Abstract
Rationale:
Although rare, occult breast cancer (OBC) originates from breast tissue. Its primary lesions cannot be identified by clinical examination or imaging; therefore, the diagnosis, treatment, and prognosis remain controversial.
Patient concerns:
This study comprised 5 female OBC patients who were admitted to the Affiliated Hospital of Guizhou Medical University for painless axillary lumps.
Diagnoses:
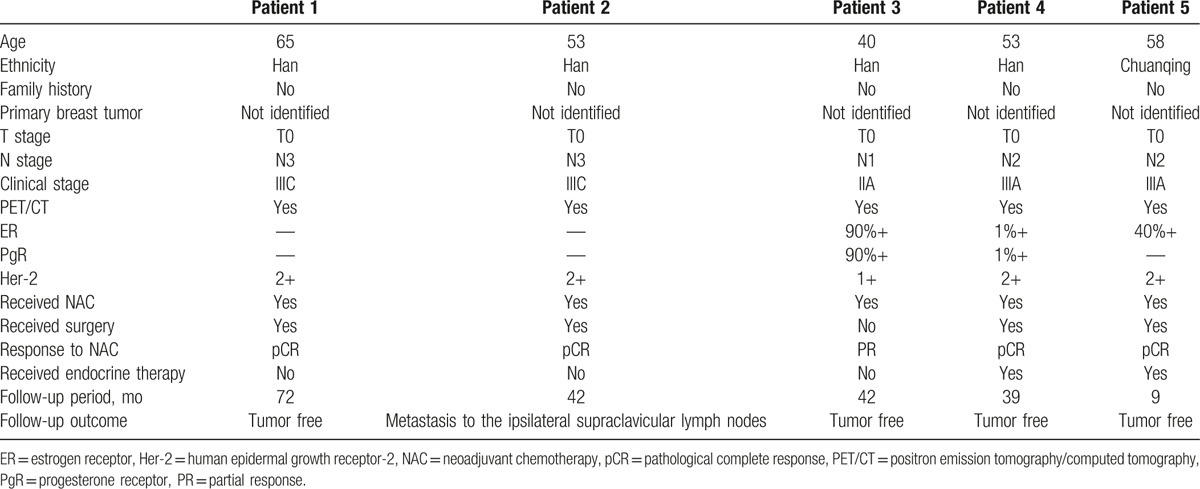
18F-flurodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) indicated metastasis in the ipsilateral axillary lymph nodes. No clear breast primary lesions were identified; other organs were also excluded as the primary site. Pathological biopsy confirmed axillary lymph node metastasis of adenocarcinoma. Immunohistochemical staining of the tumor to identify the source revealed that estrogen receptors (ERs) and progesterone receptors (PgRs) were positive in 2 cases, ER was positive and PR was negative in 1 case, and both were negative in 2 cases. Human epidermal growth factor receptor 2 was negative in all cases. All patients were diagnosed with OBC.
Interventions:
All patients underwent neoadjuvant chemotherapy (NAC). One patient did not undergo follow-up therapy. The other 4 underwent total mastectomy plus axillary lymph node dissection followed by radiotherapy. Two patients also underwent endocrine therapy.
Outcomes:
Patients were followed up for 9.0 to 72.0 months. Four achieved pathological complete response. One patient experienced metastasis to the ipsilateral supraclavicular lymph nodes 2.0 years later, which was cleared after additional treatment. The other patients were tumor free.
Lessons:
Here, we are reporting 5 cases of OBC treated with NAC that were evaluated by 18F-FDG PET/CT scans. This study suggests that NAC might lead to a positive outcome.
Keywords: 18F-FDG PET/CT, ipsilateral supraclavicular lymph nodes, metastasis, neoadjuvant chemotherapy, occult breast cancer
1. Introduction
Occult breast cancer (OBC) is a special type of breast cancer. Its initial symptoms include metastasis to the axillary lymph nodes or other parts of the body, where primary lesions cannot be identified by breast palpation or imaging; other sources can be excluded.[1] Because OBC is rare and there is a lack of clinical research with large sample sizes, there are controversies over its diagnosis and treatment. 18F-flurodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) plays a critical role in diagnosing carcinoma in patients.[2,3] Neoadjuvant chemotherapy (NAC) is one of the most comprehensive tumor therapies that have been developed since the 1980s and has been widely used in treating breast cancer,[4,5] although its use in OBC is rarely reported. In this study, OBC was detected in 5 patients by 18F-FDG PET/CT; these patients were then treated with NAC. The clinical data on these 5 patients, their diagnoses, and their treatments were analyzed and summarized retrospectively to provide references for future clinical work.
2. Case reports
2.1. Case 1
The patient was a 65-year-old Han female. In November 2010, a lump in the right axillary area was found but not considered as cause for alarm. After 27 days, the size of the lump was seen by color Doppler ultrasound to have increased significantly. The patient was admitted to our institution on December 9, 2010. The patient was otherwise healthy with no history of hypertension, diabetes, or other systemic diseases; no history of trauma or surgery; no bad habits, such as smoking or alcohol consumption; and no family history of cancer. Physical examination indicated no lump in the breasts. A hard lump of 3.0 by 3.0 cm2 was identified in the right axillary area. No swollen lymph nodes were found in the left axillary or bilateral supraclavicular areas. Breast lesions were not identified by mammography (Fig. 1A), ultrasound (Fig. 1B), or magnetic resonance imaging (MRI) (Fig. 1C). On December 17, 2010, whole-body PET/CT results showed increased metabolism of the right axillary and right supraclavicular lymph nodes, indicating tumor metastasis, while abnormal radioactive concentration was not found in any other organ (Fig. 1D). A needle puncture biopsy of the right axillary lymph nodes indicated metastatic adenocarcinoma (Fig. 6A). Immunohistochemical results were as follows: estrogen receptor (ER) (−), progesterone receptor (PgR) (−), human epidermal growth factor receptor (Her-2) (2+) (Fig. 6B–D), Fish (−), cytokeratin 7 (CK7) (+), and E-cadherin (+), which supported the diagnosis of breast cancer; carcinoembryonic antigen (CEA) (−) and CK20 (−), which excluded gastrointestinal tumors; homatropine methylbromide-45 (HMB45) (−) and soluble-protein 100 (S-100) (−), which excluded malignant melanoma; and thyroid transcription factor-1 (TTF-1) (−) and thyroglobulin (Tg) (−), which excluded thyroid or lung cancer. Based on these results, the patient was diagnosed with OBC and, after having signed an informed consent form, was treated with NAC in the form of dose-dense epirubicin in combination with cyclophosphamide (EC) followed by weekly paclitaxel as follows: 100.0 mg/m2 epirubicin (Hisun Pfizer Inc., Hangzhou, China) plus 600.0 mg/m2 intravenous cyclophosphamide (Jiangsu Shengdi Pharmaceutical Co., Ltd, Lianyungang, China) on day 1, 14.0 days per cycle, 4 cycles; a sequential regimen of 80.0 mg/m2 intravenous paclitaxel (Haikou Pharmaceutical Co., Ltd., Haikou, China) on day 1, 7.0 days per cycle, 12 cycles. The patient experienced mild side effects. She did not intend to preserve the breast and underwent total mastectomy and axillary lymph node dissection. Primary breast lesions were not found in the whole-breast biopsy. No metastasis was found in the dissected axillary lymph nodes, and the patient recovered well. After surgery, the patient underwent radiotherapy on the chest wall and supraclavicular lymph drainage area at a dose of 50.4 Gy/28 fractions. She was followed up by telephone and clinical visits until December 31, 2016. Follow-up included life status, local recurrence, and distant metastasis. Follow-up examinations were conducted every 6.0 months and included breast color ultrasound; mammography; breast MRI; head, chest, and abdominal CT; whole-body bone scan; CEA; cancer antigen 125 (CA 125); and CA 15-3. No recurrence or distant metastasis was observed (Table 1).
Figure 1.
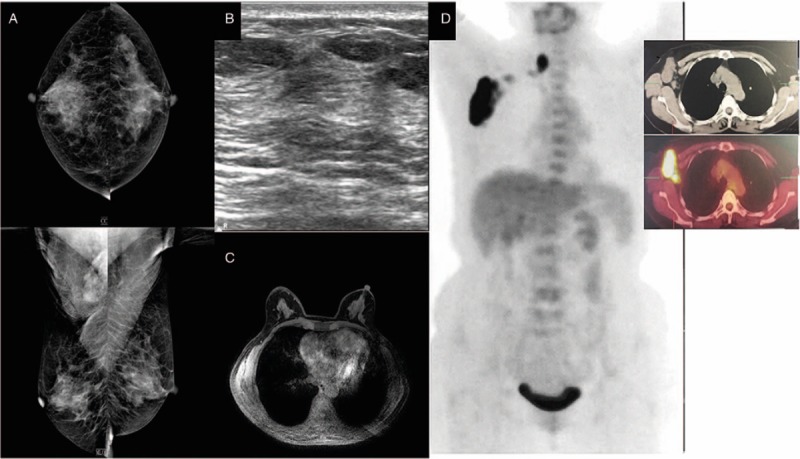
Mammography (A), breast ultrasound (B), and magnetic resonance imaging (C) do not show abnormalities in the breast. Positron emission tomography (D) shows abnormal increased flurodeoxyglucose uptake in right axillary and right supraclavicular region.
Figure 6.
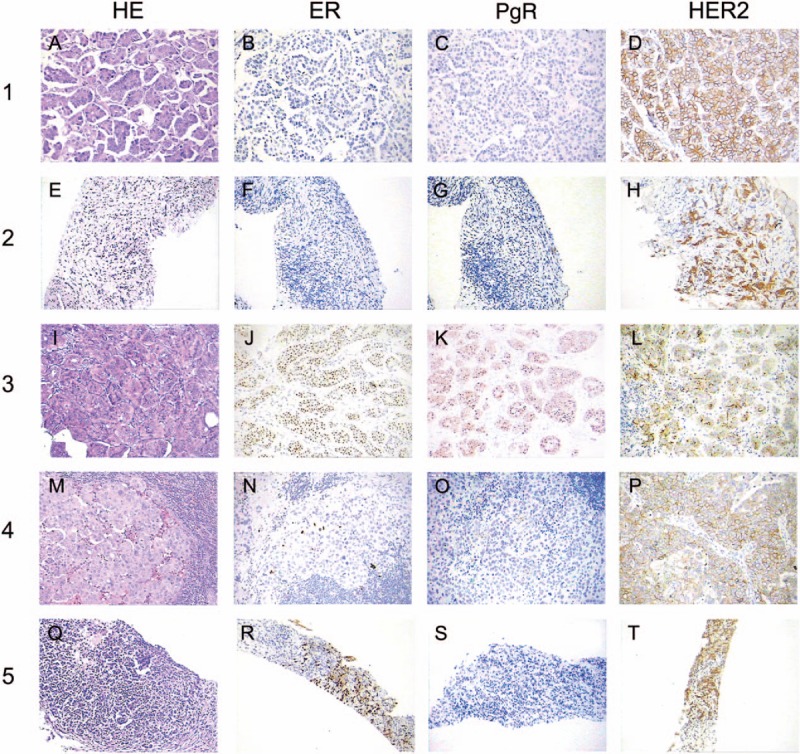
Axillary lymph node biopsy showed metastatic adenocarcinoma (hematoxylin and eosin, magnification × 200) (A, E, I, M, Q). Immunohistochemical analysis revealed tumor cells were positive for ER (J, N, R), PgR (K, O), negative for ER (B, F), PgR (C, G, S), but negative for HER2 (D, H, L, P, T) (magnification × 200).
Table 1.
Characteristics of patients and therapeutic strategies.
2.2. Case 2
The patient was a 53-year-old Han female. In May 2013, lumps were found in her left axillary and supraclavicular areas. She was admitted to our institution on May 28, 2013. The patient was healthy with no history of systemic diseases, no history of trauma or surgery, no bad habits, and no family history of cancer. Physical examination identified a hard lump of 3.0 by 2.5 cm2 in the left axillary area and a hard lump of 1.5 by 1.0 cm2 in the left supraclavicular area; however, no lump was found in the breasts and no swollen lymph nodes were identified in the right axillary or supraclavicular areas. Breast lesions were not identified by mammography (Fig. 2A), ultrasound (Fig. 2B), or MRI (Fig. 2C). Whole-body PET/CT results showed active metabolism at Region V of the left neck and lymph nodes in the left supraclavicular, infraclavicular, and axillary areas, indicating tumor metastasis, while no abnormal radioactive concentration was found in any other organ (Fig. 2D). A needle puncture biopsy of the left axillary lymph nodes indicated metastasis from invasive ductal carcinoma (Fig. 6E). Immunohistochemical results were as follows: ER (−), PgR (−), Her-2 (2+) (Fig. 6F–H), Fish (−), E-cadherin (+), CK7 (+), CEA (−), CK20 (−), HMB45 (−), S-100 (−), TTF-1 (−), and Tg (−). Based on these results, the patient was diagnosed with OBC, and after having signed an informed consent form, was treated with NAC (the same as used in case 1). The patient experienced mild side effects. PET/CT was conducted after chemotherapy, and the results showed no swollen lymph nodes or increased metabolism in the left neck and left supraclavicular and infraclavicular areas (Fig. 2E). Swollen lymph nodes in the left axillary area showed slightly increased metabolism, which was significantly reduced compared with that before radiotherapy. The patient did not intend to preserve the breast and underwent total mastectomy and axillary lymph node dissection. Primary breast lesions were not found in the whole-breast biopsy. No metastasis was found in the dissected axillary lymph nodes, and the patient recovered well. After surgery, the patient underwent radiotherapy on the chest wall and supraclavicular lymph drainage area at a dose of 50.4 Gy/28 fractions. The patient was followed up after OBC diagnosis. Two years after the treatments were completed, the patient had swollen lymph nodes in the ipsilateral supraclavicular area, which was confirmed by biopsy to be metastasis (most likely, adenocarcinoma). Immunohistochemistry results were as follows: ER (−), PR (−), and Her-2 (3+). The patient underwent targeted therapy for 1.0 year comprising 75 mg/m2 intravenous docetaxel on day 1 plus 100 mg/m2 intravenous etoposide on days 2 to 4; 21 days per cycle, 6 cycles total, with simultaneous trastuzumab first at 8.0 mg/kg, followed by 6.0 mg/kg intravenous drip on day 5. No local recurrence or distant metastasis was observed through December 31, 2016 (Table 1).
Figure 2.
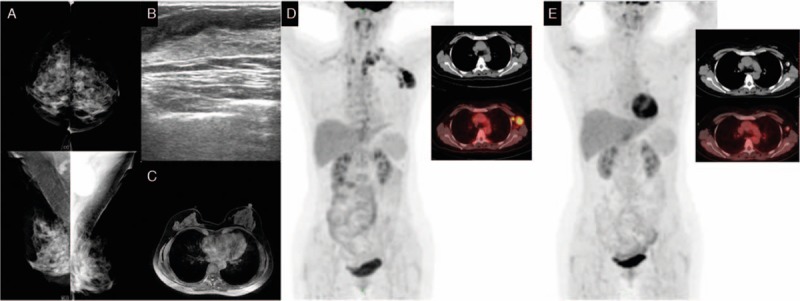
Mammography (A), breast ultrasound (B), and magnetic resonance imaging (C) do not show abnormalities in the breast. Positron emission tomography (D) shows abnormal increased FDG uptake in left axillary and left supraclavicular region. Positron emission tomography (E) does not show abnormal increased FDG uptake in the left neck and in the left supraclavicular and infraclavicular regions after neoadjuvant chemotherapy. FDG= flurodeoxyglucose.
2.3. Case 3
The patient was a 40-year-old Han female. In May 2013, a lump was found in her left axillary area, which was dissected in a local hospital 2.0 weeks later. The biopsy results indicated left axillary metastasis. Because the local hospital could not confirm the diagnosis, the patient was admitted to our institution on June 6, 2013. The patient was healthy with no history of systemic diseases, no history of trauma or surgery, no bad habits, and no family history of cancer. Physical examination indicated no lump in the breasts and no swollen lymph nodes in the bilateral axillary and supraclavicular areas. Breast lesions were not identified by mammography (Fig. 3A), ultrasound (Fig. 3B), or MRI (Fig. 3C). On June 7, 2013, whole-body PET/CT did not find abnormal radioactive concentration in any organs (Fig. 3D). Biopsy results indicated left axillary metastasis from invasive ductal carcinoma grade II (60%) and invasive papillary carcinoma (40%) (Fig. 6I), and the primary tumor was considered to originate from ipsilateral breast tissues. Immunohistochemical results were as follows: ER (+), PgR (+), Her-2 (1+) (Fig. 6J–L), CK7 (+), CEA (−), CK20 (−), HMB45 (−), S-100 (−), TTF-1 (−), and Tg (−). Based on these results, the patient was diagnosed with OBC and, after having signed an informed consent form, was treated with NAC (the same as used in case 1). The patient experienced mild side effects. She refused surgery, radiotherapy, and endocrine therapy based on personal reasons. She was followed up until December 31, 2016. The patient reported life and work as usual. No recurrence or distant metastasis was observed (Table 1).
Figure 3.
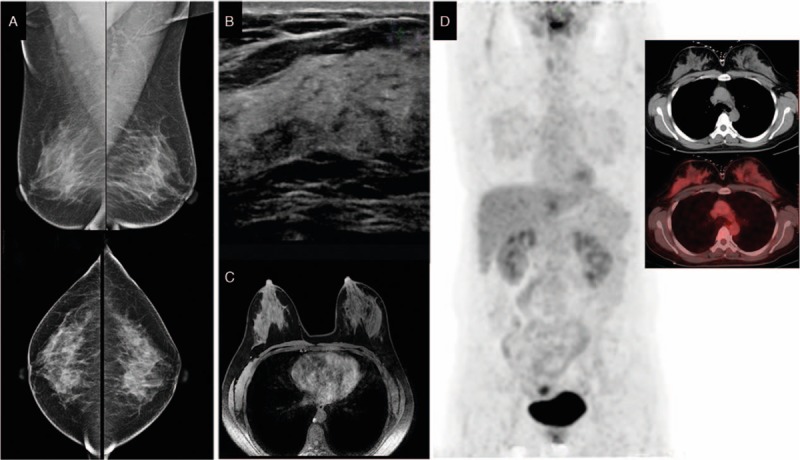
Mammography (A), breast ultrasound (B), and magnetic resonance imaging (C) do not show abnormalities in the breast. Positron emission tomography (D) does not show abnormal increased FDG uptake in left axillary region. FDG= flurodeoxyglucose.
2.4. Case 4
The patient was a 53-year-old Han female. In August 2013, a lump was found in her right axillary area, which was dissected at a local hospital 20 days later. The biopsy results indicated right axillary metastasis. Because the local hospital could not confirm the diagnosis, the patient was admitted to our institution on September 9, 2013. The patient was healthy with no history of systemic diseases, no history of trauma or surgery, no bad habits, and no family history of cancer. Physical examination indicated no lump in the breasts and no swollen lymph nodes in the bilateral axillary and supraclavicular areas. Breast lesions were not identified by mammography (Fig. 4A), ultrasound (Fig. 4B), or MRI (Fig. 4C). Whole-body PET/CT results showed increased metabolism of the right axillary lymph nodes after tumor dissection, indicating metastasis, while abnormal radioactive concentration was not found in any other organ (Fig. 4D). Biopsy results indicated right axillary metastasis (Fig. 6M). Immunohistochemical results were as follows: ER (+), PgR (+), Her-2 (2+) (Fig. 6N–P), Fish (−), E-cadherin (+), CK7 (+), CEA (−), CK20 (−), HMB45 (−), S-100 (−), TTF-1 (−), and Tg (−). Based on these results, the patient was diagnosed with OBC and, after having signed an informed consent form, was treated with NAC (the same as in case 1). The patient experienced mild side effects. PET/CT was conducted after chemotherapy, and the results showed no swollen lymph nodes or increased metabolism in the right axillary areas (Fig. 4E). The patient did not intend to preserve the breast and underwent total mastectomy and axillary lymph node dissection. Primary breast lesions were not found in the whole-breast biopsy. No metastasis was found in the dissected axillary lymph nodes, and the patient recovered well. After surgery, the patient underwent radiotherapy on the chest wall and supraclavicular lymph drainage area at a dose of 50.4 Gy/28 fractions. She also received endocrine therapy with oral administration of 2.5 mg/d letrozole. The patient was followed up until December 31, 2016. No recurrence or distant metastasis was observed (Table 1).
Figure 4.
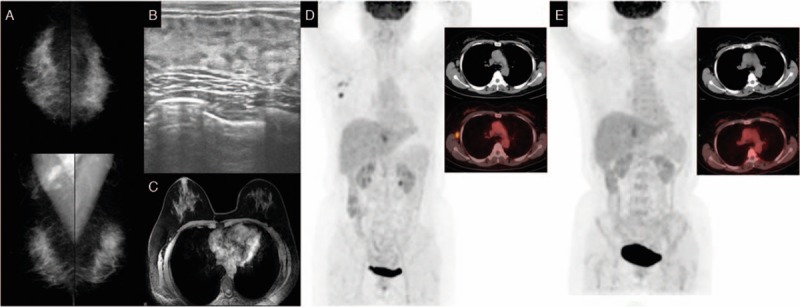
Mammography (A), breast ultrasound (B), and magnetic resonance imaging (C) do not show abnormalities in the breast. Positron emission tomography (D) shows abnormal increased FDG uptake in right axillary region. Positron emission tomography (E) does not show abnormal increased FDG uptake in the right axillary region after neoadjuvant chemotherapy. FDG= flurodeoxyglucose.
2.5. Case 5
The patient was a 58-year-old Chuanqing female. In March 2015, a lump was found in her left axillary area but was not considered as cause for alarm. One year later, the patient reported that the size of the lump had increased significantly; she was admitted to our institution on March 9, 2016. The patient was healthy with no history of systemic diseases, no history of trauma or surgery, no bad habits, and no family history of cancer. Physical examination indicated no lump in the breasts. A hard lump of 5.0 by 4.0 cm2 was identified in the left axillary area. No swollen lymph nodes were found in the right axillary area or bilateral supraclavicular areas. Breast lesions were not identified by mammography (Fig. 5A), ultrasound (Fig. 5B), or MRI (Fig. 5C). Whole-body PET/CT results showed active metabolism of the left axillary lymph nodes, indicating metastasis, while abnormal radioactive concentration was not found in any other organ (Fig. 5D). A needle puncture biopsy of the left axillary lymph nodes indicated left axillary metastatic invasive ductal carcinoma grade II (Fig. 6Q). Immunohistochemical results were as follows: ER (+), PgR (−), Her-2 (2+) (Fig. 6R–T), E-cadherin (+), CK7 (+), CEA (−), CK20 (−), HMB45 (−), S-100 (−), TTF-1 (−), and Tg (−). Based on these results, the patient was diagnosed with OBC and, after signing an informed consent form, was treated with NAC (the same as in case 1). The patient experienced mild side effects. She did not intend to preserve the breast and underwent total mastectomy and axillary lymph node dissection. Primary breast lesions were not found in the whole-breast biopsy. No metastasis was found in the dissected axillary lymph nodes, and the patient recovered well. After surgery, the patient underwent radiotherapy on the chest wall and supraclavicular lymph drainage area at a dose of 50.4 Gy/28 fractions. She also underwent endocrine therapy with oral administration of 2.5 mg/d letrozole. She was followed up until December 31, 2016. No recurrence or distant metastasis was observed (Table 1; Fig. 7).
Figure 5.
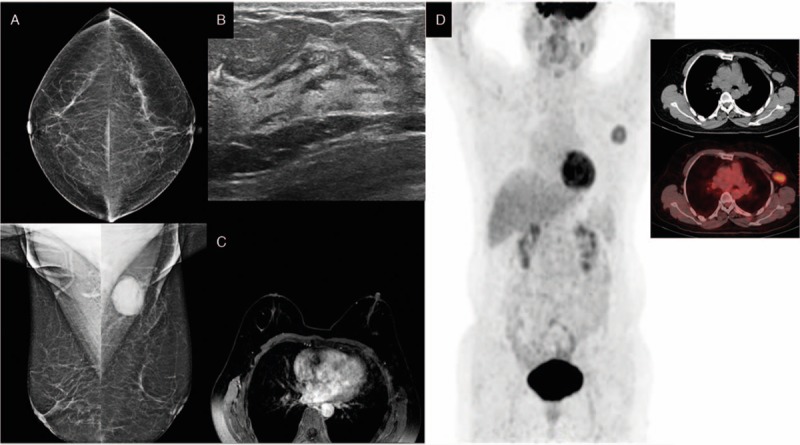
Mammography (A), breast ultrasound (B), and magnetic resonance imaging (C) do not show abnormalities in the breast. Positron emission tomography (D) shows abnormal increased FDG uptake in left axillary region. FDG= flurodeoxyglucose.
Figure 7.
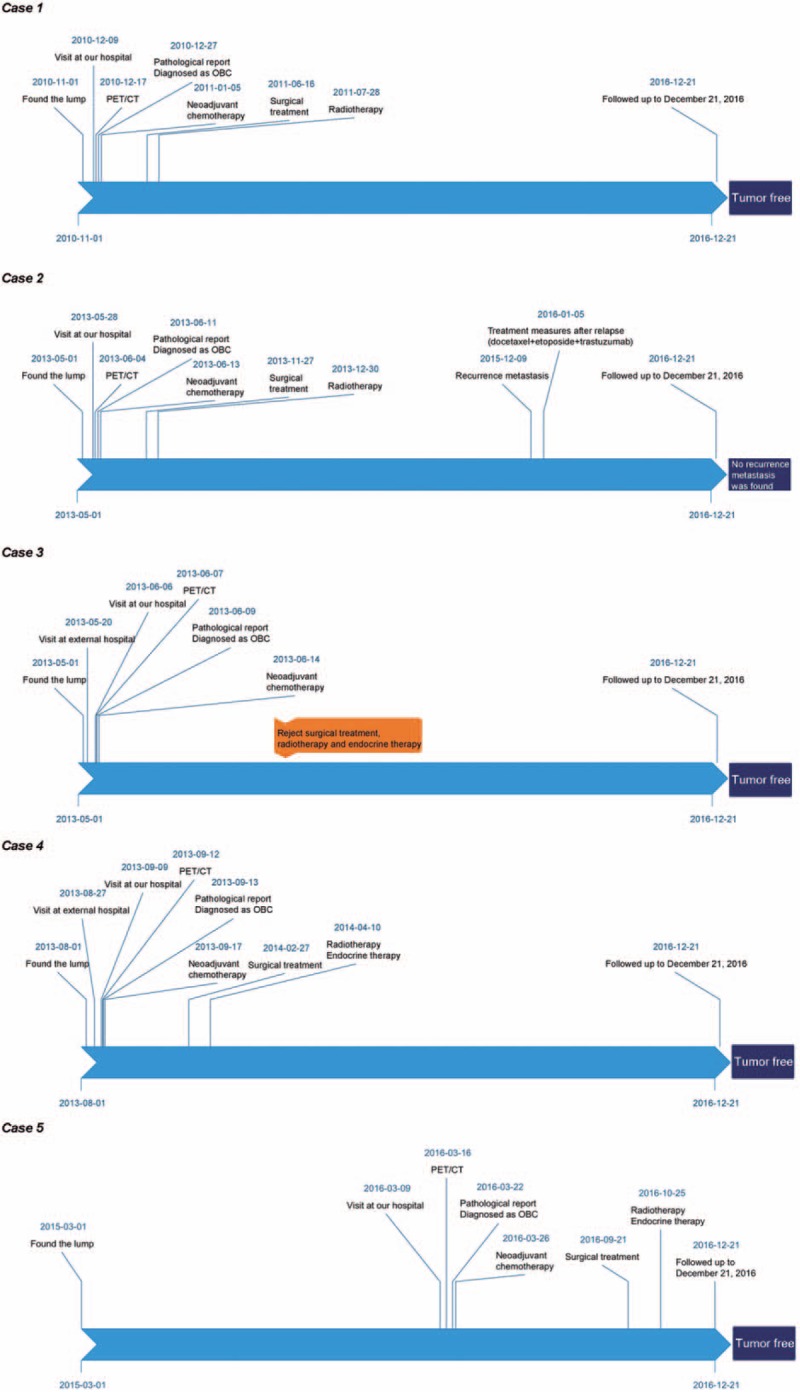
The timeline about diagnosis, treatment and clinical outcome for each patient.
3. Discussion
OBC is a rare type of breast cancer that was first reported by Halsted in 1907.[6] The incidence of OBC in China and elsewhere accounts for 0.3% to 1.0% of all breast cancer types.[7] This study reported 5 cases of patients with OBC, which accounted for ∼0.3% of all primary breast cancer cases during this time period (January 2010 to December 2016) and was consistent with data reported in the literature. Because OBC is rare and its major manifestations are swollen axillary lymph nodes, it is easily misdiagnosed and missed by both patients and physicians. In this study, 5 patients were admitted 1.0 to 12.0 months after swollen axillary lymph nodes were found; 2 patients also had swollen supraclavicular lymph nodes at the initial diagnosis, most likely disease progression resulting from late admission. Another 2 patients were referred to our institution after an axillary lymph node biopsy failed to confirm the diagnosis of OBC in their local hospitals, resulting in delays. Because confirmation is difficult, attention should be paid to patients with progressively enlarged and unexplained axillary lymph nodes, especially when metastatic adenocarcinoma is confirmed by histopathological examination, and OBC should be considered.[8] A comprehensive breast examination should be conducted to detect the primary breast lesions, including palpation, breast ultrasound, mammography, and breast MRI. Breast MRI is one of the most sensitive methods for detecting breast cancer; however, primary breast lesions were not detected by MRI in these 5 patients. To make the correct treatment decision, other types of malignant diseases, such as gastrointestinal tumor, lung carcinoma, and thyroid cancer, must be ruled out and an accurate diagnosis should be made through 18F-FDG PET/CT.
18F-FDG PET/CT is an important and widely recognized molecular imaging technique. It integrates functional and structural imaging, and can identify small lesions that are difficult to identify using other imaging techniques. The technique is mainly used in the staging, treatment effect evaluation, and diagnosis of the recurrence and metastasis of breast cancer,[9,10] but is rarely used in the diagnosis of OBC. Three cases of OBC were reported in which the primary breast lesions were detected by 18F-FDG PET/CT but not by other conventional imaging techniques.[11,12]18F-FDG PET/CT also has high specificity. Walter et al[13] reported that the sensitivity of MRI and PET/CT in detecting primary breast tumors was 89% and 63%, respectively, and the specificity was 74% and 91%, respectively; therefore, compared with conventional imaging, PET/CT imaging has the advantages of conducting a whole-body examination in 1 image and greatly improving the diagnostic accuracy of OBC by combining the high sensitivity of positron imaging with the high resolution of soft tissues from MRI. All 5 patients in this study underwent whole-body 18F-FDG PET/CT. The results showed that in addition to axillary and supraclavicular lymph nodes, no abnormal radioactivity was found in any other organ, which confirmed the diagnosis of OBC.
In addition to the aforementioned auxiliary examinations, pathological examinations and immunohistochemical indices also have high practical values in determining the source of the primary tumor and confirming the diagnosis. In this study, all 5 patients were diagnosed with metastasis in the lymph nodes by biopsy using tissue dissection or needle puncture. Immunohistochemical results were as follows: ER (+) and PgR (+/−) in 3 cases, ER (−) and PgR (−) in 2 cases, and Her-2 (−) in all cases. Other tested results were CK7 (+), CEA (−), CK20 (−), HMB45 (−), S-100 (−), TTF-1 (−), and Tg (−) in all cases, excluding the possibility of tumor metastasis from sources other than the breasts.
There is no uniform standard in the treatment of OBC because of its low incidence and a lack of direct evidence-based medicine. The National Comprehensive Cancer Network (NCCN) guidelines and a number of retrospective studies indicate that the key to OBC treatment is to diagnose breast lesions before surgery. Treatments should be based on the clinical stage and risk factors for patients with OBC having breast lesions that are confirmed by biopsy before surgery. Treatments can be based on the status of lymph node metastasis and selected according to the non-OBC stage II and III treatment guidelines for patients with OBC having no breast lesions before surgery. For local treatment, total mastectomy with axillary lymph node dissection and radiotherapy, or whole-breast radiotherapy plus axillary lymph node dissection (axillary lymph node radiotherapy can be selected) can be performed. For systemic therapy, chemotherapy, endocrine therapy, and targeted therapy can be selected. Previous studies have shown that most patients with OBC are treated with surgery followed by systemic therapy, but the prognoses varied greatly.[14,15] Among all comprehensive regimens for breast cancer, chemotherapy is one of the main methods by which to control recurrence and metastasis.
NAC has the advantages of reducing tumor stage and increasing the rates of surgical resection. Pathological complete response (pCR) after NAC is significantly associated with better survival for breast cancer patients[4]; therefore, it is widely used in treating this disease.[4,5] Few studies are available on the use of NAC in treating OBC. It was reported that a 34-year-old patient with OBC had benefitted greater from NAC. The chemotherapy regimen consisted of 4 cycles of docetaxel, trastuzumab, and pertuzumab and 3 sequential cycles of 5-fluorouracil, epirubicin, and cyclophosphamide, followed by breast-conserving surgery and axillary lymph node dissection. Postoperative biopsy showed positivity in 2 axillary lymph nodes.[16] In our study, NAC was performed on all 5 patients and was well tolerated and completed in all patients. Response to NAC is classified as clinical or pathological. The clinical measurement of the response to NAC was defined according to the Response Evaluation Criteria in Solid Tumors version 1.1. We used a breast MRI to estimate the response during NAC. Partial response (PR) is defined as at least a 30% decrease in the sum of the diameters of lymph nodes, taking as reference the baseline sum. pCR is a characteristic objectively evaluated in the surgical specimen after NAC. pCR is the absence of residual invasive cancer within the axillary lymph nodes.[17] Four of 5 patients, which is a high rate, achieved pCR.
Breast cancer is a heterogeneous disease not only in its clinical but also in its biological features. It includes several molecular subtypes with different responses to NAC. In this study, the molecular subtypes of 2 patients who achieved pCR were triple negative. In general, it is believed that the triple-negative breast cancers (TNBCs) are more likely to achieve pCR than the luminal subtype.[18,19] Two studies suggested basal-like TNBCs with pCR rates ranging from 45% to 56%.[20,21] Although 1 patient who achieved pCR had the luminal subtype, the ER and PgR expressions were low, which might have resulted in a positive response to NAC. One patient who achieved pCR was a subset of ER (+), PgR (−), and Her-2 (−), a characteristics that was the most likely to benefit from NAC.[22,23] In addition, according to NCCN guidelines, the sequential regimen consisting of dose-dense epirubicin plus cyclophosphamide followed by weekly paclitaxel is one of the preferred regimens for chemotherapy, which was used in all 5 cases. This result indicated the efficacy of a sequential regimen for OBC consisting of anthracycline plus cyclophosphamide followed by paclitaxel. Moreover, OBC, as a special type of breast cancer, might have an excellent response to NAC; therefore, further studies are needed to investigate the use of NAC and the corresponding pCR rate in OBC. For local treatment, 4 patients received surgery after completing NAC, and did not intend to preserve the breasts; therefore, all underwent total mastectomy plus axillary lymph node dissection, followed by postoperative local radiotherapy. Another patient did not receive any follow-up treatment after NAC was completed, and was closely followed up until December 31, 2016.
Although OBC is a special type of breast cancer, the major factors affecting its prognosis are similar to those of other breast cancer types,[24] such as the pathological and molecular types of the primary cancer, number of axillary lymph nodes with metastasis, presence of distant metastasis in the supraclavicular lymph nodes and other parts of the body, and choice of treatments. Two meta-analyses discuss the use of pCR as a surrogate marker for disease-free survival (DFS) and overall survival (OS) in patients with breast cancer.[25,26] Although it is not clear whether pCR can be used as a surrogate marker for DFS and OS, it has been demonstrated to best correlate with the outcome in breast cancer patients. In this study, all 5 patients were followed up. Four patients received comprehensive therapy comprising NAC, surgery, radiotherapy, and endocrine therapy. Among these 4, 3 were tumor free. In 1 patient with OBC having ER (−), PgR (−), Her-2 (−), and metastasis to the supraclavicular lymph nodes, swollen lymph nodes in the ipsilateral supraclavicular area were found 2.0 years after the treatments. Metastasis to the lymph nodes was confirmed by biopsy, and the molecular type changed to Her-2 overexpression. After chemotherapy and molecular targeted therapy, no local recurrence or distant metastasis was observed. Another patient who did not receive surgery, radiotherapy, or endocrine therapy after completing NAC was closely monitored, and no local recurrence or distant metastasis was observed.
3.1. Study limitations
Our results indicated that OBC patients could benefit from NAC; however, the limitations of our study were that the case series was small with a short follow-up period. We would like to continue to follow up with these patients to observe the long-term outcomes.
4. Conclusion
Here, we are reporting 5 cases of OBC treated with NAC that were evaluated by 18F-FDG PET/CT scans. This study suggests that NAC might lead to a positive outcome.
Acknowledgments
The authors thank the Affiliated Hospital of Guizhou Medical University for its support. They also thank the Department of Pathology at Affiliated Hospital of Guizhou Medical University for its help in obtaining the photographs.
Footnotes
Abbreviations: 18F-FDG = 18F-flurodeoxyglucose, CA = cancer antigen, CEA = carcinoembryonic antigen, CK = cytokeratin, DFS = disease-free survival, ER = estrogen receptor, Her-2 = human epidermal growth factor receptor, HMB45 = homatropine methylbromide-45, MRI = magnetic resonance imaging, NAC = neoadjuvant chemotherapy, NCCN = National Comprehensive Cancer Network, OBC = occult breast cancer, OS = overall survival, pCR = pathological complete response, PET/CT = positron emission tomography/computed tomography, PgR = progesterone receptor, PR = partial response, S-100 = soluble-protein 100, Tg = thyroglobulin, TNBCs = triple-negative breast cancers, TTF-1 = thyroid transcription factor-1.
HY and LL have contributed equally to this work.
The authors declare that they have no actual or potential conflict of interest associated with this study or its results.
References
- [1].Walker GV, Smith GL, Perkins GH, et al. Population-based analysis of occult primary breast cancer with axillary lymph node metastasis. Cancer 2010;116:4000–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nassenstein K, Veit-Haibach P, Stergar H, et al. Cervical lymph node metastases of unknown origin: primary tumor detection with whole-body positron emission tomography/computed tomography. Acta Radiol 2007;48:1101–8. [DOI] [PubMed] [Google Scholar]
- [3].Wang G, Wu Y, Zhang W, et al. Clinical value of whole-body F-18 fluorodeoxyglucose positron emission tomography/computed tomography in patients with carcinoma of unknown primary. J Med Imaging Radiat Oncol 2013;57:65–71. [DOI] [PubMed] [Google Scholar]
- [4].Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 2008;26:778–85. [DOI] [PubMed] [Google Scholar]
- [5].Guarneri V, Broglio K, Kau SW, et al. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol 2006;24:1037–44. [DOI] [PubMed] [Google Scholar]
- [6].Halsted WSI. The results of radical operations for the cure of carcinoma of the breast. Ann Surg 1907;46:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vlastos G, Jean ME, Mirza AN, et al. Feasibility of breast preservation in the treatment of occult primary carcinoma presenting with axillary metastases. Ann Surg Oncol 2001;8:425–31. [DOI] [PubMed] [Google Scholar]
- [8].Hur SM, Cho DH, Lee SK, et al. Occult breast cancers manifesting as axillary lymph node metastasis in men: a two-case report. J Breast Cancer 2012;15:359–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Champion L, Lerebours F, Alberini JL, et al. 18F-FDG PET/CT to predict response to neoadjuvant chemotherapy and prognosis in inflammatory breast cancer. J Nucl Med 2015;56:1315–21. [DOI] [PubMed] [Google Scholar]
- [10].Tabouret-Viaud C, Botsikas D, Delattre BM, et al. PET/MR in breast cancer. Semin Nucl Med 2015;45:304–21. [DOI] [PubMed] [Google Scholar]
- [11].Takabatake D, Taira N, Aogi K, et al. Two cases of occult breast cancer in which PET-CT was helpful in identifying primary tumors. Breast Cancer 2008;15:181–4. [DOI] [PubMed] [Google Scholar]
- [12].Soundararajan R, Naswa N, Karunanithi S, et al. Occult breast primary malignancy presenting as isolated axillary lymph node metastasis—early detection of primary site by 18F-FDG PET/CT. Nucl Med Rev Cent East Eur 2016;19:5–7. [DOI] [PubMed] [Google Scholar]
- [13].Walter C, Scheidhauer K, Scharl A, et al. Clinical and diagnostic value of preoperative MR mammography and FDG-PET in suspicious breast lesions. Eur Radiol 2003;13:1651–6. [DOI] [PubMed] [Google Scholar]
- [14].He M, Tang LC, Yu KD, et al. Treatment outcomes and unfavorable prognostic factors in patients with occult breast cancer. Eur J Surg Oncol 2012;38:1022–8. [DOI] [PubMed] [Google Scholar]
- [15].Sohn G, Son BH, Lee SJ, et al. Treatment and survival of patients with occult breast cancer with axillary lymph node metastasis: a nationwide retrospective study. J Surg Oncol 2014;110:270–4. [DOI] [PubMed] [Google Scholar]
- [16].Ahmed I, Dharmarajan K, Tiersten A, et al. A unique presentation of occult primary breast cancer with a review of the literature. Case Rep Oncol Med 2015;2015:102963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Choi M, Park YH, Ahn JS, et al. Evaluation of pathologic complete response in breast cancer patients treated with neoadjuvant chemotherapy: experience in a single institution over a 10-year period. J Pathol Transl Med 2017;51:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Badve S, Dabbs DJ, Schnitt SJ, et al. Basal-like and triple-negative breast cancers: a critical review with an emphasis on the implications for pathologists and oncologists. Mod Pathol 2011;24:157–67. [DOI] [PubMed] [Google Scholar]
- [19].Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 2007;13:2329–34. [DOI] [PubMed] [Google Scholar]
- [20].de Ronde JJ, Hannemann J, Halfwerk H, et al. Concordance of clinical and molecular breast cancer subtyping in the context of preoperative chemotherapy response. Breast Cancer Res Treat 2010;119:119–26. [DOI] [PubMed] [Google Scholar]
- [21].Krijgsman O, Roepman P, Zwart W, et al. A diagnostic gene profile for molecular subtyping of breast cancer associated with treatment response. Breast Cancer Res Treat 2012;133:37–47. [DOI] [PubMed] [Google Scholar]
- [22].Petruolo OA, Pilewskie M, Patil S, et al. Standard pathologic features can be used to identify a subset of estrogen receptor-positive, HER2 negative patients likely to benefit from neoadjuvant chemotherapy. Ann Surg Oncol 2017;24:2556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lips EH, Mulder L, de Ronde JJ, et al. Neoadjuvant chemotherapy in ER+ HER2- breast cancer: response prediction based on immunohistochemical and molecular characteristics. Breast Cancer Res Treat 2012;131:827–36. [DOI] [PubMed] [Google Scholar]
- [24].Montagna E, Bagnardi V, Rotmensz N, et al. Immunohistochemically defined subtypes and outcome in occult breast carcinoma with axillary presentation. Breast Cancer Res Treat 2011;129:867–75. [DOI] [PubMed] [Google Scholar]
- [25].Berruti A, Amoroso V, Gallo F, et al. Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: a meta-regression of 29 randomized prospective studies. J Clin Oncol 2014;32:3883–91. [DOI] [PubMed] [Google Scholar]
- [26].Kong X, Moran MS, Zhang N, et al. Meta-analysis confirms achieving pathological complete response after neoadjuvant chemotherapy predicts favourable prognosis for breast cancer patients. Eur J Cancer 2011;47:2084–90. [DOI] [PubMed] [Google Scholar]


