWASs have shown variable results.[13–17] Only the susceptibility loci at 9p21 (near CDKN2B-AS1) and 14q23 (near SIX6) appear to be reproducible so far. Moreover, risk variants in 3 disease loci near FOXC1, ATXN2 and TXNRD2 recently identified [12] have never been assessed by a targeted association study and the landscape of genetic factors influencing POAG in the Japanese population remain elusive.
Despite the rapid developments in the discovery of risk loci and alleles in patients with POAG, clinical characterization of the identified loci is at a preliminary stage. This is partly because the relatively low contribution of each locus to the disease (odds ratios typically range from 1.1 to 2.0) is expected to result in subtle clinical changes unique to the disease-associated single nucleotide polymorphisms (SNPs). For this reason, genotype–phenotype correlation often requires clinical data from a large number of patients. Furthermore, due to the variability in the clinical characterization of a given patient between ophthalmologists and particularly between clinics, genotype–phenotype correlation using clinical data from different clinics is complicated. Nevertheless, multicenter studies have revealed associations between risk alleles near CDKN2B-AS1 loci and vertical cup-to-disk ratio and decrease in IOP in Caucasian patients[20,21] and between a risk variant near SIX6 and peripapillary RNFL thickness in European and Chinese Singaporean patients.[22–24]
The aim of this study was test for association between POAG and the disease-associated loci in the Japanese population and to assess the genotype–phenotype correlations between risk variants and clinical features of the disease.
Materials and methods
Study subjects
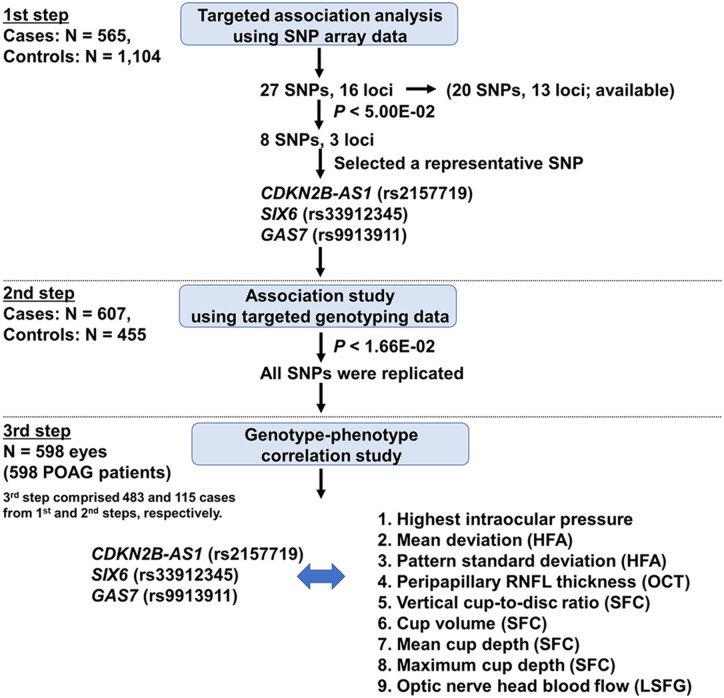
The study protocol followed the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of the Tohoku Graduate School of Medicine. All participants signed a written consent form following an explanation of the nature and possible consequences of the study. All participants in this study were at the age of 35 years or older and of Japanese residents. The experimental design, which comprises three-step analyses, is outlined in Fig 1.
Fig 1. Design of the study.
The study comprised 3 steps. In the first step, an association study of known POAG-related loci was carried out using the genome-wide SNP data from SNP array (cases) and a previous genetic study (control).[25–27] In the second step, 3 candidate risk SNPs were genotyped in the cases and controls to test for the reproducibility. Then a clinical correlation study was performed in the third step. SNP, single nucleotide polymorphism; POAG, primary open-angle glaucoma, HFA, Humphrey Field Analyzer, RNFL, retinal nerve fiber layer thickness, OCT, optical coherence tomography, SFC, stereoscopic fundus camera, LSFG, laser speckle flowgraphy.
All of the subjects with POAG were diagnosed by glaucoma specialists and fulfilled the following diagnostic criteria: presence of glaucomatous optic disk changes, including neuroretinal rim thinning, notching, or cupping; presence of visual field defect that could be attributed to the optic disk changes; and no history of secondary, angle closure, or congenital glaucoma. For the first step, all the POAG patients were recruited at the Institutes related to Tohoku University. The control subjects recruited at the Tohoku Medical Megabank Organization as a part of prospective cohort study were considered to have no POAG based on self-report. [27] In the replication study, the POAG patients were recruited by the members of Tohoku University and the Japan Glaucoma Society Omics Group (JGS-OG), whereas the control subjects were recruited at the Institutes related to Tohoku University. The outline of the case and the control subjects are summarized in S1 Table.
Genotyping
Japonica array (Toshiba, Tokyo, Japan) is a custom-designed array optimized for the Japanese population based on the information from the reference panel from 1,070 Japanese.[25,26] Genome-wide genotype data set was obtained using this SNP array for 602 patients with POAG according to the manufacturer’s instructions. Thirty-seven case samples that did not satisfy the quality control criteria (DishQC > 0.82 and call rates > 0.970) were excluded, thus resulting in a dataset comprising 565 cases. As a control, genotype data from 1,104 healthy subjects collected previously through a prospective cohort study [27] were used. Both cases and controls were genotyped using the same array at the same time.
SNP quality control was applied for the imputation procedure. We used info score using IMPUTE2, and considered a SNP with info score great than 0.9 as an acceptable well-imputed variant in this study. Autosomal SNPs that were assigned to ‘Recommended’ by the Ps_Classification program in the SNPolisher package (Affymetrix) were selected. We applied the following thresholds for quality control in further data cleaning: Hardy–Weinberg equilibrium with a P value <1.00E-04 for control samples, call rate for each SNP > 0.990, and minor allele frequencies <5.00E-02. As a result, a total of 557,352 SNPs on autosomal chromosomes passed the quality control filters and were used for whole genome imputation. Prephasing was first conducted with these SNPs by SHAPEIT (v2.r644) with options—burn 10,—prune 10, and—main 25. Genotype imputation was performed on the phased genotypes with IMPUTE2 (ver. 2.3.1) by using a phased reference panel of 1,070 healthy Japanese individuals (1KJPN panel). For IMPUTE2, the following options were used: -Ne 20000, -k_hap 1000, and -k 120.
Targeted genotyping of 3 SNPs in the second cohort was carried out by the TaqMan assay (Applied Biosystems, Carlsbad, CA, USA) and 7500 Fast Real-Time PCR systems (Applied Biosystems, Foster City, CA, USA). The call rate was over 0.990 in all of the three SNPs and was therefore considered informative.
Clinical assessment
To determine the clinical features associated with the risk variants for the disease, standardized clinical data obtained from 598 eyes of 598 patients with POAG (patients with sufficient clinical data in the primary and second cohorts were combined) seen at Tohoku University Hospital were analyzed. The details of the clinical data extracted for the genotype–phenotype correlation study are as follows. The highest recorded IOP measured with Goldman applanation, together with central corneal thickness with the use of anterior segment optical coherence tomography (OCT) (CASIA, Tomey Cooperation, Nagoya, Japan). The mean deviation and pattern standard deviation were assessed with the 24–2 test program of a Humphrey Field Analyzer (Carl Zeiss Meditec, Dublin, CA), and the worse eye from each of the subject was used for the analysis. Peripapillary RNFL thickness was measured by OCT (3D OCT 2000, Topcon, Tokyo, Japan) together with axial length (IOLMaster, Carl Zeiss, Oberkochen, Germany). Images with image quality >60 were used for the analysis in accordance with our previous investigation. [28] To assess the morphology of the optic nerve head, data from stereoscopic fundus camera photographs (nonmyd WX, Kowa Company, Nagoya, Japan) were analyzed with built-in software. The principles of stereoscopic photography have been described in detail previously. [29] The parameters reflecting optic nerve head morphology assessed in this study included vertical cup-disk ratio, cup volume, mean cup depth, and maximum cup depth. Optic nerve head blood flow was measured with laser speckle flowgraphy (LSFG, LSFG-NAVI, Softcare Co., Fukutsu, Japan). The principles of LSFG have been described in detail previously.[30,31] We used built-in software that accompanies the LSFG-NAVI device to calculate mean blur rate (MBR), a relative index of blood flow that is expressed in arbitrary units (AU). This study focused on MBR in the tissue area, since a previous investigation had shown that this measurement can be used reliably for intergroup comparisons. [32]
Statistical analysis
For the primary screening step, logistic regression analysis was applied to imputed SNPs with age and sex as covariates. For the analysis of selected POAG-associated SNPs, Fisher’s exact test was used to compare the frequency of each SNP between cases and controls in the replication study. The inverse variance weighted method was used for meta-analysis. We performed power calculations for POAG associations using the CaTS Genetic Power Calculator (http://www.sph.umich.edu/csg/abecasis/CaTS/index.html). In Step 2, the study had greater than 80% power to detect an association at an alpha level of 1.66E-02 (0.05 / 3) between POAG and SNPs in CDKN2B-AS1, SIX6 and GAS7 assuming an OR of 1.61, 1.42, 1.21 respectively, (extrapolated from Step 1) and additive genetic model.
Tests for heterogeneity to assess consistency across the primary and replication analyses were performed with Cochran’s Q test. An allele–dosage regression model was applied for genotype–phenotype correlation. Since genotypes for the selected three variants were all obtained with direct genotyping, we assumed additive/dominant/recessive genetic models where the dosage of the SNP was a variable ranging between 0, 1, 2/ 0, 1, 1 / 0, 0, 1 representing the number of copies of the risk allele carried (the T allele of rs2157719, C allele of rs33912345, and A allele of rs9913911). The analysis for highest recorded IOP was adjusted for age, sex, and central corneal thickness. IOP was adjusted for central corneal thickness because it can influence the readout. [33] The parameters for the Humphrey Field Analyzer, related to optic disk morphology, including vertical cup-disk ratio, cup volume, mean cup depth, and maximum cup depth, and optic nerve head blood flow were adjusted for age, sex, highest recorded IOP as previously reported.[34] Peripapillary RNFL thickness was adjusted for age, sex, and axial length.[23] The nominal significance level was set at P value < 5.00E-02 for each step. Bonferroni corrected P value was adopted taking multiple testing into consideration (Pcorrected). The difference was considered significant at Pcorrected < 2.50E-03 (0.05/20 SNPs) for the first step of the case-control genetic analysis and the meta-analysis, Pcorrected < 1.67E-02 (0.05/3 SNPs) for the second step of the case-control genetic analysis, and Pcorrected < 4.27E-04 (0.05/[3SNPs × 13 covariates × 3 genetic models]) for the genotype-phenotype analysis. The data were analyzed with R version 3.2.3.
Results
This study included a total of 1,172 patients with POAG and 1,559 healthy controls. DNA samples from the participants were collected by the members of Tohoku University Hospital, the Tohoku Medical Megabank Organization, and the JGS-OG. All participants in this study were of Japanese residents. The demographic characteristics of the study populations for the primary and the replication analysis are shown in Table 1.
Table 1. Demographic characteristics of the study population for the primary and replication cohorts.
| Cases | Controls | ||
|---|---|---|---|
| Primary cohorts | Genotyping | Array and imputation | Array and imputation |
| N | 565 | 1,104 | |
| Age (yr) | 64.5 ± 11.7 | 59.7 ± 14.1 | |
| Age range | 35–94 | 35–88 | |
| % female | 44.4 | 51.1 | |
| Replication cohorts | Genotyping | TaqMan assay | TaqMan assay |
| N | 607 | 455 | |
| Age (yr) | 66.3 ± 13.6 | 74.8 ± 7.8 | |
| Age range | 35–89 | 60–94 | |
| % female | 50.4 | 59.3 | |
| Combined | N | 1,172 | 1,559 |
Data are expressed as mean ± standard deviation. N: number of subjects.
Targeted analysis of previously reported POAG-associated SNPs
Initially, we focus on the targeted association study of 27 SNPs in 16 loci previously linked to POAG. All disease-associated loci were discovered originally in non-Japanese POAG cohorts, and only two of them have been convincingly reproduced in Japanese patients.[15–17] Moreover, risk variants in 3 disease loci recently identified [12] have never been assessed by a targeted association study. Among the 27 reported SNPs, the array contained 20 SNPs in 13 loci for which genotype data was available (S2 Table). Among them, data for 13 SNPs were obtained with direct genotyping and 7 SNPs were imputed at info score >0.9. Through the comparison between cases and controls, 2 SNPs in CDKN2B-AS1 and SIX6 loci showed significant correlation with POAG after multiple testing corrections (Pcorrected < 2.50E-03). In addition, 6 SNPs in 3 loci (CDKN2BAS-1, SIX6, and GAS7) showed nominal association (P < 5.00E-02). The single SNP with the lowest P value in each locus, except for the SIX6 locus, was then selected for the downstream analysis. For the SIX6 locus, two SNPs (rs33912345 and rs10483727) had a P value < 5.00E-02. We selected rs33912345 (P = 1.67E-04) for the replication study, because rs339122345 was found to alter the protein function of SIX6. [35] On the other hand, rs10483727, with a lower P value (P = 1.35E-04), was located in an intergenic region between SIX1 and SIX6, with an unknown effect on these genes. As a result, three SNPs in three genes (rs2157719 near CDKN2BAS-1, rs33912345 near SIX6, and rs9913911 near GAS7) were assessed further. These three SNPs were genotyped in independent replication cohorts comprising 607 cases and 455 controls, all from Japan. The case–control comparison found significant associations between POAG and SNPs near CDKN2B-AS1 (P = 7.38E-05), SIX6 (P = 7.20E-03), and GAS7 (P = 1.47E-02; Table 2), taking multiple testings into consideration (Pcorrected < 1.67E-02). When the data from the primary and the replication cohorts were combined and reanalyzed (Cochran’s Q test for heterogeneity, P = 0.701–0.871), increased significance levels were observed for SNPs near CDKN2B-AS1 (P = 5.78E-09), SIX6 (P = 4.33E-06) and GAS7 (P = 3.32E-04), which surpassed the significance level after correcting for multiple testings (Pcorrected < 2.50E-03).
Table 2. Summary of SNPs found to be associated with POAG by a targeted genotyping approach.
| SNP ID | rs2157719 | rs33912345 | rs9913911 |
|---|---|---|---|
| Risk allele | (T) | (C) | (A) |
| Nearest gene | CDKN2B-AS1 | SIX6 | GAS7 |
| Function | intronic | missense | Intronic |
| Chr | 9 | 14 | 17 |
| Position (hg19, bp) | 22,033,366 | 60,976,537 | 10,031,183 |
| Primary cohorts | |||
| Frequency | 0.893/0.838 | 0.832/0.772 | 0.443/0.396 |
| P | 1.42E-05 | 1.67E-04 | 9.33E-03 |
| OR (95% CI) | 1.61 (1.29–2.00) | 1.42 (1.18–1.71) | 1.21 (1.05–1.40) |
| Replication cohorts | |||
| Frequency | 0.888/0.828 | 0.820/0.772 | 0.449/0.395 |
| P | 7.38E-05 | 7.20E-03 | 1.47E-02 |
| OR (95% CI) | 1.65 (1.28–2.14) | 1.34 (1.08–1.67) | 1.24 (1.04–1.48) |
| Meta-analysis | |||
| P | 5.78E-09 | 4.33E-06 | 3.32E-04 |
| OR (95% CI) | 1.63 (1.38–1.92) | 1.38 (1.20–1.59) | 1.22 (1.09–1.37) |
| Phet | 0.871 | 0.701 | 0.828 |
Significance level was set at Pcorrected < 2.50E-03 (0.05/20 SNPs) for the first step and the meta-analysis and at Pcorrected < 1.67E-02 (0.05/3 SNPs) for the second step after Bonferroni correction. Bold texts indicate values with a statistically significant difference after the correction. Chr, chromosome; bp, base pair; Frequency, frequency of risk alleles for each case and control; OR, odds ratio; CI, confidence interval; Phet, P value of heterogeneity by Cochran’s Q test.
Clinical characterization of risk variants near CDKN2B-AS1, SIX6 and GAS7
Next, a genotype–phenotype correlation study was performed to determine the clinical features associated with the risk variants near CDKN2B-AS1, SIX6 and GAS7 that were found to be correlated with POAG in Japanese patients. Clinical data from 598 eyes of 598 patients with POAG seen at Tohoku University Hospital (from both the primary and the replication cohorts), which were obtained in identical clinical settings, were analyzed. The clinical background of the patients and the profile of the parameters assessed are summarized in Table 3. Nine quantitative traits were selected for the genotype–phenotype correlation analysis and the multivariate regression model was applied. These included the highest recorded IOP, parameters reflecting visual field sensitivity measured with the Humphrey Field Analyzer (mean deviation and pattern standard deviation), RNFL thickness obtained by OCT, parameters of optic disk morphology measured by stereoscopic fundus camera and optic nerve head blood flow measured by LSFG. The results of the correlation analysis are presented in Table 4.
Table 3. Clinical demographics of patients in the genotype–phenotype correlation study.
| Age (yr) | 64.1 ± 11.7 |
| Sex (male:female) | 264:334 |
| Axial length (mm) | 25.2 ± 1.7 |
| Central corneal thickness (μm) | 512 ± 36 |
| Highest recorded IOP (mm Hg) | 18.3 ± 5.9 |
| Humphrey Field Analyzer parameters | |
| : mean deviation (dB) | −13.4 ± 8.63 |
| : pattern standard deviation (dB) | 9.62 ± 3.78 |
| Peripapillary RNFL thickness | |
| : total (μm) | 79.3 ± 14.0 |
| : superior (μm) | 90.2 ± 21.6 |
| : temporal (μm) | 70.4 ± 17.0 |
| : inferior (μm) | 81.2 ± 20.9 |
| : nasal (μm) | 75.1 ± 15.7 |
| Vertical cup-to-disk ratio | 0.842 ± 0.075 |
| Cup volume (mm3) | 0.340 ± 0.261 |
| Mean cup depth (mm) | 0.231 ± 0.238 |
| Maximum cup depth (mm) | 0.592 ± 0.535 |
| Optic nerve head blood flow (AU) | 9.00 ± 2.37 |
AU, arbitrary units; IOP, intraocular pressure; RNFL, retinal nerve fiber layer.
Table 4. Association between genetic variants and clinical parameters.
| rs2157719 near CDKN2B-AS1 (N = 598) | |||
| Additive model | Dominant model | Recessive model | |
| Variable | P value | P value | P value |
| Highest recorded IOP (N = 597) | 5.77E-03 (-1.59 ± 0.57)* | 1.70E-04 (-6.89±1.82)* | 0.06 |
| HFA parameters | |||
| : mean deviation (N = 597) | 0.95 | 0.75 | 0.96 |
| : pattern standard deviation (N = 597) | 0.42 | 0.95 | 0.35 |
| Peripapillary RNFL thickness | |||
| : total (N = 574) | 0.17 | 0.29 | 0.23 |
| : superior (N = 574) | 0.13 | 0.85 | 0.09 |
| : temporal (N = 574) | 0.08 | 0.23 | 0.12 |
| : inferior (N = 574) | 0.47 | 0.32 | 0.62 |
| : nasal (N = 574) | 0.95 | 0.37 | 0.71 |
| Vertical cup-to-disk ratio (N = 451) | 0.66 | 0.54 | 0.76 |
| Cup volume (N = 451) | 0.95 | 0.84 | 0.89 |
| Mean cup depth (N = 451) | 0.36 | 0.69 | 0.37 |
| Maximum cup depth (N = 451) | 0.30 | 0.44 | 0.36 |
| ONH blood flow (N = 503) | 2.00E-02 (-0.54±0.23)* | 0.60 | 1.39E-02 (-0.67±0.27)* |
| rs33912345 near SIX6 (N = 596) | |||
| Additive model | Dominant model | Recessive model | |
| Variable | P value | P value | P value |
| Highest recorded IOP (N = 595) | 0.94 | 0.82 | 0.99 |
| HFA parameters | |||
| : mean deviation (N = 595) | 0.30 | 0.82 | 0.27 |
| : pattern standard deviation (N = 595) | 0.17 | 0.47 | 0.19 |
| Peripapillary RNFL thickness | |||
| : total (N = 572) | 4.68E-02 (-2.16± 1.08)* | 0.93 | 2.40E-02 (-2.82±1.24)* |
| : superior (N = 572) | 1.33E-02 (-4.11± 1.65)* | 0.28 | 1.40E-02 (-4.69±1.90)* |
| : temporal (N = 572) | 0.87 | 0.05 | 0.37 |
| : inferior (N = 572) | 0.06 | 0.85 | 3.91E-02 (-3.88±1.87)* |
| : nasal (N = 572) | 0.28 | 0.47 | 0.33 |
| Vertical cup-to-disk ratio (N = 449) | 0.70 | 0.83 | 0.72 |
| Cup volume (N = 449) | 0.42 | 0.81 | 0.41 |
| Mean cup depth (N = 449) | 0.16 | 0.24 | 0.24 |
| Maximum cup depth (N = 449) | 0.24 | 0.37 | 0.30 |
| ONH blood flow (N = 501) | 2.20E-02 (0.44 ± 0.19)* | 0.05 | 0.05 |
| rs9913911 near GAS7 (N = 596) | |||
| Additive model | Dominant model | Recessive model | |
| Variable | P value | P value | P value |
| Highest recorded IOP (N = 595) | 0.86 | 0.94 | 0.75 |
| HFA parameters | |||
| : mean deviation (N = 595) | 0.10 | 0.05 | 0.41 |
| : pattern standard deviation (N = 595) | 0.24 | 2.44E-02 (-0.87±0.38)* | 0.88 |
| Peripapillary RNFL thickness | |||
| : total (N = 572) | 0.91 | 0.82 | 0.98 |
| : superior (N = 572) | 0.83 | 0.70 | 0.99 |
| : temporal (N = 572) | 0.56 | 0.57 | 0.69 |
| : inferior (N = 572) | 0.76 | 0.79 | 0.81 |
| : nasal (N = 572) | 0.63 | 0.29 | 0.87 |
| Vertical cup-to-disk ratio (N = 449) | 0.90 | 0.94 | 0.80 |
| Cup volume (N = 449) | 4.60E-02 (0.03±0.01)* | 0.08 | 0.12 |
| Mean cup depth (N = 449) | 4.11E-02 (0.03±0.01)* | 0.07 | 0.10 |
| Maximum cup depth (N = 449) | 0.13 | 0.25 | 0.18 |
| ONH blood flow (N = 501) | 0.57 | 0.10 | 0.59 |
Significance level was set at Pcorrected < 4.27E-04 (0.05/[3 SNPs × 13 covariates × 3 genetic models]) for the genotype-phenotype analysis after Bonferroni correction. Bold texts indicate values with a statistically significant difference after the correction.
* indicates P value (β±SE)
β, changes in clinical parameters per copy of the risk allele; SE, standard error,
N, The numbers of eyes used for each variable and the risk allele for each SNP were displayed. IOP, intraocular pressure. HFA, Humphrey Field Analyzer. RNFL, retinal nerve fiber layer. ONH, Optic nerve head.
The presence of each POAG risk allele near CDKN2BAS-1 (rs2157719) was associated with a decrease in IOP of 6.89 mmHg (P = 1.70E-04; dominant model). No association was found between the other two variants and IOP. The presence of the risk allele in rs33912345 near SIX6 was nominally associated with a decrease in total peripapillary RNFL thickness of 2.16 μm (P = 4.68E-02; additive) and 2.82 μm (P = 2.40E-02; recessive). When the peripapillary RNFL was further divided into superior, inferior, nasal and temporal quadrants and assessed separately, the risk allele was nominally associated with a decrease in thickness of 4.11 μm (P = 1.33E-02; additive) and 4.69 μm (P = 1.40E-02; recessive) superiorly and 3.88 μm inferiorly (P = 3.91E-02; recessive), but not in other quadrants. A nominal association between parameters reflecting optic nerve head morphology and the risk allele near GAS7 (rs9913911) was observed. Each risk allele near GAS7 was possibly linked to an increase in cup volume of 3.00E-02 mm3 (P = 4.60E-02; additive) and mean cup depth of 3.00E-02 mm3 (P = 4.11E-02; additive). The presence of the risk allele in rs2157719 near CDKN2B-AS1 was nominally associated with a decrease in optic nerve head blood flow of 0.54 AU (P = 2.00E-02; additive) and 0.67 AU (P = 1.39E-02; recessive). The risk allele in rs33912345 near SIX6 was nominally associated with an increase in optic nerve head blood flow of 0.44 AU (P = 2.20E-02; additive). The risk allele in rs9913911 near GAS7 was nominally associated with a decrease in patter standard deviation of visual field parameters of 0.87 decibels (dB) (P = 2.44E-02; dominant). No associations were found between visual field parameters and the other two risk variants.
Discussion
By targeted analysis of a selection of reported risk SNPs in 1,172 Japanese patients with POAG and 1,559 ethnically matched controls, variants near CDKN2B-AS1 (rs2157719), SIX6 (rs33912345) and GAS7 (rs9913911) were found to be associated with the disease in the population. The association between SNPs near GAS7 and POAG was found in Japanese patients for the first time. This is only the third locus that has been associated convincingly with the disease in Japanese. The other two loci that were previously shown to be associated with the disease are CDKN2B-AS1 and SIX6 loci and both associations were confirmed in the present study. Genotype–phenotype correlation using a clinical data set derived from a single institution enabled a reliable comparison of the data from the patients and the risk variants. The results showed a unique pattern of clinical correlations for each of the risk variants in these three genes, which implies different roles of the risk genes in the development of POAG.
The results show that the disease risk variant rs2157719 near CDKN2B-AS1 was associated with decreased IOP in Japanese patients with POAG. This counterintuitive finding that an SNP linked to a lower IOP is also a risk factor for POAG, which is exacerbated by higher IOP has been reported previously in Caucasian populations.[20,21] Nevertheless, this study confirms the seemingly confusing finding in a far eastern Asian population and also assures the validity of our analysis pipeline. There are at least 3 possible explanations for the association between rs2157719 near CDKN2B-AS1 and a decrease in IOP. First, the risk alleles may confer the RGCs to become more sensitive to IOP-related glaucomatous damage. Second, the risk alleles may contribute to POAG through IOP-independent mechanisms. Lastly, although unlikely, lower IOP may be a disease risk for a subset of POAG patients with the risk alleles.
To our surprise, our study had another novel counterintuitive result. In the present analysis, the POAG risk SNP near CDKN2B-AS1 was nominally associated with decreased optic nerve head blood flow, whereas the risk SNP near SIX6 showed the inverse relationship, i.e. increased optic nerve head blood flow. On the other hand, the risk SNP near GAS7 had no association with blood flow. Reduced optic nerve head blood flow, as measured by LSFG, has been observed in patients with glaucoma and in experimental monkey models of glaucoma by us and others.[36–43] We previously reported that optic nerve head blood flow was already reduced in preperimetric glaucoma, [44] the earliest stage of glaucoma. The blood flow further declines with increased disease severity.[37,42,43] These investigations suggest that microvascular alterations in the optic nerve head may play an important role in the pathogenesis of POAG. Although the mechanisms by which different loci influence the development of glaucoma are uncertain, there are at least two potential reasons to account for the conflicting results for the association between optic nerve head blood flow and the risk alleles in CDKN2BAS-1 and SIX6. First, the risk alleles may confer the RGCs to become more sensitive to reduced ocular circulation. Second, the risk alleles may contribute to POAG through mechanisms independent of ocular circulation. Our results further highlight the complex nature of POAG, which may underlie the differences in response to treatment among patients. Our study also found that the risk allele near SIX6 (rs33912345) was nominally associated with reduced total peripapillary RNFL thickness and also with the thickness of superior and the inferior sectors in patients with POAG, a finding that was not observed for other risk variants. The findings are also in agreement with a study conducted in Singapore that found an association between the risk allele near SIX6 and reduced RNFL thickness in a cohort comprising 2,129 eyes from 1,222 subjects without glaucoma and 21 patients with glaucoma.[23] Therefore, the results of the present study are in good agreement with previously reported findings. Nevertheless, our study confirms the genotype–phenotype correlation in patients with glaucoma who actually suffer from the consequences of reduction in RNFL thickness. This finding greatly expands on the earlier study, in which the data were derived almost exclusively from normal subjects. The risk allele near GAS7, where the locus has been associated with IOP in previous studies [45,46], was found to be nominally associated with an increased cup volume and mean cup depth, as quantified from the stereoscopic fundus camera photographs but not with IOP.
The different findings for two different risk genes (SIX6 and GAS7), derived from two different imaging modalities (OCT and stereoscopic fundus camera), may imply that they have different roles in their contribution to the disease. Peripapillary RNFL thickness measured by OCT presumably reflects the number of axons projecting from the RGCs. Parameters related to optic disk morphology measured by the stereoscopic fundus camera probably reflect both the number of axons projecting from the RGCs and factors related to the extracellular matrix constituting the optic disk and the lamina cribrosa. The finding of reduced peripapillary RFNL thickness in patients with the SIX6 risk variant, but not in those with the GAS7 variant, implies that the pathologic changes in glaucoma associated with the SIX6 variant may occur primarily in the RGCs and their axons. The increased cupping with relatively preserved RNFL thickness observed in patients with the GAS7 variant implies that the primary pathologic focus may reside in the disk extracellular matrix and that axonal injury and RGC death are secondary events. Koolwijk et al., using quantitative real-time polymerase chain reaction (PCR), reported that the highest expression of GAS7 mRNA in human ocular tissues was in the lamina cribrosa, [47] a result consistent with our speculation. This suggestion could be tested further by comparing the clinical parameters in a large number of patients in the early phase of the disease before both the axons and the extracellular matrix of the optic disk degenerate in the later phase of the disease, or, alternatively, in healthy subjects.
The present study was limited by its sample size and cross-sectional design. In addition, IOP was measured in patients with POAG without discontinuing their antiglaucoma medications. The use of topical medication likely had an impact on IOP. A longitudinal study that includes a larger number of patients with POAG with complete information on pretreatment IOP is desired.
In conclusion, comprehensive SNP analysis of the known POAG-related loci in Japanese patients with POAG identified disease risk variants near CDKN2BAS-1 (rs2157719), SIX6 (rs33912345) and GAS7 (rs9913911). The genotype–phenotype correlation study suggested that the risk variants in these three genes have different effects on the glaucoma phenotype. Studying the associations between risk variants and clinical parameters is an important step toward understanding the pathology of the disease and optimizing the treatment of patients with POAG.
Supporting information
+ The case or control definition criterion was applied;—the criterion was not applied.
(DOCX)
P values from logistic regression analysis adjusted for age and sex in the GWAS are displayed (far right). Genotype method for primary screening is displayed. If the SNP is imputed, info score is displayed. NA indicates that the SNP is not included in the custom chip or was not polymorphic. Chr, chromosome, SNP, single nucleotide polymorphism; bp, base pair.
(DOCX)
(XLSX)
Acknowledgments
The authors thank Naoto Imadome, Hidetoshi Takahashi, Shota Ono, Ryosuke Wakusawa, Tomoki Yasui, Keiichi Kato, Hitoshi Okabe, Makoto Ohmura, Nobuo Maekawa, Yuya Sato, members of Japan Glaucoma Society Omics Group (Makoto Araie, Kenji Kashiwagi, Makoto Aihara, Takeshi Iwata, Fumihiko Mabuchi, Mistuko Takamoto, Mineo ozaki and Kazuhide Kawase) for their contribution in sample collection and Sakiko Fujita, Testuya Osato, Shiori Suzuki, Junko Ouchi, for technical support. Computational resources for genotype imputation were provided by the Tohoku Medical Megabank Organization supercomputer system. This research is (partially) supported by the Center of Innovation Program from Japan Science and Technology Agency, JST. The manuscript has been proof read by an English editing service (Enago Ltd., Tokyo, Japan).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This paper was supported in part by the Japan Society For The Promotion Of Science KAKENHI Grants-in-Aid for Scientific Research (B) (T.N. 26293372) and by an unrestricted grant from Senju Pharmaceutical Co. Ltd., Osaka, Japan. Part of SNP genotyping was supported by the Center of Innovation Program from Japan Science and Technology Agency, JST. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flammer J, Orgul S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–393. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki Y, Iwase A, Araie M, Yamamoto T, Abe H, Shirato S, et al. Risk factors for open-angle glaucoma in a Japanese population: the Tajimi Study. Ophthalmology. 2006;113:1613–1617. doi: 10.1016/j.ophtha.2006.03.059 [DOI] [PubMed] [Google Scholar]
- 4.Marcus MW, de Vries MM, Junoy Montolio FG, Jansonius NM. Myopia as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. Ophthalmology. 2011;118:1989–1994 e1982. doi: 10.1016/j.ophtha.2011.03.012 [DOI] [PubMed] [Google Scholar]
- 5.Nakazawa T. Ocular Blood Flow and Influencing Factors for Glaucoma. Asia Pac J Ophthalmol (Phila). 2016;5:38–44. [DOI] [PubMed] [Google Scholar]
- 6.Tielsch JM, Katz J, Sommer A, Quigley HA, Javitt JC. Family history and risk of primary open angle glaucoma. The Baltimore Eye Survey. Arch Ophthalmol. 1994;112:69–73. [DOI] [PubMed] [Google Scholar]
- 7.Klein BE, Klein R, Lee KE. Heritability of risk factors for primary open-angle glaucoma: the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 2004;45:59–62. [DOI] [PubMed] [Google Scholar]
- 8.Thorleifsson G, Walters GB, Hewitt AW, Masson G, Helgason A, DeWan A, et al. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat Genet. 2010;42:906–909. doi: 10.1038/ng.661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burdon KP, Macgregor S, Hewitt AW, Sharma S, Chidlow G, Mills RA, et al. Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1. Nat Genet. 2011;43:574–578. doi: 10.1038/ng.824 [DOI] [PubMed] [Google Scholar]
- 10.Wiggs JL, Yaspan BL, Hauser MA, Kang JH, Allingham RR, Olson LM, et al. Common variants at 9p21 and 8q22 are associated with increased susceptibility to optic nerve degeneration in glaucoma. PLoS Genet.2012;8:e1002654 doi: 10.1371/journal.pgen.1002654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gharahkhani P, Burdon KP, Fogarty R, Sharma S, Hewitt AW, Martin S, et al. Common variants near ABCA1, AFAP1 and GMDS confer risk of primary open-angle glaucoma. Nat Genet. 2014;46:1120–1125. doi: 10.1038/ng.3079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey JN, Loomis SJ, Kang JH, Allingham RR, Gharahkhani P, Khor CC, et al. Genome-wide association analysis identifies TXNRD2, ATXN2 and FOXC1 as susceptibility loci for primary open-angle glaucoma. Nat Genet. 2016;48:189–194. doi: 10.1038/ng.3482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakano M, Ikeda Y, Taniguchi T, Yagi T, Fuwa M, Omi N, et al. Three susceptible loci associated with primary open-angle glaucoma identified by genome-wide association study in a Japanese population. Proc Natl Acad Sci U S A. 2009;106:12838–12842. doi: 10.1073/pnas.0906397106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meguro A, Inoko H, Ota M, Mizuki N, Bahram S. Genome-wide association study of normal tension glaucoma: common variants in SRBD1 and ELOVL5 contribute to disease susceptibility. Ophthalmology. 2010;117:1331–1338 e1335. doi: 10.1016/j.ophtha.2009.12.001 [DOI] [PubMed] [Google Scholar]
- 15.Osman W, Low SK, Takahashi A, Kubo M, Nakamura Y. A genome-wide association study in the Japanese population confirms 9p21 and 14q23 as susceptibility loci for primary open angle glaucoma. Hum Mol Genet. 2012;21:2836–2842. doi: 10.1093/hmg/dds103 [DOI] [PubMed] [Google Scholar]
- 16.Nakano M, Ikeda Y, Tokuda Y, Fuwa M, Omi N, Ueno M, et al. Common variants in CDKN2B-AS1 associated with optic-nerve vulnerability of glaucoma identified by genome-wide association studies in Japanese. PLoS One. 2012;7:e33389 doi: 10.1371/journal.pone.0033389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takamoto M, Kaburaki T, Mabuchi A, Araie M, Amano S, Aihara M, et al. Common variants on chromosome 9p21 are associated with normal tension glaucoma. PLoS One. 2012;7:e40107 doi: 10.1371/journal.pone.0040107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Lin Y, Vithana EN, Jia L, Zuo X, Wong TY, et al. Common variants near ABCA1 and in PMM2 are associated with primary open-angle glaucoma. Nat Genet. 2014;46:1115–1119. doi: 10.1038/ng.3078 [DOI] [PubMed] [Google Scholar]
- 19.Li Z, Allingham RR, Nakano M, Jia L, Chen Y, Ikeda Y, et al. A common variant near TGFBR3 is associated with primary open angle glaucoma. Hum Mol Genet. 2015;24:3880–3892. doi: 10.1093/hmg/ddv128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burdon KP, Crawford A, Casson RJ, Hewitt AW, Landers J, Danoy P, et al. Glaucoma risk alleles at CDKN2B-AS1 are associated with lower intraocular pressure, normal-tension glaucoma, and advanced glaucoma. Ophthalmology. 2012;119:1539–1545. doi: 10.1016/j.ophtha.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 21.Pasquale LR, Loomis SJ, Kang JH, Yaspan BL, Abdrabou W, Budenz DL, et al. CDKN2B-AS1 genotype-glaucoma feature correlations in primary open-angle glaucoma patients from the United States. Am J Ophthalmol. 2013;155:342–353 e345. doi: 10.1016/j.ajo.2012.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carnes MU, Liu YP, Allingham RR, Whigham BT, Havens S, Garrett ME, et al. Discovery and functional annotation of SIX6 variants in primary open-angle glaucoma. PLoS Genet. 2014;10:e1004372 doi: 10.1371/journal.pgen.1004372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng CY, Allingham RR, Aung T, Tham YC, Hauser MA, Vithana EN, et al. Association of common SIX6 polymorphisms with peripapillary retinal nerve fiber layer thickness: the Singapore Chinese Eye Study. Invest Ophthalmol Vis Sci. 2015;56:478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo JZ, Zangwill LM, Medeiros FA, Liebmann JM, Girkin CA, Hammel N, et al. Quantitative Trait Locus Analysis of SIX1-SIX6 With Retinal Nerve Fiber Layer Thickness in Individuals of European Descent. Am J Ophthalmol. 2015;160:123–130.e121. doi: 10.1016/j.ajo.2015.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawai Y, Mimori T, Kojima K, Nariai N, Danjoh I, Saito R, et al. Japonica array: improved genotype imputation by designing a population-specific SNP array with 1070 Japanese individuals. J Hum Genet. 2015;60:581–587. doi: 10.1038/jhg.2015.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagasaki M, Yasuda J, Katsuoka F, Nariai N, Kojima K, Kawai Y, et al. Rare variant discovery by deep whole-genome sequencing of 1,070 Japanese individuals. Nat Commun. 2015;6:8018 doi: 10.1038/ncomms9018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuriyama S, Yaegashi N, Nagami F, Arai T, Kawaguchi Y, Osumi N, et al. The Tohoku Medical Megabank Project: Design and Mission. J Epidemiol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi M, Omodaka K, Maruyama K, Yamaguchi T, Himori N, Shiga Y, et al. Simulated visual fields produced from macular RNFLT data in patients with glaucoma. Curr Eye Res. 2013;38:1133–1141. doi: 10.3109/02713683.2013.807932 [DOI] [PubMed] [Google Scholar]
- 29.Yokoyama Y, Tanito M, Nitta K, Katai M, Kitaoka Y, Omodaka K, et al. Stereoscopic analysis of optic nerve head parameters in primary open angle glaucoma: the glaucoma stereo analysis study. PLoS One. 2014;9:e99138 doi: 10.1371/journal.pone.0099138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamaki Y, Araie M, Kawamoto E, Eguchi S, Fujii H. Non-contact, two-dimensional measurement of tissue circulation in choroid and optic nerve head using laser speckle phenomenon. Exp Eye Res. 1995;60:373–383. [DOI] [PubMed] [Google Scholar]
- 31.Isono H, Kimura Y, Aoyagi K, Fujii H, Konishi N. Analysis of choroidal blood flow by laser speckle flowgraphy. Nippon Ganka Gakkai Zasshi. 1997;101:684–691. [PubMed] [Google Scholar]
- 32.Aizawa N, Nitta F, Kunikata H, Sugiyama T, Ikeda T, Araie M, et al. Laser speckle and hydrogen gas clearance measurements of optic nerve circulation in albino and pigmented rabbits with or without optic disc atrophy. Invest Ophthalmol Vis Sci. 2014;55:7991–7996. doi: 10.1167/iovs.14-15373 [DOI] [PubMed] [Google Scholar]
- 33.Ehlers N, Bramsen T, Sperling S. Applanation tonometry and central corneal thickness. Acta Ophthalmol (Copenh). 1975;53:34–43. [DOI] [PubMed] [Google Scholar]
- 34.Mabuchi F, Tang S, Ando D, Yamakita M, Wang J, Kashiwagi K, et al. The apolipoprotein E gene polymorphism is associated with open angle glaucoma in the Japanese population. Mol Vis. 2005;11:609–612. [PubMed] [Google Scholar]
- 35.Carnes MU, Liu YFP, Allingham RR, Whigham BT, Havens S, Garrett ME, et al. Discovery and Functional Annotation of SIX6 Variants in Primary Open-Angle Glaucoma. Plos Genetics. 2014;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yaoeda K, Shirakashi M, Fukushima A, Funaki S, Funaki H, Abe H, et al. Relationship between optic nerve head microcirculation and visual field loss in glaucoma. Acta Ophthalmol Scand. 2003;81:253–259. [DOI] [PubMed] [Google Scholar]
- 37.Chiba N, Omodaka K, Yokoyama Y, Aizawa N, Tsuda S, Yasuda M, et al. Association between optic nerve blood flow and objective examinations in glaucoma patients with generalized enlargement disc type. Clin Ophthalmol. 2011;5:1549–1556. doi: 10.2147/OPTH.S22097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Cull GA, Piper C, Burgoyne CF, Fortune B. Anterior and posterior optic nerve head blood flow in nonhuman primate experimental glaucoma model measured by laser speckle imaging technique and microsphere method. Invest Ophthalmol Vis Sci. 2012;53:8303–8309. doi: 10.1167/iovs.12-10911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cull G, Burgoyne CF, Fortune B, Wang L. Longitudinal hemodynamic changes within the optic nerve head in experimental glaucoma. Invest Ophthalmol Vis Sci. 2013;54:4271–4277. doi: 10.1167/iovs.13-12013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maekawa S, Shiga Y, Kawasaki R, Nakazawa T. Usefulness of novel laser speckle flowgraphy-derived variables of the large vessel area in the optic nerve head in normal tension glaucoma. Clin Exp Ophthalmol. 2014;42:887–889. doi: 10.1111/ceo.12354 [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi W, Kunikata H, Omodaka K, Togashi K, Ryu M, Akiba M, et al. Correlation of optic nerve microcirculation with papillomacular bundle structure in treatment naive normal tension glaucoma. J Ophthalmol. 2014;2014:468908 doi: 10.1155/2014/468908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aizawa N, Kunikata H, Shiga Y, Yokoyama Y, Omodaka K, Nakazawa T, et al. Correlation between structure/function and optic disc microcirculation in myopic glaucoma, measured with laser speckle flowgraphy. BMC Ophthalmol. 2014;14:113 doi: 10.1186/1471-2415-14-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiga Y, Omodaka K, Kunikata H, Ryu M, Yokoyama Y, Tsuda S, et al. Waveform analysis of ocular blood flow and the early detection of normal tension glaucoma. Invest Ophthalmol Vis Sci. 2013;54:7699–7706. doi: 10.1167/iovs.13-12930 [DOI] [PubMed] [Google Scholar]
- 44.Shiga Y, Kunikata H, Aizawa N, Kiyota N, Maiya Y, Yokoyama Y, et al. Optic Nerve Head Blood Flow, as Measured by Laser Speckle Flowgraphy, Is Significantly Reduced in Preperimetric Glaucoma. Curr Eye Res. 2016:1–7. [DOI] [PubMed] [Google Scholar]
- 45.Ozel AB, Moroi SE, Reed DM, Nika M, Schmidt CM, Akbari S, et al. Genome-wide association study and meta-analysis of intraocular pressure. Hum Genet. 2014;133:41–57. doi: 10.1007/s00439-013-1349-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hysi PG, Cheng CY, Springelkamp H, Macgregor S, Bailey JN, Wojciechowski R, et al. Genome-wide analysis of multi-ancestry cohorts identifies new loci influencing intraocular pressure and susceptibility to glaucoma. Nat Genet. 2014;46:1126–1130. doi: 10.1038/ng.3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Koolwijk LM, Ramdas WD, Ikram MK, Jansonius NM, Pasutto F, Hysi PG, et al. Common genetic determinants of intraocular pressure and primary open-angle glaucoma. PLoS Genet. 2012;8:e1002611 doi: 10.1371/journal.pgen.1002611 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
+ The case or control definition criterion was applied;—the criterion was not applied.
(DOCX)
P values from logistic regression analysis adjusted for age and sex in the GWAS are displayed (far right). Genotype method for primary screening is displayed. If the SNP is imputed, info score is displayed. NA indicates that the SNP is not included in the custom chip or was not polymorphic. Chr, chromosome, SNP, single nucleotide polymorphism; bp, base pair.
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.