Supplemental Digital Content is available in the text
Keywords: cervical cancer, diabetes, prognosis
Abstract
Background:
The predictive roles of diabetes in the prognosis of many types of cancer have been well studied, but its role in predicting the prognosis of cervical cancer is still controversial. The aim of the study is to evaluate the association between diabetes/hyperglycemia and the prognosis of cervical cancer.
Methods:
We conducted a systematic review for peer-reviewed studies indexed in PubMed, Embase, Web of Science, and Wanfang published before December 2016. Hazard ratios (HRs) with 95% confidence intervals (95% CIs) were pooled in the meta-analysis.
Results:
This systematic review identified 13 studies with a total of 11,091 cervical cancer patients, of which 11 studies were included in the meta-analysis. The study indicated that diabetes was related to poorer overall survival (HR = 1.59, 95% CI: 1.35–1.87, P < .001) and poorer recurrence-free survival (HR = 1.98, 95% CI: 1.47–2.66, P < .001) in cervical cancer patients. The meta-analysis of adjusted HRs also indicated that diabetes was independently associated with poor overall survival (HR = 1.69, 95% CI: 1.38–2.05, P < .001) and poor recurrence-free survival (HR = 1.98, 95% CI: 1.47–2.66, P < .001) in cervical cancer patients. Sensitivity analysis and subgroup analyses showed similar results. No significant heterogeneity was observed for the included studies.
Conclusions:
The meta-analysis suggests that diabetes is an important predictive factor for cervical cancer prognosis, and it is linked to poorer survival of cervical cancer patients. Diabetes can serve as a useful index in the prognostic evaluation for patients with cervical cancer.
1. Introduction
Cervical cancer is one of the most common gynecological cancers in the world, and about 454,000 women are newly diagnosed with cervical cancer every year.[1,2] Though many advances have been achieved in the treatment of cervical cancer, a large proportion of patients with advanced cervical cancer still have a poor prognosis.[3,4] Thus, appropriate clinical staging before treatment is vital to improve the prognosis of cervical cancer, and patients with lower survival probability may need more intensive management. Some prognostic factors predicting the survival of cervical cancer patients have been found, whereas the clinical staging of cervical cancer is still mainly based on the clinical exam and clinical imaging.[5,6] Therefore, more useful and effective prognostic factors predicting the survival of cervical cancer patients are needed to establish a more appropriate clinical staging system for cervical cancer patients.
Diabetes is an increasingly common metabolic diseases.[7,8] Recently, the prevalence of type 2 diabetes has risen rapidly due to the epidemic of obesity.[7] Previous studies have suggested that diabetes can promote both tumorigenesis and tumor progression.[9–11] It is well known that diabetes is a risk factor of cancer. A large number of epidemiological studies have shown the predictive role of diabetes in the prognosis of many types of cancers, such as breast cancer, ovarian cancer, and colorectal cancer.[12–16] Considering the prognostic roles of diabetes, numerous studies also have investigated its predictive role in cervical cancer prognosis.[17–26] Some studies reported that diabetes was associated with poor survival in cervical cancer patients,[17,24,25] but others indicated that diabetes had no significant influence on the prognosis of cervical cancer.[20,27] Therefore, the role of diabetes in predicting the prognosis of cervical cancer is still controversial. To address this issue, we performed a systematic review and meta-analysis to comprehensively evaluate the predictive role of diabetes/hyperglycemia in cervical cancer prognosis.
2. Methods
2.1. Data sources and eligibility criteria
The PubMed, Embase, Web of Science, and Wanfang databases were searched to identify relevant studies evaluating the prognostic value of diabetes in cervical cancer. The last updated search was carried out on December 20, 2016. Google Scholar was also searched to find additional studies. The following search terms and combinations were used in keyword and subject heading searches: (diabetes or diabetic or T2DM), (cervical cancer or cervical carcinoma or cervix cancer or cervix carcinoma), and (prognosis or prognostic or survival or mortality or outcomes or outcome). There was no language limitation in the literature search. References of relevant studies were checked manually. This study was carried out under the guideline of Preferred Reporting Items for Systematic Reviews and Meta-analysis.[28]
Studies eligible for inclusion met the following criteria: (1) patients had histopathologically confirmed cervical cancer; (2) the exposure was diabetes or hyperglycemia; (3) the controls were those without diabetes or those with normal fasting blood glucose; (4) the outcomes of interests were overall survival or recurrence-free survival; (5) hazard ratios (HRs) with 95% confidence intervals (95% CIs) were reported, or data that could be transferred to risk estimates of cervical cancer prognosis were provided. Studies that did not meet the eligibility criteria were excluded. Studies with overlapping or duplicate data were also excluded.
2.2. Data extraction and quality assessment
Two investigators independently extracted data from each included study using a standardized table. Any disagreement was settled by discussion and consensus among all authors. Extracted information included: name of the first author, publication year, study design, country, characteristics of cervical cancer patients, definitions of diabetes or hyperglycemia, duration of follow-up, outcomes of interest, adjustment factors, and HRs with 95% CIs. If both unadjusted HRs and adjusted HRs were provided, only the latter were used. The study quality was assessed according to the Newcastle–Ottawa scale.[29] We evaluated the quality of included studies in terms of the representativeness of recruited cervical cancer patients, the comparability between exposed participants and nonexposed participants, and the adequate assessment of outcome. Studies that scored 6 or more were considered as high quality ones; those with scores of 5 or less were regarded as low quality.
2.3. Statistical analysis
To assess the associations between diabetes and overall survival or recurrence-free survival in cervical cancer patients, HRs with 95% CIs were pooled using meta-analysis. Heterogeneity between studies was examined by Cochran's Q test and I2.[30,31] A P value on the Q test more than 0.10 or I2 larger than 50% indicated a high degree of between-study heterogeneity and suggested the use of a random-effects model to pool HRs.[32] Otherwise, a fixed-effect model was utilized.[33] Sensitivity analyses with sequential omission of individual studies were then carried out to test the credibility of the pooled HRs. Sensitivity analysis was also carried out by omitting studies assessing the impact of hyperglycemia on the prognosis of cervical cancer patients. Subgroup analyses were conducted by sample size, adjusted status, and study quality. Publication bias was evaluated by the funnel plot and Egger's test.[34] When publication bias existed, the trim and fill method was performed.[35] STATA 12.0 was used for statistical analysis. A 2-sided P value less than 0.05 was considered to indicate statistical significance. The ethics committee was not applicable to this meta-analysis.
3. Results
3.1. Literature search and included studies
Figure 1 shows a flow chart of the study selection for the meta-analysis. A total of 1080 papers were initially identified in the literature search of 3 databases. After reviewing the titles and abstracts, 1058 obviously irrelevant studies were excluded. After full-text reading, 10 studies not meeting the inclusion criteria were excluded.[36–45] Therefore, 13 studies were included into the systematic review.[17–27,46,47] Two studies without HRs were excluded from the meta-analysis.[17,20] Finally, 11 studies[18,19,21–27,46,47] reporting data for quantitative synthesis were included in the meta-analysis (Fig. 1).
Figure 1.
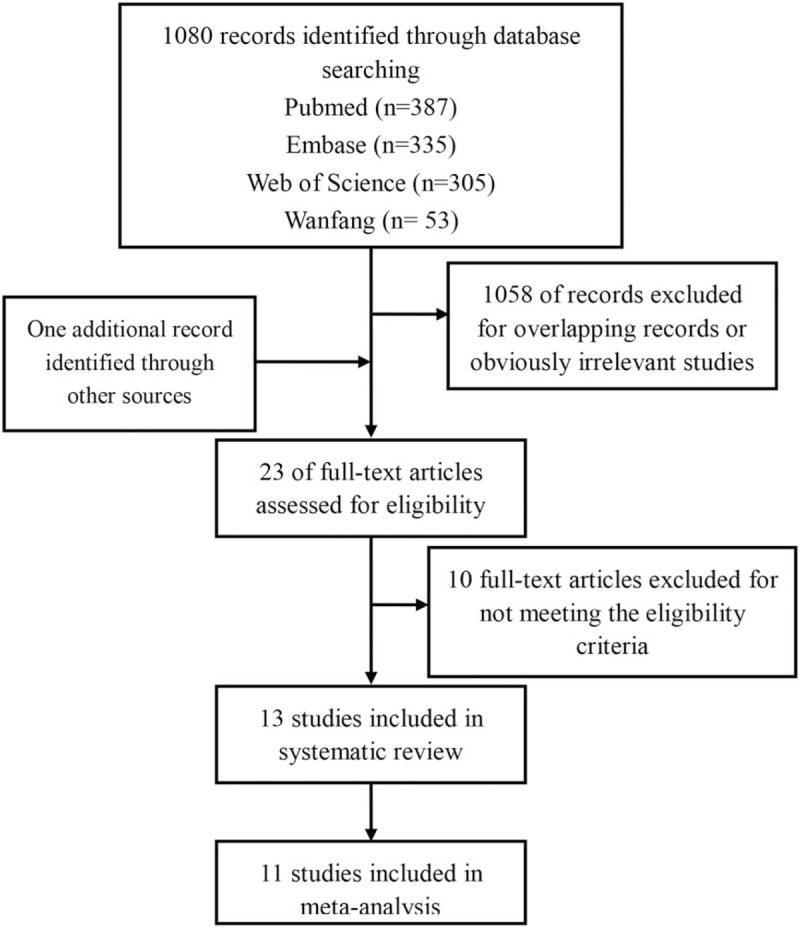
Flow chart of study selection for the meta-analysis.
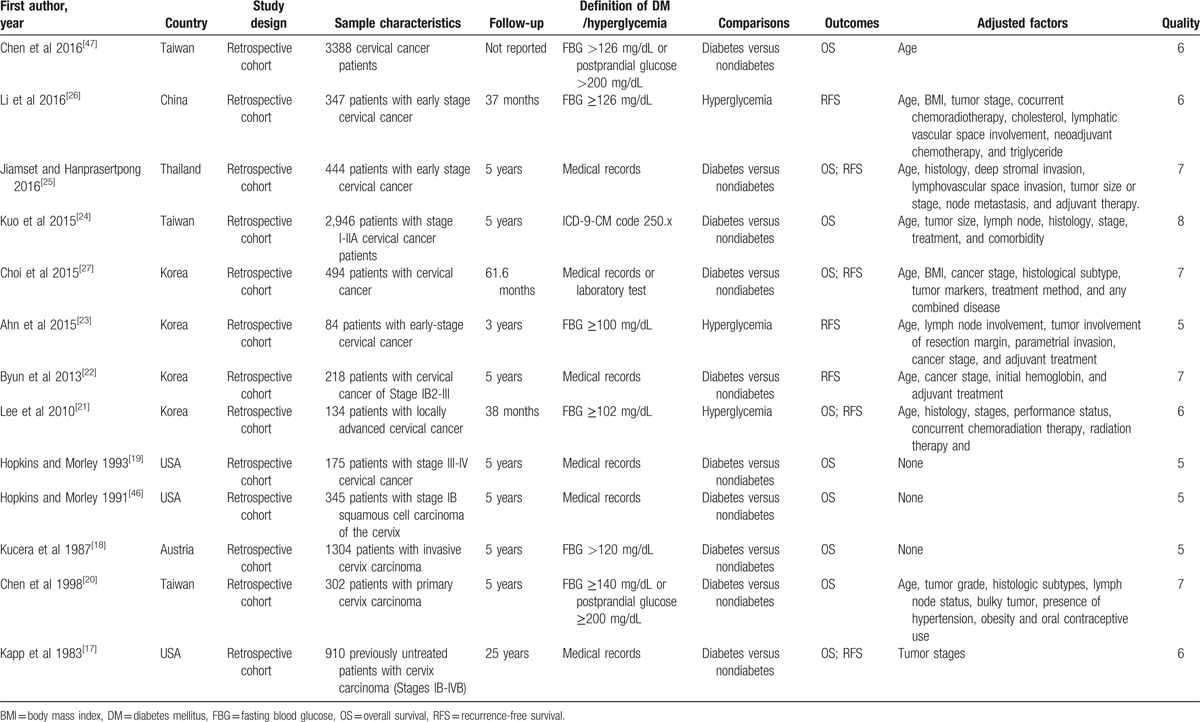
The 13 studies contained a total of 11,091 cervical cancer patients (Table 1). Table 1 summarizes the characteristics of the included studies. All studies used the retrospective cohort design. The majority of the included studies were conducted in Asia and the USA. Ten studies reported overall survival as the primary outcome of interest, and 7 studies reported recurrence-free survival. The follow-up time ranged from 3 to 25 years. Ten studies assessed the impact of diabetes on the survival of cervical cancer patients, and the rest evaluated the impact of hyperglycemia on the survival of cervical cancer patients. Kapp et al[17] reported that diabetes was associated with poor overall survival in cervical cancer after controlling for stage of disease (P = .026), but no association was found for recurrence-free survival. However, Chen et al[20] found that diabetes had no adverse effect on the overall survival of cervical cancer patients after controlling for adjusted factors. Neither of these studies reported HRs or data that could be used to calculate the HRs. The remaining 11 studies provided HRs or data that could be used to calculate the HRs, and 8 of them reported adjusted HRs. As to quality assessment, 9 studies had good quality, whereas the other 2 had suboptimal quality (Supplemental Table S1).
Table 1.
Summary of included studies on the association between diabetes and the prognosis in cervical cancer.
3.2. Meta-analysis
In the meta-analysis of overall survival, between-study heterogeneity was not obvious (P = .265; I2 = 20.7%). The meta-analysis indicated that diabetes predicted poorer overall survival in cervical cancer patients (HR = 1.59, 95% CI: 1.35–1.87, P < .001) (Fig. 2). Sensitivity analysis proved the credibility of the pooled HRs for overall survival (Supplemental Figure S1). After excluding studies addressing hyperglycemia, diabetes was still significantly associated with shorter overall survival time (HR = 1.57, 95% CI: 1.33–1.85, P < .001). In the subgroup analysis by adjusted status, after controlling other adjusted factors, diabetes independently predicted shorter overall survival time (HR = 1.69, 95% CI: 1.38–2.05, P < .001). The meta-analysis of 3 studies with unadjusted HRs also showed that diabetes was linked to shorter overall survival time (HR = 1.39, 95% CI: 1.04–1.87, P = .027). In the subgroup analysis by study quality, studies both with or without good quality suggested that diabetes could predict poorer overall survival in cervical cancer prognosis.
Figure 2.
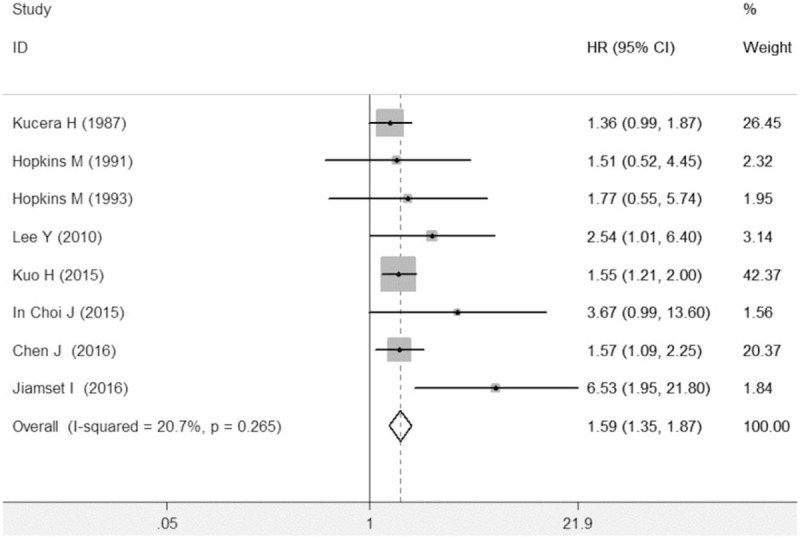
Meta-analysis indicated that diabetes predicted poorer overall survival in cervical cancer patients.
In the meta-analysis of recurrence-free survival, between-study heterogeneity was not significant (P = .745; I2 = 0%). The meta-analysis indicated that diabetes could predict poorer recurrence-free survival in cervical cancer prognosis (HR = 1.98, 95% CI: 1.47–2.66, P < .001) (Fig. 3). Sensitivity analysis proved the pooled HRs of recurrence-free survival was credible (Supplemental Figure S2). Similarly, after excluding studies assessing the impact of hyperglycemia on the prognosis of cervical cancer, diabetes could still significantly predict poorer recurrence-free survival in cervical cancer (HR = 2.09, 95% CI: 1.28–3.41, P = .003). All the included studies reported adjusted HRs. In the subgroup analysis by study quality, high-quality studies and those with suboptimal quality both suggested that diabetes could predict poorer recurrence-free survival in cervical cancer, and the pooled HRs were 1.89 (1.40–2.56; P = .000) and 4.30 (1.23–15.03; P = .022), respectively.
Figure 3.
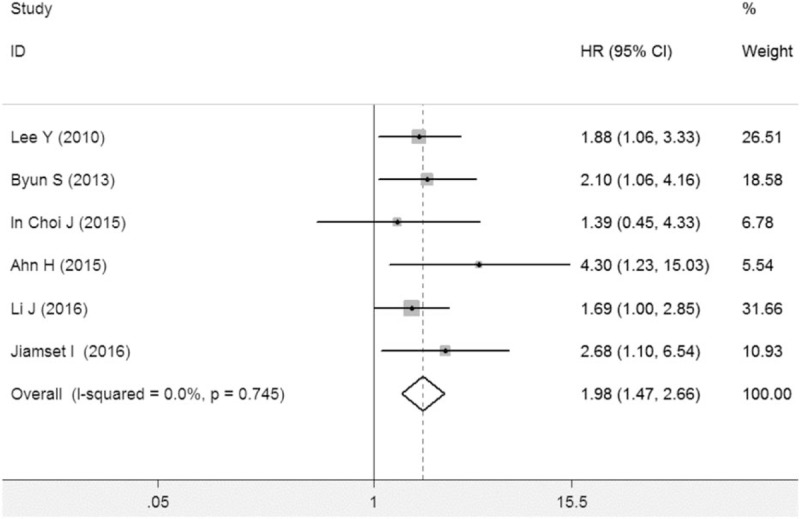
Meta-analysis indicated that diabetes predicted poorer recurrence-free survival in cervical cancer patients.
3.3. Publication bias
In the study of overall survival, there was evidence of publication bias (Supplemental Figure S3; PEggertest = .04). After using the trim and fill method of adding 4 unpublished studies, the pooled HR was 1.49 (95% CI 1.27 = 1.75; P < .001). In the meta-analysis of recurrence-free survival, no significant publication bias existed (Supplemental Figure S4; PEggertest = .23).
4. Discussion
The predictive roles of diabetes in the prognosis of many types of cancer have been well studied, but its role in predicting the prognosis of cervical cancer is still controversial. This systematic review and meta-analysis was performed to evaluate the association between diabetes and cervical cancer prognosis. Thirteen studies with a total of 11,091 cervical cancer patients were identified in the systematic review. This study indicated that diabetes could predict poor overall survival and recurrence-free survival in cervical cancer. Sensitivity analyses and subgroup analyses proved the credibility of the pooled HRs. Therefore, the systematic review and meta-analysis suggested that diabetes is an important prognostic factor in patients with cervical cancer, and it is associated with the poor survival of cervical cancer patients.
There are other prognostic factors associated with the survival of cervical cancer patients, such as FIGO stage, histologic subtypes, and some biomarkers.[48–51] In this meta-analysis, we performed subgroup analyses by the adjustment status of HRs reported by included studies. Studies reporting adjusted HRs controlled the risk of bias caused by confounders to evaluate the independent prognostic role of diabetes in cervical cancer. Upon pooling adjusted HRs, we found that diabetes was independently related to shorter overall survival (HR = 1.69, P < .001) as well as recurrence-free survival in cervical cancer patients (HR = 1.98, P < .001). Therefore, the findings above suggest that diabetes is an independent prognostic factor in cervical cancer. Since diabetes can be easily diagnosed, it can be a convenient and useful index in the prognostic evaluation of cervical cancer.
A major strength of the systematic review and meta-analysis was the large pooled sample size. Because of the inconsistent findings of the 13 included studies, a meta-analysis was necessary to summarize the predictive role of diabetes in cervical cancer prognosis. A total of 11,091 cervical cancer patients were included into the meta-analysis, which was enough to yield a reliable pooled HR and to appropriately estimate the association between diabetes and cervical cancer prognosis. Another strength was the novelty of this study. To our knowledge, this was the first meta-analysis focusing on the predictive role of diabetes in cervical cancer prognosis. Thus, the findings from the meta-analysis provided a comprehensive evaluation of diabetes as the prognostic factor of cervical cancer for the first time.
Though the prognostic role of diabetes in cervical cancer has been identified, the association between diabetes and cervical cancer risk is still poorly understood. Currently, there is still a lack of well-designed epidemiologic studies to provide evidence for the causal role of diabetes in the development of cervical cancer. In addition, few studies have explored the mechanisms underlying the prognostic role of diabetes in cervical cancer. Previous studies concluded that hyperinsulinemia in diabetes patients might explain the poor prognosis of cancer.[13,52,53] However, it is still unclear whether hyperinsulinemia can promote the development and progression of cervical cancer. Thus, further experimental studies are needed to explore the related mechanisms.
Several limitations existed in the current meta-analysis. First, all included studies were retrospective cohort studies and thus had possible risk of bias caused by residual confounders. Prospective cohort studies that are well designed and control more confounders are needed to validate recent findings via meta-analysis. Second, there were significant differences in the characteristics of recruited cervical cancer patients, such as age, follow-up period, and adjusted factors. These differences may explain the heterogeneity to some extent. However, the analysis suggested that no significant heterogeneity existed in the meta-analysis. Thus, these differences had limited influence on the association between diabetes and cervical cancer prognosis. Also, under the inclusion and exclusion criteria, most studies included in this meta-analysis were conducted in Eastern Asia, which may led to selective bias of the population and could not be corrected by literature research. Besides, the study carried out by Chen et al did not clearly state their follow-up period. However, the follow-up period is an important factor related to survival rate. Thus, the inclusion of this study may induce some extent of uncertainty in this review. Finally, various therapeutic methods were used and patients with different clinical stages were recruited in the included studies. Because of the small number of studies with a certain therapy or clinical stage, we were not unable to conduct subgroup analyses by therapies and clinical stages to identify the prognostic roles of diabetes in patients receiving a certain therapeutic method and people with different clinical stages of cancer. With more studies conducted in the future, subgroup analyses by therapies and clinical stages are needed to validate the prognostic roles of diabetes in patients receiving different types of treatment or those with different stages.
In conclusion, the meta-analysis suggests that diabetes is an important prognostic factor in patients with cervical cancer, and it is associated with poor survival in cervical cancer patients. The easy diagnosis of diabetes makes it a convenient and useful index for the prognostic evaluation of cervical cancer. However, more prospective studies with larger sample sizes are needed to validate the prognostic roles of diabetes in patients receiving different types of treatments or those with different clinical stages.
Supplementary Material
Footnotes
Abbreviations: 95%CIs = 95% confidence intervals, HRs = hazard ratios, T2DM = type 2 diabetes mellitus.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Beavis AL, Levinson KL. Preventing cervical cancer in the United States: barriers and resolutions for HPV vaccination. Front Oncol 2016;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Forouzanfar MH, Foreman KJ, Delossantos AM, et al. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet 2011;378:1461–84. [DOI] [PubMed] [Google Scholar]
- [3].Dueñas-González A, Campbell S. Global strategies for the treatment of early-stage and advanced cervical cancer. Curr Opin Obstet Gynecol 2016;28:11–7. [DOI] [PubMed] [Google Scholar]
- [4].Lapresa M, Parma G, Portuesi R, et al. Neoadjuvant chemotherapy in cervical cancer: an update. Expert Rev Anticancer Ther 2015;15:1171–81. [DOI] [PubMed] [Google Scholar]
- [5].Fields EC, Weiss E. A practical review of magnetic resonance imaging for the evaluation and management of cervical cancer. Radiat Oncol 2016;11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tsikouras P, Zervoudis S, Manav B, et al. Cervical cancer: screening, diagnosis and staging. J BUON 2016;21:320–5. [PubMed] [Google Scholar]
- [7].van Crevel R, van de Vijver S, Moore DA. The global diabetes epidemic: what does it mean for infectious diseases in tropical countries? Lancet Diabetes Endocrinol 2016;5:457–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Huang ES. Management of diabetes mellitus in older people with comorbidities. BMJ 2016;353:i2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Scappaticcio L, Maiorino MI, Bellastella G, et al. Insights into the relationships between diabetes, prediabetes, and cancer. Endocrine 2016;56:1–9. [DOI] [PubMed] [Google Scholar]
- [10].Klil-Drori AJ, Azoulay L, Pollak MN. Cancer, obesity, diabetes, and antidiabetic drugs: is the fog clearing? Nat Rev Clin Oncol 2016;14:85–99. [DOI] [PubMed] [Google Scholar]
- [11].García-Jiménez C, Gutiérrez-Salmerón M, Chocarro-Calvo A, et al. From obesity to diabetes and cancer: epidemiological links and role of therapies. Br J Cancer 2016;114:716–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang D, Zhao Y, Wang T, et al. Diabetes mellitus and long-term mortality of ovarian cancer patients. A systematic review and meta-analysis of 12 cohort studies. Diabetes Metab Res Rev 2017;33: doi: 10.1002/dmrr.2868. [DOI] [PubMed] [Google Scholar]
- [13].Vrachnis N, Iavazzo C, Iliodromiti Z, et al. Diabetes mellitus and gynecologic cancer: molecular mechanisms, epidemiological, clinical and prognostic perspectives. Arch Gynecol Obstet 2016;293:239–46. [DOI] [PubMed] [Google Scholar]
- [14].Zanders M, Vissers P, Haak H, et al. Colorectal cancer, diabetes and survival: epidemiological insights. Diabetes Metab 2014;40:120–7. [DOI] [PubMed] [Google Scholar]
- [15].De Bruijn K, Arends L, Hansen B, et al. Systematic review and meta-analysis of the association between diabetes mellitus and incidence and mortality in breast and colorectal cancer. Br J Surg 2013;100:1421–9. [DOI] [PubMed] [Google Scholar]
- [16].Voutsadakis IA. Obesity and diabetes as prognostic factors in patients with colorectal cancer. Diabetes Metab Syndr Clin Res Rev 2016;pii: S1871-4021(16)30261-2. doi: 10.1016/j.dsx.2016.12.018. [DOI] [PubMed] [Google Scholar]
- [17].Kapp DS, Fischer D, Gutierrez E, et al. Pretreatment prognostic factors in carcinoma of the uterine cervix: a multivariable analysis of the effect of age, stage, histology and blood counts on survival. Int J Radiat Oncol Biol Phys 1983;9:445–55. [DOI] [PubMed] [Google Scholar]
- [18].Kucera H, Enzelsberger H, Eppel W, et al. The influence of nicotine abuse and diabetes mellitus on the results of primary irradiation in the treatment of carcinoma of the cervix. Cancer 1987;60:1–4. [DOI] [PubMed] [Google Scholar]
- [19].Hopkins MP, Morley GW. Prognostic factors in advanced stage squamous cell cancer of the cervix. Cancer 1993;72:2389–93. [DOI] [PubMed] [Google Scholar]
- [20].Chen R-J, Chang D-Y, Yen M-L, et al. Prognostic factors of primary adenocarcinoma of the uterine cervix. Gynecol Oncol 1998;69:157–64. [DOI] [PubMed] [Google Scholar]
- [21].Lee Y-Y, Choi CH, Kim CJ, et al. Glucose as a prognostic factor in non-diabetic women with locally advanced cervical cancer (IIB–IVA). Gynecol Oncol 2010;116:459–63. [DOI] [PubMed] [Google Scholar]
- [22].Byun S, Kim J, Kim O, et al. A comparison of outcomes between concurrent chemoradiotherapy and radiotherapy alone in cancer of the uterine cervix: a single institutional experience. Eur J Gynaecol Oncol 2012;34:402–8. [PubMed] [Google Scholar]
- [23].Ahn HK, Shin JW, Ahn HY, et al. Metabolic components and recurrence in early-stage cervical cancer. Tumor Biol 2015;36:2201–7. [DOI] [PubMed] [Google Scholar]
- [24].Kuo H-Y, Lin Z-Z, Kuo R, et al. The prognostic impact of type 2 diabetes mellitus on early cervical cancer in Asia. Oncologist 2015;20:1051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jiamset I, Hanprasertpong J. Impact of diabetes mellitus on oncological outcomes after radical hysterectomy for early stage cervical cancer. J Gynecol Oncol 2016;27:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li J, Wu M-f, Lu H-w, et al. Impact of hyperglycemia on outcomes among patients receiving neoadjuvant chemotherapy for bulky early stage cervical cancer. PLoS One 2016;11:e0166612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Choi JI, Chang HK, Lee DW, et al. Does diabetes mellitus have an impact on the prognosis for patients with cervical cancer? Gynecol Oncol 2015;139:319–23. [DOI] [PubMed] [Google Scholar]
- [28].Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777–84. [DOI] [PubMed] [Google Scholar]
- [29].Wells G, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed May 16, 2016. [Google Scholar]
- [30].Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [31].Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101–29. [Google Scholar]
- [32].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [33].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease 1959;22:719–48. [PubMed] [Google Scholar]
- [34].Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Peters JL, Sutton AJ, Jones DR, et al. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med 2007;26:4544–62. [DOI] [PubMed] [Google Scholar]
- [36].Potish RA, Twiggs LB, Adcock LL, et al. Logistic models for prediction of enteric morbidity in the treatment of ovarian and cervical cancers. Am J Obstet Gynecol 1983;147:65–72. [DOI] [PubMed] [Google Scholar]
- [37].Levrant SG, Fruchter RG, Maiman M. Radical hysterectomy for cervical cancer: morbidity and survival in relation to weight and age. Gynecol Oncol 1992;45:317–22. [DOI] [PubMed] [Google Scholar]
- [38].Leath CA, Straughn JM, Kirby TO, et al. Predictors of outcomes for women with cervical carcinoma. Gynecol Oncol 2005;99:432–6. [DOI] [PubMed] [Google Scholar]
- [39].Cetina L, Garcia-Arias A, Uribe Mde J, et al. Concurrent chemoradiation with carboplatin for elderly, diabetic and hypertensive patients with locally advanced cervical cancer. Eur J Gynaecol Oncol 2008;29:608–12. [PubMed] [Google Scholar]
- [40].Shin DW, Nam JH, Kwon YC, et al. Comorbidity in disease-free survivors of cervical cancer compared with the general female population. Oncology 2008;74:207–15. [DOI] [PubMed] [Google Scholar]
- [41].Martinez-Huedo M, de Andres AL, Hernandez-Barrera V, et al. Adherence to breast and cervical cancer screening in Spanish women with diabetes: associated factors and trend between 2006 and 2010. Diabetes Metab 2012;38:142–8. [DOI] [PubMed] [Google Scholar]
- [42].Hsieh C-H, Tsai S-J, Chiou W-Y, et al. Better survival with three-dimensional conformal radiotherapy than with conventional radiotherapy for cervical cancer: a population-based study. ISRN Oncol 2013;201:729819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Han K, Pintilie M, Lipscombe LL, et al. Association between metformin use and mortality after cervical cancer in older women with diabetes. Cancer Epidemiol Prevent Biomarkers 2016;25:507–12. [DOI] [PubMed] [Google Scholar]
- [44].Stewart J, Sanson-Fisher R, Eades S. Aboriginal and Torres Strait Islander health: accuracy of patient self-report of screening for diabetes, high cholesterol and cervical cancer. Aust N Z J Public Health 2015;40:S3–6. [DOI] [PubMed] [Google Scholar]
- [45].Hanprasertpong J, Jiamset I, Geater A, et al. The effect of metformin on oncological outcomes in patients with cervical cancer with type 2 diabetes mellitus. Int J Gynecol Cancer 2017;27:131–7. [DOI] [PubMed] [Google Scholar]
- [46].Hopkins MP, Morley GW. Stage IB squamous cell cancer of the cervix: clinicopathologic features related to survival. Am J Obstet Gynecol 1991;164:1520–9. [DOI] [PubMed] [Google Scholar]
- [47].Chen JY, Chiou WK, Chou WY, et al. The impact of type 2 diabetes mellitus on mortality in hospitalized female cancer patients in Taiwan. Asia-Pacific J Clin Oncol 2013;12:e75–81. [DOI] [PubMed] [Google Scholar]
- [48].Noordhuis MG, Eijsink JJ, Roossink F, et al. Prognostic cell biological markers in cervical cancer patients primarily treated with (chemo) radiation: a systematic review. Int J Radiat Oncol Biol Phys 2011;79:325–34. [DOI] [PubMed] [Google Scholar]
- [49].Gadducci A, Guerrieri ME, Greco C. Tissue biomarkers as prognostic variables of cervical cancer. Crit Rev Oncol Hematol 2013;86:104–29. [DOI] [PubMed] [Google Scholar]
- [50].Noventa M, Ancona E, Cosmi E, et al. Usefulness, methods and rationale of lymph nodes HPV-DNA investigation in estimating risk of early stage cervical cancer recurrence: a systematic literature review. Clin Exp Metastasis 2014;31:853–67. [DOI] [PubMed] [Google Scholar]
- [51].Cui L, Shi Y, Zhang GN. Perineural invasion as a prognostic factor for cervical cancer: a systematic review and meta-analysis. Arch Gynecol Obstet 2015;292:13–9. [DOI] [PubMed] [Google Scholar]
- [52].Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol Rev 2015;95:727–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wang T, Ning G, Bloomgarden Z. Diabetes and cancer relationships. J Diabetes 2013;5:378–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


