Abstract
Background:
To synthesize and evaluate the impact of implementing post-2010 World Health Organization (WHO) prevention of mother-to-child transmission (PMTCT) guidelines on attainment of PMTCT targets.
Methods:
Retrospective and prospective cohort study designs that utilized routinely collected data with a focus on provision and utilization of the cascade of PMTCT services were included. The outcomes included the proportion of pregnant women who were tested during their antenatal clinic (ANC) visits; mother-to-child transmission (MTCT) rate; adherence; retention rate; and loss to follow-up (LTFU).
Results:
Of the 1210 references screened, 45 met the inclusion criteria. The studies originated from 14 countries in sub-Saharan Africa. The highest number of studies originated from Malawi (10) followed by Nigeria and South Africa with 7 studies each. More than half of the studies were on option A while the majority of option B+ studies were conducted in Malawi. These studies indicated a high uptake of human immunodeficiency virus (HIV) testing ranging from 75% in Nigeria to over 96% in Zimbabwe and South Africa. High proportions of CD4 count testing were reported in studies only from South Africa despite that in most of the countries CD4 testing was a prerequisite to access treatment. MTCT rate ranged from 1.1% to 15.1% and it was higher in studies where data were collected in the early days of the WHO 2010 PMTCT guidelines. During the postpartum period, adherence and retention rate decreased, and LTFU increased for both HIV-positive mothers and exposed infants.
Conclusion:
Irrespective of which option was followed, uptake of antenatal HIV testing was high but there was a large drop off along later points in the PMTCT cascade. More research is needed on how to improve later components of the PMTCT cascade, especially of option B+ which is now the norm throughout sub-Saharan Africa.
Keywords: adherence, loss to follow-up, PMTCT cascade, PMTCT options, retention rate, sub-Saharan Africa
1. Introduction
The Joint United Nations Program on human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) over the past 2 decades has documented the heavy burden and impact of HIV on mothers and infants living in resource-limited settings. Among the 260,000 new pediatric infections of HIV worldwide in 2012, 90% of new cases occurred in sub-Saharan Africa.[1] The use of antiretroviral therapy (ART) by HIV-positive mothers is the cornerstone of strategies to prevention of mother-to-child transmission (PMTCT) during the ante-partum and peri-partum periods and for the duration of breastfeeding.[2]
The World Health Organization (WHO) in 2010 revised guidelines and offered 2 options: option A and option B. Under option A, pregnant women with a CD4 ≥ 350 cells/μL receive ART prophylaxis from 14 weeks gestation through 1 week postpartum, single-dose nevirapine (NVP) at delivery, and daily lamivudine from delivery through 1 week postpartum. Their infants receive daily NVP from birth through 1 week after the cessation of breastfeeding. Women with CD4 ≤ 350 cells/μL or WHO clinical stage 3 or 4 disease are put permanently on triple-drug ART. Under option B, all women receive triple-drug ART from 14 weeks gestation through the cessation of breastfeeding, and infants receive a daily NVP or zidovudine dose from birth to 4 to 6 weeks.[2] In 2013, WHO revised its guidelines for the treatment and prevention of HIV and recommended that all pregnant and breastfeeding HIV-infected women, regardless of CD4 cell count, should continue ART for life known as “option B+” while their infants receive daily NVP or zidovudine from birth to 4 to 6 weeks.[3] Option B+ is now a norm in sub-Saharan Africa, with all the 21 global plan countries implementing it except for Nigeria as of October 2015.[4]
Effective PMTCT programmes require women and their infants to receive a cascade of interventions including uptake of antenatal services and HIV testing during pregnancy, use of ART by pregnant women living with HIV, safe child birth practices and appropriate infant feeding uptake, with infant prophylaxis, HIV testing, and other postnatal health care services following delivery.[5] The global community committed to accelerate progress for PMTCT through an initiative whose goals were to eliminate new pediatric HIV infection by 2015 and improve maternal, newborn and child health, and survival in the context of HIV.[6] Elimination of new pediatric infections has not been met, so there is a need to investigate why some programs are not effective. This systematic review of the literature was performed to evaluate the impact of implementing the WHO post-2010 PMTCT guidelines in order to inform practices which could help reach PMTCT targets.
2. Methods
2.1. Data sources
The following databases were searched for articles published from January 2010 to October 2016: Africa Wide Information, Medline, Embase, and reference lists from publications provided additional articles. The search was limited to English language journals for studies in sub-Saharan Africa. The following search terms and their variations were combined: prevention of mother to child transmission of HIV; PMTCT cascade; PMTCT options; effectiveness of PMTCT; PMTCT option A; PMTCT option B; PMTCT option B+; antiretroviral treatment; ART; antenatal care; HIV; HIV-exposed infants’ health outcomes; infant feeding; early infant diagnosis (EID), adherence; retention in care; and loss to follow-up (LTFU).
2.2. Study selection
The searched results were exported using reference management software Endnote 7.3 and duplicates were removed. The titles, abstracts, and full texts of potentially relevant studies were reviewed for eligibility. An adapted Preferred Reporting Items for Systematic and Meta-Analysis (PRISMA) flow chart was drawn (flow diagram). The selected studies had to include data collected post the WHO 2010 PMTCT guidelines. The inclusion criteria were: retrospective and prospective cohort study designs that utilized routinely collected data with a focus on provision and utilization of the cascade of PMTCT services; studies with particular interest in WHO option A, B, or B+ implementation; and studies from countries which had adopted WHO post-2010 PMTCT guidelines during the data collection period, and studies which evaluated implementation of post 2010 PMTCT guidelines. Qualitative studies, randomized controlled trials, reviews, commentaries, editorials, and modeling studies were excluded. Two independent reviewers (SGM and TM) reviewed the full text articles for inclusion, exclusion and extracted data on outlined outcomes. Ethical approval was granted from London School of Tropical Medicine and Hygiene Research Ethics Committee (Ref: 12086).
2.3. Data extraction
Data were extracted using a standardized data extraction form which summarized key information from relevant studies. The following information was extracted: proportion of pregnant women who were tested during their antenatal clinic (ANC) visits; proportion of women who tested HIV-positive; proportion of women who were already on ART before pregnancy; proportion of women who received their HIV test results; proportion of women tested for CD4 cell count; type of PMTCT option for mothers and their infants; adherence of women to ART; infant feeding methods; infant age when polymerase chain reaction (PCR) was done; mother-to-child transmission (MTCT) rate; proportion of infants reported to die or be LTFU as missing 3 consecutive clinic visits; and retention rate which is the continuous engagement from diagnosis in a package of prevention, treatment, support, and care services. In case of studies where data collection began before 2010 only post-2010 data were extracted.
3. Summary of results
3.1. Study characteristics
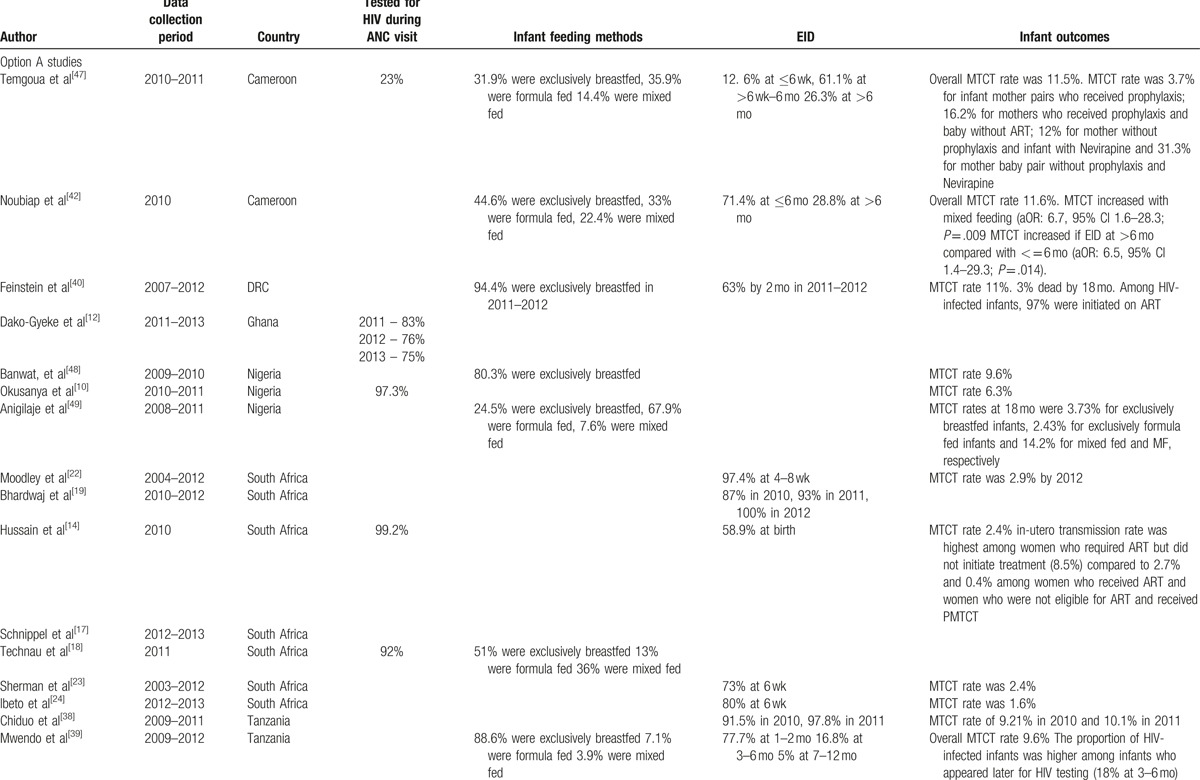
A total of 944 potentially eligible full text articles out of 2913 studies based on titles and abstracts were identified. Forty-five met the inclusion criteria (flow diagram) and they originated from 14 countries in sub-Saharan Africa (Table 1 ). The highest number of studies originated from Malawi (10) followed by Nigeria and South Africa with 7 studies each. The period of data collection was from 2010 to April 2015. The sample sizes for the selected studies ranged from 113 to 2,215,090 participants; the largest sample sizes were from the studies that utilized national data from South Africa and Ghana.
Table 1.
Uptake of PMTCT services and infant outcomes.
3.2. Uptake of ANC services
The data on HIV testing during ANC visits were extracted from 8 studies (Table 1 ) conducted in Malawi,[7–9] Nigeria,[10,11] Ghana,[12] Zimbabwe,[13] and South Africa.[14] These studies indicated a high uptake of HIV testing ranging from 75% in Nigeria to over 96% in Zimbabwe and South Africa. The high uptake of HIV testing could have been an attributable to policy changes in integrating HIV testing in ANC and shifting from opt-in to opt-out testing.
The proportion of women who were already on ART prior to ANC visits was reported in 6 studies, from Malawi,[7–9] Zimbabwe,[13] Kenya,[15] and Zambia.[16] In Zimbabwe only 7% of the women were already on ART during their ANC visits while in Malawi, implementation of option B+, resulted in an increase of women who were already on ART before pregnancy[7–9] from 30% before option B+ to 48% after option B+ adaptation. In a matched cohort study of option A and B, in 4 sites receiving external technical support for the provision of PMTCT-related care in Zambia, 48% of women were already on ART prior to their first ANC visit.[16] Findings are not representative of PMTCT service delivery in Zambia as a whole, since the sites receive technical support for the provision of PMTCT-related care from the Boston University PMTCT Integration Project through the President's Emergency Plan for AIDS Relief (PEPFAR). External support can also influence health facility characteristics and operational aspects of a facility such as capacity, location, staffing, and services provided.
Four out of the 7 studies that had information on CD4 count testing were from South Africa[17–20] and the rest from Kenya,[15] Zambia,[16] and Mozambique.[21] The proportion of women who had CD4 testing during ANC visits from Kenya, Zambia, and Mozambique was below 60% despite the fact that over the data collection period of these studies, CD4 testing was supposed to be a prerequisite to access care. In South Africa the proportion of women tested for CD4 count increased from 66% in 2010 to 76% in 2012 according to a study that utilized national data.[19]
3.3. Exposure to PMTCT options
Thirty-five (78%) studies reported on option A (Tables 1 and 2 ) and they were from the 14 representative countries with South Africa contributing 7 studies.[14,17–19,22–24] Implementation of option B+ was investigated in Nigeria,[25,26] Malawi,[7–9,27–32] Mozambique,[33] Zimbabwe,[34] and Ethiopia[35,36] in 16 studies which were synthesized. The 2 studies which reported in all 3 options (A, B, and B+) were conducted in Malawi[29] and Nigeria[25] where the data collection covered a longer period.
Table 1 (Continued).
Uptake of PMTCT services and infant outcomes.
3.4. Infant outcomes
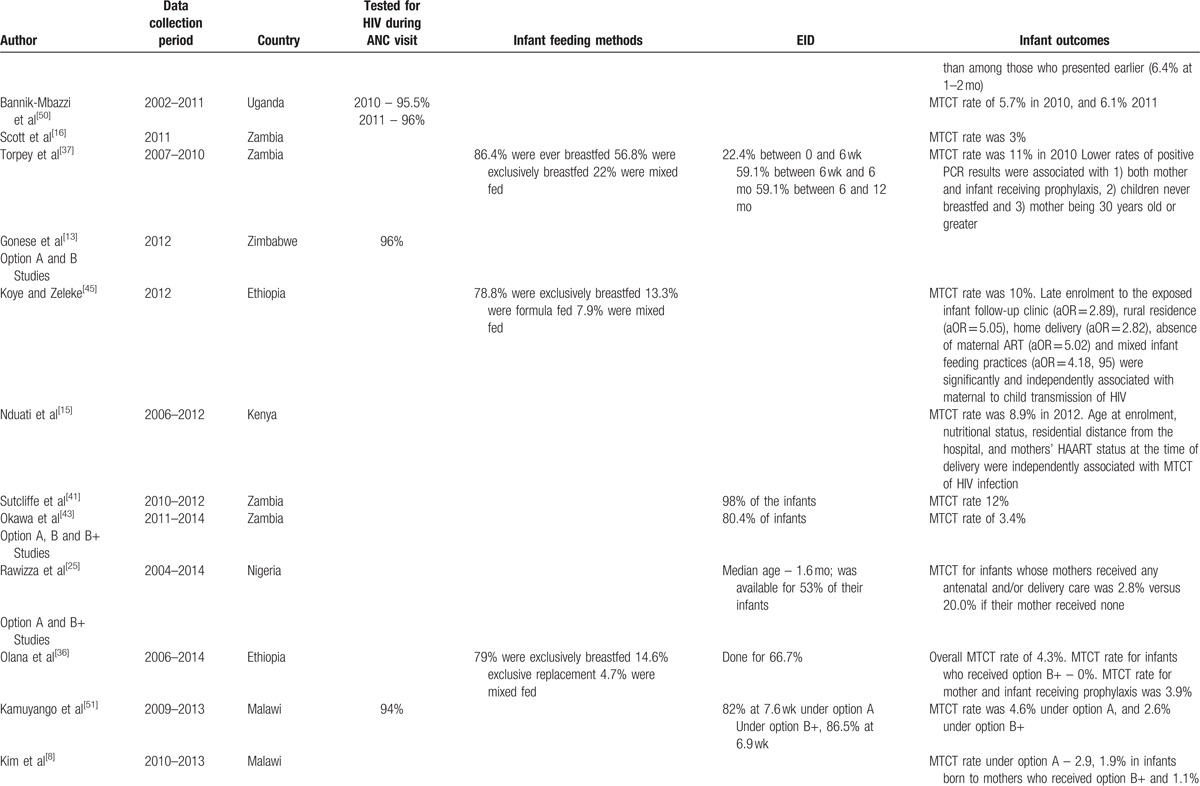
The MTCT rate was reported in 29 studies (Table 1 ) and ranged from 1.1% to 15.1%. MTCT rate was high (above 10%) mainly in studies where data were collected in the early days[37–42] of the WHO 2010 PMTCT guidelines although South Africa reported a low MTCT rate of 2% during the same period.[20] In Zambia, there was a reduction in MTCT rate from 12% in 2010[37] to 3% in a retrospective study conducted from 2011 to 2014.[43] The lowest MTCT rate of 1.1% was reported from Nigeria[26]; however, the author indicated that the study involved HIV-positive women who booked for antenatal care in a tertiary institution and were likely to be wealthier and more educated than the general population and perhaps more likely to adhere to ART and other PMTCT interventions
3.5. Exposure to PMTCT options and MTCT rate
In a study from Cameroon, under option A, the MTCT rate was 3.7% for infant mother pairs who both received prophylaxis; 16.2% when only the mothers received prophylaxis; 12% for mothers without prophylaxis whose infants received NVP; and 31.3% when neither mother or infant received prophylaxis.[44] In Zambia, lower rates of MTCT were associated with both mother and infant receiving prophylaxis: 4.2% compared to 20.1% in a no intervention group at 0 to 6 weeks.[37] In South Africa, in-utero transmission rate was highest among women who required ART but did not initiate treatment (8.5%) compared to 2.7% and 0.4% among women who received ART and women who were not eligible for ART and received prophylaxis under option A.[14]
In Ethiopia, absence of maternal ART was significantly and independently associated with maternal to child transmission of HIV (adjusted odds ratio [aOR] = 5.02, 95% CI: 2.43, 10.4).[45]
Results from 2 studies that compared option A and B+ from Ethiopia[36] and Malawi,[8] both confirmed supremacy of option B+ over A in terms of MTCT rate. In Ethiopia, none of the infants whose mothers received option B+ had a positive PCR result while the MTCT rate for those under option A was 3.9%.[36] In Malawi, the MTCT rate was 2.9% under Option A, 1.9% under option B+, and 1.1% for infants whose mothers received ART for their own health.[8]
Figure 1 shows MTCT rates at the end of data collection year and according to which option was used; only studies showing overall MTCT rates, not rates at younger ages when HIV transmission may have been ongoing in a breastfeeding population, are shown. Option A had higher MTCT rates in most of the studies under review; however, most option A studies preceded the option B+ studies. The general functions of the PMTCT programs have been improving over time with studies carried out after 2013 showing lower MTCT rates even under option A.
Figure 1.
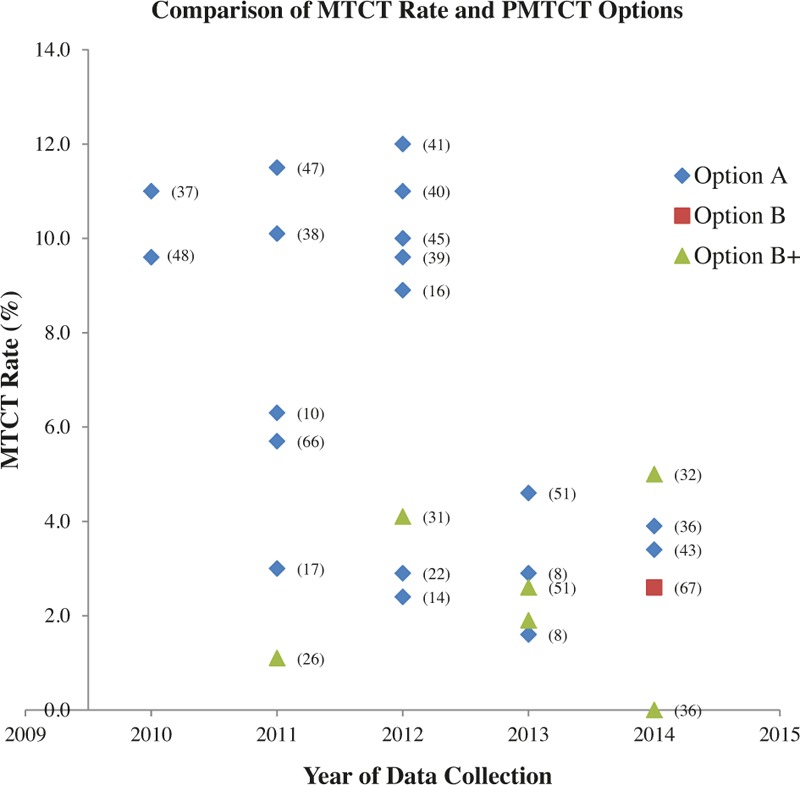
Key – The numbers in brackets are study references and the points are the MTCT rate. MTCT = mother-to-child transmission.
3.6. Timing of ART initiation and MTCT
In Kenya where an MTCT rate of 8.9% was reported, lack of maternal use of ART at the time of delivery was associated with increased risk of MTCT for infants of women who were on option A and B.[15] In Nigeria, MTCT (0.4%) was lower among women on ART before pregnancy compared to women who started ART during pregnancy or delivery which was at 2%.[46]
3.7. Impact of infant feeding on MTCT
The impact of infant feeding mode was not explored in most in the analysis of most studies under this review with only 13 studies providing results (Table 2 ). Exclusive breastfeeding is commonly measured through household surveys by asking mothers/caregivers of sample infants less than 6 months of age regarding intake in the previous day and night. However, there is a lack of uniformity of methods used for collecting exclusive breastfeeding data in the countries under review. Infant feeding methods were reported in studies from Zambia,[37] Malawi,[7] Democratic Republic of Congo (DRC),[40] Ethiopia,[36,45] Cameroon,[42,47] Nigeria,[26,46,48,49] South Africa,[18] and Tanzania.[39] High levels of exclusive breastfeeding were reported, with Malawi reporting that 99% of HIV-exposed infants being exclusively breastfed in the first 6 months.
MTCT of HIV was significantly higher with mixed feeding (aOR: 6.7, 95% CI 1.6–28.3; P = .009) in Cameroon under option A exposure.[42] Similarly in Nigeria, MTCT was higher for mixed fed infants at 14.2% compared to 3.73% for exclusively breastfed infant and 2.43% for exclusively formula-fed infants at 18 months.[49] This was consistent with a study in Ethiopia where mixed infant feeding practices were significantly and independently associated with MTCT of HIV (aOR = 4.18, 95% CI: 1.59, 10.99).[45]
Lower rates of MTCT were found in children who never breastfed in Zambia at 2.5% at 0 to 6 weeks compared to 6.5% those who had been breastfed under option A exposure.[37]
3.8. Early Infant diagnosis
EID of HIV by PCR was reported in 20 studies from 8 countries (Table 1 ). The uptake of EID ranged from less than 60% in Nigeria[25] and Zambia[37] to 100% in 2012 according to South African national data where it increased from 87% in 2010.[19] The age at which PCR was done ranged from 4 weeks to 18 months. In South Africa, 80% of exposed infants had PCR results at 6 weeks,[24] whereas in Malawi 52% underwent testing at 6 to 12 weeks and 28% tested at 12 months.[31]
Table 1 (Continued).
Uptake of PMTCT services and infant outcomes.
3.9. Impact of age at first PCR on MTCT
Age at first PCR had an impact on the MTCT of HIV-exposed infants. In Tanzania, the proportion of HIV-infected infants was higher among infants who appeared later for HIV testing (18% at 3–6 months) than among those who presented earlier (6.4% at 1–2 months) under option A implementation.[39] The Tanzanian observations were in agreement with the Ethiopian study which reported that late enrolment to the exposed infant follow-up clinic was significantly and independently associated with MTCT of HIV (aOR = 2.89, 95% CI: 1.35, 6.21).[45]
3.10. ART initiation of HIV-positive infants
ART initiation of HIV-positive infants is a key stage of the PMTCT cascade. In this review, it was reported in 3 studies from DRC,[40] Zambia,[41] and Nigeria.[11] Among HIV-infected infants in DRC, 97% enrolled in 2011 to 2012 were initiated on ART; this was an increase from 61% for infants enrolled in 2007 to 2008.[40] Lower rates were reported in Zambia where 67% of infants who tested positive started ART by the end of the study[41] and Nigeria where 75% of HIV-positive infants were initiated on ART.[11]
3.11. Retention in care
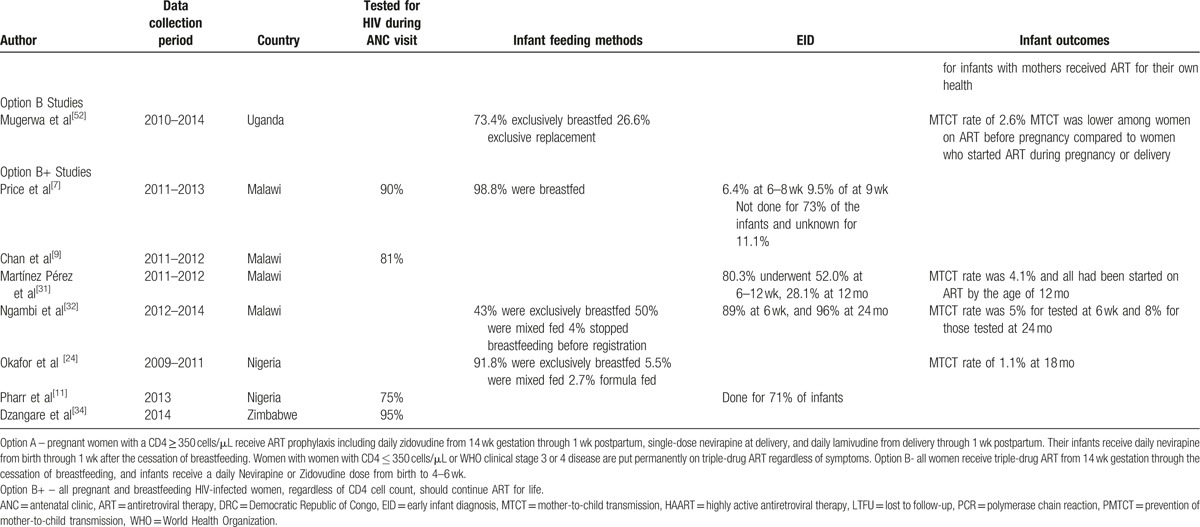
Retention in routine maternal-infant HIV care of HIV exposed infants was explored 3 studies from Zambia, Malawi, and Rwanda. In Zambia, the retention rate of HIV-exposed infants under option A, at 6 months after delivery was 62% compared to 30% of HIV-unexposed infants under the same ongoing routine care conditions.[16] In Malawi, 72% of HIV-exposed infants remained in care after 12 months under option B+,[31] whereas in Rwanda under option B, infants’ 12-month retention was 81% (95% CI: 76%, 86%).[53]
In Malawi, the retention in HIV care of women initiated on option B+ was 85%, compared to 93% after 2 years among those initiated on ART because of clinical or CD4 cell count criteria.[29] These results were consistent with the findings from Rwanda where mothers eligible for ART for their own health were better retained across all the time periods, 66% (CI: 59%, 73%), compared with those not eligible and receiving ART solely for PMTCT, 47% (CI: 37%, 57%), at 12 months, P < .001.[53] Another Malawian study highlighted that initiation of ART on the same day as HIV diagnosis was independently associated with reduced retention in the first 6 months (aOR 2.27; 95% CI: 1.34–3.85; P = .002) in under option B+.[9]
In a retrospective record review of women presenting to antenatal care or maternal and child health services at 34 health facilities in rural Zimbabwe, retention in ART care after 6 months of option B+ initiation was 83%.[34] In contrast, retention for Rwandan mothers exposed to option B was 68% at 6 weeks postdelivery, decreasing to 58% by 12 months.[53]
3.12. Lost to follow-up (LTFU)
Data on the magnitude of LTFU (missing 3 consecutive clinic visits) along the PMTCT cascade were reported in 9 studies (Table 2 ) from Malawi,[27,28,51] Tanzania,[38] Kenya,[15] Nigeria,[10,25] and Ethiopia.[35,36] In the studies from Malawi and Ethiopia, this was explored in the context of option B+ while in the other countries it was explored under option A.
Table 2.
Retention rate, adherence, and magnitude of LTFU∗.
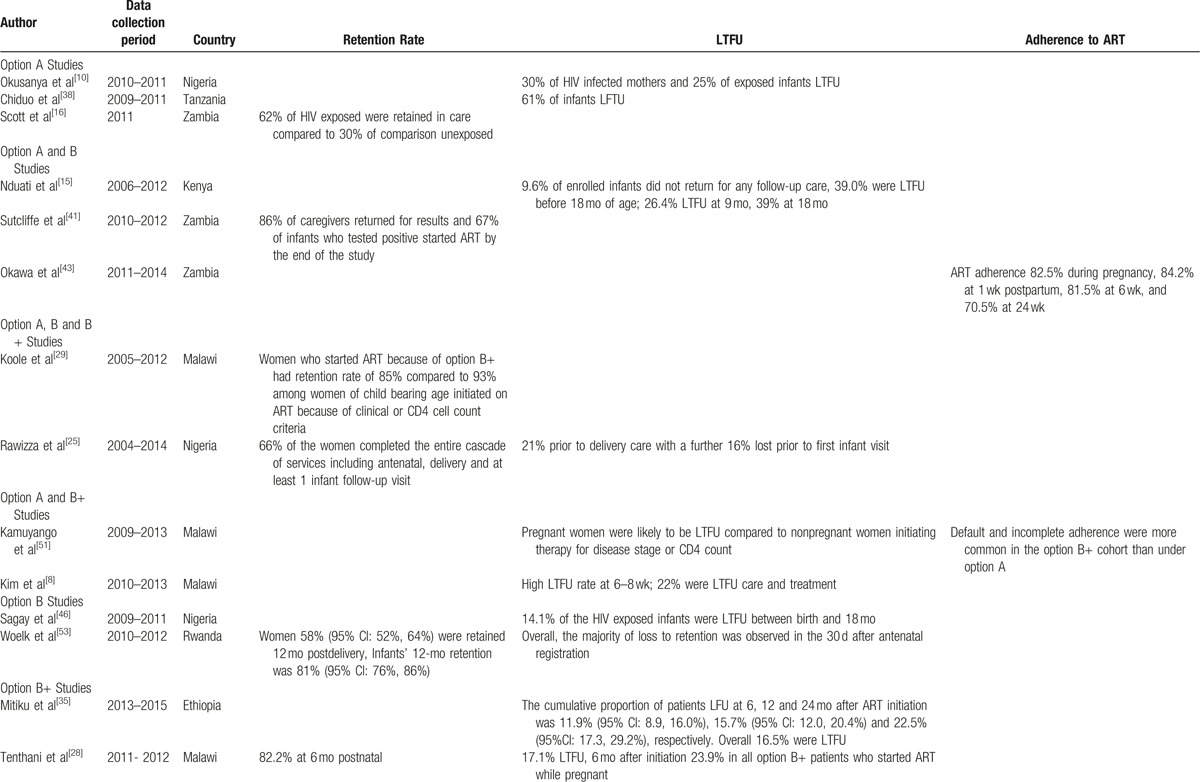
Table 2 (Continued).
Retention rate, adherence, and magnitude of LTFU∗.
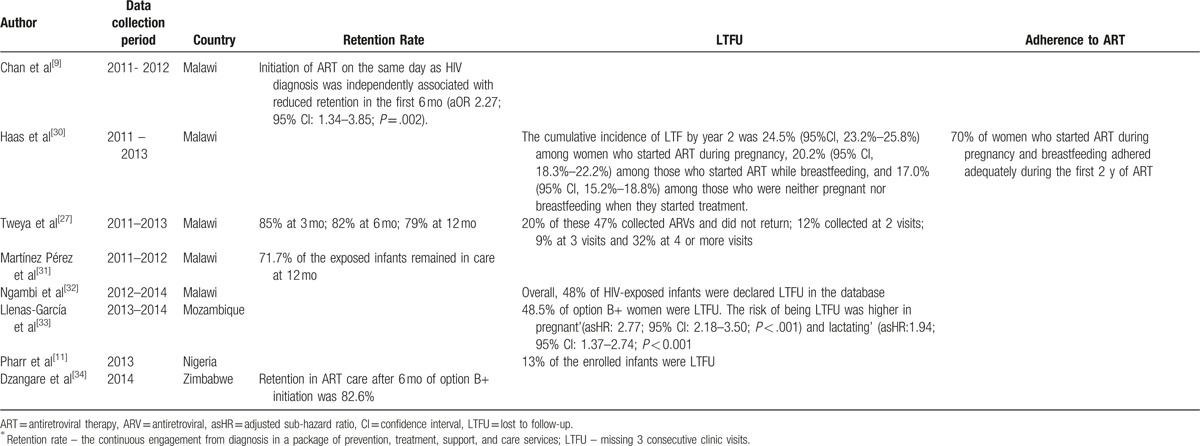
In Tanzania,[38] 61% of infants receiving treatment were LTFU at the time of review, despite the high proportion of guardians and parents who returned for PCR results (92% in 2010 and 98% in 2011). The results were consistent with a study from Malawi were 48% of the HIV-exposed infants were declared LTFU in the database although 96% of the them in the cohort had their PCR test done at 24 months.[54] However, Nigeria reported low rates of LTFU for HIV-infected infants of less than 15%[11,46] despite that the studies evaluated different PMTCT options. In Kenya, LTFU increased with the age of infants with 9.6% of enrolled infants not returning for any follow-up care, 26.4% dropping out by 9 months and 39% by 18 months of age.[15]
In Ethiopia under option B+ implementation, the cumulative proportions of women LTFU at 6, 12, and 24 months were 12%, 15%, and 23%, respectively.[35] Similar results were reported in Malawi where cumulative incidence of LTFU by year 2 was 24.5% (95%CI, 23.2%–25.8%) among women who started ART during pregnancy, 20.2% (95% CI, 18.3%–22.2%) among those who started ART while breastfeeding, and 17.0% (95% CI, 15.2%–18.8%) among those who were neither pregnant nor breastfeeding when they started treatment.[30]
The independent risk factors for LTFU were younger maternal age at ART initiation, missing CD4 cell count at ART initiation, ART initiation on the same day of diagnosis, and starting ART at hospital.[35] The risk of being LTFU was higher in “B+ pregnant” (adjusted sub-hazard ratio [asHR]: 2.77; 95% CI: 2.18–3.50; P < .001) and “B+ lactating” (asHR: 1.94; 95% CI: 1.37–2.74; P < .001) compared to women on ART for their own health in Mozambique.[33]
3.13. Adherence
Adherence to ART drugs for PMTCT by the mothers was reported in 2 studies (Table 2 ) from Malawi[51] and Zambia.[43] Adherence during the postpartum period in Zambia[43] ranged from 71% to 84% at different times postpartum. The results of the study were consistent with the Malawian study were 70% of women exposed to option B+, who started ART during pregnancy and breastfeeding adhered adequately during the first 2 years of ART.[30] However, default and incomplete adherence were more common in the option B+ compared to the option A in Malawi,[51] where 4% of women in the option B+ cohort had less than 95% adherence compared to 2% for the option A cohort.
4. Discussion
The global plan toward the elimination of new HIV infections among children by 2015 and keeping their mothers alive prioritized 21 countries in sub-Saharan Africa. The studies in the review originated from 14 of the priority countries. The studies show a continued decline in the incidence of HIV among children, as indicated by low MTCT rates, but the target of a 90% reduction by 2015 was not met, reduction in incidence being only 76% in the 21 priority countries.[55] South Africa reported low MTCT rates under option A implementation[24] even from the results of the facility-based cross-sectional study conducted in 2010.[56] This could have been as a result of their health system which is better resourced than in most of the countries under review.
Many of the studies on the impact of infant feeding methods and exposure to various ART regimens were conducted before the 2010 WHO PMTCT.[55,57] Although infant feeding results were available from only a minority of studies in this review, the results of the review are in agreement with the findings which led to the current guidelines. In the countries under review breastfeeding is very common, and breastfed infants have an increased risk of MTCT of HIV.[58] Mixed feeding is also a common practice in sub-Saharan Africa and is an additional risk for postnatal HIV transmission.[59] As revealed by the studies from Tanzania[39] and Ethiopia,[45] where there was increase in MTCT over time, continued breastfeeding in the face of low adherence to ART treatment, is a risk.
Option B+ which initiates lifelong ART to all pregnant and breastfeeding women is now widespread in sub-Saharan Africa, with all the 22 global plan countries implementing it except for Nigeria. As of October 2015, option B+ was being nationally implemented in 14 (Angola, Burundi, Cameroun, Chad, Ethiopia, Kenya, Lesotho, Malawi, South Africa, Swaziland, Tanzania, Uganda, Zambia, and Zimbabwe) out of the 21 countries in sub-Saharan Africa, and scale-up continues in 6 countries (Botswana, Cote d’Ivoire, DRC, Ghana, Mozambique, and Namibia).[4] In this review, Implementation of option B+ was investigated in Nigeria,[25,26] Malawi,[7–9,27–32] Mozambique,[33] Zimbabwe,[34] and Ethiopia.[35,36] Most studies came from Malawi since they were the pioneers of this option; hence, there is a need to explore the impact of implementing the option B+ guidelines and identifying missed opportunities along the PMTCT cascade in other countries which have adopted it.
The implementation of option B+ in Malawi resulted in a 5-fold increase in the numbers of pregnant women being enrolled on ART. Nonetheless, default and incomplete adherence were more common in option B+ implementation.[43,51] These results were consistent with a qualitative study conducted in Tanzania among mothers who were put on option B+ during pregnancy who indicated various reasons for poor adherence to ART, which included lack of motivation to continue ART after weaning the child and protecting the child from becoming infected, stigma, and poverty.[60] There is also a need to adjust operational practices related to quality of counseling as it has been indicated to be a predictor of adherence to treatment.[61] Moreover, a recent study suggested that retention in all postnatal programs, including those outside the context of HIV, is poor globally, elaborating the need for evidence-based intervention strategies and further research on the drivers of disengagement.[62]
The retention in care of HIV-infected and lactating mothers under option B+ was poor and driven by early losses.[9,27–29,34,53] Hence, implementation of option B+ requires that policy makers rethink ways of ensuring optimal adherence to ART for maximal suppression of viral replication and avoidance of drug resistance. One possibility is to explore the use of cell phone SMS which has been found to be useful in Africa for improving the quality of care and follow-up of people with HIV/AIDS.[63,64]
The consolidated guidelines recommend that HIV-exposed infants be tested for HIV between 6 and 8 weeks, at the end of breastfeeding, and at any point they present with illness.[3] However, in the studies under review the retention rate tended to decrease during the postpartum period.[16,27–29,32] This reduction in postpartum retention rate implies that HIV-exposed infants will be detached from the health system, thus missing opportunities for PCR testing. A systematic review of mostly sub-Saharan countries found that about a 3rd of HIV-exposed children in standard PMTCT programs fall out of care in 3 months after delivery and a further 45% stop care after their first HIV test.[65] Furthermore, low rates of infected infants are initiated on ART despite being PCR-tested,[32,38] raising concerns on the benefits of EID and the referral to care and treatment of these infected infants. Similar results were reported by Chatterjee et al,[66] in their descriptive analysis of national EID programs in 4 countries where only 22% to 38% of infected infants were initiated on ART. There is a need for strategic and technical developments for ensuring that drop-out rates along the PMTCT cascade are minimal.
4.1. Limitations of the study
The major limitation of the review is that the results of most of the study findings may not be representative of the general health care systems in the countries reviewed as only 2 studies from Ghana and South Africa used national data for analysis. There are also profound variations in the implementation of PMTCT programs across countries. For instance, in Nigeria MTCT was reported to be 1.1%,[26] which was largely attributed to the study population which comprised wealthier and more educated women than the general population. There is a research gap of evaluating PMTCT interventions using large cohorts that can be generalized to the whole population.
The limitations of the data sources of this review are mainly due to the retrospective nature posing problems of incomplete recordings. Moreover, the data used in these studies were originally collected for different purposes from this review and therefore hard to compare data when a lot of time points differ because of different timings of key outcomes. However, the advantage of using routinely collected data is that research will be conducted in a timely and cost-efficient manner as data are already collected and available for analysis.[67]
All the studies reported challenges in the documentation of routine services, linkage of HIV diagnosis to care, and active follow-up of those enrolled in care. The data collection systems often lack immediacy as many are paper-based with records completed at each facility and collated centrally. This is likely to be in challenge in most sub-Saharan countries; and therefore, there is a need to improve management of health information systems through the use of modern technology.
5. Conclusion
Irrespective of which option was followed, uptake of antenatal HIV testing was high but there was a large drop off along later points in the PMTCT cascade. More research is needed on how to improve later components of the PMTCT cascade, especially of option B+ which is now the norm throughout sub-Saharan Africa.
There is research gap for studies that investigated the full cascade of interventions that include uptake of antenatal services and HIV testing during pregnancy, use of ART by pregnant women living with HIV, appropriate infant feeding, uptake of infant HIV testing, and other postnatal health care services in the context of the PMTCT option B+ interventions. In view of the gap there is a need for implementation research evaluating real world effectiveness of the 2010 WHO PMTCT guidelines specifically option B+.
Acknowledgments
The authors thank the SEARCH (Sustainable Evaluation through Analysis of Routinely Collected HIV data) Project funded by the Bill & Melinda Gates Foundation grant number OPP1084472 for the support.
Footnotes
Abbreviations: AIDS = acquired immunodeficiency syndrome, ANC = antenatal clinic, ART = antiretroviral therapy, DRC = Democratic Republic of Congo, EID = early infant diagnosis, HIV = human immunodeficiency virus, LTFU = lost to follow-up, MTCT = mother-to-child transmission, NVP = nevirapine, PMTCT = prevention of mother-to-child transmission, WHO = World Health Organization.
Authorship: SGM conceived the idea, carried out the review, and wrote the manuscript. TM and PM appraised the quality of a portion of the included studies for quality control. SF and JT critically reviewed drafts and approved the final manuscript.
Funding/support: The study was supported by the SEARCH (Sustainable Evaluation through Analysis of Routinely Collected HIV data) Project funded by the Bill & Melinda Gates Foundation grant number OPP1084472.
The authors have no conflicts of interest to disclose.
References
- [1].UNAIDS. Global plan towards the elimination of New HIV infections among children by 2015 and keeping their mothers alive. 2011. http://www.unaids.org/sites/default/files/media_asset/20110609_JC2137_Global-Plan-Elimination-HIV-Children_en_1.pdf. Accessed August 17, 2015. [Google Scholar]
- [2].UNAIDS. Global HIV/AIDS response epidemic update and health sector progress towards universal access. 2011. http://www.unaids.org/sites/default/files/media_asset/20111130_UA_Report_en_1.pdf. Accessed August 17, 2015. [Google Scholar]
- [3].UNAIDS. Countdown to Zero: Global Plan Towards the Elimination of new HIV Infections among Children by 2015 and Keeping their Mothers Alive 2011–2015. Geneva: 2011. file:///C:/Users/lsh1405741/Downloads/elimination-of-new-hiv-among-children-in-africa-by-2015-08112013.pdf. Accessed August 17, 2015. [Google Scholar]
- [4]. The Interagency Task Team on the Prevention and Treatment of HIV Infection in Pregnant Women MaC. Option B+ countries and PMTCT regimen 2015. Available from: http://emtct-iatt.org/b-countries-and-pmtct-regimen/. [Accessed Aug 17, 2016] [Google Scholar]
- [5].Padian NS, et al. HIV prevention transformed: the new prevention research agenda. Lancet 2011;378:269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].UNAIDS, Countdown to Zero: Global Plan Towards the Elimination of new HIV Infections among Children by 2015 and Keeping their Mothers Alive 2011–2015. 2011: Geneva. http://www.unaids.org/sites/default/files/media_asset/20121211_Women_Out_Loud_en_1.pdf. Accessed December 22, 2015. [Google Scholar]
- [7].Price AJ, et al. Uptake of prevention of mother-to-child-transmission using option B+ in northern rural Malawi: a retrospective cohort study. Sex Transm Infect 2014;90:309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kim MH, Ahmed S, Hosseinipour MC, et al. The impact of option b+ on the antenatal PMTCT Cascade in Lilongwe, Malawi. J Acquir Immune Defic Syndr 2015;68:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chan AK, et al. Same day HIV diagnosis and antiretroviral therapy initiation affects retention in Option B+ prevention of mother-to-child transmission services at antenatal care in Zomba District, Malawi. J Int AIDS Soc 2016;19:20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Okusanya BO, Ashimi AO, Aigere EO, et al. Scaling up prevention of PMTCT of HIV infection to primary health centres in Nigeria: findings from two primary health centres in North West Nigeria. Afr J Reprod Health 2013;17:130–7. [PubMed] [Google Scholar]
- [11].Pharr JR, et al. Linkage to care, early infant diagnosis, and perinatal transmission among infants born to HIV-infected Nigerian mothers: evidence from the healthy beginning initiative. J Acquir Immune Defic Syndr 2016;72(Suppl 2):S154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dako-Gyeke P, Dornoo B, Ayisi Addo S, et al. Towards elimination of mother-to-child transmission of HIV in Ghana: an analysis of national programme data. BMC Int J Equity Health 2016;15: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gonese E, et al. Is Zimbabwe ready to transition from anonymous unlinked sero-surveillance to using prevention of mother to child transmission of HIV (PMTCT) program data for HIV surveillance?: results of PMTCT utility study. BMC Infect Dis 2016;16:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hussain A, Moodley D, Naidoo S, et al. Esterhuizen pregnant women's access to PMTCT and ART services in South Africa and implications for universal antiretroviral treatment. PLoS Med 2011;6:e27907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nduati EW, et al. Outcomes of prevention of mother to child transmission of the human immunodeficiency virus-1 in rural Kenya-a cohort study. BMC Public Health 2015;15:1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Scott CA, et al. Uptake, outcomes, and costs of antenatal, well-baby, and prevention of mother-to-child transmission of HIV services under routine care conditions in Zambia. PLoS One 2013;8:e72444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schnippel K, Mongwenyana C, Long LC, et al. Delays, interruptions, and losses from prevention of mother-to child transmission of HIV services during antenatal care in Johannesburg, South Africa: a cohort analysis. BMC Infect Dis 2015;6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Technau KG, et al. Timing of maternal HIV testing and uptake of prevention of mother-to-child transmission interventions among women and their infected infants in Johannesburg, South Africa. J Acquir Immune Defic Syndr 2014;65:e170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bhardwaj S, Barron P, Pillay Y, et al. Elimination of mother-to-child transmission of HIV in South Africa: rapid scale-up using quality improvement. S Afr Med J 2014;104:239–43. [DOI] [PubMed] [Google Scholar]
- [20].Hussain A, et al. Pregnant women's access to PMTCT and ART services in South Africa and implications for universal antiretroviral treatment. PLoS One 2011;6:e27907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gimbel S, et al. What does high and low have to do with it? Performance classification to identify health system factors associated with effective prevention of mother-to-child transmission of HIV delivery in Mozambique. J Int AIDS Soc 2014;17:18828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Moodley P, Parboosing R, Moodley D. Reduction in perinatal HIV infections in KwaZulu-Natal, South Africa, in the era of more effective prevention of mother to child transmission interventions (2004–2012). J Acquir Immune Defic Syndr 2013;63:410–5. [DOI] [PubMed] [Google Scholar]
- [23].Sherman GG, et al. Laboratory information system data demonstrate successful implementation of the prevention of mother-to-child transmission programme in South Africa. S Afr Med J 2014;104(3 Suppl 1):235–8. [DOI] [PubMed] [Google Scholar]
- [24].Ibeto M, Giddy J, Cox V. Closing the gaps: steps towards elimination of mother-to-child transmission of HIV. S Afr J HIV Med 2014;15:107–9. [Google Scholar]
- [25].Rawizza HE, et al. Loss to follow-up within the prevention of mother-to-child transmission care cascade in a large ART program in Nigeria. Curr HIV Res 2015;13:201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Okafor I, Eqwu U, Obi S, et al. Virtual elimination of mother-to-child transmission of human immunodeficiency virus in mothers on highly active antiretroviral therapy in Enugu, South-Eastern Nigeria. Ann Med Health Sci Res 2014;4:615–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tweya H, et al. Understanding factors, outcomes and reasons for loss to follow-up among women in Option B+ PMTCT programme in Lilongwe, Malawi. Trop Med Int Health 2014;19:1360–6. [DOI] [PubMed] [Google Scholar]
- [28].Tenthani L, et al. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women (‘Option B+’) in Malawi. AIDS 2014;28:589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Koole O, et al. Improved retention of patients starting antiretroviral treatment in Karonga District, northern Malawi, 2005–2012. J Acquir Immune Defic Syndr 2014;67:e27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Haas AD, Msukwa MT, Egger M, et al. Adherence to antiretroviral therapy during and after pregnancy: cohort study on women receiving care in Malawi's Option B+ Program. Clin Infect Dis 2016;63:1227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Martínez Pérez G, Metcalf C, Garone D, et al. HIV testing and retention in care of infants born to HIV-infected women enrolled in ‘Option B+’, Thyolo, Malawi. Public Health Action 2014;4:102–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ng’ambi WF, et al. Follow-up and programmatic outcomes of HIV-exposed infants registered in a large HIV centre in Lilongwe, Malawi: 2012–2014. Trop Med Int Health 2016;21:995–1002. [DOI] [PubMed] [Google Scholar]
- [33].Llenas-Garcia J, et al. Retention in care of HIV-infected pregnant and lactating women starting ART under Option B+ in rural Mozambique. Trop Med Int Health 2016;21:1003–12. [DOI] [PubMed] [Google Scholar]
- [34].Dzangare J, et al. HIV testing uptake and retention in care of HIV-infected pregnant and breastfeeding women initiated on ’Option B+’ in rural Zimbabwe. Trop Med Int Health 2016;21:202–9. [DOI] [PubMed] [Google Scholar]
- [35].Mitiku I, et al. Factors associated with loss to follow-up among women in Option B+ PMTCT programme in northeast Ethiopia: a retrospective cohort study. J Int AIDS Soc 2016;19:20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Olana T, Bacha T, Worku TW, et al. Early infant diagnosis of HIV infection using DNA-PCR at a referral center: an 8 years retrospective analysis BMC. AIDS Res Ther 2016;13:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Torpey K, et al. Analysis of HIV early infant diagnosis data to estimate rates of perinatal HIV transmission in Zambia. PLoS One 2012;7:e42859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chiduo MG, Mmbando BP, Theilgaard ZP, et al. Early infant diagnosis of HIV in three regions in Tanzania; successes and challenges. BMC Public Health 2013;13:910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mwendo EM, et al. Effectiveness of prevention of mother-to-child HIV transmission programmes in Kilimanjaro region, northern Tanzania. Trop Med Int Health 2014;19:267–74. [DOI] [PubMed] [Google Scholar]
- [40].Feinstein L, Edmonds A, Chalachala JL, et al. Temporal changes in the outcomes of HIV-exposed infants in Kinshasa, Democratic Republic of Congo during a period of rapidly evolving guidelines for care (2007–2013). AIDS 2014;28:301–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sutcliffe CG, van Dijk JH, Hamangaba F, et al. Turnaround time for early infant HIV diagnosis in rural Zambia: a chart review. PLoS One 2014;9:e87028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Noubiap JJ, Bongoe A, Demanou SA. Mother-to-child transmission of HIV: findings from an Early Infant Diagnosis program in Bertoua, Eastern Cameroon. Pan Afr Med J 2013;15:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Okawa S, Chirwa M, Ishikawa N, et al. Longitudinal adherence to ART drugs for PMTCT of HIV in Zambia. BMC Pregnancy Childbirth 2015;15:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Saounde Temgoua EM, et al. HIV-1 early infant diagnosis is an effective indicator of the prevention of mother-to-child transmission program performance: experience from Cameroon. Curr HIV Res 2015;13:286–91. [DOI] [PubMed] [Google Scholar]
- [45].Koye DN, Zeleke BM. Mother-to-child transmission of HIV and its predictors among HIV-exposed infants at a PMTCT clinic in northwest Ethiopia. BMC Public Health 2013;13:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sagay AS, et al. Mother-to-child transmission outcomes of HIV-exposed infants followed up in Jos North-Central Nigeria. Curr HIV Res 2015;13:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Saounde TEM, Nkenfou CN, Zoung-Kanyi Bissek AC, et al. HIV-1 early infant diagnosis is an effective indicator of the prevention of mother-to-child transmission program performance: experience from Cameroon. Curr HIV Res 2016;13:286–91. [DOI] [PubMed] [Google Scholar]
- [48].Banwat SB, Ochekpe NA, Auta A, et al. Anti retroviral drug prophylaxis in prevention of mother-to-child transmission of HIV infection in a treatment centre in Jos, Nigeria. J Pharm Bioresourc 2015;11:93–100. [Google Scholar]
- [49].Anigilaje EA, et al. HIV-free survival according to the early infant-feeding practices; a retrospective study in an anti-retroviral therapy programme in Makurdi, Nigeria. BMC Infect Dis 2015;15:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bannink-Mbazzi F, Lowicki-Zucca M, Ojom L, et al. High PMTCT program uptake and coverage of mother, their partners, and babies in Northern Uganda: achievements and lesson learnt over 10 years of implementation (2002–2011). J Acquir Immune Defic Syndr 2013;62:138–45. [DOI] [PubMed] [Google Scholar]
- [51].Kamuyango AA, et al. One-year outcomes of women started on antiretroviral therapy during pregnancy before and after the implementation of Option B+ in Malawi: a retrospective chart review. World J AIDS 2014;4:332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mugerwa JN, Namukwaya Z, Kekitiinwa A, et al. Early Infection Among Ugandan HIV-Exposed Infants Whose Mothers Received Option B+ vs Option A, In: International Antiviral Society, Boston, Massachusetts, USA, March 3–6, 2014. [Google Scholar]
- [53].Woelk GB, Ndatimana D, Behan S, et al. Retention of mothers and infants in the prevention of mother-Tochild transmission of HIV programme is associated with individual and facility-level factors in Rwanda. J Int AIDS Soc 2016;19:20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ng’ambi WF, Ade S, Harries AD, et al. Follow-up and programmatic outcomes of HIV-exposed infants registered in a large HIV centre in Lilongwe, Malawi: 2012–2014. Trop Med Int Health 2016;21:995–1002. [DOI] [PubMed] [Google Scholar]
- [55].World Health Organization. New data on the prevention of mother-to-child transmission of HIV and their policy implications: conclusions and recommendations. Technical Consultation on behalf of the UNFPA/UNICEF/WHO/UNAIDS Inter-Agency Task Team on Mother-to-Child Transmission of HIV. Geneva: WHO, 2001. http://apps.who.int/iris/bitstream/10665/66851/1/WHO_RHR_01.28.pdf. Accessed September 20, 2015. [Google Scholar]
- [56].Goga AE, et al. First population-level effectiveness evaluation of a national programme to prevent HIV transmission from mother to child, South Africa. J Epidemiol Commun Health 2015;69:240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Little KM, et al. A review of evidence for transmission of HIV from children to breastfeeding women and implications for prevention. Pediatr Infect Dis J 2012;31:938–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Coutsoudis A, et al. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis 2004;189:2154–66. [DOI] [PubMed] [Google Scholar]
- [59].Iliff PJ, et al. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS 2005;19:699–708. [DOI] [PubMed] [Google Scholar]
- [60].Ngarina M, et al. Reasons for poor adherence to antiretroviral therapy postnatally in HIV-1 infected women treated for their own health: experiences from the Mitra Plus study in Tanzania. BMC Public Health 2013;13:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ebuy H, Henock Y, Mussie A. Level of adherence and predictors of adherence to the Option B+ PMTCT programme in Tigray. Northern Ethiop Int J Infect Dis 2015;33:123–9. [DOI] [PubMed] [Google Scholar]
- [62].Myer L, Phillips TK. Beyond Option B+”: understanding antiretroviral therapy (ART) adherence, retention in care and engagement in ART services among pregnant and postpartum women initiating therapy in Sub-Saharan Africa. J Acquir Immune Defic Syndr 2017;75(Suppl 2):S115–22. [DOI] [PubMed] [Google Scholar]
- [63].Siedner MJ, Santorino D, Lankowski AJ, et al. A combination SMS and transportation reimbursement intervention to improve HIV care following abnormal CD4 test results in rural Uganda: a prospective observational cohort study. BMC Med Global Health 2015;13:160.DOI 10.1186/s12916-015-0397-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Chang LW, Njie-Carr V, Kalenge S, et al. Perceptions and acceptability of mHealth interventions for improving patient care at a community-based HIV/AIDS clinic in Uganda: a mixed methods study. AIDS Care 2014;25:874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Sibanda EL, et al. The magnitude of loss to follow-up of HIV-exposed infants along the prevention of mother-to-child HIV transmission continuum of care: a systematic review and meta-analysis. AIDS 2013;27:2787–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Chatterjee A, et al. Implementing services for Early Infant Diagnosis (EID) of HIV: a comparative descriptive analysis of national programs in four countries. BMC Public Health 2011;11:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Grzeskowiak LE, Gilbert AL, Morrison JL. Methodological challenges in using routinely collected health data to investigate long-term effects of medication use during pregnancy. Ther Adv Drug Saf 2012;4:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]


