Abstract
Background
Little is known about the frequency of ongoing HIV transmission within U.S. African immigrant communities.
Methodology
We used HIV surveillance and partner services data to describe African-born persons newly reported with HIV infection in King County (KC), WA from 1/1/2010–12/31/2013. We performed phylogenetic clustering analysis of HIV-1 pol to identify putative transmission events within this population.
Results
From 2010–2013, 1,148 KC adults were reported with HIV, including 102 (9%) born in Africa. Forty-one African-born cases were interviewed and reported diagnosis after arrival in the U.S. Fourteen (34%) reported ≥1 negative test prior to diagnosis, and 9 (26%) reported ≥1 negative test after U.S. arrival. Pol genotypes were available for 7 of these 9. For 2 of these 7, a KC case was the nearest phylogenetic neighbor; 2 others were infected with subtype B virus.
Discussion
We found substantial evidence of ongoing HIV transmission in the African community of KC.
Keywords: Migrants, HIV, African, subtypes, immigrants
Introduction
There is increasing recognition of the disproportionately large impact of HIV on African-born residents of the U.S.1–4 Previous studies and surveillance data suggest that the HIV epidemic among African-born people in the U.S. reflects the epidemic in Africa: primarily heterosexual transmission, with a much larger proportion of HIV-infected women than has been observed in other populations in the U.S.1–6 While many HIV-infected African people diagnosed in the U.S. were likely exposed to HIV before arriving in this country, data from Western Europe suggest that there is some level of local transmission within African migrant destination countries, including the U.S.7–10 Molecular epidemiology can complement clinical and surveillance data to provide additional insight into the region of HIV exposure.11,12 We undertook the current study both to estimate the degree of local HIV transmission in newly diagnosed African-born people reported with HIV, and to describe HIV testing patterns in this population, in King County (KC), WA.
Methods
In WA, providers, medical facilities and laboratories are required to report HIV diagnoses to the local health jurisdiction. In KC, Public Health–Seattle and KC (PHSKC) collects genotypic sequences from WA laboratories conducting genotypic sequencing for the Molecular HIV Surveillance component of the National HIV Surveillance System. The HIV/AIDS case report includes country of birth, time since arrival in U.S., and self-reported HIV testing history.
PHSKC routinely offers persons newly reported with HIV assistance with partner notification. Foreign-born persons are asked questions about HIV testing history after arrival in the U.S., and sexual behavior related to travel abroad. Non-English speaking patients are interviewed with a telephone interpretation service.
We analyzed surveillance and partner services interview data for adults (18 years or older newly reported with HIV in KC from 2010–2013. We used CDC HIV surveillance definitions for HIV transmission categories. We present descriptive statistics.
HIV gene sequence analysis
Phylogenetic analyses are now a common approach to studying HIV transmission, as viruses with closely related gene sequences can be inferred to represent transmission clusters. We used phylogenetic analysis of HIV gene sequences to identify potential transmission clusters that may represent local transmission among African-born residents. We used a longer timeframe for this analysis than for our analyses with HIV surveillance and partner services data (2010–2013), including HIV pol sequences collected between January 1, 2008 and February 28, 2014. See digital supplemental content for more detailed information about our analysis.
We identified phylogenetic clusters as ≥2 sequences with a shared ancestral node, and assessed the robustness of each cluster with FastTree likelihood-based support values13 and maximum pairwise genetic distances using ClusterPicker.14 The University of Washington Institutional Review Board approved the use of PHSKC records for this study.
Results
From 2010–2013, 1,127 cases were reported as newly diagnosed on or after Nov 1, 2009, including 97 (9%) cases among African-born persons. These 97 patients accounted for 49% of 197 black persons newly reported with HIV during the study period.
Case investigations determined that 36 (37%) of these 97 cases had been previously diagnosed elsewhere, and 33 of these reported previous diagnosis before arrival in the U.S. Of the remaining 61 cases who were presumably newly diagnosed in KC, 48 (79%) were interviewed. Across these three groups (newly reported, presumably newly diagnosed, and interviewed patients), approximately 60% were women, and mean age was 41–43 years (Table I). Among total cases, patients had spent a mean of 4.7 years in the U.S. prior to diagnosis. Most patients were from East African countries (86% of total newly reported cases), primarily from Ethiopia and Kenya (45% and 19% of all cases, respectively). Over 80% of patients had no HIV transmission risk identified or reported; presumably most of these acquired HIV through heterosexual contact. Among interviewed cases, 15 (31.3%) were non-English speakers.
Table I.
Participant characteristics: Total reported, not previously diagnosed, and interviewed for partner services, African-born PLWHA in King County, WA, 2010–2013*
| Patient characteristic | Total N=97 |
Total not previously diagnosed N=61 |
Interviewed N=48 |
|||
|---|---|---|---|---|---|---|
|
| ||||||
| N or mean (median) | % (or range) | N or mean (median) | % (or range) | N or mean (median) | % (or range) | |
| Gender | ||||||
| Men | 35 | 36.1 | 24 | 39.3 | 20 | 41.6 |
| Women | 62 | 63.9 | 37 | 60.7 | 28 | 58.3 |
| Age, years | 41.8 | 22.3 – 62.4 | 42.3 | 23.1–62.2 | 42.2 | 23.1 – 62.2 |
| Years from arrival in the U.S. to HIV case report | 4.6 (0.6) | 8 days-26.7 years | 6.9 (5.1) | 8 days – 26.7 years | 7.3 (5.7) | 8 days – 26.7 years |
| Region of birth^ | ||||||
| East Africa | 83 | 85.6 | 50 | 82.0 | 39 | 81.3 |
| West Africa | 8 | 8.3 | 6 | 9.8 | 5 | 10.4 |
| Middle Africa | 4 | 4.1 | 3 | 4.9 | 3 | 6.3 |
| Southern or North Africa | 2 | 2.0 | 2 | 3.2 | 1 | 2.1 |
| HIV transmission category | ||||||
| Heterosexual sexual contact | 11 | 11.3 | 8 | 13.1 | 6 | 12.5 |
| No risk reported or no risk identified | 84 | 86.6 | 51 | 83.6 | 40 | 83.3 |
| Male to male sexual contact | 3 | 3.1 | 2 | 3.3 | 2 | 4.2 |
| HIV Subtypes** | ||||||
| A1 | 14 | 23.3 | 13 | 28.9 | 10 | 28.6 |
| B | 3 | 5.0 | 3 | 6.7 | 3 | 8.6 |
| C | 34 | 56.7 | 21 | 46.7 | 15 | 42.9 |
| D or G | 4 | 6.7 | 4 | 8.9 | 4 | 11.5 |
| CRF02AG | 5 | 8.3 | 4 | 8.9 | 3 | 8.6 |
Includes cases ≥18 years of age diagnosed on or after Nov 1, 2009
Categorization of region based on United Nations Statistics Division classification of continental regions19
N for subtypes: Total=60, Total not previously diagnosed=45, Interviewed cases=35
Thirty-nine percent of total cases, and 52% of interviewed cases, were diagnosed with AIDS within three months of HIV diagnosis (Table II). Among interviewed cases, 45% had a CD4 count less than 200 at the time of diagnosis.
Table II.
Morbidity and mortality outcomes for African-born PLWHA in King County, WA, 2010–2013
| Outcome | Total N=97 |
Interviewed N=48 |
||
|---|---|---|---|---|
|
| ||||
| N or mean | % or range | N or mean | % | |
| Diagnosed with AIDS within 3 months of HIV diagnosis | 38 | 39.2 | 25 | 52.1 |
| Death during study period | 1 | 1.0 | 0 | 0 |
| First CD4 count < 200* | 31 | 33.3 | 21 | 44.7 |
| Mean CD4 count at diagnosis (median)* | 305 (289) | 7–991 | 279 (234) | 7–991 |
Four patients overall and 1 interviewed were missing CD4 count at diagnosis
Among the 48 interviewed PLWHA, 7 reported HIV diagnosis before arrival in the U.S. and were excluded from further analysis. Of the remaining 41, the most frequently reported reason for testing was because of symptoms or illness (N=16, 43%, Table III). Among this group of interviewed cases, only 14 (34%) reported ever having tested for HIV prior to their diagnosis. Eleven (29%) of 35 PLWHA who were asked if they had ever been HIV tested before arriving in the U.S. reported they had. Of the 34 PLWHA who provided complete date of U.S. arrival, 9 (26%) reported testing HIV negative since their arrival in the U.S.
Table III.
Reason for HIV testing and previous HIV testing history among interviewed, newly diagnosed PLWHA, King County, WA, 2010–2013
| Women N=25 |
Men N=16 |
Total N=41 |
||||
|---|---|---|---|---|---|---|
|
| ||||||
| N | % | N | % | N | % | |
| Reason for HIV testing at time of diagnosis* | ||||||
| Symptoms/illness | 8 | 36.4 | 8 | 53.3 | 16 | 43.2 |
| Routine testing or provider recommended testing | 6 | 27.3 | 2 | 13.3 | 8 | 21.6 |
| Exposed to HIV | 5 | 22.7 | 1 | 6.7 | 6 | 16.2 |
| Prenatal screening | 1 | 4.6 | 0 | N/A | 1 | 2.7 |
| Other | 2 | 9.1 | 4 | 26.7 | 6 | 16.2 |
| Any negative HIV test prior to diagnosis | 10 | 40.0 | 4 | 25.0 | 14 | 34.2 |
| Any negative test before arriving in US^ | 8 | 36.4 | 3 | 23.0 | 11 | 28.6 |
| Previous negative HIV test in U.S.** | 7 | 31.8 | 2 | 16.7 | 9 | 26.4 |
Four patients (3 women and 1 man) were missing reason for testing and are excluded.
Six patients (3 women and 3 men) were missing information on testing before arrival in the U.S. and are excluded
Seven patients (3 women and 4 men) were missing date of U.S. arrival and are excluded
N/A= Not applicable
The 41 interviewed patients not previously diagnosed identified 45 sex partners, 6 (13.3%) of whom had been diagnosed with HIV infection prior to the partner services investigation. Of these six, three had been interviewed and all were African-born. Nine (23.1%) partners not known to be previously HIV-infected were newly HIV tested as a result of the investigations, four (44%) of whom tested HIV positive. Of these four, 3 (75%) were African-born.
Our phylogenetic analysis was based on 112 African-born KC residents with an available HIV-1 pol sequence. Of these, 42 (30%) shared a most recent common ancestor with an individual from KC, while the remaining 70 individuals were related most closely to pol sequences obtained from individuals in Africa. The 42 African-born KC residents linked to other KC residents made up 22 putative transmission pairs/clusters (1 cluster of 4, 4 clusters of 3, 17 pairs).
Assessing the validity of these 22 putative transmission pairs/clusters depends on defining ad-hoc thresholds for genetic distance and/or node support. Only 7 putative pairs/clusters (of the 22) would pass both conservative distance and support thresholds (<3.0% distance and >0.90 node support. The 12 clusters which met either of the ad-hoc thresholds included 23 (21%) of the 112 African-born PLWHA with sequences available. We assessed epidemiologic links between individuals in 9 putative transmission pairs/clusters which passed genetic distance and/or support thresholds and for whom interview data were available; 3 (33%) clusters included an individual who identified at least one other cluster member as a sexual partner. Of 7 individuals with a pol sequence available and a previous negative test in the U.S., 2 were in putative Seattle-based transmission clusters (Figure 1). Two other African-born individuals with a previous negative test in the U.S. were infected with subtype B, offering additional sequence-based evidence for local HIV transmission.
Figure 1.
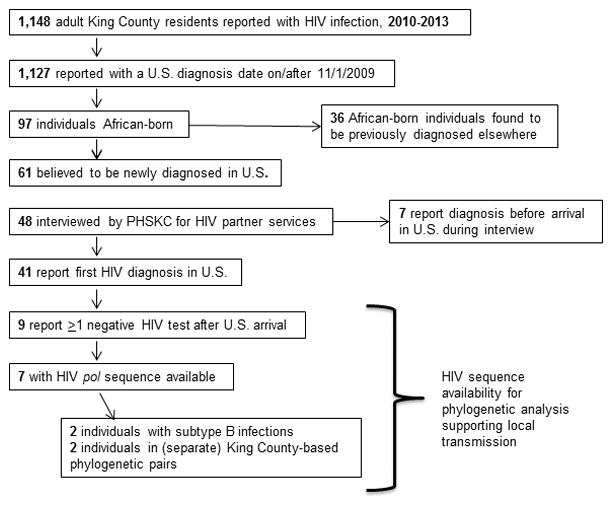
Study flow diagram
Discussion
We found that almost half of all cases of newly reported HIV among blacks in KC, WA 2010–13 occurred in African-born persons, and among those who were not known to be HIV-infected before arrival in the U.S., 26% reported having tested HIV negative in the U.S. This finding was corroborated by phylogenetic analyses, which linked 21% of African-born cases to a non-subtype B case reported in KC. Thirty-nine percent of African-born cases were diagnosed with AIDS within 3 months of their HIV diagnosis, a measure commonly used to indicate late HIV diagnosis, and less than half had ever been HIV tested before diagnosis. These findings demonstrate significant levels of ongoing HIV transmission among local African-born persons, as well as the inadequacy of existing efforts to test persons born in Africa.
Studies from the UK using different approaches have estimated that approximately one-third of UK African-born PLWHA acquired HIV in the UK.7,9 Wiewel et al. recently estimated that among African-born persons interviewed for partner services from 2006–2012 in NYC, 34% likely acquired HIV in the U.S.15 We believe that the remarkable consistency of these results with our findings strengthens the evidence that a significant proportion of HIV-positive members of the African community are likely acquiring HIV after arrival in the U.S.
Our study had several limitations. First, among interviewed patients, we excluded patients who reported diagnosis with HIV before arriving in the U.S. If we were to include these 7 patients in our denominator of interviewed patients, it would slightly decrease the proportion of patients who we believe acquired HIV in the U.S. Secondly, although we think that most people who had previously tested HIV-negative in the U.S. acquired HIV in the U.S., HIV acquisition may have occurred during travel outside the U.S. While we asked many patients about sex in the context of travel, for some this information was missing. Some other persons may have misreported their HIV testing history, but the fact that our findings are consistent with prior work and our phylogenetic analyses suggests that our findings are valid. Additionally, interviewed PLWHA were somewhat more likely to have been diagnosed with AIDS within three months of HIV diagnosis than other PLWHA. This may be because hospitalized individuals are easier to locate. As such, interviewed cases may reflect a group of patients who were more likely to be hospitalized. Finally, our estimate of the proportion of cases likely infected in the U.S. is based on a small number of interviewed patients, and as such, may somewhat over- or underestimate the proportion who acquired HIV in the U.S.
In summary, we found that African-born persons in KC, WA are at high risk for late HIV diagnoses, and that 26% of HIV cases in African-born persons likely occur as a result of transmission within the U.S. These findings highlight the need to consistently collect country of origin of persons with HIV, to identify African-born persons as a priority population for HIV prevention, and to develop culturally appropriate interventions to increase HIV testing among persons from Sub-Saharan Africa.
Supplementary Material
Table IV.
Putative transmission pairs or clusters, based on phylogenetic analysis of HIV-1 pol sequences.
| N linked individuals | Pairwise distance* | Node support* | Putative transmission cluster | HIV-1 Subtype | Region(s) of origin | Previous negative test in U.S. for one partner? | Epidemiologic link in putative transmission clusters?** |
|---|---|---|---|---|---|---|---|
| 2 (one pair) | 0.0011 | 1 | yes | C | Africa, North America | no | no |
| 2 | 0.0030 | 1 | yes | G | Africa | no | yes |
| 2 | 0.0040 | 0.998 | yes | C | Africa | no | no |
| 2 | 0.0050 | 1 | yes | A1 | Africa | yes | no |
| 2 | 0.0060 | 1 | yes | C | Africa, North America | no | yes |
| 3 (cluster) | 0.0081 | 1 | yes | C | Africa | no | yes |
| 2 | 0.0200 | 1 | yes | CRF02AG | Africa, North America | no | no |
| 2 | 0.0259 | 0.893 | yes | C | Africa | no | ^ |
| 2 | 0.0296 | 0.880 | yes | CRF02AG | Africa | yes | no |
| 2 | 0.0334 | 0.720 | no | A1 | Africa | no | |
| 3 | 0.0359 | 0.516 | no | A1 | Africa, Asia, North America | no | |
| 2 | 0.0369 | 0.881 | no | C | Africa | no | |
| 2 | 0.0381 | 0.827 | no | C | Africa | no | |
| 2 | 0.0391 | 0.718 | no | CRF02AG | Africa | no | |
| 2 | 0.0422 | 0.997 | yes | H | Africa | no | ^ |
| 3 | 0.0423 | 0.012 | no | C | Africa | no | |
| 2 | 0.0472 | 0.483 | no | CRF02AG | Africa | no | |
| 3 | 0.0487 | 0.998 | yes | C | Africa, North America | no | ^ |
| 2 | 0.0530 | 0.375 | no | G | Africa, Europe | no | |
| 4 | 0.0530 | 0.972 | yes | CRF02AG | Africa, North America | no | no |
| 2 | 0.0539 | 0.208 | no | A1 | Africa | no | |
| 2 | 0.0551 | 0.118 | no | A1 | Africa | no |
Values which meet genetic distance or node support thresholds are shown in bold and are italicized.
Epidemiologic link defined as two individuals who acknowledged sexual contact with other individuals in the transmission pair/cluster
Partner services data were not available for at least one patient; epidemiologic links cannot be determined for these clusters
Acknowledgments
This work was supported by NIAD K01 AI095060 (to R.P.K.), NIAID R01 AI108490 (to J.T.H.), NIAID K23 AI11523791 (to L.A.B.), and by a developmental grant from the University of Washington Center for AIDS Research (CFAR), an NIH funded program under award number P30AI027757 that is supported by the following NIH Institutes and Centers (NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, NIDDK).
Footnotes
Compliance with ethical standards: The authors declare that they have no conflict of interest.
Research involving human participants and/or animals: This article does not contain any studies with animals performed by any of the authors.
Informed consent: The University of Washington Institutional Review Board approved the use of Public Health – Seattle and King County records for this study, including a waiver of consent.
References
- 1.Ashton C, Bernhardt SA, Lowe M, Mietchen M, Johnston J. Comparison of HIV/AIDS rates between U.S.-born Blacks and African-born Blacks in Utah, 2000 - 2009. The open AIDS journal. 2012;6:156–162. doi: 10.2174/1874613601206010156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerani RP, Kent JB, Sides T, et al. HIV among African-born persons in the United States: a hidden epidemic? J Acquir Immune Defic Syndr. 2008;49(1):102–106. doi: 10.1097/QAI.0b013e3181831806. [DOI] [PubMed] [Google Scholar]
- 3.Sides TL. An epidemiologic update on the HIV/AIDS epidemic in Minnesota. Minn Med. 2003;86(6):33–37. [PubMed] [Google Scholar]
- 4.Blanas DA, Nichols K, Bekele M, Lugg A, Kerani RP, Horowitz CR. HIV/AIDS among African-born residents in the United States. Journal of immigrant and minority health/Center for Minority Public Health. 2013;15(4):718–724. doi: 10.1007/s10903-012-9691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akinsete OO, Sides T, Hirigoyen D, et al. Demographic, clinical, and virologic characteristics of African-born persons with HIV/AIDS in a Minnesota hospital. AIDS Patient Care STDS. 2007;21(5):356–365. doi: 10.1089/apc.2006.0074. [DOI] [PubMed] [Google Scholar]
- 6.Johnson AS, Hu X, Dean HD. Epidemiologic differences between native-born and foreign-born black people diagnosed with HIV infection in 33 U.S. states, 2001–2007. Public Health Rep. 2010;125(Suppl 4):61–69. doi: 10.1177/00333549101250S410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns FM, Arthur G, Johnson AM, Nazroo J, Fenton KA. United Kingdom acquisition of HIV infection in African residents in London: more than previously thought. AIDS. 2009;23(2):262–266. doi: 10.1097/QAD.0b013e32831c546b. [DOI] [PubMed] [Google Scholar]
- 8.Desgrees du Lou A, Pannetier J, Ravalihasy A, et al. HIV acquisition after arrival in France among sub-Saharan African migrants living with HIV in Paris area. Estimations from the ANRS PARCOURS study; Paper presented at: International AIDS Society; July, 2015; Vancouver, BC. 2015. [Google Scholar]
- 9.Rice BD, Elford J, Yin Z, Delpech VC. A new method to assign country of HIV infection among heterosexuals born abroad and diagnosed with HIV. AIDS. 2012;26(15):1961–1966. doi: 10.1097/QAD.0b013e3283578b80. [DOI] [PubMed] [Google Scholar]
- 10.Sabin CA, Smith CJ, Gumley H, et al. Late presenters in the era of highly active antiretroviral therapy: uptake of and responses to antiretroviral therapy. AIDS. 2004;18(16):2145–2151. doi: 10.1097/00002030-200411050-00006. [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal I, Smith M, Tatt ID, et al. Evidence for onward transmission of HIV-1 non-B subtype strains in the United Kingdom. J Acquir Immune Defic Syndr. 2006;41(2):201–209. doi: 10.1097/01.qai.0000179430.34660.11. [DOI] [PubMed] [Google Scholar]
- 12.Lemoh C, Ryan CE, Sekawi Z, et al. Acquisition of HIV by African-born residents of Victoria, Australia: insights from molecular epidemiology. PLoS One. 2013;8(12):e84008. doi: 10.1371/journal.pone.0084008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price MN, Dehal PS, Arkin AP. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5(3):e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ragonnet-Cronin M, Hodcroft E, Hue S, et al. Automated analysis of phylogenetic clusters. BMC bioinformatics. 2013;14:317. doi: 10.1186/1471-2105-14-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiewel EW, Torian LV, Hanna DB, Bocour A, Shepard CW. Foreign-Born Persons Diagnosed with HIV: Where are They From and Where Were They Infected? AIDS and behavior. 2014 doi: 10.1007/s10461-014-0954-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


