Abstract
Randomized controlled trials have demonstrated that laparoscopic surgery for rectal cancer is safe and can accelerate recovery without compromising oncological outcomes. However, such a surgery is technically demanding, limiting its application in nonspecialized centers. The operational features of a robotic system may facilitate overcoming this limitation. Studies have reported the potential advantages of robotic surgery. However, only a few of them have featured the application of this surgery in patients with advanced rectal cancer undergoing neoadjuvant chemoradiation therapy (nCRT).
From January 2012 to April 2015, after undergoing nCRT, 40 patients with mid or low rectal cancer were operated using the robotic approach at our institution. Another 38 patients who were operated using the conventional laparoscopic approach were matched to patients in the robotic group by sex, age, the body mass index, and procedure. All operations were performed by a single surgical team. The clinicopathological characteristics and short-term outcomes of these patients were compared. To assess the effect of the learning curve on the outcomes, patients in the robotic group were further subdivided into 2 groups according to the sequential order of their procedures, with an equal number of patients in each group. Their outcome measures were compared.
The robotic and laparoscopic groups were comparable with regard to pretreatment characteristics, rectal resection type, and pathological examination result. After undergoing nCRT, more patients in the robotic group exhibited clinically advanced diseases. The complication rate was similar between the 2 groups. The operation time and the time to the resumption of a soft diet were significantly prolonged in the robotic group. Further analysis revealed that the difference was mainly observed in the first robotic group. No significant difference was observed between the second robotic and laparoscopic groups.
Although the robotic approach may offer potential advantages for rectal surgery, comparable short-term outcomes may be achieved when laparoscopic surgery is performed by experienced surgeons. However, our results suggested a shorter learning curve for robotic surgery for rectal cancer, even in patients who exhibited more advanced disease after undergoing nCRT.
Keywords: laparoscopic surgery, learning curve, neoadjuvant chemoradiation therapy, rectal cancer, robotic surgery
1. Introduction
Several prospective randomized trials have demonstrated that laparoscopic surgery for rectal cancer is a safe and feasible procedure. The quality of the surgical specimen and the long-term oncological outcomes of laparoscopic surgery are equivalent to those of open surgery; however, recovery, physiological function, and other short-term outcome measures improve after laparoscopic surgery.[1–4] However, laparoscopic surgery for rectal cancer is technically demanding, limiting its application in nonspecialized centers. The anatomical confinement of the deep pelvis, restricted movement of the rigid instruments, amplification of the tremor from the fulcrum effect, and unstable image provided by the hand-held camera contribute to the difficulty of this procedure.[5] The influence of these factors is more pronounced for mid and low rectal cancer. This observation is reflected by a conversion rate as high as 22% for laparoscopic surgery for rectal cancer, as reported previously.[6–8]
Neoadjuvant chemoradiation therapy (nCRT) for rectal cancer has been shown to reduce the local recurrence rate and increase the sphincter preservation rate.[9–11] Performing nCRT in patients with T3, T4, or N-positive rectal cancer has become a clinical routine in most institutions.[12] However, its post-treatment effects, such as tissue fibrosis and edema, further contribute to the difficulty of the laparoscopic procedure.
Robotic surgery for rectal cancer is an emerging technique. A robotic system facilitates precise dissection and maneuvering in a narrow space, such as the pelvis, through a combination of motion scaling and intuitive manipulation.[1,3,7,13] It may overcome the limitations of laparoscopic surgery for rectal cancer. Studies have demonstrated equivalent outcomes and potential benefits of this robotic approach.[5,14,15] Other studies have specified that robotic surgery for rectal cancer results in more favorable outcomes in patients with unfavorable clinical characteristics, such as obesity, male sex, receiving nCRT, and tumors in the lower two-thirds of the rectum.[13,16] However, no solid evidence demonstrating the superiority of the robotic procedure over the conventional laparoscopic procedure is available to support its general adoption for rectal surgery, particularly considering its high cost.[17–19]
In addition to conventional laparoscopic surgery, robotic surgery for rectal cancer was initiated at our institute from 2012 onward. After accumulating experience from selected cases, we began expanding our indication to more complicated cases, such as those involving patients with mid or low rectal cancer and patients undergoing nCRT. In this study, we analyzed the clinical outcomes of this patient subset to determine whether the advantages of robotic surgery are conferred to them.
2. Methods
2.1. Patient selection
Patients with an adenocarcinoma of the mid or low rectum (5–10 and <5 cm from the anal verge, respectively, as measured through colonoscopy or rigid proctoscopy) and those with a clinical stage of T3–4 or N-positive according to the Union for International Cancer Control–American Joint Committee on Cancer Tumor-Node-Metastasis Classification System, Seventh Edition, were operated on after administering nCRT to them. All these patients, except for those with contraindications to prolonged pneumoperitoneum or apparent cancer invasion to adjacent structures on preoperative images, were considered for minimally invasive surgery. After a thorough discussion with the attending surgeons about the potential advantages and drawbacks of the robotic and laparoscopic procedures, patients consented to either procedure according to their own choice. Patients with distant metastases, histologies other than adenocarcinoma, a history of other malignancies, signs of acute intestinal obstruction or perforation, familial adenomatous polyposis coli, hereditary nonpolyposis colorectal cancer, active inflammatory bowel disease, or an American Society of Anesthesiologists class greater than III were excluded from this study.
Between January 2012 and April 2015, 40 patients were operated on by using the DaVinci Si HD Robotic System (Intuitive Surgical Inc., Sunnyvale, CA) at our institution. These patients were included in the robotic group. For further comparison and for assessing the effect of the learning curve for the robotic approach, patients in the robotic group were further subdivided into the first (Robot 1) and second (Robot 2) groups according to the sequential order of their procedures, with 20 in each group. Nevertheless, the inclusion and exclusion criteria remained constant throughout the study.
During the same period, another 38 patients with an adenocarcinoma of the mid or low rectum who were operated on by using the conventional laparoscopic approach after receiving nCRT were matched to patients in the robotic group by age, sex, the body mass index, and procedure. For comparison with the robotic group, these patients were selected from our prospectively maintained database and were included in the laparoscopic (LPS) group. The inclusion and exclusion criteria remained constant for both groups of patients. The robotic and laparoscopic operations were performed by a single surgical team. This study was approved by the Joint Institutional Review Board of Taipei Medical University (TMU-JIRB No. 201310028).
2.2. Neoadjuvant therapy and perioperative management
nCRT was administered according to our institutional guidelines. The radiotherapy regimen was 50.4 Gy for 5.5 weeks, including 45 Gy in 25 fractions to the pelvis and a 5.4-Gy boost in 3 fractions to the primary tumor. The patients also received 2 cycles of daily intravenous boluses of fluorouracil (400 mg/m2) and leucovorin (20 mg/m2) for 3 days in the first and fifth weeks of radiotherapy. Before and after administering nCRT, staging workup was performed using thoracoabdominal computed tomography and pelvic magnetic resonance imaging. Specifically, pelvic magnetic resonance imaging was performed for defining the T and N status. Surgery was performed 6 to 8 weeks after nCRT completion.
In this study, the patients received the same standardized protocol of perioperative care, including antibiotic prophylaxis, bowel preparation, thrombotic prophylaxis, analgesic care, and diet resumption. Mechanical bowel preparation was achieved by the administration of oral sodium phosphate preparations on the day before surgery and by an enema of sodium phosphate preparations in the morning of the surgery day. Antibiotic prophylaxis was achieved by the intravenous administration of a single dose of 1 g cefazolin. Prophylaxis for deep vein thrombosis was achieved by applying antiembolic stockings and intermittent pneumatic compression without the routine administration of heparin. Oral intake was allowed after the return of bowel movement, typically the passage of flatus, and was advanced to a soft diet gradually daily.
2.3. Operative procedure of robotic surgery for rectal cancer
Patients were placed in a modified lithotomy position with the head down at 30° and the right side down at 20°. We used 6 ports for the procedure. A 12-mm paraumbilical trocar was inserted to create a port for the camera. Four 8-mm da Vinci trocars were inserted at the right lower, right upper, left upper, and left lower abdomen. A 12-mm port was inserted at the right lateral abdomen to create a port to be used by the assistant surgeon.
After lymph node dissection, the inferior mesenteric artery was divided at its root. The inferior mesenteric vein was divided at approximately the same level. The splenic flexure was mobilized to facilitate a tension-free anastomosis, as required. Pelvic dissection was performed according to the principles of total mesorectal excision (TME). The tumor-bearing bowel segment was eventually resected through endoscopic stapling or intersphincteric resection, and bowel continuity was restored using the intracorporeal double stapling technique or transanal hand-sewn suture.
2.4. Operative procedure of laparoscopic surgery for rectal cancer
In the laparoscopic procedure, patients were positioned similar to that in the robotic procedure. We used a 4-port approach. A 12-mm supraumbilical trocar was inserted to create a port for the camera. Another 12-mm trocar was inserted at the right lower abdomen and two 5-mm trocars were inserted at the right lateral and left lower abdomen. The inferior mesenteric artery was ligated close to its origin by using clips and was then divided. The inferior mesenteric vein was divided at approximately the same level. Complete splenic flexure mobilization was performed to achieve a tension-free anastomosis, as required. Pelvic dissection was performed according to the principles of TME. The tumor-bearing bowel segment was resected through endoscopic stapling or intersphincteric resection. Bowel anastomoses were performed using the intracorporeal double stapling technique or the transanal hand-sewn suture.
2.5. Outcome measures
The operation time and intraoperative blood loss were recorded. The operation time was defined as the time between the initial skin incision and completion of wound closure. Conversion was defined as the unintended extension of laparotomy beyond the routine incision length (5 cm) necessary for specimen retrieval. A diverting stoma was created at the discretion of the surgeon. Bowel continuity was restored after the completion of adjuvant chemotherapy. For all patients, adjuvant chemotherapy was administered 4 to 6 weeks after rectal resection.
The histopathological parameters of the surgical specimens, including proximal and distal resection margin, circumferential resection margin (CRM), and the number of lymph nodes harvested, were recorded to assess the quality of surgery. The CRM was considered positive if cancer cells were observed microscopically within 1 mm of the CRM.[3] The response to nCRT was classified using the tumor regression grade scale proposed by Dworak et al.[20]
Morbidity and mortality events that occurred 30 days postoperatively were recorded. For specificity, anastomotic leakage was defined as the clinical or radiological evidence of a defect of the integrity of the anastomotic site.[21]
2.6. Statistical analysis
Categorical variables were compared using the Chi-squared or Fisher exact test, as appropriate. Continuous variables were compared using the Student t test or analysis of variance, as appropriate. P < .05 was considered statistically significant. Regional differences were isolated using post hoc comparison with the Tukey test. Statistical analyses were performed using Statistical Package for Social Science for Windows, Version 17.0 (SPSS, Inc., Chicago, IL).
3. Results
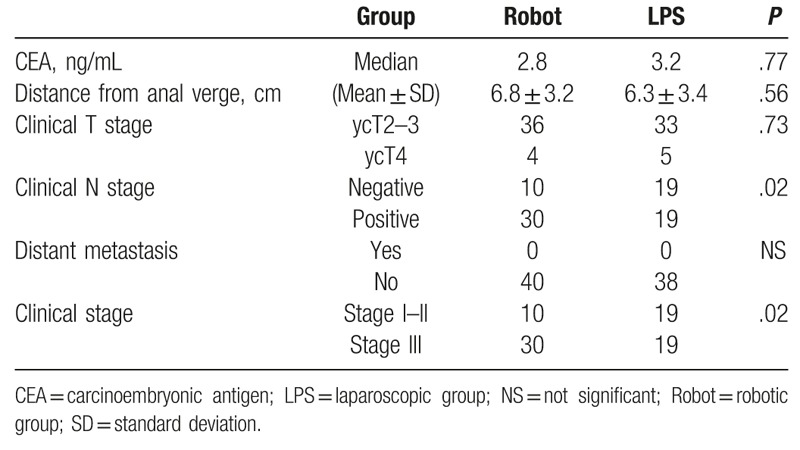
The pretreatment characteristics of patients are listed in Table 1. The robotic and LPS groups were comparable with regard to baseline demographics and clinical parameters. After undergoing nCRT, patients in both groups were comparable with regard to the levels of the carcinoembryonic antigen, distance of the lesion from the anal verge, and number of clinical T stages. No distant metastasis was detected in both groups. However, in the robotic group, more patients exhibited N-positive cancer (P = .02) and consequently clinical stage III cancer (P = .02) (Table 2).
Table 1.
Clinical characteristics of patients before undergoing neoadjuvant chemoradiation therapy (n = 78).
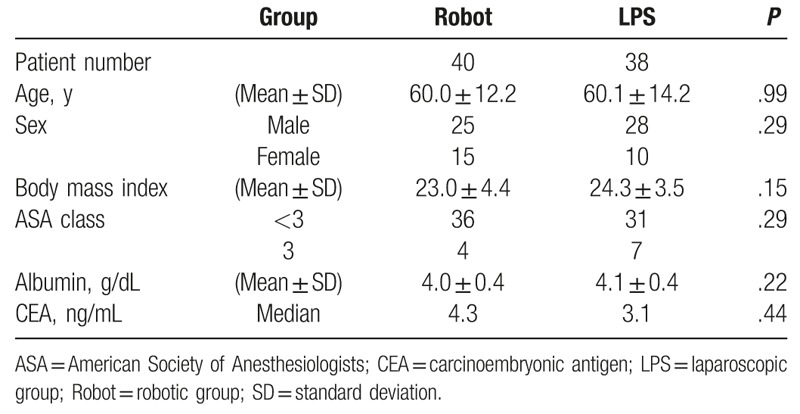
Table 2.
Clinicopathological characteristics of patients after undergoing neoadjuvant chemoradiation therapy (n = 78).
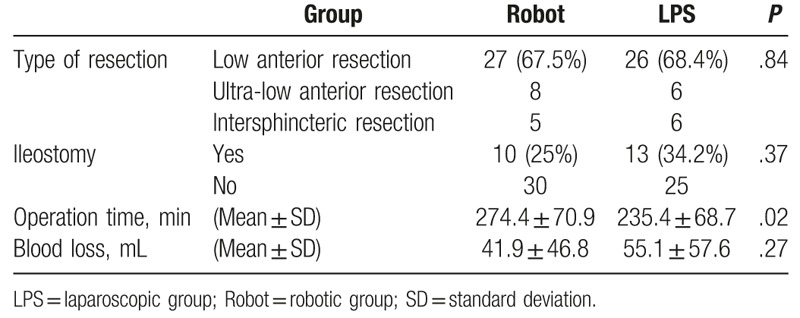
The most frequently performed procedure was low anterior resection (Table 3; 67.5% and 68.4% in the robotic and LPS groups, respectively). Anastomoses of all low and ultra-low anterior resections were performed using the double stapling technique, whereas anastomoses of all intersphincter resections were performed using the hand-sewn suture. The diverting ileostomy creation rate did not significantly vary between the groups (25% and 34.2% in the robotic and LPS groups, respectively). The operation time was significantly longer (i.e., approximately 40 minutes longer) in the robotic group than in the LPS group (P = .02). The estimated blood loss did not vary significantly between the groups. No conversion was noted in all patients.
Table 3.
Operative parameters of patients (n = 78).
The 2 groups were similar with regard to the tumor size and treatment effect of nCRT (Table 4). A total of 82.1% (64/78) of patients showed a certain degree of response to nCRT; however, no patient experienced complete regression in our study. The proximal and distal resection margins did not differ significantly between the groups. A total of 5.3% of patients in the LPS group showed CRM involvement; however, none of the patients in the robotic group showed this involvement, although the difference was nonsignificant. The distribution of pathological tumor and nodal stages was similar between the groups. The mean numbers of lymph nodes harvested were 16.7 in the robotic group (range: 4–46) and 15.6 (range: 6–29) in the LPS group. Although the number of lymph nodes harvested tended to be more in the robotic group, the difference did not reach statistical significance.
Table 4.
Pathological parameters of surgical specimens (n = 78).

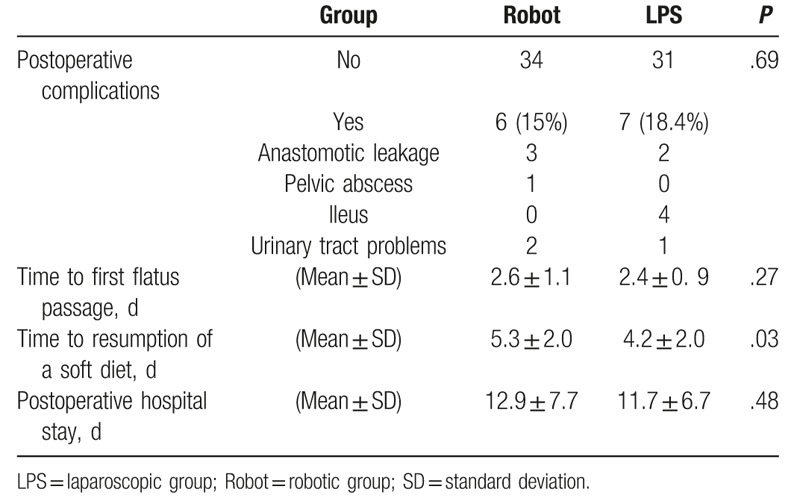
Common procedure-related complications included anastomotic leakage, pelvic abscess, ileus, and urinary tract problems (Table 5). Moreover, the affected proportion of patients was similar in both groups (15% and 18.4% in the robotic and LPS groups, respectively). The time to passage of flatus was also similar between the groups. However, the time to resumption of a soft diet was delayed by approximately 1 day in the robotic group (P = .03). The postoperative hospital stay was also longer in the robotic group, although this finding was not statistically significantly.
Table 5.
Postoperative outcomes of patients (n = 78).
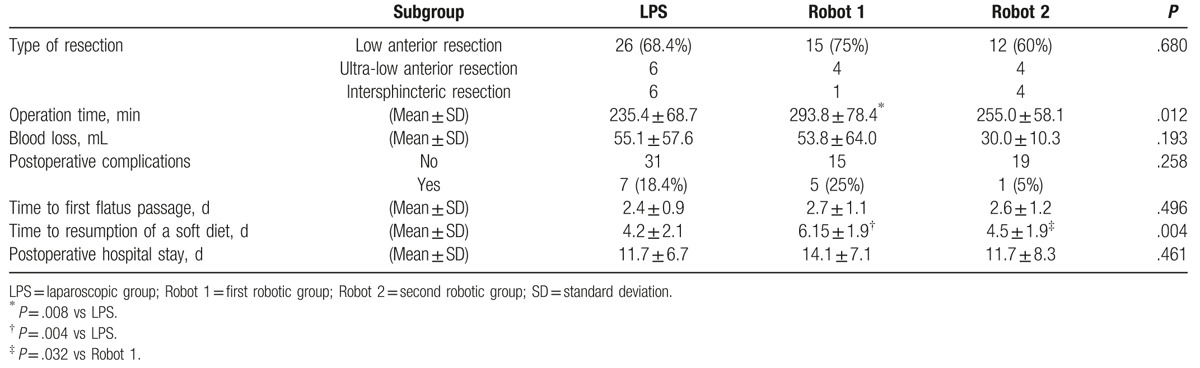
To assess the effect of the learning curve on perioperative outcomes, the LPS, Robot 1, and Robot 2 groups were compared (Table 6). Similar operative procedures were performed in the 3 surgery groups. Nevertheless, the operation time was significantly longer (approximately 58 minutes longer) in the Robot 1 group than in the LPS group (P = .008). Similarly, the operation time was markedly longer (approximately 39 minutes longer) in the Robot 1 group than in the Robot 2 group, although the difference did not reach statistical significance (P = .183). By contrast, a much smaller difference was observed in the operation time between the LPS and Robot 2 groups (approximately 20 minutes, P = .560). The estimated blood loss was less in the Robot 2 group than in the other 2 groups. However, the difference did not reach statistical significance. Similarly, although the complication rate did not vary significantly among the 3 groups (18.4% for LPS, 25% for Robot 1, and 5% for Robot 2), the rate tended to be lower in the Robot 2 group. The time to passage of flatus was also similar among the 3 groups. However, the time to resumption of a soft diet was significantly delayed by more than 1.5 days in the Robot 1 group compared with that in the LPS and Robot 2 groups (P = .004; LPS vs Robot 1, P = .004; Robot 1 vs Robot 2, P = .032; LPS vs Robot 2, P = .919). The postoperative hospital stay was also longer in the Robot 1 group, although this finding was not statistically significant.
Table 6.
Perioperative parameters in the 3 groups (n = 78).
4. Discussion
Randomized controlled trials have demonstrated that the oncological outcomes of laparoscopic surgery for rectal cancer are comparable to those of open surgery.[2,4,12] With the advent of robotic surgery, studies have been conducted to evaluate whether its application can overcome the limitations of laparoscopic surgery for rectal cancer. In the first study comparing robotic and laparoscopic anterior rectal resection, Patriti et al[22] demonstrated that robotic resection resulted in a significantly shorter operation time and a significantly lower conversion rate. Moreover, other clinical and oncological outcomes were similar.[22] In a review of 3013 robotic rectal resections, Staderini et al[13] reported that despite a longer operation time, robotic surgery for rectal cancer was associated with lower conversion rates and higher preservation of autonomic function. Moreover, other clinicopathological characteristics were similar between the robotic and LPS groups.[13] Other systemic reviews have also reported that the surgical outcomes of robotic rectal resection are comparable to those of the laparoscopic procedure; however, robotic surgery offers potential benefits, such as a shorter postoperative hospital stay, higher quality of the specimen, lower overall complication rate, and more favorable functional results.[5,23–25] However, the proportion of patients receiving nCRT in these studies was variable and relatively low, ranging from 2.3% to 45.9%.[3,5,13,22–26] Because nCRT is an integral part of modern treatment for advanced rectal cancer and both treatment effect of nCRT and anatomical location of rectal cancer may render surgery more technically challenging, we conducted this study to further explore the role of robotic and laparoscopic surgery in patients receiving nCRT. Our results revealed that both approaches were feasible and equally effective. The operation time for robotic surgery was initially longer. However, after a relatively short learning curve, the short-term outcomes of robotic surgery for rectal cancer were comparable to those of laparoscopic surgery performed by experienced surgeons.
Several factors have been proposed to explain why the robotic approach is more advantageous than the laparoscopic approach for rectal surgery.[3,15] The wristed instruments enable ambidextrous capability and intuitive manipulation by the surgeon. The camera system provides a stable 3D high-definition image. The robotic third arm provides steady retraction and exposure. The combination of these functions facilitates accurate anatomical dissection in the narrow pelvis and theoretically may enable a higher preservation of pelvic autonomic functions. The advantages may be more pronounced under specific conditions, such as lower rectal tumors, male or obese patients, and those undergoing preoperative radiotherapy.[5,15,25] Nevertheless, controversial results have been reported regarding the advantages of robotic surgery. For example, Park et al[19] compared the outcomes of robotic and laparoscopic resections for rectal cancer and concluded that robotic surgery failed to offer any oncologic or clinical benefit despite the high cost. Melich et al[18] also reported similar clinicopathological outcomes for laparoscopic and robotic rectal resections.
A clear CRM is highly important because a positive margin increases the risk of local recurrence by 3 to 4 times.[4,27] Therefore, the rate of CRM involvement is applied as a parameter for assessing the quality of robotic and laparoscopic rectal resection. The rate in our study was comparable to the reported rate of 0% to 11.7% and 0% to 10% for robotic and laparoscopic surgery, respectively.[2–4,13,19,22] Moreover, when focusing on the subgroup of patients with advanced rectal cancer undergoing nCRT, the rate in our study was favorably comparable with the reported rates of 7.1% to 16.4% and 4.6% to 16% for robotic and laparoscopic surgery, respectively, in previous studies.[11,16] However, we did not observe a higher CRM clearance rate in the robotic group, as many reports did. A study proposed that the higher CRM positive rate might reflect the greater technical difficulty associated with laparoscopic rectal resection.[11] However, experience in laparoscopic rectal surgery may overcome this limitation. Similar rates of positive CRM for robotic and laparoscopic rectal resections have also been reported in other studies.[13,19,22]
The number of lymph nodes harvested is another parameter frequently adopted to evaluate the oncological quality of the surgical procedures. Studies have reported a significantly higher number of lymph nodes harvested for the robotic approach.[11,26] In our study, the mean number in both groups was higher than the requirement for accurate pathological staging and was comparable to the reported numbers of 10 to 32 and 11 to 23 for the robotic and laparoscopic groups, respectively, in other studies.[3,6–9,11,13,16,18,19,22,26,28] Considering that the number of lymph nodes may decrease after nCRT, the present findings were even more favorably comparable with previous findings in patients undergoing nCRT.[14,16] However, our result indicated that a similar number of lymph nodes were harvested in the robotic and LPS groups, consistent with previous reports.[7,13,16,18,19,22,28]
The conversion rate of a minimally invasive procedure reflects its technical complexity. Moreover, achieving a low conversion rate is important because converted patients are more likely to develop complications and local recurrence.[29,30] Although excellent results can be achieved in specialized centers,[2] the conversion rate for laparoscopic surgery for rectal cancer typically ranges from 1.8% to 22% and can be as high as 28% in patients with rectal cancer undergoing nCRT.[7–9] Most studies have reported a lower conversion rate for robotic procedures.[6,8,13,19,22,28,31] In our study, no conversion was noted in both groups. This was not uncommon for robotic surgery but was favorably comparable with the conversion rate of laparoscopic surgery for rectal cancer in previous studies.[3,4,7,11,19,22,28]
Although nCRT is an established risk factor for the complications, the complication rate in our study was comparable to the previously reported rates of 10.7% to 41.3% and 12.2% to 32.8% for robotic and laparoscopic rectal resections, respectively.[6–8,11,13,18,22] Moreover, in the present study, no significant difference was observed between the 2 groups. Furthermore, the complication rate tended to be lower in the Robot 2 group, indicating the potential of the robotic procedure to further reduce the complication rate. However, this finding is not supported by that of Akmal et al,[32] who reported that the complication rate did not differ significantly between 2 consecutive groups of patients undergoing robotic TME.
Considering the aforementioned observations, it can be inferred that when performed by experienced laparoscopic surgeons, the surgical quality and short-term outcomes of laparoscopic rectal resection are virtually equivalent to those of its robotic counterpart. In our study, the comparison results for other parameters were in agreement with this corollary. The distal resection margin and diverting ileostomy creation rate in our study were also comparable to those reported previously.[3,4,7,9,13,14,19,22,28] Moreover, in our study, the results did not differ significantly between the laparoscopic and robotic groups. Comparative studies have reported equivalent performance for laparoscopic and robotic rectal resections.[18,19] Our results provided further evidence that although the robotic procedure offers potential advantages to overcome the limitations of the laparoscopic procedure, comparable outcomes can still be achieved when technically demanding laparoscopic rectal resection is performed by an experienced surgical team.
A disadvantage of robotic surgery is the prolonged operation time, mainly attributed to the time required to dock the robotic system, change instruments, and undock the system if a change is required in the patient's position. In previous studies, the reported operation time widely varied, ranging from 182 to 485.8 minutes and from 140 to 374.3 minutes for robotic and laparoscopic rectal resections, respectively. However, generally, a longer operation time was reported for the robotic procedure.[1–3,7–9,11,13,14,19,26,33,34] In our study, the mean operation time was significantly longer in the Robot 1 group than in the LPS group. However, the mean operation time shortened markedly in the Robot 2 group, approaching that in the LPS group. Similar trends were also observed for the estimated blood loss and complication rate. This finding may be attributed to the effect of the learning curve. A previous study suggested that the learning curve for laparoscopic rectal resection is steep, and that 30 to 70 cases are required to overcome the learning phase.[35] Jeong et al[12] reported an even higher number of 50 to 80 cases. By contrast, the learning curve for robotic rectal resection has been reported to be shorter. It is generally agreed that 15 to 35 cases are required for surgeons to be proficient in robotic rectal resection.[8,35,36] The shorter learning curve is mainly attributable to the aforementioned advantageous operational features of the robotic system.[14,15,37] Akmal et al[32] divided 80 consecutive patients undergoing robotic TME into 2 groups and found that clinicopathological characteristics did not differ significantly between the groups; this finding implied that experienced laparoscopic surgeons achieved proficiency for robotic surgery within 40 cases. Melich et al[18] reported that after the initial 41 cases, a surgeon naive to minimally invasive surgery for rectal cancer performed the robotic procedure faster than the laparoscopic procedure, mainly because of expedited pelvic dissection. Our data showed obvious progress in several outcome measures after the initial 20 cases of robotic surgery. The outcome measures in the subsequent 20 cases were equivalent to those of the laparoscopic procedures. More than 1000 laparoscopic procedures have been performed by our team. Notably, patients in our robotic group had more clinically advanced diseases than those in the LPS group. Our results support a shorter learning curve for robotic rectal resection, even in patients receiving nCRT. Moreover, we believe that extensive experience in laparoscopic surgery may attenuate the learning curve of robotic surgery because of transferrable skill sets and familiarity with regional anatomy.[18,32,35]
In the present study, the mean distal resection margin in both robotic and LPS groups was less than the generally accepted 2 cm. This margin may still be considered adequate, however, as nCRT leads to regression of the intramural tumor spread.[38] The postoperative hospital stay in our study was markedly longer than that in previous series.[1,8,9,11,18,19,26,28] A shorter hospital stay has been recognized as a potential advantage of robotic surgery. By contrast, the mean postoperative hospital stay in our Robot 1 group was longer than that in the LPS group, although no such difference was observed in the Robot 2 group. The difference might be attributed to psychosocial reasons and the procedure per se because an identical and constant perioperative management protocol was adopted for all patients throughout the study. The longer postoperative hospital stay is also a health care system specific concern. In Taiwan, the admission cost is covered by the National Health Insurance program. Therefore, patients may opt to stay in the hospital longer until subjectively satisfactory recovery is attained, particularly after paying the extra fee for robotic surgery. Moreover, this was more common during the early period of robotic surgery before we were confident with its postoperative clinical course.
One of the major limitations of our study is the retrospective design. The patient number was small, and patient selection was not random, although we obviated the discrepancy by matching patients by age, sex, the body mass index, and procedure. Conducting a randomized study for comparing robotic and laparoscopic surgery is almost impossible because the cost of robotic surgery for rectal cancer is approximately 3 times higher than that of laparoscopic surgery according to previous reports.[8,19] Considering this reason, a previous comparative study abandoned its original randomized design.[28] In our study, in the robotic group, significantly more patients had positive nodes and advanced clinical stages. The outcomes of robotic surgery might be negatively biased. However, similar outcomes were achieved by the robotic procedure despite the bias, which is compatible with the previous expectation of the advantageous role of robotic surgery in difficult cases.[7,28] The outcomes may also be biased because of the personal preference of the surgeons and patients. For example, despite a similar time to flatus for all patients and a constant perioperative management protocol, the time to the resumption of a soft diet was prolonged in the Robot 1 group. In our study, operations were performed by highly experienced laparoscopic surgeons. Therefore, the results may not be extrapolated to surgeons with less experience. The benefits of robotic surgery may also be mitigated in comparison with laparoscopic surgery.[7] The short-term outcome measures reported in the present study may not persist in the long-term analysis, as was the case in previous comparative studies of laparoscopic and open surgery for rectal cancer.[12,39] Finally, we did not compare the functional result of patients, which may be more favorably preserved after robotic surgery.[1,3]
In conclusion, robotic surgery for patients with advanced rectal cancer undergoing nCRT is safe and feasible. Furthermore, although robotic procedure was performed in a relatively limited number of patients with more clinically advanced diseases, the results indicated that the perioperative outcomes of robotic surgery may be comparable to those of laparoscopic surgery. In this regard, the previous experience of the laparoscopic surgical team may have an additive role. However, considering the extra financial and time expenses of the robotic procedure, this novel technology should be selectively applied; thus, additional efforts should be devoted toward searching for the specific subset of patients who may benefit from the robotic procedure.
Footnotes
Abbreviations: CRM = circumferential resection margin, nCRT = neoadjuvant chemoradiation therapy, TME = total mesorectal excision.
The authors report no conflicts of interest.
References
- [1].Ferrara F, Piagnerelli R, Scheiterle M, et al. Laparoscopy versus robotic surgery for colorectal cancer: a single-center initial experience. Surg Innov 2016;23:374–80. [DOI] [PubMed] [Google Scholar]
- [2].Kang SB, Park JW, Jeong SY, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 2010;11:637–45. [DOI] [PubMed] [Google Scholar]
- [3].Kim CN, Bae SU, Lee SG, et al. Clinical and oncologic outcomes of totally robotic total mesorectal excision for rectal cancer: initial results in a center for minimally invasive surgery. Int J Colorectal Dis 2016;31:843–52. [DOI] [PubMed] [Google Scholar]
- [4].van der Pas MH, Haglind E, Cuesta MA, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013;14:210–8. [DOI] [PubMed] [Google Scholar]
- [5].Rodriguez-Sanjuan JC, Gomez-Ruiz M, Trugeda-Carrera S, et al. Laparoscopic and robot-assisted laparoscopic digestive surgery: present and future directions. World J Gastroenterol 2016;22:1975–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kim NK, Kang J. Optimal total mesorectal excision for rectal cancer: the role of robotic surgery from an expert's view. J Korean Soc Coloproctol 2010;26:377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kwak JM, Kim SH, Kim J, et al. Robotic vs laparoscopic resection of rectal cancer: short-term outcomes of a case-control study. Dis Colon Rectum 2011;54:151–6. [DOI] [PubMed] [Google Scholar]
- [8].Mak TW, Lee JF, Futaba K, et al. Robotic surgery for rectal cancer: a systematic review of current practice. World J Gastrointest Oncol 2014;6:184–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Denoya P, Wang H, Sands D, et al. Short-term outcomes of laparoscopic total mesorectal excision following neoadjuvant chemoradiotherapy. Surg Endosc 2010;24:933–8. [DOI] [PubMed] [Google Scholar]
- [10].Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–40. [DOI] [PubMed] [Google Scholar]
- [11].Serin KR, Gultekin FA, Batman B, et al. Robotic versus laparoscopic surgery for mid or low rectal cancer in male patients after neoadjuvant chemoradiation therapy: comparison of short-term outcomes. J Robot Surg 2015;9:187–94. [DOI] [PubMed] [Google Scholar]
- [12].Jeong SY, Park JW, Nam BH, et al. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol 2014;15:767–74. [DOI] [PubMed] [Google Scholar]
- [13].Staderini F, Foppa C, Minuzzo A, et al. Robotic rectal surgery: state of the art. World J Gastrointest Oncol 2016;8:757–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Huang CW, Yeh YS, Ma CJ, et al. Robotic colorectal surgery for laparoscopic surgeons with limited experience: preliminary experiences for 40 consecutive cases at a single medical center. BMC Surg 2015;15:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sun Y, Xu H, Li Z, et al. Robotic versus laparoscopic low anterior resection for rectal cancer: a meta-analysis. World J Surg Oncol 2016;14:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].de Jesus JP, Valadao M, de Castro Araujo RO, et al. The circumferential resection margins status: a comparison of robotic, laparoscopic and open total mesorectal excision for mid and low rectal cancer. Eur J Surg Oncol 2016;42:808–12. [DOI] [PubMed] [Google Scholar]
- [17].Kim CW, Kim CH, Baik SH. Outcomes of robotic-assisted colorectal surgery compared with laparoscopic and open surgery: a systematic review. J Gastrointest Surg 2014;18:816–30. [DOI] [PubMed] [Google Scholar]
- [18].Melich G, Hong YK, Kim J, et al. Simultaneous development of laparoscopy and robotics provides acceptable perioperative outcomes and shows robotics to have a faster learning curve and to be overall faster in rectal cancer surgery: analysis of novice MIS surgeon learning curves. Surg Endosc 2015;29:558–68. [DOI] [PubMed] [Google Scholar]
- [19].Park EJ, Cho MS, Baek SJ, et al. Long-term oncologic outcomes of robotic low anterior resection for rectal cancer: a comparative study with laparoscopic surgery. Ann Surg 2015;261:129–37. [DOI] [PubMed] [Google Scholar]
- [20].Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 1997;12:19–23. [DOI] [PubMed] [Google Scholar]
- [21].Rahbari NN, Weitz J, Hohenberger W, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery 2010;147:339–51. [DOI] [PubMed] [Google Scholar]
- [22].Patriti A, Ceccarelli G, Bartoli A, et al. Short- and medium-term outcome of robot-assisted and traditional laparoscopic rectal resection. JSLS 2009;13:176–83. [PMC free article] [PubMed] [Google Scholar]
- [23].Kim JY, Kim NK, Lee KY, et al. A comparative study of voiding and sexual function after total mesorectal excision with autonomic nerve preservation for rectal cancer: laparoscopic versus robotic surgery. Ann Surg Oncol 2012;19:2485–93. [DOI] [PubMed] [Google Scholar]
- [24].Kang J, Yoon KJ, Min BS, et al. The impact of robotic surgery for mid and low rectal cancer: a case-matched analysis of a 3-arm comparison: open, laparoscopic, and robotic surgery. Ann Surg 2013;257:95–101. [DOI] [PubMed] [Google Scholar]
- [25].Scarpinata R, Aly EH. Does robotic rectal cancer surgery offer improved early postoperative outcomes? Dis Colon Rectum 2013;56:253–62. [DOI] [PubMed] [Google Scholar]
- [26].Feroci F, Vannucchi A, Bianchi PP, et al. Total mesorectal excision for mid and low rectal cancer: laparoscopic vs robotic surgery. World J Gastroenterol 2016;22:3602–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Birbeck KF, Macklin CP, Tiffin NJ, et al. Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg 2002;235:449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Baik SH, Kwon HY, Kim JS, et al. Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol 2009;16:1480–7. [DOI] [PubMed] [Google Scholar]
- [29].Law WL, Poon JT, Fan JK, et al. Comparison of outcome of open and laparoscopic resection for stage II and stage III rectal cancer. Ann Surg Oncol 2009;16:1488–93. [DOI] [PubMed] [Google Scholar]
- [30].Chan AC, Poon JT, Fan JK, et al. Impact of conversion on the long-term outcome in laparoscopic resection of colorectal cancer. Surg Endosc 2008;22:2625–30. [DOI] [PubMed] [Google Scholar]
- [31].Xiong B, Ma L, Zhang C, et al. Robotic versus laparoscopic total mesorectal excision for rectal cancer: a meta-analysis. J Surg Res 2014;188:404–14. [DOI] [PubMed] [Google Scholar]
- [32].Akmal Y, Baek JH, McKenzie S, et al. Robot-assisted total mesorectal excision: is there a learning curve? Surg Endosc 2012;26:2471–6. [DOI] [PubMed] [Google Scholar]
- [33].deSouza AL, Prasad LM, Marecik SJ, et al. Total mesorectal excision for rectal cancer: the potential advantage of robotic assistance. Dis Colon Rectum 2010;53:1611–7. [DOI] [PubMed] [Google Scholar]
- [34].Park YA, Kim JM, Kim SA, et al. Totally robotic surgery for rectal cancer: from splenic flexure to pelvic floor in one setup. Surg Endosc 2010;24:715–20. [DOI] [PubMed] [Google Scholar]
- [35].Jimenez-Rodriguez RM, Rubio-Dorado-Manzanares M, Diaz-Pavon JM, et al. Learning curve in robotic rectal cancer surgery: current state of affairs. Int J Colorectal Dis 2016;31:1807–15. [DOI] [PubMed] [Google Scholar]
- [36].Bokhari MB, Patel CB, Ramos-Valadez DI, et al. Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc 2011;25:855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jimenez-Rodriguez RM, Diaz-Pavon JM, de la Portilla de Juan F, et al. Learning curve for robotic-assisted laparoscopic rectal cancer surgery. Int J Colorectal Dis 2013;28:815–21. [DOI] [PubMed] [Google Scholar]
- [38].Bujko K, Rutkowski A, Chang GJ, et al. Is the 1-cm rule of distal bowel resection margin in rectal cancer based on clinical evidence? A systematic review. Ann Surg Oncol 2012;19:801–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Andersson J, Angenete E, Gellerstedt M, et al. Health-related quality of life after laparoscopic and open surgery for rectal cancer in a randomized trial. Br J Surg 2013;100:941–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


