Abstract
Background:
Colorectal cancer (CRC) is one of the most common cancers all over the world, but its epidemiology is obviously different in various regions.
Methods:
The treatment of CRC also has varying characteristics due to differences in economy, geography, disease onset, chemotherapy, and other factors, although international guidelines are used to guide the treatment of CRC in China.
Results:
This paper summarizes the current status of CRC treatment, including surgical therapy, neoadjuvant radiotherapy and chemotherapy, postoperative chemotherapy, targeted therapy, maintenance therapy, and immunotherapy, according to the clinical situation in China, so as to provide better therapy and improve clinical practice for patients with CRC.
Conclusion:
This research shows that the treatment of colorectal cancer continues to progress, and the patient's efficacy and quality of life have improved.
Keywords: chemotherapy, colorectal cancer, maintenance therapy, radiotherapy, targeted therapy
1. Introduction
Worldwide, colorectal cancer (CRC) is the third most commonly diagnosed cancer in men and the second in women. It is estimated that there were 1.4 million new cases of CRC in 2012, accounting for 9.9% of the global cancer burden. Meanwhile, 693,900 deaths occurred, making it the fourth most common cause of cancer-related mortality. Mortality from CRC is higher in men than in women, with significant regional differences.[1,2] In China, CRC has the fifth highest incidence among all cancers in men and the fourth in women, and the fifth highest mortality in both men and women.[3]
In 2011, the incidence of CRC was 15 to 25/100,000 population in China, and the mortality was 7 to 13/100,000 population with an upward trend. The incidence in urban areas was higher than that in rural areas, and was higher in more economically developed areas, with a recent rapid increase in the southeast coastal region.[3,4]
Risk factors for CRC include age, male sex,[5] familial adenomatous polyposis, and hereditary nonpolyposis colorectal cancer such as Lynch syndrome, sporadic colorectal cancer, adenomatous polyphoid individual or family history, inflammatory bowel disease, diabetes and insulin resistance, drinking, obesity, smoking, red meat and high fat intake, pelvic radiotherapy, and so on.[6]
Comprehensive treatment of CRC has certain characteristics due to the different epidemiology of CRC and specific conditions within China. The current status of CRC treatment in China is summarized in this review to better guide clinical practice.
2. The current status of treatment in China
In China, the treatment decision-making is usually based on the National Comprehensive Cancer Network,[7] European Society for Medical Oncology,[8] and Chinese Society of Clinical Oncology guidelines and is considered in light of the specific circumstances in China such as patients’ socioeconomic status, biological behavior of the tumor, and patients’ expected tolerance to treatment. So far, surgical procedures are the most common treatment methods, and other approaches such as radiotherapy, chemotherapy, targeted therapy, and immunotherapy play a role in improving the therapeutic efficacy, enhancing the control rate in patients with locally advanced rectal cancer, and extending survival and improving quality of life for patients with metastatic CRC (mCRC).
2.1. Neoadjuvant therapy
The purpose of neoadjuvant radiotherapy and chemotherapy is to reduce the risk of local recurrence, tumor shrinkage to achieve a R0 resection, or downsize the clinical staging to enhance the opportunity for surgical resection, and maintain low rectal sphincter function to increase the anus preservation rate, thereby prolonging patients’ disease-free survival (DFS).[9]
Neoadjuvant chemoradiotherapy in China is suitable for locally advanced rectal cancer with a distance <12 cm from anus.[10] Neoadjuvant chemotherapy is suitable for rectal cancer at a distance of >12 cm from the anus. Continuous intravenous infusion of 5-fluorouracil (5-FU), 5-FU plus leucovorin (LV), single-agent capecitabine, reduced-dose capecitabine plus oxaliplatin (XELOX), or oxaliplatin plus 5-FU plus LV (FOLFOX) are considered to be the preferred chemotherapy regimens.
Radiotherapy is not recommended for stage I rectal cancer, but neoadjuvant radiotherapy can be utilized for stage II to III rectal cancer.[11,12] The precise radiotherapy for rectal cancer used in China, e.g., three-dimensional conformal radiotherapy techniques (3D-CRT) or intensity modulated radiotherapy techniques (IMRT), can reduce the radiation fields of the small intestine. A Chinese clinical practice study has shown that IMRT is significantly superior to 3D-CRT (P = .005).[13] It is recommended that the pelvic dose is DT 45.0–50.4 Gy/25–28 times, and conventional fractionated radiation is recommended for advanced rectal cancer.[14]
In China, the FOWARC randomized, multicenter, phase III trial showed that, compared with 5-FU combined with radiotherapy, modified FOLFOX6 (mFOLFOX6) with concurrent radiotherapy resulted in a higher rate of pathologic complete response (pCR) and downstaging.[15] The pCR rates were 14.0%, 27.5%, and 6.6%, and downstaging rates were 37.1%, 56.4%, and 35.5% in the 5-FU-radiotherapy, mFOLFOX6-radiotherapy, and mFOLFOX6 groups, respectively. A phase II study showed IMRT with concurrent XELOX in patients with locally advanced rectal cancer also had a high pCR rate.[16] These studies have shown that use of oxaliplatin can further enhance the curative effect and prolong DFS and overall survival (OS).[15–17] The specific regimens determined based on the patient's physical tolerance and preoperative clinical stage in China.
In recent years, CRC patients with liver or pulmonary metastases have undergone surgical resection after receiving conversion therapy under the guidance of the multidisciplinary team (MDT).[18] Compared with 5-FU, LV, plus irinotecan (FOLFIRI), the response rate and radical resection rate of metastases can be significantly improved by oxaliplatin, irinotecan, 5-FU, plus LV (FOLFOXIRI), thereby prolonging PFS.[19] For patients who have better performance status and hope for a surgical opportunity by receiving intensive chemotherapy, FOLFOXIRI can be selected primarily, but it is not recommended for patients with poor performance status.
Rectal cancer is mainly characterized by local recurrence, while liver, lung, and other distant metastases mainly occur in colon cancer. Infusional 5-FU, 5-FU plus LV, single-agent capecitabine, FOLFOX, XELOX, FOLFIRI (5-FU, LV, plus irinotecan), or FOLFOXIRI (oxaliplatin, irinotecan, 5-FU, plus LV) and combined targeted drug therapy such as bevacizumab or cetuximab (for RAS and BRAF wild-type) are regarded as the optimal chemotherapy regimens.[8,20]
In a clinical study performed at Zhongshan Hospital, Shanghai, China, the results confirmed that cetuximab plus chemotherapy in patients with KRAS wild-type unresectable CRC liver metastases had higher objective response rate (ORR) and median OS than the chemotherapy alone group; the ORR was 57.1 versus 29.4, and median OS was 30.9 versus 21.0 months, respectively.[21] Therefore, for CRC patients with initially unresectable metastases, cetuximab combined with chemotherapy improved the resectability (Table 1).
Table 1.
Summary of clinical studies of neoadjuvant therapy for colorectal cancer in China.
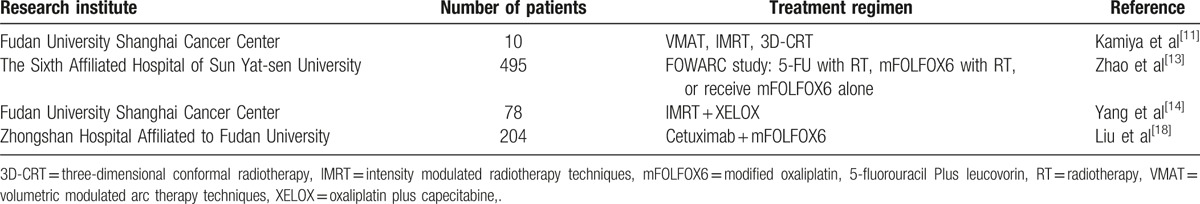
If metastatic lesions are converted to resectable foci, surgical treatment can be planned. If metastatic lesions are unable to be treated by R0 resection after conversion treatment, maintenance therapy or systemic therapy can be considered in order to reduce the toxicity of continuous high-intensity combined chemotherapy.
The results of multiple clinical studies in China have revealed that neoadjuvant radiotherapy and chemotherapy used in the majority of patients with CRC can significantly downsize the stage and results in surgical opportunities for an efficacy rate of surgery combined with radiotherapy and chemotherapy of 70% to 80%.
2.2. Surgical therapy
Surgical resection is one of the most effective treatments for CRC. Currently, laparoscopic resection is an important method of CRC surgery in China. Minimally invasive resection can accelerate recovery of gastrointestinal function after surgery, shorten the hospital stay, and does not adversely affect long-term survival.[22,23]
A number of studies have confirmed that, compared with open surgery, blood loss was less in laparoscopic resection (90 mL vs 100 mL, P = .001), and a shorter duration of hospital stay (9 days vs 10 days, P < .001). However, operating times were longer in laparoscopic resection group (180 min vs 140 min, P < .001).[24,25] The 5-year OS for laparoscopic surgery for CRC was not significantly different from open surgery (70% vs 66%, P = .395), but there were short-term benefits.[25,26]
Robotic surgery for CRC offers better view than open surgery and laparoscopic surgery. Compared with the other 2 surgical approaches, robotic surgery reduces the risk of hospital mortality, and bowel movements resume earlier. However, the costs of robotic surgery are larger and less benefits. In addition, there is a greater expense for patients. Of course, further studies and data are needed.[27]
Robot-assisted surgery for CRC is at the initial stage of use in China, but it can be used for rectal and sigmoid colon resection.[28] For local invasion and distant metastasis in CRC patients, robot-assisted surgery can also be used for combined resection. Several hospitals in China have utilized the da Vinci Surgical System, which has proved to be safe and feasible.[29]
All patients with initially unresectable mCRC are divided into 2 groups according to whether there is potential for radical resection of metastases, and such patients are supported by a continuum of care under the guidance of the MDT. The liver is the most common site of CRC metastases. For initially resectable CRC liver metastases, a study from the Chinese Academy of Medical Sciences demonstrated that the optimal surgical strategy was simultaneous colorectal and hepatic resection.[30] The 1-, 2-, and 3-year OS rates in the simultaneous resection group were 77%, 59%, and 53%, and in the staging resection group were 67%, 42%, and 10%, respectively. The median DFS were 19.1 months in the simultaneous resection group and 8.8 months in the staging resection group. The study showed that simultaneous hepatic resection was safe and improved the DFS and OS for patients with CRC liver metastases.
For initially resectable metastatic colon cancer, complete mesocolic excision surgery is recommended routinely. Colectomy plus regional lymph node dissection is recommended for colon cancer of cT1∼4N0∼2M0 clinical staging with no need for emergency treatment of symptoms.[31] For patients with colon cancer of cT1∼4N0∼2M0 accompanied by symptoms requiring emergency treatment, such as ileus, perforation, or hemorrhage that causes obstruction, stage I resection and anastomosis with or without proximal protective colostomy, stage I tumor resection combined with proximal colostomy plus distal closure, stage II resection after fistulation, or stage II resection after stent implantation is recommended.[32]
2.3. Adjunctive therapy
Postoperative adjuvant chemotherapy for CRC can reduce distant tumor metastases by eradicating circulating tumor cells and micrometastases, thereby improving the 5-year survival rate.
Adjuvant chemotherapy is generally based on a 5-FU chemotherapy regimen; LV can enhance the efficacy of 5-FU. For stage I to II colon cancer without high-risk factors, postoperative chemotherapy is not recommended. High-risk factors include bowel obstruction, T4 disease, poorly differentiated tumor, vascular or neural invasion, positive incisal margin or unknown margin, insufficient safe distance of the incisal margin, and <12 lymph nodes via pathological examination. For patients with stage II disease with high-risk factors, chemotherapy is recommended, namely 5-FU plus LV, capecitabine, mFOLFOX6, or XELOX, among which combination chemotherapy with oxaliplatin has the best curative effect and gives most benefit to patients.[15,17,33]
For all stage II disease patients, mismatch repair protein (MMR) or high-level microsatellite instability (MSI-H) should be detected. A study reported at the American Society of Clinical Oncology (ASCO) Gastrointestinal Cancers Symposium showed a correlation between MMR and clinicopathological characteristics in Chinese CRC patients (Chen G, 2017, unpublished data). The rates of MMR deletion (dMMR) in stages I, II, III, and IV disease were 9.7%, 16.5%, 8.5%, and 3.9%, respectively. The study suggested that dMMR was less frequent in Chinese patients, and stage II patients with dMMR had the highest rate among these patients, so may have a better prognosis.
For stage II and III CRC patients, a single-center retrospective study showed that KRAS status predicted the prognosis for treatment with adjuvant chemotherapy and suggested whether patients would benefit from adjuvant FOLFOX therapy.[34] The results showed that, in KRAS mutation patients, the 3-year DFS rate was 78.0% versus 69.2% for adjuvant chemotherapy compared with no chemotherapy. In contrast, in the KRAS wild-type group the 3-year DFS was 84.3% versus 82.0%, respectively. Therefore, Chinese CRC patients with KRAS mutation had a poor prognosis, but adjuvant FOLFOX therapy was beneficial, especially in stage III CRC.
A clinical trial for stage II and III colon cancer that applied intraportal chemotherapy plus adjuvant chemotherapy with mFOLFOX6 demonstrated that risk of tumor recurrence was reduced by 34% and DFS was improved. The 3-year DFS rate was 85.2% in the intraportal chemotherapy plus mFOLFOX6 group and 75.6% in the mFOLFOX6 alone group (P = .030), the 3-year metastasis-free survival rate was 87.6% and 78% (P = .035), respectively, and the distant metastases rate was 12.7% versus 22.7%, respectively (P = .044). Specifically, stage III CRC patients benefited significantly from intraportal chemotherapy plus adjuvant chemotherapy in China.[35]
Clinical trials of adjuvant chemotherapy with monoclonal antibodies failed to demonstrate the effectiveness of targeted drugs used in adjuvant setting. The recommended regimen of concurrent radiotherapy and chemotherapy for use in China is: radiotherapy plus 5-FU, maintained for 5 to 7 days per week; or radiotherapy plus capecitabine with capecitabine taken twice daily for 5 days per week.
2.4. Targeted therapy
Screening for CRC has not been widely implemented in China, resulting most of disease detected in late. Chemotherapy for mCRC is superior to optimum supportive therapy in terms of prolonging survival and improving quality of life. However, multiple clinical trials have confirmed that, compared with chemotherapy alone, targeted therapy combined with chemotherapy significantly improves PFS and OS in patients with mCRC. Moreover, the earlier treatment is started, the more that patients will benefit.
In line with the Asian consensus on mCRC,[36] the most common regimens used in China are FOLFIRI, mFOLFOX6, XELOX, FOLFOXIRI, bevacizumab, or cetuximab, combined with raltitrexed or regorafenib.
CRC patients should be diagnosed by histology of the primary tumor or metastases, and the enough radiological imaging. Before the patients started systemic treatment, biomarker testing was required to assist oncology decision making, such as RAS, BRAF testing. Postoperative tumor tissue or endoscopic biopsy were carried out for biomarker testing for CRC patients, to improve the accuracy of the test results.[37]
Bevacizumab plus chemotherapy can be used as first-line or second-line treatment for patients with no prior history of bevacizumab use, thereby benefiting most patients. In a multicenter phase II study of Chinese patients with mCRC, the median PFS was 8.5 months in the bevacizumab plus FOLFIRI group and 5.1 months in the FORFIRI alone group, the median OS was 15.2 and 11.3 months, respectively. The results suggest that bevacizumab combined with chemotherapy has better survival rates than chemotherapy alone for Chinese mCRC patients.[38]
TAILOR was a randomized, multicenter trial of cetuximab combined with FOLFOX4 versus FOLFOX4 alone in the treatment of RAS wild-type mCRC in China reported at the ASCO Gastrointestinal Cancers Symposium (Qin and Li, 2017, unpublished data). PFS was 9.2 months in the group receiving cetuximab combined with FOLFOX4 and 7.4 months in the group receiving FOLFOX4 alone and the median OS was 20.7 versus 17.8 months, respectively. Therefore, cetuximab combined with chemotherapy is recommended for Chinese mCRC patients with wild-type RAS and BRAF as a standard-of-care first-line treatment regimen, with improved efficacy and survival benefit.
Some studies have shown a correlation with the efficacy of cetuximab in the primary tumor site of RAS wild-type mCRC patients.[39] In first-line treatment, cetuximab with chemotherapy had a significantly higher PFS (9.1 vs 6.2 months, P = .002) and OS (28.9 vs 20.1 months, P = .036) than chemotherapy alone in left-sided colon cancer patients, but these rates were not significant for PFS (5.6 vs 5.7 months, P = .904) and OS (25.1 vs 19.8 months, P = .553) in right-sided colon cancer.
In CRYSTAL trial, the group of cetuximab combined with FOLFIRI had a higher median PFS (12 vs 8.9 months, P < .001) and OS (28.7 vs 21.7 months, P = .002) than chemotherapy alone in left-sided CRC, these rates in right-sided CRC were PFS (8.1 vs 7.1 months, P = .66) and OS (18.5 vs 15 months) than chemotherapy alone.[40]
In FIRE-3 study, cetuximab plus FOLFIRI group had higher OS (38.3 vs 28 months, P = .002) but same PFS (10.7 vs 10.7 months, P = .38) than bevacizumab plus FOLFIRI group in left-sided CRC. In contrast, among FIRE-3 study patients with right-sided CRC, the PFS was 7.6 vs 9 months (P = .11) and OS was 18.3 vs 23 months (P = .27) for cetuximab plus FOLFIRI versus bevacizumab plus FOLFIRI, respectively.[41,42]
In CALGB 80405 study, cetuximab combined chemotherapy had higher OS (39.3 vs 32.6 months, P = .05) than bevacizumab combined chemotherapy in left-sided CRC. In contrast, among CALGB 80405 study patients with right-sided CRC, the PFS was 7.5 versus 10.2 months (P = .007) and OS was 13.7 versus 29.2 months (P = .11) for cetuximab combined chemotherapy versus bevacizumab combined chemotherapy, respectively.[43,44]
These studies confirmed that the prognosis of mCRC with the primary tumor located on the right side was significantly poorer than for that located on the left side, and epidermal growth factor receptor (EGFR) antibody used in patients with right-sided mCRC received only a small benefit. The efficacy of vascular endothelial growth factor monoclonal antibody was superior to that of EGFR monoclonal antibody, and cetuximab was better for left-sided mCRC with RAS wild-type.[42]
Some studies have demonstrated that panitumumab used in KRAS wild-type patients with mCRC is not inferior to cetuximab in terms of OS.[45,46] In ASPECCT study, median OS was 10.2 months in the group of panitumumab, and 9.9 months in the group of cetuximab. Median PFS was 4.2 months in the group of panitumumab and 4.4 months in the group of cetuximab. Before the application of cetuximab or panitumumab, the routine detection of RAS and B-RAF status is recommended.[8] Most wild-type KRAS patients can benefit from treatment with cetuximab and panitumumab.[47] The common use of cetuximab in China may be related to the fact that panitumumab is not widely available.
Regorafenib has been approved by the Food and Drug Administration as a third-line treatment, which can be used after failure of fluorouracil, oxaliplatin, and irinotecan treatment. An Asian clinical study conducted mainly in China (CONCUR) has shown the efficacy of regorafenib.[48] The study included 204 patients from 25 hospitals, to receive oral regorafenib 160 mg daily or placebo on days 1 to 21 of each 28-day cycle. Median OS was 8.8 months in the regorafenib group versus 6.3 months in the placebo group (P = .00016) and median PFS was 3.2 versus 1.7 months (P < .0001), respectively. The effect of regorafenib on prolonging survival time in Asian patients was superior to that in western populations.
Several trials have compared the safety and efficacy of fruquintinib in mCRC patients.[49] In the phase Ib study, the median PFS was 5.8 months, and the median OS was 8.88 months. In the phase II study, the median PFS was 4.73 months in the fruquintinib group versus 0.99 months in the placebo group (P < .001)
A phase II registration trial of famitinib (Xu, 2015, unpublished data) showed that the median PFS was 2.8 and 1.5 months in the famitinib group and control group, respectively. Famitinib improved the PFS and had a good safety and tolerability profile in advanced CRC patients. Unfortunately, OS was not improved in this trial.
With targeted therapy progressing for CRC, such as vemurafenib for mCRC with BRAF V600E mutation,[50] and further studies of fruquintinib for mCRC, we expect new breakthroughs in targeted therapy for CRC.
2.5. Maintenance therapy for CRC
Maintenance therapy is mainly for mCRC, and is necessary for most patients. When the best curative effect for mCRC has been achieved with first-line therapy and the disease is stable, continuous treatment with low intensity and low toxicity drugs is used to prolong the PFS, reduce adverse effects, delay the recurrence time of tumor-related symptoms and improve patients’ quality of life.
The drugs used in maintenance therapy for mCRC in China are described below. Single-agent capecitabine has been studied in a phase III trial to evaluate the efficacy and safety of maintenance therapy with capecitabine versus observation in mCRC patients. The median PFS in the capecitabine and observation groups was 6.43 and 3.43 months, respectively (P < .001), and the median OS was 25.63 and 23.3 months, respectively.[51] 5-FU plus LV, and bevacizumab combined with capecitabine can be used as maintenance therapy.[52] For RAS and BRAF wild-type patients, cetuximab can be adopted for maintenance therapy.
Given China's economic status, many patients in the first-line treatment phase are unable to be treated with targeted drugs for financial reasons. For such patients, single-agent capecitabine can be given as maintenance therapy.[53]
2.6. Immunization therapy
Recently, immunization therapy has made great progress with promising results. The sensitivity of radiotherapy and chemotherapy can be enhanced by immunization therapy. Cell-mediated immunotherapy has been widely used in China, including cytokine-induced killer cells,[54] dendritic cells and cytokine-induced killer cells,[55] dendritic cells, in vitro dendritic cell-activated T cells, activated T cells, and natural killer cells.[56] Studies have shown that cell-mediated immunotherapy in combination with chemotherapy was safe, well tolerated, and improved the OS of CRC patients. Further clinical trials are needed to confirm the results. CRC immunotherapy in China is being explored, and favorable clinical trial results are expected to be published. In 2015, the clinical results of anti-PD-1 antibody immunotherapy for advanced colorectal cancer under the guidance of MMR gene status has been announced by Deng Le in ASCO 2015 conference, and immune-related (objective response rate) ORR and PFS at 20-week administration of PD-1 (pembrolizumab 10 mg/kg, every 2 weeks) has been pointed out; ORR values in dMMR CRC, pMMR CRC, and dMMR groups were respectively 40%, 0%, and 71%; PFS were respectively 78%, 11%, and 67%; the objective response rate could be significantly ameliorated, and progression-free survival was improved, simultaneously indicating that MMR can predict the clinical benefit by adopting checkpoint blockade immunization therapy.[57] However, MMR defect is only about 10% to 15% in stage II to III colorectal cancer, while the miss rate of MMR in metastatic colorectal cancer is <5%.[58] PD-1 pathways include that inhibitory coreceptor programmed death 1 (PD-1) is expressed on T, B and NK immune cells, its ligand PD-L1 (B7-H1) is displayed on cancer cells and antigen-presenting cells as well as PD-L2 (B7-DC) is selectively expressed on activated monocytes and dendritic cells. PD-1 pathway is a definitive immunosuppressive mediator in the local tumor microenvironment. The drug is designed to block PD-1 or PD-L1 to open gate in anti-tumor immunity, starting mechanism of endogenous effect, thereby causing cell death.[59] The new study has shown that in colorectal cancer patients with high expression of PD-L1 and MSI-H, PFS is significantly prolonged when compared with that of previous adjuvant chemotherapy by anti-PD-1 antibody immunotherapy, which is expected to replace the adjuvant chemotherapy in this part of patients.[60] Further clinical trials are needed to confirm the results. Colorectal cancer immunotherapy in China is being explored, and great clinical trial results are expected to be published in the world.
The optimal supportive treatment for CRC includes 3-step pain management, nutritional support, and psychological intervention, the need for which exists throughout the disease course, and is an important factor for improving treatment efficacy and patients’ quality of life.
3. Conclusion
The incidence and mortality from CRC are increasing in China, so it is important to develop effective therapies that are suitable for Chinese population. The MDT principle should be utilized throughout the treatment schedule for each patient. It is recommended that a comprehensive assessment is done by combining disease onset characteristics, disease development trends, and prognosis, and selecting treatment according to the current domestic and international guidelines or evidence-based medicine to formulate the most appropriate holistic treatment strategy for each patient.
This review has described the current status of CRC treatment in China. Approximately 50% of patients are in the middle and late stages at the time of diagnosis, and recurrence and metastasis still occur after operation. Safe and effective surgery, radiotherapy, and chemotherapy, as well as maintenance therapy focused on capecitabine, have shown clinical benefit. With new breakthroughs achieved in precision medical treatment, the prognosis can be further improved by chemotherapy combined with targeted therapy, such as bevacizumab plus chemotherapy in right-sided colon cancer,[61] vemurafenib,[50] fruquintinib.[49] Meanwhile, immunization therapy is expected to make great strides in treatment of CRC, such as anti-programmed death 1(PD-1) antibody and cytotoxic T-lymphocyte antigen 4 (CTLA-4) immunotherapy for advanced colorectal cancer.[57,62]
Footnotes
Abbreviations: 3D-CRT = three-dimensional conformal radiotherapy techniques, 5-FU = 5-fluorouracil, ASCO = American Society of Clinical Oncology, CRC = colorectal cancer, CTLA-4 = cytotoxic T-lymphocyte antigen 4, DFS = disease-free survival, dMMR = MMR deletion, FOLFIRI = 5-FU, LV, plus irinotecan, FOLFOX = oxaliplatin plus 5-FU plus LV, FOLFOXIRI = oxaliplatin, irinotecan, 5-FU, plus LV, IMRT = intensity modulated radiotherapy techniques, LV = leucovorin, mCRC = metastatic CRC, MDT = multidisciplinary team, mFOLFOX6 = modified FOLFOX6, MMR = mismatch repair protein, MSI-H = high-level microsatellite instability, ORR = objective response rate, OS = overall survival, pCR = pathologic complete response, PD-1 = programmed death 1, XELOX = capecitabine plus oxaliplatin.
The authors declare that they have no conflict of interest.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [3].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [4].Yang J, Du XL, Li ST, et al. Characteristics of differently located colorectal cancers support proximal and distal classification: a population-based study of 57,847 patients. PLoS One 2016;11:e0167540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kim SE, Paik HY, Yoon H, et al. Sex- and gender-specific disparities in colorectal cancer risk. World J Gastroenterol 2015;21:5167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Provenzale D, Gupta S, Ahnen DJ, et al. Genetic/Familial high-risk assessment: Colorectal Version 1. 2016, NCCN Clinical Practice Guidelines in oncology. J Natl Compr Canc Netw 2016;14:1010–30. [DOI] [PubMed] [Google Scholar]
- [7].Benson AB, 3rd, Venook AP, Bekaii-Saab T, et al. Rectal cancer, Version 2.2015. J Natl Compr Canc Netw 2015;13:719–28. [DOI] [PubMed] [Google Scholar]
- [8].Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386–422. [DOI] [PubMed] [Google Scholar]
- [9].Guan X, Jiang Z, Ma T, et al. Radiotherapy dose led to a substantial prolongation of survival in patients with locally advanced rectosigmoid junction cancer: a large population based study. Oncotarget 2016;7:28408–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cercek A, Goodman KA, Hajj C, et al. Neoadjuvant chemotherapy first, followed by chemoradiation and then surgery, in the management of locally advanced rectal cancer. J Natl Compr Canc Netw 2014;12:513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kamiya T, Uehara K, Nakayama G, et al. Early results of multicenter phase II trial of perioperative oxaliplatin and capecitabine without radiotherapy for high-risk rectal cancer: CORONA I study. Eur J Surg Oncol 2016;42:829–35. [DOI] [PubMed] [Google Scholar]
- [12].Huang M, Lin J, Yu X, et al. Erectile and urinary function in men with rectal cancer treated by neoadjuvant chemoradiotherapy and neoadjuvant chemotherapy alone: a randomized trial report. Int J Colorectal Dis 2016;31:1349–57. [DOI] [PubMed] [Google Scholar]
- [13].Zhao J, Hu W, Cai G, et al. Dosimetric comparisons of VMAT, IMRT and 3DCRT for locally advanced rectal cancer with simultaneous integrated boost. Oncotarget 2016;7:6345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yang Y, Feng L, Wang Y, et al. A dosimetric analysis of preoperative intensity-modulated and image-guided radiation therapy with and without simultaneous integrated boost for locally advanced rectal cancer. Technol Cancer Res Treat 2015;14:557–63. [DOI] [PubMed] [Google Scholar]
- [15].Deng Y, Chi P, Lan P, et al. Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the Chinese FOWARC multicenter, open-label, randomized three-arm phase III trial. J Clin Oncol 2016;34:3300–7. [DOI] [PubMed] [Google Scholar]
- [16].Zhu J, Liu F, Gu W, et al. Concomitant boost IMRT-based neoadjuvant chemoradiotherapy for clinical stage II/III rectal adenocarcinoma: results of a phase II study. Radiat Oncol 2014;9:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rödel C, Graeven U, Fietkau R, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2015;16:979–89. [DOI] [PubMed] [Google Scholar]
- [18].Liu W, Zhou JG, Sun Y, et al. The roleof neoadjuvant chemotherapy for resectable colorectal liver metastases: a systematic review and meta-analysis. Oncotarget 2016;7:37277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 2014;371:1609–18. [DOI] [PubMed] [Google Scholar]
- [20].Kataoka M, Kanda M, Ishigure K, et al. The COMET Open-label Phase II study of neoadjuvant FOLFOX or XELOX treatment combined with molecular targeting monoclonal antibodies in patients with resectable liver metastasis of colorectal cancer. Ann Surg Oncol 2017;24:546–53. Erratum in: Ann Surg Oncol. 2016;23:1064. [DOI] [PubMed] [Google Scholar]
- [21].Ye LC, Liu TS, Ren L, et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J Clin Oncol 2013;31:1931–8. [DOI] [PubMed] [Google Scholar]
- [22].Zhu DJ, Chen XW, OuYang MZ, et al. Three surgical planes identified in laparoscopic complete mesocolic excision for right-sided colon cancer. World J Surg Oncol 2016;14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang YW, Huang LY, Song CL, et al. Laparoscopic vs open abdominoperineal resection in the multimodality management of low rectal cancers. World J Gastroenterol 2015;21:10174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zeng WG, Liu MJ, Zhou ZX, et al. Outcome of laparoscopic versus open resection for transverse colon cancer. J Gastrointest Surg 2015;19:1869–74. [DOI] [PubMed] [Google Scholar]
- [25].Zhou ZX, Zhao LY, Lin T, et al. Long-term oncologic outcomes of laparoscopic vs open surgery for stages II and III rectal cancer: a retrospective cohort study. World J Gastroenterol 2015;21:5505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Guo C, Zhang Z, Ren B, et al. Comparison of the long-term outcomes of patients who underwent laparoscopic versus open surgery for rectal cancer. J BUON 2015;20:1440–6. [PubMed] [Google Scholar]
- [27].Lee MG, Chiu CC, Wang CC, et al. Trends and outcomes of surgical treatment for colorectal cancer between 2004 and 2012—an analysis using National Inpatient Database. Sci Rep 2017;7:2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Xu J, Qin X. Expert consensus on robotic surgery for colorectal cancer (2015 edition). Chin J Cancer 2016;35:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Xu JM, Wei Y, Wang XY, et al. Robot-assisted one-stage resection of rectal cancer with liver and lung metastases. World J Gastroenterol 2015;21:2848–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li Y, Bi X, Zhao J, et al. Simultaneous hepatic resection benefits patients with synchronous colorectal cancer liver metastases. Chin J Cancer Res 2016;28:528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sheng QS, Pan Z, Chai J, et al. Complete mesocolic excision in right hemicolectomy: comparison between hand-assisted laparoscopic and open approaches. Ann Surg Treat Res 2017;92:90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Huang X, Lv B, Zhang S, et al. Preoperative colonic stents versus emergency surgery for acute left-sided malignant colonic obstruction: a meta-analysis. J Gastrointest Surg 2014;18:584–91. [DOI] [PubMed] [Google Scholar]
- [33].Ku G, Tan IB, Yau T, et al. Management of colon cancer: resource-stratified guidelines from the Asian Oncology Summit 2012. Lancet Oncol 2012;13:e470–81. [DOI] [PubMed] [Google Scholar]
- [34].Deng Y, Wang L, Tan S, et al. KRAS as a predictor of poor prognosis and benefit from postoperative FOLFOX chemotherapy in patients with stage II and III colorectal cancer. Mol Oncol 2015;9:1341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chang W, Wei Y, Ren L, et al. Randomized controlled trial of intraportal chemotherapy combined with adjuvant chemotherapy (mFOLFOX6) for Stage II and III colon cancer. Ann Surg 2016;263:434–9. [DOI] [PubMed] [Google Scholar]
- [36].Cheng AL, Li J, Vaid AK, et al. Adaptation of international guidelines for metastatic colorectal cancer: an Asian consensus. Clin Colorectal Cancer 2014;13:145–55. [DOI] [PubMed] [Google Scholar]
- [37].Van Cutsem E, Cervantes A, Nordlinger B, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25(Suppl):iii1–9. [DOI] [PubMed] [Google Scholar]
- [38].Cao R, Zhang S, Ma D, et al. A multi-center randomized phase II clinical study of bevacizumab plus irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) compared with FOLFIRI alone as second-line treatment for Chinese patients with metastatic colorectal cancer. Med Oncol 2015;32:325. [DOI] [PubMed] [Google Scholar]
- [39].Wang F, Bai L, Liu TS, et al. Right- and left-sided colorectal cancers respond differently to cetuximab. Chin J Cancer 2015;34:384–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment formetastatic colorectal cancer. N Engl JMed 2009;360:1408–17. [DOI] [PubMed] [Google Scholar]
- [41].Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:1065–75. [DOI] [PubMed] [Google Scholar]
- [42].Tejpar S, Stintzing S, Ciardiello F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol 2017;3:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Venook A, Niedzwiecki D, Lenz HJ, et al. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients ( pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J Clin Oncol 2014;32: abstr LBA 3. [Google Scholar]
- [44].Arnold D, Lueza B, Douillard JY, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomised trials. Ann Oncol 2017;28:1713–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Price T, Kim TW, Li J, et al. Final results and outcomes by prior bevacizumab exposure, skin toxicity, and hypomagnesaemia from ASPECCT: randomized phase 3 non-inferiority study of panitumumab versus cetuximab in chemorefractory wild-type KRAS exon 2 metastatic colorectal cancer. Eur J Cancer 2016;68:51–9. [DOI] [PubMed] [Google Scholar]
- [46].Price TJ, Peeters M, Kim TW, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol 2014;15:569–79. [DOI] [PubMed] [Google Scholar]
- [47].Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:4706–13. [DOI] [PubMed] [Google Scholar]
- [48].Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2015;16:619–29. [DOI] [PubMed] [Google Scholar]
- [49].Xu RH, Li J, Bai Y, et al. Safety and efficacy of fruquintinib in patients with previously treated metastatic colorectal cancer: a phase Ib study and a randomized double-blind phase II study. J Hematol Oncol 2017;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hong DS, Morris VK, El Osta B, et al. Phase IB study of vemurafenib in combination with irinotecan and cetuximab in patients with metastaticColorectal cancer with BRAFV600E mutation. Cancer Discov 2016;6:1352–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Luo HY, Li YH, Wang W, et al. Single-agent capecitabine as maintenance therapy after induction of XELOX (or FOLFOX) in first-line treatment of metastatic colorectal cancer: randomized clinical trial of efficacy and safety. Ann Oncol 2016;27:1074–81. [DOI] [PubMed] [Google Scholar]
- [52].Yalcin S, Uslu R, Dane F, et al. Bevacizumab + capecitabine as maintenance therapy after initial bevacizumab + XELOX treatment in previously untreated patients with metastatic colorectal cancer: phase III 'Stop and Go’ study results—a Turkish Oncology Group Trial. Oncology 2013;85:328–35. [DOI] [PubMed] [Google Scholar]
- [53].Wen F, Yao K, Du ZD, et al. Cost-effectiveness analysis of colon cancer treatments from MOSIAC and No. 16968 trials. World J Gastroenterol 2014;20:17976–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zhao H, Wang Y, Yu J, et al. Autologous cytokine-induced killer cells improves overall survival of metastatic colorectal cancer patients: results from a Phase II clinical trial. Clin Colorectal Cancer 2016;15:228–35. [DOI] [PubMed] [Google Scholar]
- [55].Lin T, Song C, Chuo DY, et al. Clinical effects of autologous dendritic cells combined with cytokine-induced killer cells followed by chemotherapy in treating patients with advanced colorectal cancer: a prospective study. Tumour Biol 2016;37:4367–72. [DOI] [PubMed] [Google Scholar]
- [56].Du XH, Liu HL, Li L, et al. Clinical significance of immunotherapy with combined three kinds of cells for operable colorectal cancer. Tumour Biol 2015;36:5679–85. [DOI] [PubMed] [Google Scholar]
- [57].Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Nordholm-Carstensen A, Krarup PM, Morton D, et al. Danish Colorectal Cancer Group. Mismatch repair status and synchronous metastases in colorectal cancer: a nationwide cohort study. Int J Cancer 2015;137:2139–48. [DOI] [PubMed] [Google Scholar]
- [59].Lipson EJ, Forde PM, Hammers HJ, et al. Antagonists of PD-1 and PD-L1 in cancer treatment. Semin Oncol 2015;42:587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Dunne PD, McArt DG, O’Reilly PG, et al. Immune-derived PD-L1 gene expression defines a subgroup of stage II/III colorectal cancer patients with favorable prognosis who may be harmed by adjuvant chemotherapy. Cancer Immunol Res 2016;4:582–91. [DOI] [PubMed] [Google Scholar]
- [61].Ulivi P, Scarpi E, Chiadini E, et al. Right- vs. left-sided metastatic colorectal cancer: differences in tumor biology and bevacizumab efficacy. Int J Mol Sci 2017;18:pii: E1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Syed Khaja AS, Toor SM, El Salhat H, et al. Intratumoral FoxP3 + Helios + Regulatory T cells upregulating immunosuppressive molecules are expanded in human colorectal cancer. Front Immunol 2017;8:619. [DOI] [PMC free article] [PubMed] [Google Scholar]


