Abstract
There is no known useful clinical parameter that can specifically predict a biliary stricture and differentiate it from other related complications after living donor liver transplantations (LDLT). The aims of this study were to determine whether the changes of liver enzymes can predict postoperative biliary stricture apart from other complications. We reviewed the medical records of 203 patients who underwent LDLT with duct to duct anastomosis from 2008 to 2010. The longitudinal changes of liver enzyme over time and the occurrence of complication were evaluated. A total of 124 patients had no complication up to 2 years after LDLT, and 74 patients had complications including biliary stricture and graft rejection. Complications developed more frequently in patients who's alkaline phosphatase (ALP) and gamma-glutamyl transpeptidase (GGT) did not return to the baseline plateau at 30 days after LDLT (ALP; P = .045, GGT; P = .047). Aspartate transaminase (AST) and alanine transaminase (ALT) increased continuously until the diagnosis of complication in both stricture and rejection groups with more rapid increase in enzymes in the rejection versus stricture group (P < .05). In addition, AST and ALT were 2-fold higher in the rejection than the stricture group at the diagnosis of each complication (AST; P < .05, ALT; P < .05). The increasing slope and final levels of AST and ALT are potentially helpful parameters to differentiate rejection and stricture, the 2 most common posttransplantation complications.
Keywords: biliary stricture, changes of liver enzymes, graft rejections, living donor liver transplantation, liver transplantation
1. Introduction
Living donor liver transplantation (LDLT) has been widely accepted as a therapeutic option for end-stage liver disease because of the shortage of deceased donor organs.[1] Despite improvements in surgical techniques and immunosuppressive therapies, biliary complications remain the major causes of morbidity and mortality after LDLT.[2,3] Biliary complications occur more frequently in LDLT than deceased donor liver transplantation[4] because of a small diameter of the anastomotic bile duct, anatomical biliary diversity, complex surgical procedures, and local ischemia of the peribiliary plexus.[3,5,6] The incidence of bile duct strictures reportedly ranges from 12% to 24% of all LDLT patients,[6–8] and the incidence of anastomotic strictures is higher than that of nonanastomotic strictures.
Since many years, endoscopic and percutaneous approaches have largely replaced surgery as the primary management of biliary strictures after LT regardless of repeated interventions. Endoscopic management has the advantage over percutaneous approaches because of easy endoscopic accessibility and less invasiveness. The success rates of endoscopic treatment for LDLT ranges from 60% to 75% for anastomotic strictures and 25% to 33% for nonanastomotic strictures.[3,9–12]
Several studies reported that various factors, including stricture morphology and the tip shape of distal bile duct, were associated with the outcomes in endoscopic management of biliary stricture.[12–15] Our previous study reported that prompt endoscopic interventions after the detection of biliary strictures may improve the success rate of endoscopic management.[15] Laboratory parameters are required for early detection, since the confirmative diagnosis of biliary stricture relies on endoscopic retrograde cholangio-pancreatography (ERCP) or magnetic resonance cholangiopancreatography. Although elevated liver enzymes could provide a diagnostic clue, they are not sensitive or accurate enough to the detection of biliary stricture.[16] No single parameter including the possible relationship between cytokine levels and biliary complication[17–19] is satisfactory for the detection of biliary stricture and its differentiation from other complications. We hypothesized that the complication after LDLT is predicted by the changes of liver enzymes. Therefore, the aims of the study were to determine whether the change of liver enzymes predict postoperative biliary stricture and distinguish it from other complications.
2. Material and methods
2.1. Patients
We conducted retrospective cohort study with adult patients who underwent LDLT with duct to duct anastomosis at Samsung Medical Center, Seoul, Korea from January 2008 to December 2010. The patient records were anonym zed and deidentified prior to analysis. None of the transplant donors were from a vulnerable population. This study was approved by the Institutional Review Board of Samsung Medical Center (SMC 2011-01-046). Patients with the following conditions were excluded: patients undergoing additional operation within 3 months; patients with recurrence of hepatocellular carcinoma; patients dying from causes unrelated to transplantation within 3 months; patients with recurrence of underlying disease within 3 months; lack of outpatient department visit after discharge; and history of previous liver transplantation.
2.2. Methods
The presence of a biliary stricture suspected by symptoms such as jaundice or an itching sensation, laboratory abnormalities such as elevated liver enzymes or hyperbilirubinemia, and dilated bile duct on abdominal ultrasonography or computed tomography (CT) were confirmed by ERCP or magnetic resonance cholangiopancreatography. Liver biopsy was performed in patients with unexplained elevated liver enzymes or hyperbilirubinemia. Pathognomonic features of biopsy specimens confirmed graft rejection or autoimmune hepatitis. The initial procedure (ERCP or liver biopsy) was selected according to the discretion of clinician based on clinical situation. The other procedure was performed step by step in case of no recovery after 1 procedure.
The results of all laboratory tests including total bilirubin, aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), and gamma-glutamyl transpeptidase (GGT) after transplant were evaluated. Laboratory test was usually performed daily or every other day during hospital admission and at every outpatient clinic visit after discharge at the institution. The general follow-up schedule was at a weekly interval for the 1st month after discharge, a 2 weekly interval for the 2nd month after discharge, and finally a 1 to 2 month interval. Patients were followed up to a maximum 2 years after transplantation. The patients were divided into 2 groups according to the recovery of serum ALP and GGT levels at 30 days after transplantation. Patients with ALP normalization (male < 128 U/L, female < 98 U/L) and GGT recovery (<90 U/L) at 30 days after transplantation were designated as the recovery group. Patients without ALP normalization and GGT recovery at 30 days after transplantation were assigned to the nonrecovery group.
2.3. Statistical analysis
The difference in baseline characteristics among groups was evaluated using chi-squared test or Fisher exact test for categorical variables and analysis of variance (ANOVA) for continuous variables as appropriate. We use the Scheffe test to determine which means actually differ if the null hypothesis was rejected. Mixed models of fixed and random effects were used to compare the level of change in the liver enzymes over 40 days follow-up time after LDLT. The observation period was based on recovery trends in the group without complication. We used the Kaplan–Meier method to assess the risk of biliary complications of LDLT by liver enzymes recovery status. Complication-free period after transplantation was calculated from day 1 of transplantation to the date of 1st diagnosed complication. Specific liver enzyme predictors of the development of biliary stricture prior to clinical diagnosis were identified by comparing enzyme levels among the complication groups using mixed models for overall patterns and ANOVA for each time point. Statistical analyses were performed using STATA 12.0 (Stata Corp, College Station, TX). P < .05 was considered statistically significant.
3. Results
During the study period, 255 adult LDLT with duct to duct anastomosis were performed. Fifty-two patients with the following conditions were excluded: additional operation within 3 months (17 patients); recurrence of hepatocellular carcinoma (16 patients); operation-unrelated death within 3 months (10 patients); recurrence of underlying disease within 3 months (4 patients); no follow-up after discharge (3 patients); and retransplantation (2 patients). Of the 79 patients with complication, 58 were confirmed with biliary stricture, 16 graft rejection, 2 biliary leakage, 1 bile duct stone, and 2 autoimmune hepatitis. Five patients with biliary leakage, bile duct stone, and autoimmune hepatitis were excluded from the analysis due to small sample size (Fig. 1). The baseline characteristics of the 198 patients are described in Table 1. There was no significant difference of profiles including patient age, patient gender, donor age, donor gender, operation time, ischemic time, donor duct number, anastomosis number, and ductoplasty between groups. The smallest size of donor duct was significantly different between the stricture and no complication groups (5.5 vs 6.5 mm, P < .01). The time from transplantation to confirmed complication was not significantly different between groups (P = .87): median time was 207.5 days (IQR 91–304) in stricture group and 163.5 days (IQR 81.5–373) in rejection group.
Figure 1.
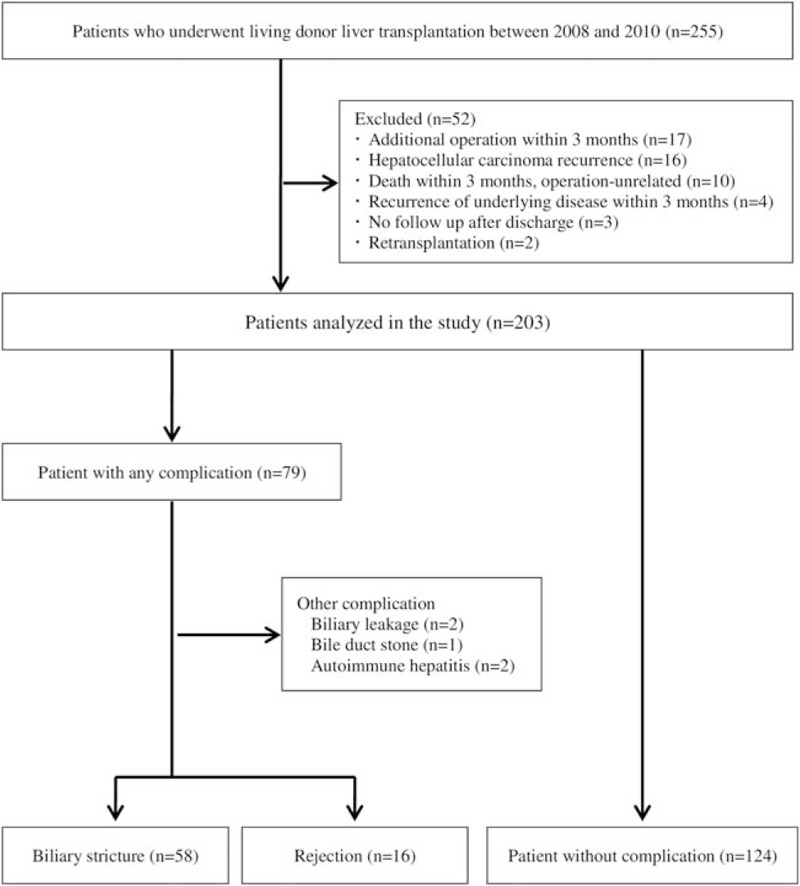
Flow diagram of 255 patients who underwent living donor liver transplantation with duct to duct anastomosis.
Table 1.
Characteristics of the study participants.
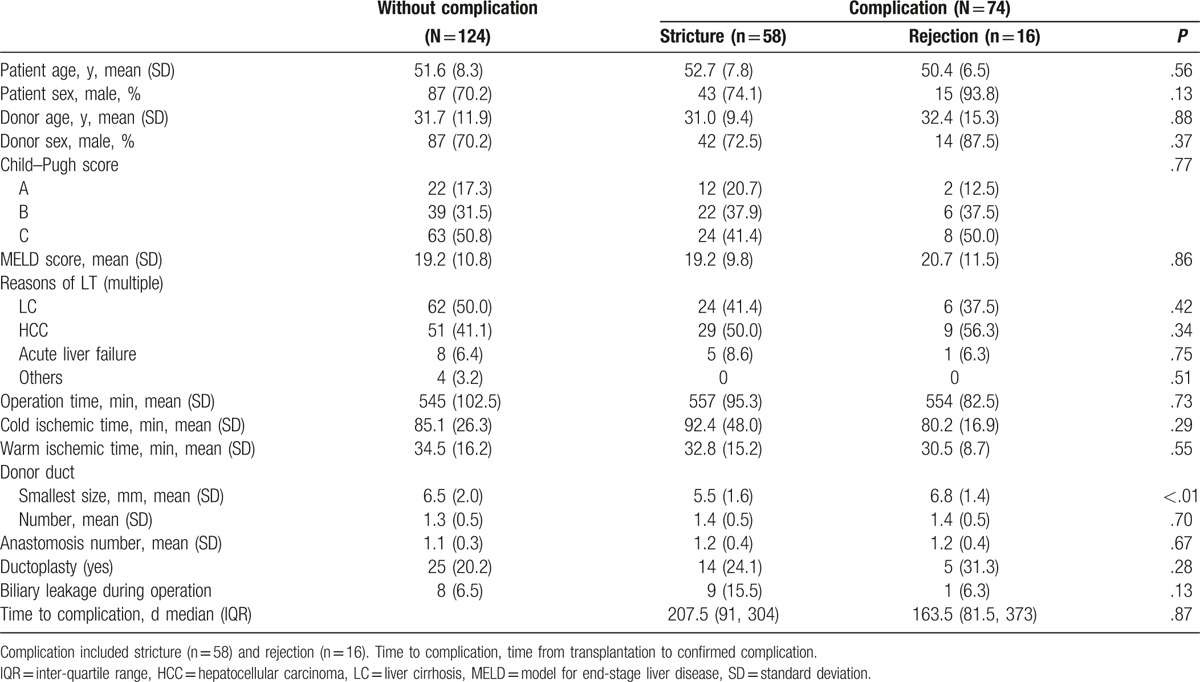
The change of serum liver enzymes for 40 days after transplantation is shown in Fig. 2. Serum AST and ALT levels were markedly elevated immediately after transplantation and gradually decreased to normal in all groups. Serum total bilirubin levels were decreased to normal gradually after transplantation in all groups. Serum ALP levels increased continuously after transplantation in all groups. Serum ALP levels tended to increase more in the stricture group. Serum GGT levels after transplantation were initially increased and decreased subsequently, with a tendency to later decrease in the stricture group, as compared to the other groups.
Figure 2.
Changes of liver enzymes for 40 days after living donor liver transplantation. Complication included stricture (n = 58) and rejection (n = 16). w/o complication, patients without complication. Each unit is U/L for AST, ALT, ALP, and GGT, and mg/dL for total bilirubin, respectively. ALP = alkaline phosphatase, ALT = alanine transaminase, AST = aspartate transaminase, GGT = gamma-glutamyl transpeptidase.
We assessed the incidence of complications according to ALP and GGT recovery status at 30 days after transplantation (Fig. 3). There was a significantly higher incidence of complication in the ALP and GGT nonrecovery group than the ALP and GGT recovery group, respectively (P = .032, P = .018).
Figure 3.
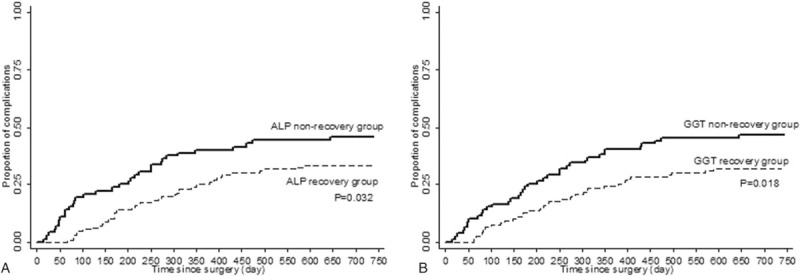
Proportion of subjects with the occurrence of postoperative complications by ALP and GGT recovery status for 30 days after living donor liver transplantation. Within 30 days after the transplantation, patients with ALP normalization (male < 128 U/L, female < 98 U/L) and GGT recovery (<90 U/L) were designed as ALP recovery group and GGT recovery group, respectively. Patients without ALP normalization and GGT recovery were designed as ALP nonrecovery group and GGT nonrecovery group, respectively. ALP = alkaline phosphatase, ALT = alanine transaminase, AST = aspartate transaminase, GGT = gamma-glutamyl transpeptidase.
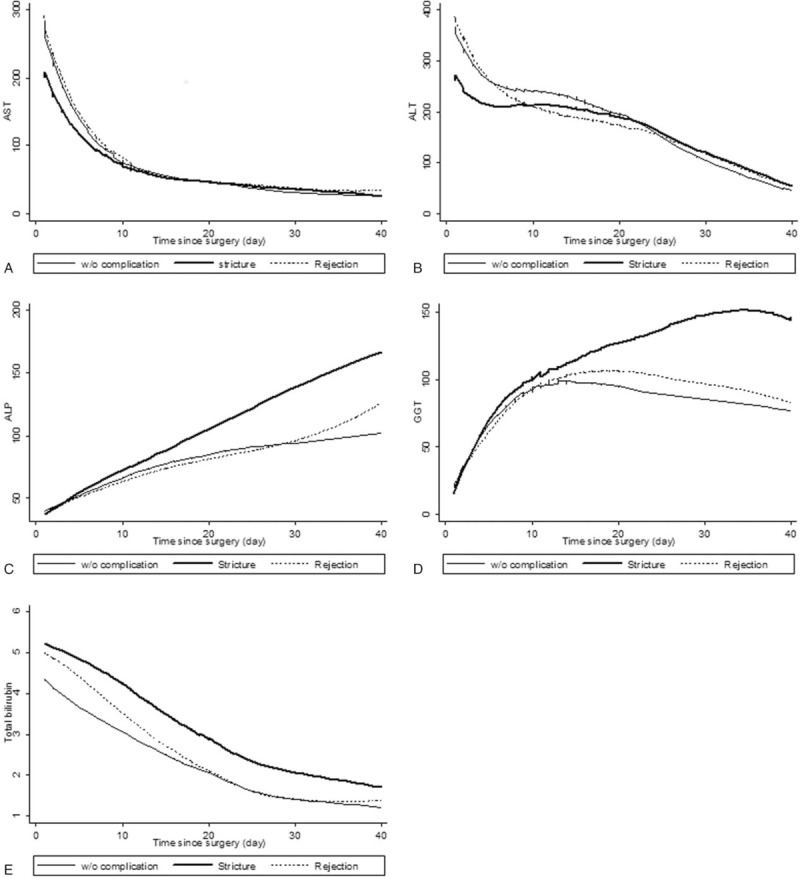
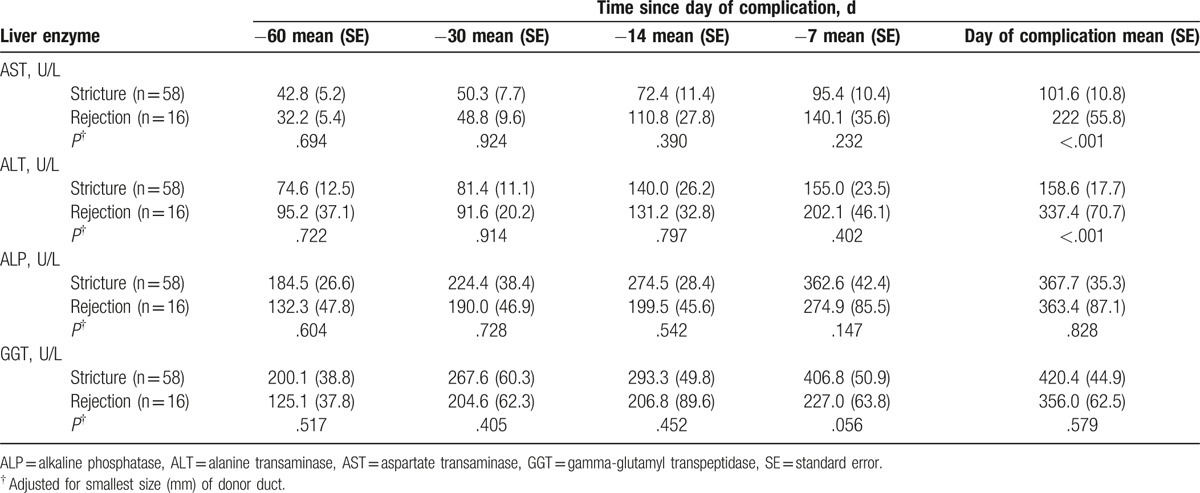
The change of liver enzymes was analyzed for 60 days before the confirmed complication (Fig. 4). In stricture and rejection groups, liver enzymes were continuously increased until time to confirm the complication. Although serum ALP and GGT levels had no difference in slopes between both groups, the rejection group had steeper slopes of serum AST and ALT levels than the stricture group (P < .05). Liver enzyme levels were analyzed at 60-, 30-, 14-, and 7-days before the confirmed complication and the day of confirmed complication (Table 2). On the day of confirmed complication, the rejection group showed a 2-fold elevation of serum AST and ALT levels than the stricture group (AST; 222 ± 55.8 vs 101.6 ± 10.8, P < .05 and ALT; 337.4 ± 70.7 vs 158.6 ± 17.1, P < .05).
Figure 4.
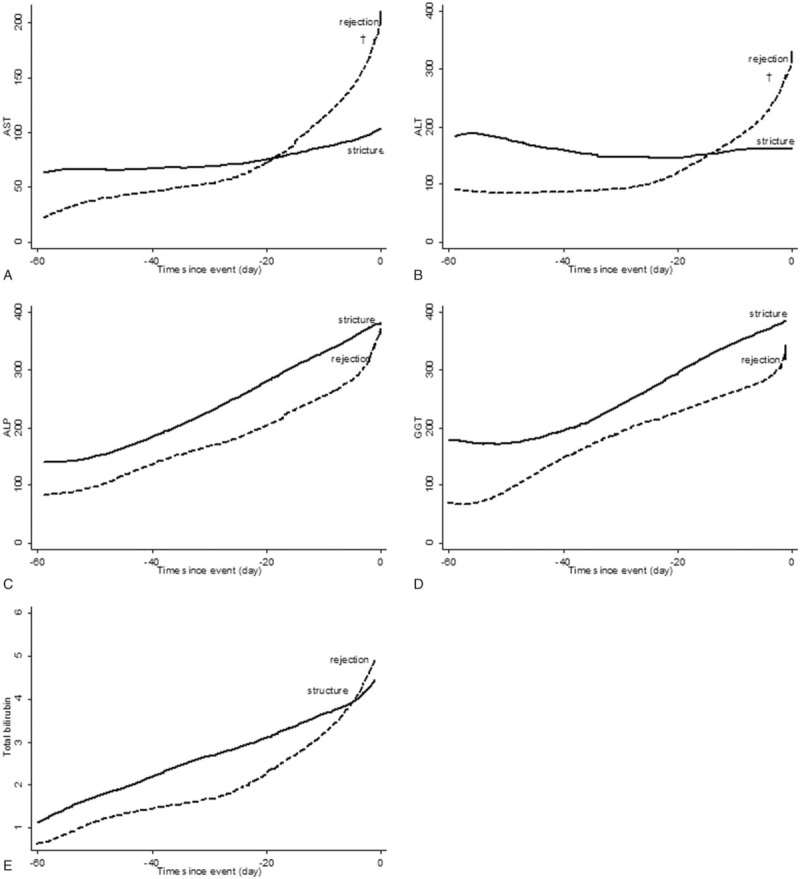
Changes of liver enzymes for 60 days before confirmed complication. Each unit is U/L for AST, ALT, ALP, and GGT, and mg/dL for TB, respectively. †Significantly difference of slopes between stricture and rejection (P < .05). ALP = alkaline phosphatase, ALT = alanine transaminase, AST = aspartate transaminase, GGT = gamma-glutamyl transpeptidase, TB = total bilirubin.
Table 2.
Liver enzymes at 60-, 30-, 14-, and 7-days before confirmed complication and at the day of confirmed complication.
4. Discussion
We investigated the change of liver enzymes and complication occurrence after LDLT. Serum AST and ALT levels gradually decreased to normal after marked elevation in all groups. Serum ALP and GGT levels increased continuously after transplantation. The ALP and GGT recovery status for 30 days after transplantation indicated that the ALP and GGT nonrecovery group had a significantly higher complication incidence than the ALP and GGT recovery group. In the stricture group, serum ALP levels tended to have greater increases and serum GGT levels tended to decrease later than other groups. Before diagnosis of complication, serum AST and ALT levels had significantly different slopes and levels between the stricture and rejection groups.
Many previous studies reported the change of liver enzymes after orthotropic liver transplantation. Marked elevation of transaminases is reported in the 1st hours after liver reperfusion, with a peak at the 1st or 2nd day after transplantation. Unless acute rejection occurs, transaminase levels are normalized within 1 week.[20] The increase of cholestasis parameter, including GGT and bilirubin, was initiated from day 2 after transplantation with a peak after 10 to 16 days. The damage of bile canaliculi caused by ischemia-reperfusion during liver transplantation could explain this difference in biochemical changes.[21] In our study of patients who underwent only LDLT, the same changes of liver enzymes were observed in the noncomplication group.
Biliary stricture after liver transplantation could be suspected in patients with abnormal liver enzymes levels on routine measurements. Diagnosis of biliary stricture based on liver enzymes levels alone is very challenging, because recurrence of the underlying diseases or graft rejection could also lead to elevation of liver enzymes levels. Invasive techniques, such as ERCP or percutaneous transhepatic cholangiography, are still the gold standard. Liver biopsy is required to exclude other causes of cholestasis and diagnose graft rejection. Although many laboratory parameters have been studied as early, noninvasive markers, there is no single parameter to detect the complication after LT. Few studies evaluated the significance of serum liver enzymes levels on routine measurement during the follow-up period. Some reports indicated that liver enzyme values may provide a clue to presence of posttransplant biliary stricture.[22–24] Other studies reported that liver enzyme values are not sensitive or accurate enough to detect any biliary complications after LT.[16] The drawback of their study was that the analysis and comparison of liver enzyme values was conducted at the time of complication diagnosis and regular follow-up. On the other hand, we collected data on liver enzymes immediately after LT and at every follow-up until the 1st diagnosis of complication or maximum 2 years.
ALP and GGT recovery status for 30 days after transplantation suggested a significant difference in complication occurrence within 2 years between the recovery and nonrecovery groups. These findings may suggest that the posttransplantation complication has been initiated from 30 days after LDLT. A recent study reported that the biliary complication group had significantly increased levels of IL-2, IL-12, and IL-4 on posttransplantation day 7.[17] However, there has been no sufficient evidence to explain mechanisms that the patients of this group have a high incidence of complication including biliary stricture and graft rejection. Close surveillance will be required in patients of this group until the sufficient data are presented in further studies.
Serum AST and ALT levels were significantly different between biliary stricture and graft rejection at the day of complication. However, this single value could not differentiate biliary stricture from graft rejection. Also, CT imaging is difficult to diagnose the biliary stricture after transplantation, because dilation of the bile duct may develop more slowly and also because the bile duct may be filled with epithelial casts.[24] Therefore, it is not easy to decide the next diagnostic step such as ERCP and liver biopsy. Our study found that the slope of AST and ALT level change was significantly different between groups. Based on this finding, liver biopsy may be considered 1st in patients with a steep slope of serum AST and ALT change and ERCP in patients with a gradual slope.
Strengths of the present study included sequential measures of serum liver enzymes during routine follow-up. Hence, the diagnosis and differentiation of posttransplantation complications was based on serial changes in liver enzymes levels rather than at discrete points. Limitations were mainly related to retrospective data collection. A lack of information of subjective symptoms made it impossible to estimate the exact time at which complications developed. Second, even though information on major factors that influence the liver enzymes was collected, our study was unable to examine additional factors that might have confounded the relationship between liver enzyme change and complication, including noncompliance of immunosuppressive drug use, alcohol consumption, and physical activity. Third, the mean follow-up period in our study was <2 years, which may not have been sufficient to determine the onset of long-term outcomes. The study follow-up period was probably sufficient to determine whether the change of liver enzymes predict biliary stricture, since most biliary strictures occur within 1 year of transplantation (median 207.5 days), and the finding was in agreement with previous studies.[14,15,25]
In conclusion, the change of serum liver enzymes can be used as noninvasive and nonexpensive ancillary markers to distinguish biliary stricture from graft rejection. Complications developed more frequently in patients whose ALP and GGT did not return to the baseline plateau at 30 days after LDLT. The different slope and final level of AST and ALT could be helpful parameters to differentiate rejection from stricture, the 2 most common complications after LDLT. Further prospective studies are needed to validate our finding and develop a detection and prediction model of posttransplantation complication using the change of serum liver enzyme levels.
Footnotes
Abbreviations: ALP = alkaline phosphatase, ALT = alanine transaminase, AST = aspartate transaminase, ERCP = endoscopic retrograde cholangio-pancreatography, GGT = gamma-glutamyl transpeptidase, LDLT = living donor liver transplantation.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Nadalin S, Malago M, Radtke A, et al. Current trends in live liver donation. Transpl Int 2007;20:312–30. [DOI] [PubMed] [Google Scholar]
- [2].Verdonk RC, Buis CI, Porte RJ, et al. Anastomotic biliary strictures after liver transplantation: causes and consequences. Liver Transpl 2006;12:726–35. [DOI] [PubMed] [Google Scholar]
- [3].Shah SA, Grant DR, McGilvray ID, et al. Biliary strictures in 130 consecutive right lobe living donor liver transplant recipients: results of a Western center. Am J Transpl 2007;7:161–7. [DOI] [PubMed] [Google Scholar]
- [4].Hwang S, Lee SG, Sung KB, et al. Long-term incidence, risk factors, and management of biliary complications after adult living donor liver transplantation. Liver Transpl 2006;12:831–8. [DOI] [PubMed] [Google Scholar]
- [5].Yoshimoto T, Yazumi S, Hisatsune H, et al. Crane-neck deformity after right lobe living donor liver transplantation. Gastrointest Endosc 2006;64:271. [DOI] [PubMed] [Google Scholar]
- [6].Gondolesi GE, Varotti G, Florman SS, et al. Biliary complications in 96 consecutive right lobe living donor transplant recipients. Transplantation 2004;77:1842–8. [DOI] [PubMed] [Google Scholar]
- [7].Liu CL, Lo CM, Chan SC, et al. Safety of duct-to-duct biliary reconstruction in right-lobe live-donor liver transplantation without biliary drainage. Transplantation 2004;77:726–32. [DOI] [PubMed] [Google Scholar]
- [8].Dulundu E, Sugawara Y, Sano K, et al. Duct-to-duct biliary reconstruction in adult living-donor liver transplantation. Transplantation 2004;78:574–9. [DOI] [PubMed] [Google Scholar]
- [9].Shah JN, Ahmad NA, Shetty K, et al. Endoscopic management of biliary complications after adult living donor liver transplantation. Am J Gastroenterol 2004;99:1291–5. [DOI] [PubMed] [Google Scholar]
- [10].Tashiro H, Itamoto T, Sasaki T, et al. Biliary complications after duct-to-duct biliary reconstruction in living-donor liver transplantation: causes and treatment. World J Surg 2007;31:2222–9. [DOI] [PubMed] [Google Scholar]
- [11].Tsujino T, Isayama H, Sugawara Y, et al. Endoscopic management of biliary complications after adult living donor liver transplantation. Am J Gastroenterol 2006;101:2230–6. [DOI] [PubMed] [Google Scholar]
- [12].Yazumi S, Yoshimoto T, Hisatsune H, et al. Endoscopic treatment of biliary complications after right-lobe living-donor liver transplantation with duct-to-duct biliary anastomosis. J Hepatobiliary Pancreat Surg 2006;13:502–10. [DOI] [PubMed] [Google Scholar]
- [13].Chok KS, Chan SC, Cheung TT, et al. A retrospective study on risk factors associated with failed endoscopic treatment of biliary anastomotic stricture after right-lobe living donor liver transplantation with duct-to-duct anastomosis. Ann Surg 2014;259:767–72. [DOI] [PubMed] [Google Scholar]
- [14].Seo JK, Ryu JK, Lee SH, et al. Endoscopic treatment for biliary stricture after adult living donor liver transplantation. Liver Transplant 2009;15:369–80. [DOI] [PubMed] [Google Scholar]
- [15].Lee YY, Gwak GY, Lee KH, et al. Predictors of the feasibility of primary endoscopic management of biliary strictures after adult living donor liver transplantation. Liver Transplant 2011;17:1467–73. [DOI] [PubMed] [Google Scholar]
- [16].Thuluvath PJ, Pfau PR, Kimmey MB, et al. Biliary complications after liver transplantation: the role of endoscopy. Endoscopy 2005;37:857–63. [DOI] [PubMed] [Google Scholar]
- [17].Kim JM, Kim JH, Lee SY, et al. Prediction of biliary complications after living-donor liver transplantation based on serum cytokine profile. Transplant Proc 2014;46:861–4. [DOI] [PubMed] [Google Scholar]
- [18].Nakagiri T, Inoue M, Minami M, et al. Immunology mini-review: the basics of T(H)17 and interleukin-6 in transplantation. Transplant Proc 2012;44:1035–40. [DOI] [PubMed] [Google Scholar]
- [19].Shivakumar P, Campbell KM, Sabla GE, et al. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J Clin Invest 2004;114:322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Biasi F, Bosco M, Chiappino I, et al. Oxidative damage in human liver transplantation. Free Radic Biol Med 1995;19:311–7. [DOI] [PubMed] [Google Scholar]
- [21].Cutrin JC, Cantino D, Biasi F, et al. Reperfusion damage to the bile canaliculi in transplanted human liver. Hepatology 1996;24:1053–7. [DOI] [PubMed] [Google Scholar]
- [22].Hintze RE, Abou-Rebyeh H, Adler A, et al. Endoscopic therapy of ischemia-type biliary lesions in patients following orthotopic liver transplantation. Z Gastroenterol 1999;37:13–20. [PubMed] [Google Scholar]
- [23].Shastri YM, Hoepffner NM, Akoglu B, et al. Liver biochemistry profile, significance and endoscopic management of biliary tract complications post orthotopic liver transplantation. World J Gastroenterol 2007;13:2819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zoepf T, Maldonado-Lopez EJ, Hilgard P, et al. Diagnosis of biliary strictures after liver transplantation: which is the best tool? World J Gastroenterol 2005;11:2945–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kim TH, Lee SK, Han JH, et al. The role of endoscopic retrograde cholangiography for biliary stricture after adult living donor liver transplantation: technical aspect and outcome. Scand J Gastroenterol 2011;46:188–96. [DOI] [PubMed] [Google Scholar]


