Abstract
Although mutations in the proinsulin gene (INS) are the second most common cause of neonatal diabetes mellitus, the natural history of β-cell death and the most appropriate treatments remains unknown. We describe the management and outcome of two sisters with INS-mediated diabetes (S1 and S2) and suggest that more intensive insulin treatment of S2 may have resulted in better clinical outcomes. S1 was diagnosed with diabetes after presenting with serum glucose of 404 mg/dL (22.4 mmol/L) and started multiple daily insulin injections at age 4 months, followed by continuous subcutaneous insulin infusion (CSII) at age 42 months. S1 had positive genetic testing at age 4 months for the GlyB8Ser or Gly32Ser mutation in proinsulin. S2 had positive research-based genetic testing, age 1 month, before she had consistently elevated blood glucose levels. Continuous glucose monitoring revealed abnormal excursions to 200 mg/dL. Low-dose insulin therapy was initiated at age 2.5 months via CSII. At age-matched time points, S2 had higher C-peptide levels, lower hemoglobin A1c values, and lower estimated doses of insulin as compared with S1. Earlier, more intensive insulin treatment was associated with higher C-peptide levels, decreased insulin dosing, and improved glycemic control. Initiating exogenous insulin before overt hyperglycemia and maintaining intensive insulin management may reduce the demand for endogenous insulin production and may preserve β-cell function. Studies accumulating data on greater numbers of participants will be essential to determine whether these associations are consistent for all INS gene mutations.
Keywords: diabetes mellitus, genetic testing, insulin infusion systems, permanent neonatal diabetes mellitus, predictive genetic testing
We present the management and outcome of two sisters with INS gene–mediated neonatal diabetes to suggest that earlier initiation of intensive insulin therapy may improve long-term glycemic control.
Neonatal diabetes mellitus (NDM) is a group of heterogeneous conditions characterized by permanent or transient hyperglycemia diagnosed within the first 6 months of life [1]. Single-gene mutations are most often the cause of NDM, as in the case of KCNJ11-, ABCC8-, and INS-related diabetes. Mutations in the INS gene are the second leading cause of NDM [2] and are clinically similar to type 1 diabetes. Those affected present with persistent hyperglycemia, need exogenous insulin therapy, and are typically pancreatic islet autoantibody negative. Maintaining adequate glycemic control in people with diabetes is critical to lessening risk for diabetes-related complications [3], but uncertainty remains about the most appropriate approach to insulin replacement therapy in those with mutations in the INS gene. Here we describe two siblings in a family with four generations of INS-mediated neonatal diabetes, present the differing treatment approaches, and compare the resulting courses of care.
1. Methods
We present a family with four linear generations of INS-mediated neonatal diabetes. Using standard clinical assays [C-peptide, hemoglobin A1c (HbA1c), total daily insulin dose, continuous glucose monitoring], we explored the management and clinical outcomes of two sisters in the youngest generation, S1 and S2. This family consented to participate in the University of Chicago Monogenic Diabetes Registry. The University of Chicago Institutional Review Board has approved this study.
A. Total Daily Dose Calculations
Continuous subcutaneous insulin infusion (CSII) downloads were available for S1 from age 44 months onward and for S2 from 2.5 months onward. When available, total daily units from CSII downloads were used in age-matched comparisons (Table 1). Before CSII initiation, S1 was on once-daily long-acting insulin (detemir) from age 4 months to 13 months and long-acting plus rapid-acting insulin from 13 months to 44 months. While she was on multiple daily injections, estimations were made for total daily dose (TDD) calculations if the exact dose was not noted in S1’s medical record. For example, detemir 4 U in the morning and 3 U in the evenings, plus lispro with meals (without specific dosing) would be estimated as (total basal units × 2), so an estimated 14 total units daily. Multiple regimens were noted in each age range (Table 1), and therefore TDD was averaged across all regimens noted in each age range (e.g., used 1.01 U/kg/d at 30.8 months of age and 0.84 U/kg/d at 34.2 months of age; average = 0.93 U/kg/d).
Table 1.
Age-Matched Comparisons of Average HbA1c (%), Insulin TDD (U/kg/d), and Proinsulin for S1 and S2 From Birth to 5 years
| Age, mo |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 to 6 | 6 to 12 | 12 to 18 | 18 to 24 | 24 to 30 | 30 to 36 | 36 to 42 | 42 to 48 | 48 to 54 | 54 to 60 | |
| S1 | ||||||||||
| HbA1c, % (mmol/mol) | 9.8a (84) | 7.8 (62) | 8.0 (64) | 8.6 (70) | 8.9 (74) | 8.5 (69) | 9.2 (77) | 8.7 (72) | 8.2 (72) | 9.9 (85) |
| Insulin total daily dose, U/kg/d | 0.29b | 0.37b,c | 0.57c | 0.61c | 0.85c | 0.93c | 0.79c | 0.69 | 0.69 | 0.78 |
| C-peptide, pmol/mL (reference 0.3 to 2.35) | 0.5 | 0.86 | 0.26 | 0.07 | 0.11 | 0.13 | ||||
| Proinsulin, pmol/L (reference 3 to 20) | 5.1 | 1.3 | 1.0 | |||||||
| S2 | ||||||||||
| HbA1c, % (mmol/mol) | 4.5a (26) | 5.7 (39) | 5.6 (38) | 6.1 (43) | 5.6 (38) | 6.3 (45) | 6.6 (49) | 6.8 (51) | 6.7 (50) | 7.6 (60) |
| Insulin total daily dose, U/kg/d | 0.06 | 0.04 | 0.06 | 0.11 | 0.11 | 0.10 | 0.16 | 0.19 | 0.2 | 0.2 |
| C-peptide, pmol/mL (reference 0.3 to 2.35) | 0.71 | 0.34 | 0.74 | 0.65 | ||||||
| Proinsulin, pmol/L (reference 3 to 20) | 7.7 | 5.7 | 8.4 | 5.8 | ||||||
HbA1c values <6 months are underestimated due to fetal hemoglobin. All HbA1c values reported to one decimal place.
S1 was on only long-acting insulin from diagnosis (4 months) to ~10 months of age.
Estimated TDD (see Methods for details).
2. Results and Case Report
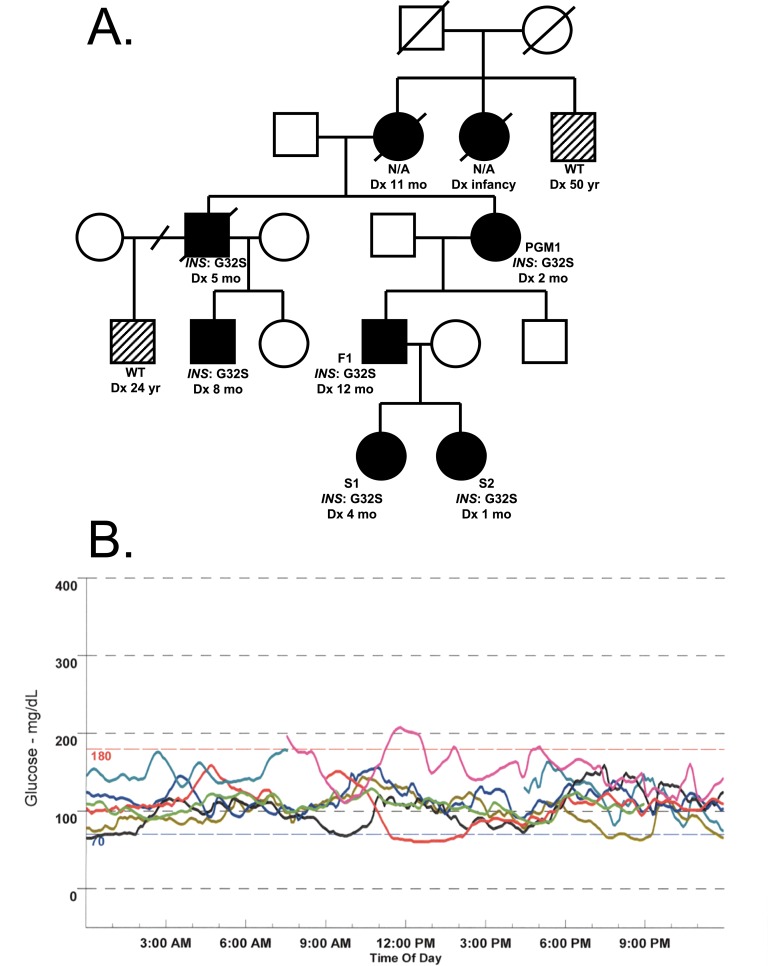
Multiple generations were diagnosed with diabetes early in life (Fig. 1A). Linkage analysis followed by Sanger sequencing revealed p.Gly32Ser heterozygous mutations in the INS gene in several family members, and the family has been previously described [4].
Figure 1.
(A) Pedigree. Squares represent males, circles represent females. Black circles and squares indicate neonatal diabetes with diagnosis age and genotype noted underneath. Half black half white circles and squares indicate diabetes that is not consistent with neonatal diabetes. Diagonal slashes through squares or circles indicate deceased subjects. Diagonal slash through the branch indicates divorced partners. Dx, diagnosis; WT, wildtype. (B) Initial continuous glucose monitor data for S2 from Medtronic iPro 2.0A.
The paternal grandmother of S1 and S2 (PGM1) was diagnosed with diabetes at 2 months of age and treated with insulin. The current daily dose of insulin for PGM1 is 0.85 U/kg/d. The father of S1 and S2 (F1) was diagnosed with diabetes at 1 year of age and treated with insulin. The current daily dose of insulin for F1 is 1.09 U/kg/d.
S1 was born full term [37 weeks’ gestational age, 2764 g, 44th percentile, appropriate for gestational age (AGA), 50.8 cm] after an uncomplicated pregnancy and delivery. Their mother did experience hyperglycemia during a pregnancy with a different partner, but hyperglycemia was not noted for the pregnancy of S1 or her sister S2. Blood glucose values on the day of birth were normal.
Intermittent mild hyperglycemia was detected by caregivers on a home glucometer from age 1 month to age 4 months. Long-acting insulin was initiated at 4 months, 11 days of age after S1 presented with serum glucose of 404 mg/dL (22.4 mmol/L). Genetic testing for S1 was performed at 4 months of age via Sanger sequencing and confirmed the same heterozygous mutation found in various other family members. Diluted fast-acting insulin was added at 13 months of age, and U100 fast-acting insulin was in use by 21 months of age. Glycemic control was consistently suboptimal, and she transitioned to continuous subcutaneous insulin infusion therapy at age 44 months. S1’s growth was stable, with her height from age 2 to 5 years trending between the 30th and 50th percentile, weight between the 40th and 60th percentile, and body mass index between the 50th and 80th percentile for age [5].
Four years after the birth of S1, S2 was born full term [37 weeks’ gestational age, 3120 g, 73rd percentile, AGA, 50.8 cm] after an uncomplicated pregnancy and delivery. Blood glucose values on the day of birth were normal. Genetic testing was performed at 1 month of age via Sanger sequencing, which confirmed the same heterozygous mutation present in S1 and other family members. Although she was asymptomatic, a continuous glucose monitor was placed for 7 days, which revealed abnormal glucose excursions to ≥140 mg/dL (7.8 mmol/L), including one >200 mg/dL (11.1 mmol/L) (Fig. 1B). Low-dose exogenous insulin therapy was initiated at 2.5 months of age in the hopes of preserving β-cell function (CSII, basal rate of 0.025 U/h from 08:30 to 21:00, with no basal insulin overnight). Like S1’s, S2’s growth was stable, with her height from age 2 to 5 years trending between the 30th and 50th percentile, weight between the 20th and 50th percentile, and body mass index between the 25th and 60th percentile for age [5].
Age-matched average daily doses of insulin (U/kg/d), average HbA1c values, and proinsulin levels are displayed in Table 1. Age-matched C-peptide values and serial HbA1c values are displayed in Fig. 2. S2 has consistently held lower HbA1c values, needed lower TDD, and had higher C-peptide and proinsulin production at all age-matched time points compared with S1. Glycemic variability is shown via blood glucose meter downloads from S1 and S2 in Fig. 3.
Figure 2.
C-peptide (bars) and HbA1c (lines) values for first 5 years of life for sisters S1 (blue) and S2 (red). *HbA1c values <6 months are underestimated due to fetal hemoglobin.
Figure 3.
Age-matched comparison of blood glucose (BG) meter downloads between sisters S1 (top) and S2 (bottom) at 4 years 11 months of age (CareLink software, Medtronic).
3. Discussion
Presentation of patients with INS mutations is variable, including variable age at onset, variable phenotypes consistent with permanent neonatal diabetes mellitus, as in this case, or transient neonatal diabetes mellitus (TNDM) or maturity onset diabetes of the young. Patients may present with heterozygous, homozygous, or compound heterozygous genotypes. Figure 1A shows varying ages of diagnosis during infancy for the same INS gene mutation in this family (all were diagnosed between 1 and 12 months of age). Ranges of diagnosis age in INS gene mutations have been discussed previously [2, 4], suggesting that other factors may modify diagnosis age. More functional data are needed to explain this variability.
INS mutations lead to abnormal sequences in preproinsulin, which may impair prohormone convertase cleavage sites, disulfide bond formation, premature termination, or abnormal folding. These misfolded proteins are retained in the endoplasmic reticulum (ER) of the β-cell, inhibiting normal function and probably leading to β-cell death via the unfolded protein response through ER stress [6, 7]. Providing exogenous insulin therapy early probably reduces the demand for endogenous insulin production, thereby decreasing the amount of misfolded proinsulin that is produced and retained in the ER. This effect may allow a decrease in β-cell death and preservation of some amount of endogenous insulin production and overall β-cell function [8]. Preserved β-cell function, such as during the “honeymoon period,” may provide some clinical benefits. Another example includes patients with renewed β-cell function (measured as C-peptide) after islet cell transplantation, who experience improved glucose counterregulation, less frequent hypoglycemia unawareness, improved insulin sensitivity, and improved glycosylated hemoglobin values [9]. The data detailed in this report suggest that earlier insulin treatment of S2 may have contributed to increased C-peptide levels, decreased insulin dosing, and improved glycemic control as compared with S1.
The frequent eating patterns of infants, need for small insulin doses, and decreased injection burden on caregivers indicate CSII as the preferred method of insulin delivery. Although it has been shown to reduce hypoglycemia and overall insulin requirement in pediatric patients with diabetes [10], initiating CSII therapy at a very young age can be challenging for many reasons [11]. Potential barriers include difficulty in keeping CSII pods or infusion sets on an active infant and obtaining insurance approval for needed supplies. However, this case shows that it is possible to initiate pump therapy quickly and effectively with infants as young as 2 months of age.
Infants are unable to verbally communicate with care providers, which can delay a diagnosis of new-onset diabetes. This delay increases the risk of diabetic ketoacidosis (DKA), which has been associated with morbidity in infants [12]. Previous research has shown that early detection of diabetes in children allowed a milder onset with lower risk of diagnosis hospitalization and DKA [13]. For families with known history of neonatal diabetes, performing predictive genetic testing at birth helps predict the clinical course and probably lessens the risk for development of DKA.
Performing genetic testing allows targeted therapy in NDM genes such as sulfonylurea-responsive KCNJ11 and ABCC8 [14]. Although people with INS gene mutations are likely to remain insulin dependent, the current study suggests that early administration of insulin may lead to preserved β-cell function, decreased insulin dose, and improved glycemic control, potentially lessening the risk for diabetes-related complications later in life.
There are limitations to this study. Although S1 and S2 are sisters and were raised in the same environment, we acknowledge that factors other than earlier initiation and better adherence to her insulin regimen may have contributed to improved glycemic control in S2. It is possible that the family was more proficient at diabetes care by the time S2 was diagnosed, because difficulties with optimal diabetes management could lead to more pronounced effects on the β-cells, such as glucotoxicity. It is also possible that the insulin needs of each sister simply may have been different, because we know that diabetes can be a heterogeneous condition, even among related individuals. Other factors could include any number of genetic differences that may have influenced the rate of β-cell death. Furthermore, because of her multiple daily injection regimen, total daily doses were estimated for S1 from age 13 months to 44 months, potentially overestimating insulin usage during this time period. However, both sisters were on CSII from ~3.5 to 5 years of age, so these time points may be more directly comparable. Social barriers made it difficult for this family to adhere to this complicated medication regimen. We acknowledge that these barriers may have contributed to variability in glycemic control and insulin usage.
This unique family offers an opportunity to further investigate the most appropriate course of care for people with diabetes caused by INS gene mutations. This report describes how predictive genetic testing and early insulin administration can lead to personalized therapy and improved care quality for people with INS-NDM.
Acknowledgments
We thank the family for their participation in our research studies (http://monogenicdiabetes.uchicago.edu).
Financial Support: This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (http://dx.doi.org/10.13039/100000062) of the National Institutes of Health (http://dx.doi.org/10.13039/100000002) through grants supporting the University of Chicago Diabetes Research and Training Center (P30DK020595), S.A.W.G.’s K23 Award (K23DK094866), R01 DK104942 to L.H.P., and CTSA UL1 TR000430, as well as by grants from the American Diabetes Association (http://dx.doi.org/10.13039/100000041; 1-11-CT-41 to L.H.P. and 1-17-JDF-008 to S.A.W.G.) and gifts from the Kovler Family Foundation.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AGA
- appropriate for gestational age
- CSII
- continuous subcutaneous insulin infusion
- DKA
- diabetic ketoacidosis
- ER
- endoplasmic reticulum
- F1
- father of sister 1 and sister 2
- HbA1c
- hemoglobin A1c
- NDM
- neonatal diabetes mellitus
- PGM1
- paternal grandmother of sister 1 and sister 2
- S1
- sister 1
- S2
- sister 2
- TDD
- total daily dose.
References and Notes
- 1.Greeley SAW, Naylor RN, Philipson LH, Bell GI. Neonatal diabetes: an expanding list of genes allows for improved diagnosis and treatment. Curr Diab Rep. 2011;11(6):519–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edghill EL, Flanagan SE, Patch AM, Boustred C, Parrish A, Shields B, Shepherd MH, Hussain K, Kapoor RR, Malecki M, MacDonald MJ, Støy J, Steiner DF, Philipson LH, Bell GI, Hattersley AT, Ellard S; Neonatal Diabetes International Collaborative Group . Insulin mutation screening in 1,044 patients with diabetes: mutations in the INS gene are a common cause of neonatal diabetes but a rare cause of diabetes diagnosed in childhood or adulthood. Diabetes. 2008;57(4):1034–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. [DOI] [PubMed] [Google Scholar]
- 4.Støy J, Edghill EL, Flanagan SE, Ye H, Paz VP, Pluzhnikov A, Below JE, Hayes MG, Cox NJ, Lipkind GM, Lipton RB, Greeley SA, Patch AM, Ellard S, Steiner DF, Hattersley AT, Philipson LH, Bell GI; Neonatal Diabetes International Collaborative Group . Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci USA. 2007;104(38):15040–15044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention NC for HS. CDC growth charts: United States. 2000 Available at: http://www.cdc.gov/growthcharts/. Accessed 8 November 2017.
- 6.Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, Takata K, Koizumi A, Izumi T. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J Clin Invest. 1999;103(1):27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park SY, Ye H, Steiner DF, Bell GI. Mutant proinsulin proteins associated with neonatal diabetes are retained in the endoplasmic reticulum and not efficiently secreted. Biochem Biophys Res Commun. 2010;391(3):1449–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Absood A, Gandomani B, Zaki A, Nasta V, Michail A, Habib PMW, Hodish I. Insulin therapy for pre-hyperglycemic beta-cell endoplasmic reticulum crowding. PLoS One. 2013;8(2):e54351 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3567120&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rickels MR, Kong SM, Fuller C, Dalton-Bakes C, Ferguson JF, Reilly MP, Teff KL, Naji A. Improvement in insulin sensitivity after human islet transplantation for type 1 diabetes. J Clin Endocrinol Metab. 2013;98(11):E1780–E1785 http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=24085506&retmode=ref&cmd=prlinks%5Cnpapers3://publication/doi/10.1210/jc.2013-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jakisch BI, Wagner VM, Heidtmann B, Lepler R, Holterhus PM, Kapellen TM, Vogel C, Rosenbauer J, Holl RW; German/Austrian DPV Initiative and Working Group for Paediatric Pump Therapy . Comparison of continuous subcutaneous insulin infusion (CSII) and multiple daily injections (MDI) in paediatric Type 1 diabetes: a multicentre matched-pair cohort analysis over 3 years. Diabet Med. 2008;25(1):80–85. [DOI] [PubMed] [Google Scholar]
- 11.Phillip M, Battelino T, Rodriguez H, Danne T, Kaufman F; European Society for Paediatric Endocrinology, Lawson Wilkins Pediatric Endocrine Society, International Society for Pediatric and Adolescent Diabetes, American Diabetes Association, European Association for the Study of Diabetes . Use of insulin pump therapy in the pediatric age-group: consensus statement from the European Society for Paediatric Endocrinology, the Lawson Wilkins Pediatric Endocrine Society, and the International Society for Pediatric and Adolescent Diabetes, endorsed by the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2007;30(6):1653–1662 http://www.ncbi.nlm.nih.gov/pubmed/17372151. [DOI] [PubMed] [Google Scholar]
- 12.Letourneau LR, Carmody D, Wroblewski K, Denson AM, Sanyoura M, Naylor RN, Philipson LH, Greeley SAW. Diabetes presentation in infancy: high risk of diabetic ketoacidosis. Diabetes Care. 2017;40(10):e147–e148 http://www.ncbi.nlm.nih.gov/pubmed/28779000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barker JM, Goehrig SH, Barriga K, Hoffman M, Slover R, Eisenbarth GS, Norris JM, Klingensmith GJ, Rewers M; DAISY study . Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes Care. 2004;27(6):1399–1404. [DOI] [PubMed] [Google Scholar]
- 14.Pearson ER, Flechtner I, Njølstad PR, Malecki MT, Flanagan SE, Larkin B, Ashcroft FM, Klimes I, Codner E, Iotova V, Slingerland AS, Shield J, Robert JJ, Holst JJ, Clark PM, Ellard S, Søvik O, Polak M, Hattersley AT; Neonatal Diabetes International Collaborative Group . Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;355(5):467–477 http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=16885550&retmode=ref&cmd=prlinks%5Cnpapers2://publication/doi/10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]