Abstract
Background
Metabolic syndrome (MetS) is a highly prevalent condition that identifies individuals at risk for type 2 diabetes mellitus and atherosclerotic cardiovascular disease. Prevention of these diseases relies on early detection and intervention in order to preserve pancreatic β-cells and arterial wall integrity. Yet, the clinical criteria for MetS are insensitive to the early-stage insulin resistance, inflammation, cholesterol and clotting factor abnormalities that characterize the progression toward type 2 diabetes and atherosclerosis. Here we report the discovery and initial characterization of an atypical new biomarker that detects these early conditions with just one measurement.
Methods
Water T2, measured in a few minutes using benchtop nuclear magnetic resonance relaxometry, is exquisitely sensitive to metabolic shifts in the blood proteome. In an observational cross-sectional study of 72 non-diabetic human subjects, the association of plasma and serum water T2 values with over 130 blood biomarkers was analyzed using bivariate, multivariate and logistic regression.
Results
Plasma and serum water T2 exhibited strong bivariate correlations with markers of insulin, lipids, inflammation, coagulation and electrolyte balance. After correcting for confounders, low water T2 values were independently and additively associated with fasting hyperinsulinemia, dyslipidemia and subclinical inflammation. Plasma water T2 exhibited 100% sensitivity and 87% specificity for detecting early insulin resistance in normoglycemic subjects, as defined by the McAuley Index. Sixteen normoglycemic subjects with early metabolic abnormalities (22% of the study population) were identified by low water T2 values. Thirteen of the 16 did not meet the harmonized clinical criteria for metabolic syndrome and would have been missed by conventional screening for diabetes risk. Low water T2 values were associated with increases in the mean concentrations of 6 of the 16 most abundant acute phase proteins and lipoproteins in plasma.
Conclusions
Water T2 detects a constellation of early abnormalities associated with metabolic syndrome, providing a global view of an individual’s metabolic health. It circumvents the pitfalls associated with fasting glucose and hemoglobin A1c and the limitations of the current clinical criteria for metabolic syndrome. Water T2 shows promise as an early, global and practical screening tool for the identification of individuals at risk for diabetes and atherosclerosis.
Electronic supplementary material
The online version of this article (10.1186/s12967-017-1359-5) contains supplementary material, which is available to authorized users.
Keywords: Metabolic syndrome; Insulin resistance; Inflammation, dyslipidemia, magnetic resonance relaxometry; T2, transverse relaxation time, type 2 diabetes mellitus; Atherosclerosis, cardiovascular disease
Background
Metabolic syndrome (MetS) is one of the most prevalent public health problems of the twenty-first century [1–3]. In the US, approximately one-third of adults and half of those ≥ 60 years of age have MetS [2, 3]. Previously called insulin resistance syndrome or syndrome X, MetS can be defined in two ways [4–6]: (i) generally, as a constellation of abnormalities that includes insulin resistance, glucose intolerance, abdominal obesity, elevated blood pressure, dyslipidemia, and/or a pro-inflammatory, pro-thrombotic state, and (ii) specifically, as a set of clinical criteria and cutoffs. The definitions and criteria for MetS have been the subject of considerable debate [4–9]. The Cardiometabolic Think Tank was convened in 2014 in an attempt to develop a consensus on affirmed and emerging concepts as well as recommendations regarding MetS [5]. The concepts are sound, but questions remain about how best to capture the heterogeneity of MetS, particularly the subtypes, stages and unmeasured or residual risk factors [5].
The primary importance of MetS is in identifying individuals at increased risk for type 2 diabetes and atherosclerotic cardiovascular disease [5, 6, 10–14]. The prevention of these diseases hinges on early detection and intervention in order to preserve pancreatic β-cell function and the integrity of the arterial wall [15, 16]. Yet, the clinical criteria and cutoffs for MetS appear to be insensitive to the early-stage metabolic abnormalities that put individuals at risk.
In the progression toward type 2 diabetes, the hallmark early abnormality is insulin resistance [17]. Because of the compensatory hypersecretion of insulin by intact β-cells, fasting glucose levels remain in the normal range during early-stage insulin resistance [17–22]. By the time an individual develops impaired fasting glucose (≥ 100 mg/dL)—one criterion for MetS—a significant decline in β-cell function has already occurred. This decline is characterized by a loss in first-phase insulin secretion [23]. In the VA Genetic Epidemiology Study, individuals with impaired fasting glucose and impaired glucose tolerance averaged a 70% decline in pancreatic insulin secretion compared with individuals with normal glucose tolerance, after correcting for variable degrees of insulin sensitivity [24]. Since the primary goal of type 2 diabetes prevention is preserving β-cell function [25], the glucose criterion of MetS is inadequate for the early detection of diabetes risk.
Elevated fasting triglyceride level, another MetS criterion, provides an alternative marker of early insulin resistance. However, the cutoff value may not be properly calibrated. In a study of 178 normoglycemic adults from New Zealand, 42% of whom had insulin resistance, the optimal triglyceride cutoff value was 1.5 mM (133 mg/dL), rather than 1.7 mM (150 mg/dL) as specified in the MetS criteria. The 1.5 mM cutoff was rigorously calibrated against the euglycemic clamp, a direct measure of insulin sensitivity [26]. Waist circumference is another MetS criterion related to insulin resistance and obesity. However, as acknowledged by the Cardiometabolic Health Alliance, waist circumference is an imperfect gauge of the ectopic lipid deposition and visceral adiposity associated with type 2 diabetes risk [5].
With respect to atherosclerosis, early plaque formation is driven by inflammation and cholesterol deposition in the arterial wall [16, 27]. Yet, the MetS clinical criteria do not include markers of inflammation and elevated cholesterol [4]. The prothrombotic state, characterized by increased fibrinogen, platelet and PAI-1 levels, also contribute to plaque progression and the triggering of cardiovascular events [28–30]. The MetS criteria do not include these measures either. One possible solution is to expand the harmonized definition of MetS into a larger, more comprehensive biomarker panel. However, with added measurements comes complexity and cost, which can render the use of biomarker panels impractical for population screening and front-line clinical monitoring. There is an unmet need for simpler, more effective approaches.
Here we present an atypical new biomarker for MetS that does not rely on direct measures of glucose, triglycerides, waist circumference, cholesterol, inflammatory markers or biomarker panels. Rather, it is based on the motional properties of water—by far, the most abundant molecule in the blood. Changes in the rotational and translational diffusion of water in plasma or serum can be monitored by T2, the transverse relaxation time constant. It can be measured using a simple benchtop implementation of nuclear magnetic resonance relaxometry [31].
This approach exploits the unique properties of water as a metabolic surveillance system, as water molecules form hydrogen bonds with virtually every protein and lipoprotein in plasma or serum. Each protein affects water T2 in a specific manner, depending its molecular weight, shape, water-binding properties and concentration [31]. A shift in the concentrations of a cassette of proteins and lipoproteins, as occurs in MetS, alters water mobility and reduces water T2 values. Thus, water T2 simultaneously monitors the net response to changes in many blood proteins, providing a global view of an individual’s metabolic state with just one measurement. The measurement can be made in a few minutes using a small volume of unmodified human plasma or serum, and requires no chemical reagents or reactions. Pending further testing and validation, water T2 offers a surprisingly powerful, yet practical new tool for detecting metabolic syndrome and monitoring cardiometabolic health.
Methods
Study design
This was a biomarker discovery study with an observational, cross-sectional design. Initially, it was designed to test the hypothesis that serum water T2 was associated with markers of insulin, glucose and lipid metabolism. Phase 1 was designed to collect data on 29 non-diabetic human subjects. The target number of subjects was derived from a power calculation with α = 0.05, β = 0.2 (statistical power of 0.8) and a correlation coefficient of 0.5 [32, 33]. The actual number of subjects analyzed in Phase 1 was 28.
Analysis of the Phase 1 data led to the observation that, in addition to insulin-, glucose- and lipid-related markers, water T2 appeared to be correlated with inflammatory markers. So Phase 2 of the study added an expanded set of inflammatory and acute phase markers. The target number of 38 subjects for Phase 2 was derived from a power calculation with α = 0.05, β = 0.1 (statistical power of 0.9) and a correlation coefficient of 0.5 [32, 33]. The actual number of subjects analyzed in Phase 2 was 44.
Therefore, the total number of subjects analyzed and reported here was 72: 28 in Phase 1 and 44 in Phase 2. Many biomarkers were collected in both Phase 1 and 2, while some were collected only in Phase 2.
Subject recruitment
Human subject volunteers were recruited with prior written informed consent into two protocols approved by the Institutional Review Board of the University of North Texas Health Science Center in Fort Worth (UNTHSC). One protocol recruited adult subjects from the student and staff population of UNTHSC, including spouses, friends and associates. The second protocol recruited Fort Worth community members enrolled in the Health & Aging Brain Study at UNTHSC [34]. Exclusion criteria for the current study included diabetes (HbA1C ≥ 6.5, fasting plasma glucose ≥ 125 mg/dL or prior history/diagnosis), active acute or chronic illness (C-reactive protein > 10 or history/diagnosis), history of bleeding disorders or difficulty donating blood, confirmed or suspected pregnancy from medical history, or not fasting for 12 h. Inclusion criteria were ages 18 and up. A total of 87 subjects were enrolled in the study, with 72 of the 87 subjects qualified according to the inclusion and exclusion criteria.
All subjects completed a comprehensive medical history form and a follow-up interview prior to the day of blood draw. On the morning of the blood draw, anthropometric measurements (height, weight, waist circumference, blood pressure and heart rate) were taken by the study nurse, and urine samples were screened for microalbuminuria using Chemstrip Micral (Roche Diagnostics).
Blood collection
Fasting blood samples were drawn at 7 a.m. by the study nurse following a standard order-of-draw protocol. For plasma preparation, blood was drawn into BD Vacutainer lavender-top tubes containing K2EDTA as the anticoagulant. For serum used for NMR and viscosity measurements, blood was drawn into plain glass red-top tubes lacking any gel separator or clot activators (BD models 366,441 and 366,430) to avoid potential interference in the NMR and viscosity measurements. Every effort was made to collect enough blood to perform all planned measurements. However, there were instances where the amount of blood collected from a given subject was not sufficient or samples were rejected by the testing lab due to hemolysis or other reasons. That variability, along with a few laboratory errors (instrument malfunction, data not collected or accidentally overwritten), accounted for the test-to-test differences in sample size (n) for the measurements listed in the tables. No attempts were made to interpolate or fill in missing data.
Blood sample processing, analysis and bio-banking
The plasma and serum samples were processed immediately after each blood draw. The serum samples were allowed to clot for 30 min, while plasma samples were being centrifuged. The first spin was at 3380 rpm (1590×g) for 10 min at room temperature to pellet and remove blood cells, followed by a second spin of the supernatant at 3800 rpm (2361×g) for 15 min to remove residual cells or debris. The presence of residual platelets was ruled out by dynamic light scattering analysis of each twice-centrifuged sample using a Wyatt Mobius instrument. The water T2 measurements were performed in triplicate on a sample of fresh plasma followed immediately by three repeats on fresh serum such that all water T2 measurements were completed within 2 h after the blood draw. Likewise, viscosity was measured on fresh serum and plasma samples within a few hours of the blood draw using a VISCOLab3000 instrument [35]. Aliquots of fresh serum were sent on ice to Atherotech, Inc. for Vertical Autoprofile (VAP) advanced lipoprotein testing and to determine LDL-P, hs-CRP, GGT, homocysteine, and Lp(a). Other aliquots of fresh plasma and serum were temporarily stored at 4°C prior to being sent the same day to Quest or Labcorp for diagnostic testing. Plasma aliquots for amino acid analysis, glucagon, fibrinogen, free fatty acids and proinsulin were frozen immediately after preparation and stored at − 80 °C prior to shipment to Quest. Other individual aliquots of plasma and serum were frozen at − 80 °C for subsequent in-house analysis using the following assay kits: apolipoprotein E concentration (Abcam, Ab108813), ORAC antioxidant capacity (Cell Biolabs, STA-345), protein carbonyl content (Cell Biolabs, STA-307), HNE (Cell Biolabs, STA-838), phospholipids (Wako Diagnostics, Assay Kit C), α2-macroglobulin (Abcam, ab108888), PAI-1 (Abcam, ab184863), neutrophil elastase (Abcam ab119553), soluble fibronectin (Abcam, ab181419), l-lactate (Abcam ab65331), endotoxin (Thermo Scientific, PI88282), and staphylococcus enterotoxin (Creative Diagnostics, DEIA-CL032).
Cytokines were assayed using the V-PLEX platform from Meso Scale Discovery, with a customized human cytokine plate for IL-6, IL-1β, TNF-α and IL-10 (MSD, K151AOH-1). Adiponectin and Factor VII were assayed using MSD Plate K151BXC-1, and sICAM-1, using MSD Kit K151SUD-1. All tests using − 80 °C frozen specimens were performed on samples that underwent only one freeze–thaw cycle.
Benchtop nuclear magnetic resonance relaxometry
Measurements of T2, the transverse relaxation time constant, were performed at 37 °C using a Bruker Minispec mq20 benchtop time-domain NMR instrument equipped with a 10 mm variable temperature probe (Model H20-10-25-AVGX). The 10 mm-diameter sample tube included a 3 mm coaxial insert (Norell NI10CCI-B), and the insert was filled to a sample height of 1 cm, corresponding to a sample volume of ~ 50 μL.
The modified Carr–Purcell–Meiboom–Gill (CPMG) pulse sequence we employed for T2 measurements is illustrated in Figure 1 of Ref. [31]. In our experience, a critical factor in obtaining high quality NMR relaxometry data with aqueous samples is to avoid radiation damping, particularly when higher magnetic fields and/or larger sample volumes are used (e.g., 10 mm tubes without a coaxial insert). The magnitude of the radiation damping depends on the particular instrument and probe design. We determined that the sample size in the coaxial insert was sufficiently small in this instrument to avoid radiation damping. Thus, it was not necessary to use the optional composite 180° pulse and Δ delay shown in the pulse sequence. Other experimental aspects pertinent to NMR data collection and analysis are detailed in Ref. [31].
Statistical analysis
The bivariate correlations, multiple and logistical regression analyses, categorical means comparisons, receiver operator characteristic curves and principal components analysis with variable clustering were performed using JMP Pro version 13.1 (SAS, Inc.) and GraphPad Prism v. 6.05 (GraphPad Software, Inc.). The guiding principles for the statistical analyses were derived largely from the books by Motulsky and Huber [36, 37]. Regression residuals were analyzed in GraphPad Prism using the strategy outlined by Klingenberger [38].
The bivariate correlation coefficients were calculated using three complementary methods: Pearson product-moment r, Spearman ρ, and the Huber M-value [36, 37]. These three estimators involve different assumptions about the data, and thus, have different strengths and weaknesses. Included among the assumptions for the Pearson r analysis is that both measures are sampled from a Gaussian distribution [36]. Analysis of the variables in this study revealed that more than half were not Gaussian distributed, as assessed using the D’Agostino–Pearson omnibus normality test implemented in GraphPad. However, a natural log transformation corrected the problem in nearly all cases (see Table 3, footnote c).
Table 3.
Bivariate Huber correlation coefficients for water T2
| Biomarkera,b,c | n | Plasma water T2 | n | Serum water T2 |
|---|---|---|---|---|
| Insulin and glucose markers | ||||
| McAuley Indexd | 70 | + 0.64**** | 69 | + 0.70**** |
| Insulin C-peptide | 70 | − 0.65**** | 69 | − 0.45**** |
| HOMA-IRd (insulin c-peptide) | 70 | − 0.64**** | 69 | − 0.46**** |
| Insulin | 70 | − 0.57**** | 69 | − 0.60**** |
| HOMA-IRd (insulin) | 70 | − 0.56**** | 69 | − 0.58**** |
| QUICKId | 70 | + 0.60**** | 69 | + 0.57**** |
| FIRId | 70 | − 0.58**** | 69 | − 0.59**** |
| Glucose/insulin ratiod | 70 | + 0.55**** | 69 | +0.58**** |
| Glucose | 70 | − 0.28* | 69 | − 0.28* |
| HbA1c | 69 | − 0.54**** | 69 | − 0.43*** |
| Proinsulin | 42 | − 0.53*** | 43 | − 0.60*** |
| Protein, viscosity and liver function markers | ||||
| Total protein, serum | 69 | − 0.56**** | 68 | − 0.79**** |
| Serum globulins | 69 | − 0.53**** | 68 | − 0.65**** |
| Serum viscosity | 65 | − 0.30* | 67 | − 0.45*** |
| Total protein, plasma | 41 | − 0.55*** | 42 | − 0.72**** |
| Plasma globulins | 41 | − 0.66**** | 42 | − 0.69**** |
| Plasma viscosity | 51 | − 0.47*** | 52 | − 0.56**** |
| Alanine aminotransferase (ALT) | 52 | − 0.37** | 50 | − 0.35** |
| Lipid and lipoprotein markers | ||||
| Apolipoprotein B (apoB) | 70 | − 0.55**** | 69 | − 0.52**** |
| Non-high-density lipoprotein cholesterol | 70 | − 0.52**** | 69 | − 0.52**** |
| Low-density lipoprotein cholesterol (LDL-C) | 70 | − 0.50**** | 69 | − 0.53**** |
| LDL/HDL ratio | 70 | − 0.54**** | 69 | − 0.58**** |
| Total cholesterol | 70 | − 0.50**** | 69 | − 0.51**** |
| LDL particle number (LDL-P) | 70 | − 0.52**** | 68 | − 0.54**** |
| Triglycerides (TG) | 70 | − 0.54**** | 69 | − 0.54**** |
| TG/HDL ratio | 70 | − 0.46**** | 69 | − 0.49**** |
| Phospholipids | 65 | − 0.44*** | 66 | − 0.41** |
| Very low-density lipoprotein-chol. (VLDL-C) | 63 | − 0.44*** | 62 | − 0.49**** |
| Intermediate-density lipoprotein-chol. (IDL-C) | 63 | − 0.39** | 62 | − 0.50**** |
| Remnant-cholesterol (Rem-C)e | 63 | − 0.44*** | 62 | − 0.53**** |
| Apo B/Apo A–I ratio | 63 | − 0.53**** | 62 | − 0.56**** |
| Inflammation and blood cell markers | ||||
| White blood cell count (WBC) | 69 | − 0.58**** | 68 | − 0.47**** |
| Neutrophil count | 69 | − 0.41*** | 68 | − 0.37** |
| Lymphocyte count | 69 | − 0.40*** | 68 | − 0.36** |
| C-reactive protein (CRP) | 69 | − 0.51**** | 68 | − 0.31** |
| Serum % globulins | 69 | − 0.46**** | 68 | − 0.50**** |
| Plasma % globulins | 41 | − 0.56*** | 42 | − 0.45** |
| Fibrinogen | 43 | − 0.65**** | 44 | − 0.40** |
| Complement C3c | 40 | − 0.52*** | 41 | − 0.44** |
| Complement C4c | 40 | − 0.59**** | 41 | − 0.43** |
| Electrolyte markers | ||||
| Lactate | 41 | − 0.53*** | 42 | − 0.49*** |
| Anion Gap, uncorrected | 69 | − 0.55**** | 68 | − 0.43*** |
| Anion Gap, corrected for [albumin] | 68 | − 0.44*** | 67 | − 0.39*** |
| Cl− + CO2 (HCO3 −) | 69 | + 0.36** | 68 | + 0.30* |
* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001
aAll blood samples were collected in the early morning following a 12-h overnight fast
bThis table includes only those biomarkers that were unambiguously correlated with both plasma and serum water T2. Results for markers that correlated with plasma or serum water T2, but not both, are discussed in the text and provided in Additional file 1: Tables S1 and S2. Ambiguous correlations are discussed in “Methods” section
cMany of the variables were natural-log transformed in order to meet the normality condition, an assumption inherent to the Pearson correlation. The correlation coefficients reported here and in Additional file 1: Tables S1 and S2 are for the ln-transformed variables, except the McAuley Index and QUICKI, as these indices are inherently ln-transformed. Other variables that were normally distributed and analyzed without ln transformation were plasma and serum T2, total serum and plasma protein, serum and plasma globulins and % globulins, serum and plasma viscosity, HbA1c, LDL-C, LDL-P, total C, apolipoprotein B, lymphocyte and platelet counts, lactate, and complement C3c
dAs defined elsewhere: McAuley Index [26], HOMA-IR [66, 67], FIRI [68], QUICKI [69], and G/I ratio [70]
eRemnant cholesterol is defined as intermediate-density lipoprotein (IDL) plus VLDL3, as determined using the vertical autoprofile method [71]
Another key assumption of the Pearson and Spearman correlations is that there are no outliers. Pearson is especially sensitive to outliers [36, 37, 39], which can lead to an over- or under-estimation of correlation coefficients. The Huber M-value has the distinct advantage of being robust to outliers [37]. Therefore, we chose to not eliminate any outliers, with the caveat that the Pearson and Spearman coefficients would be interpreted together with the Huber M-values and with careful inspection of the scatter plots.
In most cases, all three correlation coefficients had comparable values. Specific cases where outliers appeared to cause a significant under-estimation of the Pearson or Spearman coefficients included HbA1c, serum % globulins, VLDL-C, Rem-C, platelet count, lymphocyte count, RDW, complement C4c, and anion gap, uncorrected. Cases where outliers may have caused an overestimation of Pearson and Spearman values were asparagine, PAI-1, and haptoglobin. For these reasons, the Huber M-value was taken as the single-best estimate of the correlation, especially in cases where outliers had an influence.
The use of all three methods was particularly useful when correlations were ambiguous, i.e., when one method yielded a statistically significant result, while others did not. One notable example was body-mass-index or BMI, where a weak but statistically significant Pearson correlation was observed with both plasma and serum water T2. However, the Spearman and Huber M-value coefficients were weaker and not statistically significant. Inspection of the scatter plots revealed that the Pearson analysis was heavily influenced by a single outlier point that fell well outside of the Huber 95% confidence ellipse. This point corresponded to the subject in this study with the highest BMI and one of the lowest water T2 values. However, this study contained mostly non-obese individuals. Therefore, a proper assessment of the possible correlation between BMI and water T2 values in the context of obesity will require the study of a larger number of obese subjects. In the current study population, water T2 did not correlate with BMI.
Multiple linear regression models were built from Gaussian-distributed variables using the stepwise analysis feature in JMP v. 13.1. Potential predictor variables were chosen from the output of the bivariate analyses (Table 3, Additional file 1: Tables S1, S2), and from the principal components analyses with variable clustering, i.e., the most representative variable in each cluster. The stepwise analysis provided starting points for the exploration of different models and the reduction of possibilities. Acceptable models satisfied all three of the following criteria: (1) the p values for all predictor variables in the model were significant at α = 0.05, (2) the model avoided overfitting, as assessed using k-fold cross validation with k = 10, and (3) the adjusted R2 was maximized, within the constraints of criteria (1) and (2). The highest number of predictor variables in our models was five, including the y-intercept. This number is consistent with the rule of thumb that models should contain no more than 1 predictor variable for every 8–10 observables (subjects).
For the comparison of two means, unpaired two-tailed t-tests were used to assess significance, assuming equal variances and that the data were sampled from a Gaussian distribution. Five tests to confirm equal variances (O’Brien, Brown–Forsythe, Levene, Bartlett and 2-sided F-test) were performed as implemented in JMP 13.1. In one case specified in Table 4, equal variances could not be confirmed, so significance was assessed using the Welch test instead of the t test.
Table 4.
Mean plasma water T2 values for conditions and measures associated with early metabolic syndrome
| Conditions and measuresa | Cutoff value | Mean plasma T2 (ms) ± S.E. | |||
|---|---|---|---|---|---|
| No | Yes | Δb | Odds ratiob | ||
| Hyperinsulinemia (H) | Any of 3 below | 796.1 ± 7.9 | 728.7 ± 8.4 | 67.4**** | 9.5 (2.9–32.5)**** |
| High fasting insulinc | ≥ 10.0 μIU/mL | 786.6 ± 7.4 | 721.7 ± 10.2 | 65.0**** | |
| High insulin C-peptidec | ≥ 2.3 mg/mL | 780.7 ± 8.0 | 733.1 ± 11.1 | 47.6*** | |
| Low McAuley Indexd | ≤ 6.07 | 778.1 ± 7.2 | 718.1 ± 13.3 | 60.0*** | |
| Dyslipidemia (D) | Any of 3 below | 798.1 ± 8.6 | 734.3 ± 8.1 | 63.8**** | 5.8 (2.3–15.6)**** |
| High non-HDL-Cc | ≥ 149 mg/dL | 782.4 ± 7.9 | 729.8 ± 10.9 | 52.6*** | |
| Small, dense LDL | Pattern B/AB | 778.0 ± 8.1 | 736.4 ± 11.6 | 41.6** | |
| High LDL-Pc | ≥ 1408 nM | 779.1 ± 8.0 | 734.2 ± 11.5 | 44.9** | |
| Inflammation (I)e | Any of 3 below | 808.3 ± 9.4 | 738.4 ± 7.3 | 69.9**** | 10.4 (3.2–35)**** |
| High CRPc | ≥ 2.5 mg/L | 778.4 ± 7.6 | 725.1 ± 12.9 | 53.2*** | |
| High WBC countc | ≥ 6.92 × 103/μL | 780.3 ± 8.3 | 737.3 ± 10.8 | 43.0** | |
| High serum globulinsc | ≥ 2.9 g/dL | 788.5 ± 7.7 | 725.8 ± 9.7 | 62.7**** | |
| Electrolyte abnormal | Any of 3 below | 784.8 ± 10.3 | 747.3 ± 8.8 | 37.5** | 1.6 (1.1–2.3)* |
| Low (Cl− + CO2)f | ≤ 126 meq/L | 776.5 ± 9.0 | 745.6 ± 10.3 | 30.8* | |
| High anion gapc | ≥ 17.8 meq/L | 777.8 ± 7.9 | 731.6 ± 11.6 | 46.2** | |
| High anion gap corr.g | ≥ 17.7 meq/L | 776.8 ± 8.1 | 742.0 ± 11.3 | 34.8* | |
| Metabolic syndrome | Ref. [4] | 776.0 ± 7.4 | 721.6 ± 14.1 | 54.5** | 2.7 (1.2–5.9)** |
| H + ≥ 1 other condition | See above | 796.8 ± 7.6 | 725.8 ± 8.3 | 71.1**** | 15 (4–63)**** |
| H + ≥ 2 other conditions | See above | 792.6 ± 6.9 | 716.5 ± 9.0 | 76.1**** | 17 (4–73)**** |
| H + D + I | See above | 786.5 ± 6.5 | 705.0 ± 10.6 | 81.4**** | 24 (5–-124)**** |
* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001
aThis analysis included variables collected in both Phase 1 and Phase 2 of this study (n = 72)
bMean difference (Δ) and odds ratio (95% confidence limits) for the mean difference shown one column to the left
cTop tertile of subjects in this study
dObtained using formula in abstract of ref [26], with insulin = 12.2 μIU/mL and triglyceride = 1.5 mM as input
eFor this row, the Welch test was used in place of the t test, as equal variances could not be confirmed
fTotal measured anions, where ~ 95% of total CO2 is HCO3 −; bottom tertile of subjects in this study
gAnion gap corrected for albumin concentration using regression residuals; top tertile of subjects in this study
Receiver operator characteristic (ROC) curves were generated and evaluated using JMP 13.1. For determining the sensitivity, specificity and cutoff values for plasma and serum water T2, the McAuley Index value of ≤ 6.07 was chosen as the categorical reference standard for early insulin resistance [26]. The equation for the McAuley Index is provided in the abstract of Ref. [26], and the input values of fasting insulin and triglyceride are provided in Table 3 (“Method A”) of Ref. [26]. The optimal ROC cutoff points for plasma and serum water T2 were those that fell closest to the [0,1] coordinate, i.e., those which intersected with or closely approached the gray 45° tangent line shown for each ROC curve.
Results
Characteristics of the study population
The clinical characteristics of the human study population are presented in Table 1. Overall, this was a fairly diverse group of asymptomatic, non-diabetic adult volunteers spanning a wide age range. The gender distribution was approximately equal. The 72 subjects included 35 white, 23 Asian, 10 Hispanic and 4 African American individuals. The inclusion and exclusion criteria are specified in “Methods” section. As shown in Table 1, the mean values for the diagnostic markers fell near the middle of their normal reference ranges. With respect to glucose markers, 47 of the 72 subjects were normoglycemic by American Diabetes Association criteria [40], with both fasting glucose < 100 mg/dL and HbA1c < 5.7%. The remaining 25 subjects had glucose and/or HbA1c values consistent with prediabetes.
Table 1.
Characteristics of the study population, n = 72
| Parameter | Mean ± S.D.a | Rangea | Reference valuesb |
|---|---|---|---|
| Age | 39.5 ± 15.3 | 23–80 | n/a |
| Gender | n/a | 34 female, 38 male | n/a |
| Body-mass index (kg/m2) | 26.1 ± 4.9 | 18.2–45.1 | < 25 normal weight, 25–30 overweight, > 30 obese |
| Plasma T2 (ms) | 764.4 ± 58.7 | 631–887 | ≥ 745.0c |
| Serum T2 (ms) | 818.4 ± 56.7 | 692–927 | ≥ 811.8c |
| Glucose (mg/dL) | 90.9 ± 7.7 | 71–115 | < 100 non-diabetic 100–124 (pre-diabetic) |
| HbA1c (%) | 5.5 ± 0.3 | 4.7–6.2 | < 5.7 (non-diabetic) 5.7–6.4 (pre-diabetic) |
| Insulin C-peptide (ng/mL) | 2.0 ± 0.9 | 0.7–5.1 | 0.8–3.9 (> 2.85, IRd) |
| Insulin (μU/mL | 9.1 ± 6.0 | 2.2–40.1 | 2.0–19.6 (> 12.2, IRd) |
| Total serum protein (g/dL) | 7.1 ± 0.4 | 6.2–8.0 | 6.1–8.1 |
| Serum albumin (g/dL) | 4.5 ± 0.3 | 3.6–5.1 | 3.6–5.1 |
| Serum globulins (g/dL) | 2.7 ± 0.4 | 1.8–3.3 | 1.9–3.7 |
| Triglycerides (mg/dL) | 117.6 ± 60.0 | 42–321 | < 150 |
| Total cholesterol (mg/dL) | 187.0 ± 41.0 | 97–291 | < 200 |
| HDL-C (mg/dL) | 53.3 ± 12.7 | 31–85 | ≥ 40 (male); ≥ 50 (female) |
| LDL-C (mg/dL) | 111.1 ± 34.7 | 42–191 | < 130 |
| WBC count (×103/μL) | 6.5 ± 1.6 | 3.9–11.2 | 3.8–10.8 |
| Neutrophil count (×103/μL) | 3.6 ± 1.2 | 1.8–7.3 | 1.5–7.8 |
| hs-CRP (mg/L) | 2.3 ± 2.3 | 0.1–9.6 | < 3.0 (low-to-average CV risk) 3.0–10.0 (high CV risk) > 10.0 (infection/illness) |
| Sodium (mmol/L) | 139.0 ± 2.7 | 131–146 | 135–146 |
| Potassium (mmol/L) | 4.2 ± 0.3 | 3.5–4.8 | 3.5–5.3 |
| Total CO2, serum (mmol/L) | 24.0 ± 2.3 | 16–29 | 19–30 |
aAll blood samples were collected in the early morning after a 12-h overnight fast
bReference values from Quest Diagnostics and Atherotech, except where noted
cCutoff for normoglycemic population established in this study
dInsulin cutoff from McAuley et al. [26]; insulin C-peptide cutoff established by linear regression with inulin
Bivariate correlations between water T2 and blood biomarkers
The plasma and serum water T2 values were Gaussian distributed and exhibited high coefficients of variation (7–8%) across the study cohort (Table 1). This high variance did not result from imprecision in the NMR relaxometry measurements, as the coefficient of variation for multiple repeats on a single subject averaged < 1%. Rather, the high variance was caused by subject-to-subject biological variation reflecting the range of metabolic health among the subjects.
To identify the specific factors governing this variation, up to 130 diagnostic blood biomarkers were measured for each subject and correlated with plasma and serum water T2 values. As detailed in Methods, the study was conducted in two phases, with many biomarkers measured in both phases (n = 72), and some additional biomarkers measured only in Phase 2 (n = 44). While a number of markers showed significant correlations with plasma water T2 (Table 2, middle column), many others did not (Table 2, right column). Among those showing no correlation were albumin and sodium, markers of a subject’s hydration status. In addition, markers related to paramagnetic ions or their binding proteins (transferrin, total iron, total iron binding capacity, percent iron saturation, ferritin and ceruloplasmin) showed no correlation with plasma or serum T2, in spite of the inherent sensitivity of T2 to changes in paramagnetic ions. Thus, in this study population, variation in water T2 was not associated with variation in the subject’s hydration state or iron/copper status.
Table 2.
Biomarkers measured in this study; TD-NMR markers: plasma water T2, serum water T2
| Category | Correlation with plasma water Ta2 | No correlation with plasma water Ta2 |
|---|---|---|
| Insulin resistance, diabetes and anthropometric markers | Fasting insulin, insulin C-peptide, proinsulin, HbA1c, glucose, HOMA-IR, QUICKI, FIRI, G/I ratio, McAuley Index | Body-mass index, waist circumference, age, resting heart rate |
| Protein, viscosity liver function and amino acid markers | Total serum and plasma protein, serum and plasma globulins, % globulins, viscosity, ALT, GGT, Ser, Asn, Gln, Thr, Tyr | Serum and plasma albumin, IgG, IgM, IgA, AST, homocysteine, and 30 other amino acid and amino acid metabolites |
| Lipid and lipoprotein markers | Total cholesterol, non-HDL-C, LDL-C, LDL-P, VLDL-C, IDL-C, remnant-C, apoB, phospholipids, TG, TG/HDL ratio | Lp(a), HDL-C, HDL2, HDL3, apoAI, apoE, omega-3 index, DHA, AA, EPA, LpPLA2, serum turbidity (O.D.550 nm), free fatty acids |
| Inflammation, acute phase proteins, blood cell and oxidative stress markers | hs-CRP, WBC, neutrophils, lymphocytes, platelets, RDW, MCH, sedimentation rate, fibrinogen, complement C3c, C4c, plasminogen activator inhibitor-1 (PAI-1), α1-acid glycoprotein, interleukin-6 | RBC, HCT, Hb, MCHC, MCV, mean PLT volume, HNE, ORAC total antioxidant capacity, monocytes, eosinophils, basophils, haptoglobin, fibronectin, transferrin, ceruloplasmin, total iron, TIBC, % iron sat., ferritin, sICAM1, adiponectin, factor VII, uric acid, neutrophil elastase, endotoxin, staph. enterotoxin, α2-macroglobulin, α1-antitrypsin, IL-10, TNFα, IL-1β |
| Electrolyte markers | Lactate, Cl−, Cl− + CO2 (bicarbonate), anion gap, anion gap corrected for albumin | Sodium, potassium, calcium |
| Kidney and thyroid markers | BUN, creatinine, eGFR, thyroid stimulating hormone (TSH), free T4 |
Bivariate scatterplots for the correlation between plasma water T2 and insulin C-peptide, the McAuley Index, total serum protein concentration, LDL-cholesterol, triglycerides and complement C3 (measured clinically as its stable conversion product C3c) are displayed in Fig. 1. The bivariate normal density ellipse, indicated in red, provides a visual indicator of the Huber correlation at the 95% confidence level. The Huber M-value has the advantage of being robust to outliers, as compared with the Pearson r and the Spearman ρ values [37, 41]. This enabled us to analyze the correlations without excluding any real or perceived outliers, as detailed in “Methods” section.
Fig. 1.
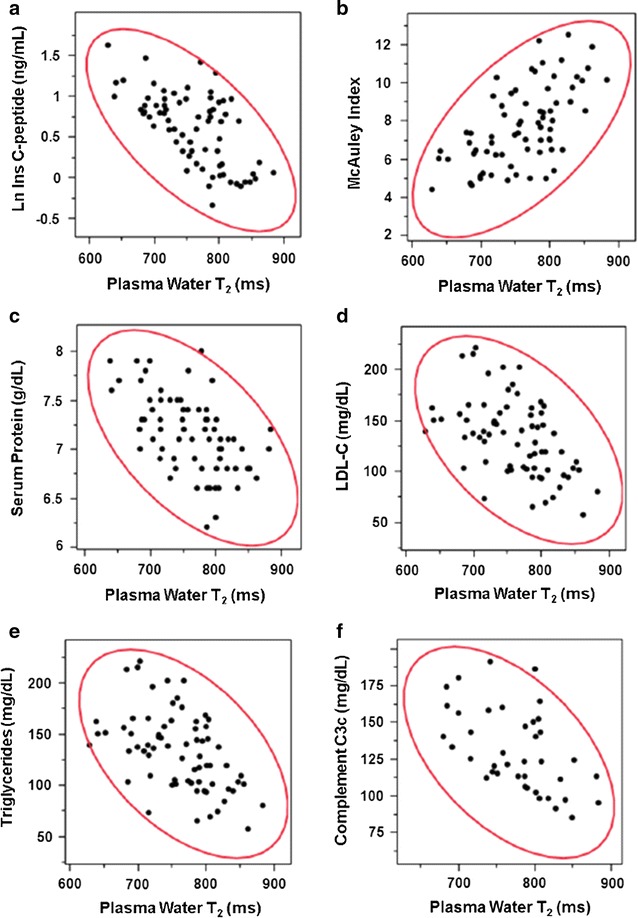
Scatterplots for the bivariate correlations between plasma water T2 values and 6 diagnostic markers: a natural log of insulin C-peptide; b McAuley Index; c total serum protein; d low-density lipoprotein cholesterol; e triglycerides; f complement C3c. Each filled black circle represents a data point for an individual human subject. The red ellipse represents the Huber bivariate normal density at the 95% confidence level
Table 3 lists the statistically significant bivariate Huber correlation coefficients for biomarkers that were unambiguously correlated with both plasma and serum water T2. Strong correlations (0.5–0.7) were observed between water T2 and fasting insulin C-peptide, insulin, and proinsulin, as well as indices derived from insulin plus glucose, or insulin plus triglycerides (Table 3, first section). Note that the correlation with fasting glucose, while statistically significant, is considerably weaker (~ 0.3). Also, strong correlations were observed with protein markers, specifically plasma and serum globulins, total serum and plasma proteins, plasma and serum viscosity, as well as alanine aminotransferase, a marker of liver function (Table 3, second section). However, no significant correlations were observed with plasma and serum albumin, which account for ~ 60% of the total plasma and serum protein mass.
Correlation coefficients of ~ 0.4 to 0.6 were observed between water T2 and markers of cholesterol- and triglyceride-rich lipoproteins (Table 3, third section). Moreover, correlations were observed with markers of inflammation and coagulation (Table 3, fourth section), especially white blood cell count, fibrinogen, complement C3c and C4c, and C-reactive protein. Finally, statistically significant correlations were observed with electrolyte markers, namely lactate, total measured anions (Cl− + HCO3 −) and the anion gap (Table 3, last section). Electrolyte abnormalities have been associated with insulin resistance, inflammation and high blood pressure in the National Health and Nutritional Examination Survey [42–44].
The association between plasma and serum water T2 was very strong, with a correlation coefficient of 0.8. However, some biomarkers correlated with plasma, but not serum, water T2 or vice versa. Those that correlated only with plasma water T2 include platelet and monocyte counts, red cell distribution width, mean corpuscular volume, mean corpuscular hemoglobin, serine, asparagine, glutamine, threonine, β-alanine, chloride, gamma glutamyl transferase, erythrocyte sedimentation rate, plasminogen activator inhibitor-1, α1-acid glycoprotein and interleukin-6. Those that correlated only with serum water T2 included immunoglobulin G, lipoprotein-associated phospholipase A2, red blood cell count, tyrosine and 3-methyl histidine. Full sets of Pearson, Spearman and Huber correlation coefficients are provided in Additional file 1: Tables S1 and S2 for plasma water T2 and serum water T2, respectively.
The overall pattern of correlations is consistent with key elements of insulin resistance and the metabolic syndrome, namely hyperinsulinemia, dyslipidemia, pro-inflammation, pro-coagulation, and electrolyte imbalances. In all five categories, plasma and serum water T2 values were inversely correlated with those metabolic abnormalities.
Of note, water T2 measurements did not correlate with body-mass index or waist circumference, at least in this mostly non-obese population. Also, plasma and serum water T2 did not correlate with free fatty acid levels or with age. The mean plasma water T2 value was lower in women than men, but this difference was not statistically significant (752.1 vs. 775.3, p = 0.104). By contrast, the mean serum water T2 values were nearly identical in women and men (817.0 vs. 819.8, p = 0.838). The possible gender difference seen with plasma but not serum water T2 could be attributed to a higher level of fibrinogen observed for women vs. men, although the gender difference in fibrinogen did not reach statistical significance (289.4 vs. 255.9, p = 0.091).
The inflammatory markers showed weaker correlation coefficients with serum water T2 (~ 0.3 to 0.5; Table 3) as compared with plasma water T2 (~ 0.4 to 0.7, Table 3). The primary difference between plasma and serum is the absence of fibrinogen or Factor I in serum [45], providing an explanation for the weaker correlations observed for serum water T2. Fibrinogen is a key reporter that connects plasma water T2 with inflammation and coagulation status. However, fibrinogen is not the only such reporter, as discussed below.
Principal components analysis with variable clustering
The observed bivariate correlations led us to consider factors that may contribute directly to the variation in plasma and serum water T2, as well as those that may be indirectly linked through another variable or a network of variables. Human blood plasma is a complex mixture containing hundreds of different proteins and lipoproteins as well as numerous small molecule metabolites. At first thought, de-convoluting these myriad variables would seem hopelessly complex. However, water mobility, and hence water T2, is affected mainly by its binding to macromolecules, as the influence of small molecule metabolites such as glucose is negligible [46]. Moreover, the sixteen most abundant proteins and lipoproteins in plasma (albumin, IgG, transferrin, fibrinogen, IgA, α2-macroglobulin, apolipoprotein AI, α1-antitrypsin, complement C3, IgM, haptoglobin, apolipoprotein B, α1-acid glycoprotein, apolipoprotein E, complement C4, and ceruloplasmin) account for > 99% of the total plasma protein mass. Thus, identifying the primary variables that contribute to water T2 is not an intractable problem.
We used three approaches to reduce the complexity of the network and tease apart variables that contribute independently and/or additively to the variation in plasma and serum water T2. The first approach utilized a principal components analysis with variable clustering [47]. This algorithm reduced the dimensionality by identifying clusters of variables that are most closely related. The results of one such analysis are presented in Additional file 1: Table S3. The statistical clusters correspond largely to the categories of markers based on physiological considerations. In the example shown in Additional file 1: Table S3, cluster 1 represents insulin and glucose markers; clusters 2–4, protein and viscosity markers; cluster 5, lipid and lipoprotein markers, clusters 6–8, inflammation markers; and cluster 9, electrolyte markers. One benefit of this analysis was to define the “most representative variable” in each category, which served as a starting point for building multiple regression models.
Multiple regression analysis
The second approach to reducing the complexity of the variable network used multiple regression to control for the effect of confounders and identify variables that have independent contributions to plasma water T2. The parameters for four of the best multiple regression models for plasma water T2 are provided in Additional file 1: Table S4. Model 1 was derived using variables collected in both phases of the study (72 subjects), while Models 2–4 included at least one variable that was measured only in Phase 2 (44 subjects). These models accounted for approximately two-thirds to three-fourths of the variation in plasma water T2. Attempts to add more variables to the models resulted in overfitting, as assessed using k-fold cross validation and described in “Methods” section.
The primary independent contributors to plasma water T2 were (1) insulin c-peptide, (2) total serum or plasma protein, plasma globulins, or plasma viscosity, (3) total cholesterol or apolipoprotein B, and (4) white blood cell count or fibrinogen. In general, one biomarker from each of four categories (insulin, proteins, lipids and inflammation) had independent contributions to the variation in plasma water T2. A key observation was that plasma water T2 was correlated with markers of insulin resistance or metabolic syndrome, even after correcting for total serum or plasma protein, serum or plasma globulins, or plasma viscosity. Attempts to correct for BMI and age did not yield statistical significance for those variables. Likewise, variables in the electrolyte category did not display a contribution to plasma water T2 independent of the other four categories. However, lactate could be substituted for insulin c-peptide or insulin in models for serum water T2. Multiple regression models for serum water T2 were similar to those for plasma water T2. Examples are presented in Additional file 1: Table S5.
Categorical and logistic regression analyses
The third approach used categorical, rather than continuous, variables to compare means and assess the additivity of contributions to plasma water T2. As shown in Table 4, the subjects were categorized as having or not having hyperinsulinemia, dyslipidemia, inflammation or electrolyte abnormalities, using three measures of each condition. In addition, the subjects were categorized as having or not having clinically-defined metabolic syndrome. The differences in mean plasma water T2 values were computed for each of the measures and conditions. The differences were greater when two or more conditions were combined. The largest difference in mean plasma water T2 values was observed for subjects who had hyperinsulinemia plus dyslipidemia plus inflammation (Table 4, last row). Thus, the lowest plasma water T2 values were observed in subjects who had multiple elements of early metabolic syndrome, as the effect on T2 has both independent and additive components.
To further assess the dose–response relationship between the number of metabolic conditions and plasma water T2, the subjects were divided into quintiles with respect to T2 values, and the average number of conditions for each quintile was calculated on a scale of 0–4. The average number of conditions increased from 0.71 (top quintile) to 1.57, 2.36, 2.79, and 3.57 for the 4th, 3rd, 2nd and lowest quintiles of plasma water T2, respectively. The lower the T2 value, the greater the number of conditions associated with metabolic syndrome.
To further quantify the association of plasma water T2 with these conditions, logistic regression models were constructed with each of the conditions in Table 4 serving as the categorical outcome. Each model was adjusted for potential confounders, specifically BMI, age and gender. For hyperinsulinemia, the unit odds ratio for plasma water T2 was 1.034 (95% confidence limits 1.016–1.053, p < 0.0001). Thus, the observed 67.4 ms decrease in mean plasma water T2 increased the odds of hyperinsulinemia by a factor of 1.03467.4 = 9.5. The corresponding unit odds ratio for serum water T2 and hyperinsulinemia was 1.026 (1.011–1.041, p < 0.0001). When dyslipidemia was the categorical outcome, the unit odds ratios were 1.028 (1.013–1.044, p < 0.0001) and 1.030 (1.015–1.047, p < 0.0001) for plasma and serum water T2, respectively. This translated into an odds ratio of 5.8 for the decrease in mean plasma water T2 associated with dyslipidemia (Table 4).
Even higher unit odds ratios were observed when inflammation was the categorical outcome: 1.034 (1.017–1.052, p < 0.0001) and 1.040 (1.019–1.061, p < 0.0001) for plasma and serum water T2, respectively. Thus, the observed 69.9 ms decrease in mean plasma water T2 translated into a 10.4-fold increase in the odds of having inflammation (Table 4). Smaller, but statistically significant odds ratios were observed when the categorical outcome was electrolyte abnormalities. Subjects who met the clinical criteria for metabolic syndrome as a whole had lower T2 values and higher odds ratios as well (Table 4).
The large decrease in mean water T2 values and high odds ratios provide further evidence of the strong association between water T2 and elements of the metabolic syndrome.
Sensitivity and specificity from ROC analysis
As an initial attempt to assess the sensitivity and specificity of water T2 for detecting elements of early metabolic syndrome, we performed a receiver operator characteristic curve (ROC) analysis. Insulin resistance, as defined by the McAuley Index, was used as the reference standard. The McAuley Index combines input from fasting triglycerides and fasting insulin and thus, captures two related elements of MetS. Moreover, the McAuley Index was rigorously calibrated with 178 normoglycemic subjects using a direct measure of insulin sensitivity, obtained from the euglycemic clamp, as the outcome variable [26]. Thus, it was calibrated to detect the earliest stage of insulin resistance. We categorized the 47 normoglycemic subjects in the current study using the McAuley Index cutoff of ≤ 6.07, derived using a fasting insulin of 12.2 μU/mL and a fasting triglyceride of 1.5 mM (133 mg/dL) as input variables [26]. As illustrated by the blue curve in Fig. 2, plasma water T2 detects early insulin resistance with a sensitivity of 100% and a specificity of 87% at a cutoff value of ≤ 745.0 ms. The area under the curve (AUC) is 0.96. By contrast, HbA1c and glucose—the tools most widely used for diabetes screening and risk assessment—show lower values of area-under-the-curve (AUC) and lower combinations of sensitivity and specificity (Fig. 2, red and yellow, respectively). To detect early insulin resistance with 100% sensitivity, the HbA1c cutoff would have to be lowered to 5.4, which would lead to poor specificity and a 42% false positive rate.
Fig. 2.
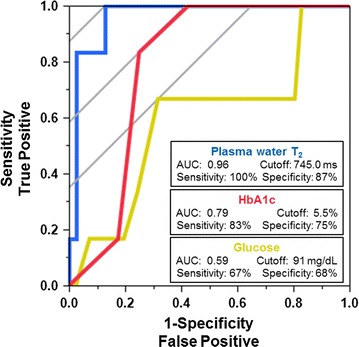
Receiver operator characteristic (ROC) curves to quantify the ability of different diagnostic tests to detect early insulin resistance (metabolic syndrome), as defined by the McAuley Index. Blue (left curve): plasma water T2; Red (middle curve): hemoglobin A1c; Yellow (right curve): glucose. An ideal ROC curve follows the left and top axes and intersects with the (0,1 coordinate)
For plasma water T2, the likelihood ratio for a positive test result (LR+) was 7.8. The likelihood ratio for a negative result (LR−) was zero.
Serum water T2 yielded ROC curve parameters similar to those of plasma: AUC = 0.94 with a sensitivity of 100% and specificity of 80% at a cutoff value of ≤ 811.8 ms. The LR+ and LR− values were 5.0 and zero, respectively.
As a further exercise, we used the harmonized clinical criteria for metabolic syndrome [4] as the reference standard for ROC analysis, instead of the McAuley Index. For plasma water T2, this analysis yielded sensitivity and specificity values of 73% at a cutoff value of 745 ms and an AUC = 0.75. For serum water T2, the values were similar: 71% sensitivity and 69% specificity at a cutoff of 804.8 ms and an AUC = 0.71.
Identification of early metabolic abnormalities using water T2
Using the plasma and serum water T2 cutoff values of 745.0 and 811.8 ms, along with the current criteria for prediabetes [40] and metabolic syndrome [4], the subjects in this study were classified into metabolic stages, as shown in Fig. 3. Of the total of 72 subjects, 31 had normal metabolism, while the other 41 had early metabolic abnormalities, prediabetes and/or metabolic syndrome defined by clinical criteria. Of the 41, 25 had prediabetes, and 10 of those 25 met the clinical criteria for metabolic syndrome. The remaining 16 did not have prediabetes, but had plasma or serum water T2 values below the cutoffs. Of those 16, only three met the clinical criteria for metabolic syndrome. Therefore, plasma and serum water T2 uniquely identified 13 subjects (18% of the study population) with metabolic abnormalities that would have gone undetected by the current clinical definitions of prediabetes and metabolic syndrome.
Fig. 3.
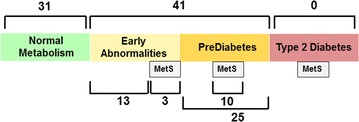
The distribution of the 72 subjects in this study with respect to metabolic abnormalities. Thirty-one subjects had no identifiable metabolic abnormalities (“Normal Metabolism”), while 41 had early abnormalities, prediabetes and/or clinically-defined metabolic syndrome (MetS). Twenty-five of the 41 met the ADA criteria for prediabetes, and 10 of those 25 also met the harmonized clinical criteria for MetS [4]. The remaining 16 subjects with “Early Abnormalities” were identified by low water T2 values in the absence of prediabetes. Only three of those 16 met the clinical criteria for MetS. Thus, water T2 uniquely identified 13 individuals with abnormalities consistent with early metabolic syndrome
Further examination of the 13 normoglycemic subjects with low plasma or serum water T2 values yielded the following observations:
-
A.
Three of the 13 subjects had overt compensatory hyperinsulinemia, with fasting insulin above 12.2 μIU/mL [26] and insulin C-peptide in the top tertile. All three subjects had subclinical inflammation as well.
-
B.
Another 3 of the 13 subjects showed evidence of hyperinsulinemia, but missed the fasting insulin cutoff of 12.2 μIU/mL; two of the three also missed the McAuley Index cutoff < 6.07 [26]. However, they had insulin and insulin c-peptide levels in the top tertiles of the subjects in this study. Two of these three subjects showed evidence of inflammation.
-
C.
Two subjects had low or moderate insulin levels, but insulin c-peptide in the top quartile. The mismatch between insulin vs. insulin c-peptide likely results from rapid hepatic insulin clearance rates. Insulin is cleared by the liver, whereas insulin c-peptide is cleared more slowly by the kidney [48]. Subjects with rapid insulin hepatic clearance may have a limited capacity to sustain insulin levels high enough to compensate for tissue insulin resistance. These subjects may be prone to develop impaired glucose tolerance. In addition to elevated insulin c-peptide, both subjects showed evidence of inflammation.
-
D.
Three subjects showed no evidence of hyperinsulinemia, as monitored by insulin, c-peptide or the McAuley Index, but exhibited high levels of proinsulin, with ratios of proinsulin/insulin c-peptide in the top tertile. This pattern points to a defect in the enzymatic conversion of proinsulin to insulin and insulin c-peptide, which has been observed in non-diabetic and diabetic subjects [49–53]. High proinsulin levels are predictive of incident type 2 diabetes and insulin resistance in diabetes [49, 51, 52]. In addition, these subjects showed signs of dyslipidemia and inflammation.
-
E.
The remaining two subjects had no apparent elevations in insulin, insulin c-peptide or proinsulin. One subject had elevated total cholesterol, LDL-cholesterol and LDL particle number, but not triglycerides or triglyceride-related markers. In addition, this subject had levels of white blood cells and neutrophils in the top tertile, but C-reactive protein and serum globulins were unremarkable. This subject appeared to have a type of dyslipidemia and subclinical inflammation unrelated to insulin resistance. The remaining subject had only three abnormalities besides a low serum T2: elevated lipoprotein (a), an increased anion gap and serum globulins in the top quartile.
Thus, the 13 normoglycemic subjects with low water T2 had a heterogeneous set of early metabolic abnormalities (hyperinsulinemia, dyslipidemia and/or inflammation) that were undetected by the clinical criteria for MetS. This observation illustrates the limitations of the clinical definition of MetS, and the unique power of water T2 to detect this condition.
Identifying the principal drivers of low water T2 in metabolic syndrome
To assess the role of the 16 most abundant plasma proteins and lipoproteins in metabolic syndrome, the plasma and serum water T2 cutoffs of ≤ 745.0 and ≤ 811.8 ms were used to classify the study subjects into those with and without the syndrome. The percent differences in the mean plasma protein concentrations for the two groups of subjects are displayed in Fig. 4. Subjects with metabolic syndrome, here defined by low water T2, displayed statistically significant increases in the mean concentrations of fibrinogen, complement C3c, haptoglobin, apolipoprotein B, α1-acid glycoprotein and complement C4c, as well as total plasma proteins and globulins (Fig. 4, black bars). By contrast, there were no significant changes in the concentrations of the other 10 proteins: albumin, IgG, transferrin, IgA, α2-macroglobulin, apolipoprotein AI (HDL), α1-antitrypsin, IgM, apolipoprotein E and ceruloplasmin (Fig. 4, grey bars). Thus, low T2 values and metabolic syndrome are characterized by increases in the concentrations of a specific subset of acute phase proteins and lipoproteins.
Fig. 4.
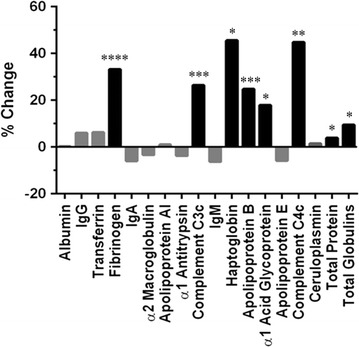
The percent change in mean plasma protein concentration between the low vs. high water T2 groups, using the ROC-established cutoffs defined in the text. The 16 most abundant proteins in plasma are listed from left to right in order of decreasing concentration, with total protein and total globulin concentrations listed at the far right. Together, these 16 proteins accounted for > 99% of the total plasma protein mass. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Discussion
This report describes the serendipitous discovery of a new biomarker for early metabolic syndrome and its initial characterization in human subjects. The results revealed the strong correlations between plasma and serum water T2 values and markers of five conditions related to metabolic syndrome: hyperinsulinemia, dyslipidemia, pro-inflammation and pro-coagulation states, and electrolyte imbalances. The correlations were observed in a cohort of otherwise healthy, non-diabetic, mostly non-obese adults. Based on multiple and logistic regression analyses, these conditions had independent and/or additive contributions to the lowering of water T2. Water T2 values were driven lower by increases in the concentrations of 6 of the 16 most abundant proteins and lipoproteins in human plasma. Five of the six were positive acute phase proteins—markers of innate immunity—and the other was apolipoprotein B, a major protein component of cholesterol- and triglyceride-rich lipoprotein particles.
The five most abundant acute phase proteins associated with lower plasma water T2—fibrinogen, complement C3, haptoglobin, α1-acid glycoprotein and complement C4—have been the focus of several prospective epidemiological studies. The Framingham Study helped to establish fibrinogen as a risk factor for cardiovascular disease [28], and the Insulin Resistance Atherosclerosis Study demonstrated the relationship between fibrinogen and insulin resistance syndrome (metabolic syndrome) [29]. In addition to its effects on thrombogenesis and platelet aggregation, fibrinogen affects the rheology of blood flow by making the blood more viscous [30], which may explain part of the mechanism by which it lowers plasma blood T2 values. In a study of hospital patients, most of whom had severe lung disease (carcinoma, metastases, infectious or inflammatory diseases), an inverse correlation was observed between fibrinogen and plasma T2 [54]. The interpretation was that fibrinogen was monitoring the inflammatory status of the patient, rather than the presence or absence of cancer [54]. Those observations are consistent with the current study, even though the current subjects do not have any serious acute or chronic illnesses. The current study highlights the exquisite sensitivity of water T2 to detect subtle, subclinical inflammation, even in subjects who are otherwise healthy.
In a prospective study [55], fibrinogen, complement C3, C4 and haptoglobin were associated with insulin resistance and incident type 2 diabetes, but not α1-antitrypsin, ceruloplasmin or orosomucoid. This pattern of selective increases in some acute phase proteins, but not others, is generally consistent with the pattern of changes detected here by plasma water T2. The current observations highlight the unique capability of water T2 to detect changes in a cassette of co-regulated acute phase proteins using just one measurement.
Moreover, plasma and serum water T2 were inversely correlated with the concentrations of apolipoprotein B and apo B-containing lipoproteins. These abundant nanoparticles constitute the largest molecular assemblies in plasma and serum, ranging from ~ 20 nm diameter for cholesterol-rich LDL, to ~ 60 to 100 nm for triglyceride-rich VLDL. Increased concentrations of apo B-containing lipoprotein particles should cause a profound decrease in water mobility and lowering of water T2, as water binds to a larger number of particles. Through the elevation of plasma triglycerides, insulin resistance causes a remodeling of LDL particles, resulting in a larger number of smaller, denser particles [56, 57]. At a given cholesterol level, this increase in particle number provides additional surface area for water molecules to bind and thus, could lower water T2 values. In addition, the accumulation of remnant lipoprotein particles in the blood that occurs with insulin resistance could have a similar effect on lowering T2.
A curious observation was the lack of correlation between water T2 and HDL-cholesterol or apolipoprotein A-I, even though HDL-C was inversely correlated with triglyceride levels. A possible explanation is that low HDL-C is not a prominent feature of early metabolic syndrome, as typified by the subjects in this study, but becomes more prominent in later stages of MetS when triglyceride levels tend to be higher, such as overt type 2 diabetes. This distinction is important, as low HDL-C is one of the five clinical criteria for metabolic syndrome.
As expected, water T2 values were inversely correlated with measures of the bulk properties of plasma and serum, i.e., viscosity and total protein concentration. However, after correcting for bulk factors using multiple regression, water T2 remained independently associated with markers of hyperinsulinemia, dyslipidemia and inflammation. Thus, water T2 is driven by non-specific changes in bulk factors as well as specific changes in individual proteins tied to different aspects of metabolism. A model for how metabolic syndrome reduces water T2 is presented in Fig. 5.
Fig. 5.
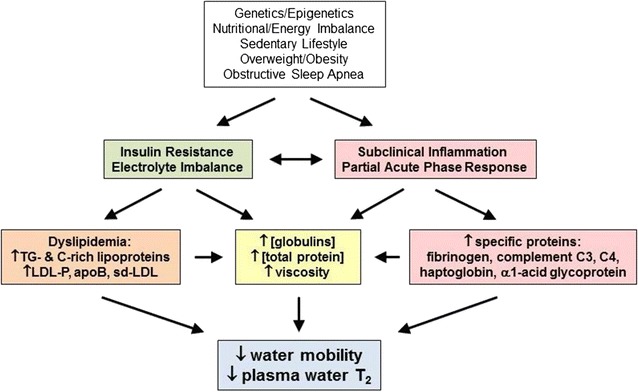
A model for the linkage between genetic and environmental factors, metabolic abnormalities, blood biomarkers, and plasma and serum water T2 values
Plasma and serum water T2 showed a remarkably high sensitivity and specificity for detecting early insulin resistance in subjects with normal fasting glucose and HbA1c levels. The sensitivity was 100%, with no false negatives. The specificity was 86% for plasma and 80% for serum, as water T2 detected two individuals with cardio-metabolic abnormalities apparently unrelated to early insulin resistance.
The profile of sensitivity and specificity for water T2 makes for a good screening test, which is not the same as a diagnostic test [58]. Screening is beneficial when (i) the disease is serious, (ii) treatment before symptoms is more effective than treatment delayed until after symptoms, and (iii) the prevalence of the detectable pre-clinical phase is high [59]. Type 2 diabetes and atherosclerotic cardiovascular disease meet those criteria. A good screening test is inexpensive, easy to administer, reliable, reproducible, has minimal discomfort and is valid, i.e., sensitive and specific. Water T2 meets those criteria. Unlike disease risk surveys, where risk scores are based on broad population averages, water T2 provides a personalized assessment of metabolic health status. Pending further testing and validation, water T2 appears to be a promising screening test for identifying those at risk for prediabetes, type 2 diabetes, atherosclerotic cardiovascular disease, and possibly even Alzheimer’s disease. Approximately one-third of Alzheimer’s cases are preventable, and insulin resistance and diabetes are among the modifiable risk factors [60–64].
The measurement of water T2 in human blood is surprisingly simple and practical [31]. The test uses a small volume (~ 50 μL) of unmodified plasma or serum, and requires no reagents, chemical reactions or sample manipulations. The data collection takes ~ 3 min, and the analysis is quick and straightforward. Currently, the measurement is made in a benchtop NMR relaxometry device about the size of a toaster oven, but even smaller devices for this purpose could be designed [31, 65]. Although this study used a research-grade instrument, a clinical instrument designed for the diagnosis of sepsis and blood coagulation in critical care units is commercially available from T2 Biosystems, Inc. (Lexington, Massachusetts, USA). It can perform the measurements described here. It is feasible to perform this test in point-of-care settings like primary care clinics.
Limitations of the study
This biomarker discovery study provides an initial assessment of the metabolic information content of plasma and serum water T2 in non-diabetic human subjects. It needs to be followed up by a series of validation steps: in longitudinal cohorts, in the postprandial state, against direct measures of insulin sensitivity, in larger populations and in response to therapeutic interventions, as explained below.
This cross-sectional study does not provide direct evidence for the ability of plasma or serum water T2 to predict future risk for type 2 diabetes or cardiovascular disease. However, the individual acute phase proteins and lipoproteins monitored by water T2 have been shown, in prior prospective studies, to be associated with incident type 2 diabetes and cardiovascular events. Future work should assess water T2 in longitudinal cohorts.
All blood testing was performed on subjects who underwent a 12-h overnight fast. The current results provide little or no direct insight into postprandial metabolism or glucose tolerance following an oral glucose load. Thus, T2 will need to be correlated with results from oral glucose tolerance tests.
This study employed indirect measures of insulin resistance based on fasting insulin, insulin c-peptide, proinsulin, triglycerides and indices derived from them, most notably the McAuley Index. An important next step is to validate plasma and serum water T2 values against direct measures of tissue insulin resistance.
The correlation of water T2 with BMI and waist circumference in the context of obesity was inconclusive, as this study enrolled mainly non-obese subjects. Further work needs to be done to evaluate the association of water T2 with BMI and central obesity. Likewise, the relationship of water T2 with blood pressure warrants further evaluation.
While the 72 subjects employed in this biomarker discovery study provided sufficient statistical power to identify biologically important correlation coefficients greater than ~ 0.25, a future study with a larger number of subjects will permit a more comprehensive statistical analysis. The main benefit of a larger study would be the generation of multiple and logistical regression models that can accommodate a larger number of predictor variables. Remarkably, we were able to account for three-fourths of the variation in water T2 with up to five predictor variables The high sensitivity and large variance in water T2 made that possible. However, a study with a larger number of subjects may be able to account for other independent predictors of water T2.
Conclusions
Water T2 from benchtop NMR relaxometry offers a new tool for detecting individuals with metabolic syndrome. Its advantages can be summarized in three words: early, global and practical. It detects the earliest abnormalities, capturing a global view of an individual’s metabolic health, with one simple measurement. Water T2 should be a central component of personalized strategies to assess metabolic health and prevent type 2 diabetes and atherosclerotic cardiovascular disease.
Authors’ contributions
MDR and IM contributed to study design and subject recruitment, collected and analyzed the NMR data and some biomarker measurements, and edited the manuscript. They contributed equally to this work. SD managed the blood draw protocols and database, performed many of the in-house biomarker assays and edited the manuscript. VP and KVG performed some of the in-house assays and edited the manuscript. KB performed the phlebotomy, clinical and anthropometric measurements and edited the manuscript. RV, LJ and SO managed and oversaw the subject recruitment and data collection for subjects from the Health & Aging Brain Study and edited the manuscript. DPC oversaw the study and study design, performed the statistical analyses, and drafted/edited the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors thank Tori Conger for performing the cytokine measurements using the Meso Scale Discovery platform. We are indebted to Drs. Myron Jacobson and Raz Shaikh for reviewing early drafts of the manuscript and providing constructive feedback. Special thanks are warranted for Dr. Alok Dwivedi, a biostatistician who offered recommendations that strengthened and clarified the statistical analyses. Finally, we thank Dr. Brian Gladue, who—on multiple occasions—provided expert guidance with human subjects protections and IRB protocols.
Competing interests
The University of North Texas Health Science Center, Fort Worth has applied for a patent related to the methods described in this study, with D.P.C. and M.D.R. as co-inventors.
Availability of data
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study received prior approval and annual reviews from the Institutional Review Board of the University of North Texas Health Science Center, Fort Worth, Protocol Numbers 2012-083 and 2013-205. All enrolled subjects received and signed an IRB-approved consent form to participate after an in-person interview and opportunity to ask questions about the study.
Funding
This work was supported by institutional start-up funds (to D.P.C.) from the University of North Texas Health Science Center, Fort Worth and the Texas Tech University Health Sciences Center El Paso, as well as a grant from the Garvey Texas Foundation. The Health & Aging Brain Study, which provided some of the subjects and data for this study, is supported by NIA/NIH Grant R01AG039389 (to S.O.) and the UNT Health Science Center. Additional funding was received from the Hogg Foundation, the Institute for Aging and Alzheimer’s Disease Research and the Alzheimer’s Disease Center, University of Texas Southwestern Medical Center, Dallas.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AA
arachidonic acid
- ALT
alanine aminotransferase
- ApoB, apoA–I or apoE
apolipoprotein B, A–I or E concentration
- Asn
asparagine
- AST
aspartate aminotransferase
- AUC
area under the curve in ROC analysis
- BMI
body-mass index
- BUN
blood urea nitrogen
- C3c
complement C3c, a proteolytic fragment of complement C3 measured in diagnostic tests
- C4c
complement C4c, a proteolytic fragment of complement C4 measured in diagnostic tests
- Cl−
chloride anion
- CO2
total carbon dioxide in serum, ~ 95% of which is in the form of bicarbonate ion or HCO3 −
- CPMG
Carr–Purcell–Meiboom–Gill NMR pulse sequence to measure T2
- DHA
docosahexaenoic acid
- EDTA
ethylene-diamine-tetra-acetic-acid
- eGFR
estimated glomerular filtration rate
- EPA
eicosapentaeneoic acid
- FIRI
fasting insulin resistance index
- GGT
gamma glutamyl transferase or transpeptidase
- G/I ratio
fasting glucose-to-insulin ratio
- HABS
Health & Aging Brain Study at the UNT Health Science Center, Fort Worth
- HbA1C
percent glycated hemoglobin
- HCT
hematocrit
- HDL2 or HDL3
high-density lipoprotein, subfractions 2 or 3, cholesterol concentration
- HDL-C
high-density lipoprotein cholesterol concentration
- HDL-P
high-density lipoprotein particle number concentration
- Hb
hemoglobin concentration
- HNE
4-hydroxynonenal, a product of lipid peroxidation
- HOMA2-%B
homeostatic model assessment version 2, % beta cell function
- HOMA2-%S
homeostatic model assessment version 2, % insulin sensitivity
- HOMA2-IR
homeostatic model assessment version 2, insulin resistance index (see https://www.dtu.ox.ac.uk/homacalculator for HOMA2 definitions)
- hs-CRP
C-reactive protein concentration measured using high-sensitivity assay
- IDL-C
intermediate-density lipoprotein cholesterol concentration
- IgA, IgG, IgM
immunoglobulins A, G or M
- IL-6, -1β, or -10
interleukin-6, -1β, or -10
- LDL-C
low density lipoprotein cholesterol concentration
- LDL-P
low density lipoprotein particle number concentration
- Lp(a)
lipoprotein (a) cholesterol concentration
- LpPLA2
lipoprotein-associated phospholipase A2
- LR+
likelihood ratio for a positive test
- LR−
likelihood ratio for a negative test
- M-value
correlation coefficient from Huber M-value estimator
- MCH
mean corpuscular hemoglobin (per cell)
- MCHC
mean corpuscular hemoglobin concentration (per unit volume of red cells)
- MCV
mean corpuscular volume
- MDA
malondialdehyde
- MetS
metabolic syndrome
- NMR
nuclear magnetic resonance
- O.D.550
optical density at 550 nm
- ORAC
oxygen radical absorbance capacity, a measure of anti-oxidant capacity
- PAI-1
plasminogen activator inhibitor-1
- PLT
platelets
- QUICKI
quantitative insulin sensitivity check index
- RBC
red blood cell count
- ROC
receiver operator characteristic
- Ser
serine
- T2
transverse or spin–spin relaxation time constant obtained by NMR relaxometry
- T2a
regression residuals from a linear fit of plasma or serum water T2 vs. serum albumin
- T2c
regression residuals from a linear fit of plasma or serum water T2 vs. serum cholesterol
- T2g
regression residuals from a linear fit of plasma or serum water T2 vs. serum globulins (globulins = total serum protein − serum albumin)
- T2p
regression residuals from a linear fit of plasma or serum water T2 vs. total serum protein
- T2v
regression residuals from a linear fit of plasma or serum water T2 vs. viscosity
- TG
triglyceride or triacylglycerol concentration
- Thr
threonine
- TIBC
total iron binding capacity
- Tyr
tyrosine
- r
Pearson correlation coefficient, parametric
- ρ or rS
Spearman correlation coefficient, non-parametric
- R2
square of the Pearson correlation coefficient
- RDW
red cell distribution width
- Remnant-C
remnant lipoprotein particle cholesterol concentration
- sICAM1
soluble intercellular adhesion molecule 1
- T4
thyroxine or tetraiodothyronine (thyroid hormone)
- TD-NMR
time-domain nuclear magnetic resonance
- TG
serum triglyceride concentration
- TNFα
tumor necrosis factor alpha
- TSH
thyroid stimulating hormone
- [UA]
unmeasured anion concentration, in meq/L
- [UC]
unmeasured cation concentration, in meq/L
- VAP
Vertical AutoProfile test, Atherotech
- VLDL-C
very low density lipoprotein cholesterol concentration
- WBC
white blood cell count
Additional file
Additional file 1: Table S1. Correlation coefficients for plasma water T2-provides a full set of Pearson, Spearman and Huber correlation coefficients for biomarkers that have a statistically significant correlation with plasma water T2. Table S2. Correlation coefficients for Serum water T2-provides a full set of Pearson, Spearman and Huber correlation coefficients for biomarkers that have a statistically significant correlation with serum water T2. Table S3. Principal components analysis with variable clustering-provides clusters of related variables and most representative variable in each cluster. Table S4. Multiple regression models for plasma water T2-provides parameter details for the best multiple regression models for plasma water T2. Table S5. Multiple regression models for Serum water T2-provides parameter details for the best multiple regression models for serum water T2.
Footnotes
Michelle D. Robinson and Ina Mishra contributed equally to this work
Electronic supplementary material
The online version of this article (10.1186/s12967-017-1359-5) contains supplementary material, which is available to authorized users.
Contributor Information
Michelle D. Robinson, Email: michelle.robinson@boehringer-ingelheim.com
Ina Mishra, Email: ina.mishra@live.unthsc.edu.
Sneha Deodhar, Email: sneha.deodhar@unthsc.edu.
Vipulkumar Patel, Email: vipulkumar.patel@ttuhsc.edu.
Katrina V. Gordon, Email: katrina.gordon@unthsc.edu
Raul Vintimilla, Email: raul.vintimilla@unthsc.edu.
Kim Brown, Email: kim.brown@unthsc.edu.
Leigh Johnson, Email: leigh.johnson@unthsc.edu.
Sid O’Bryant, Email: sid.obryant@unthsc.edu.
David P. Cistola, Email: david.cistola@ttuhsc.edu
References
- 1.Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: prevalence in worldwide populations. Endocrinol Metab Clin North Am. 2004;33:351–375. doi: 10.1016/j.ecl.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313:1973–1974. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 3.Moore JX, Chaudhary N, Akinyemiju T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev Chronic Dis. 2017;14:E24. doi: 10.5888/pcd14.160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alberti KG, Eckel RH, Grundy SM. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 5.Sperling LS, Mechanick JI, Neeland IJ, Herrick CJ, Després J, Ndumele CE, Vijayaraghavan K, Handelsman Y, Puckrein GA, Araneta MRG, Blum QK, Collins KK, Cook S, Dhurandhar NV, Dixon DL, Egan BM, Ferdinand DP, Herman LM, Hessen SE, Jacobson TA, Pate RR, Ratner RE, Brinton EA, Forker AD, Ritzenthaler LL, Grundy SM. The cardiometabolic health alliance. J Am Coll Cardiol. 2015;66:1050–1067. doi: 10.1016/j.jacc.2015.06.1328. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM. Metabolic syndrome update. Trends Cardiovasc Med. 2016;26:364–373. doi: 10.1016/j.tcm.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal. Diabetes Care. 2005;28:2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 8.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 9.Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48. doi: 10.1186/1741-7015-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanson RL, Imperatore G, Bennett PH, Knowler WC. Components of the “metabolic syndrome” and incidence of type 2 diabetes. Diabetes. 2002;51:3120–3127. doi: 10.2337/diabetes.51.10.3120. [DOI] [PubMed] [Google Scholar]
- 11.Laaksonen DE, Lakka HM, Niskanen LK, Kaplan GA, Salonen JT, Lakka TA. Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am J Epidemiol. 2002;156:1070–1077. doi: 10.1093/aje/kwf145. [DOI] [PubMed] [Google Scholar]
- 12.Lorenzo C, Okoloise M, Williams K, Stern MP, Haffner SM, San Antonio Heart Study The metabolic syndrome as predictor of type 2 diabetes: the San Antonio heart study. Diabetes Care. 2003;26:3153–3159. doi: 10.2337/diacare.26.11.3153. [DOI] [PubMed] [Google Scholar]
- 13.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 14.Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119:812–819. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 15.Alberti KGMM, Zimmet P, Shaw J. International Diabetes Federation: a consensus on type 2 diabetes prevention. Diabetic Med. 2007;24:451–463. doi: 10.1111/j.1464-5491.2007.02157.x. [DOI] [PubMed] [Google Scholar]
- 16.Napoli C, Crudele V, Soricelli A, Al-Omran M, Vitale N, Infante T, Mancini FP. Primary prevention of atherosclerosis. Circulation. 2012;125:2363–2373. doi: 10.1161/CIRCULATIONAHA.111.085787. [DOI] [PubMed] [Google Scholar]
- 17.DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM: a balanced overview. Diabetes Care. 1992;15:318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- 18.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 19.Weyer C, Tataranni PA, Bogardus C, Pratley RE. Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Diabetes Care. 2001;24:89–94. doi: 10.2337/diacare.24.1.89. [DOI] [PubMed] [Google Scholar]
- 20.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 21.Ahren B, Pacini G. Islet adaptation to insulin resistance: mechanisms and implications for intervention. Diabetes Obes Metab. 2005;7:2–8. doi: 10.1111/j.1463-1326.2004.00361.x. [DOI] [PubMed] [Google Scholar]
- 22.Kim SH, Reaven GM. Insulin resistance and hyperinsulinemia: you can’t have one without the other. Diabetes Care. 2008;31:1433–1438. doi: 10.2337/dc08-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B. Impaired fasting glucose and impaired glucose tolerance. Diabetes Care. 2007;30:753–759. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- 24.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the veterans administration genetic epidemiology study. Diabetes. 2006;55:1430–1435. doi: 10.2337/db05-1200. [DOI] [PubMed] [Google Scholar]
- 25.DeFronzo RA, Abdul-Ghani MA. Preservation of beta-cell function: the key to diabetes prevention. J Clin Endocrinol Metab. 2011;96:2354–2366. doi: 10.1210/jc.2011-0246. [DOI] [PubMed] [Google Scholar]
- 26.McAuley KA, Williams SM, Mann JI, Walker RJ, Lewis-Barned NJ, Temple LA, Duncan AW. Diagnosing insulin resistance in the general population. Diabetes Care. 2001;24:460–464. doi: 10.2337/diacare.24.3.460. [DOI] [PubMed] [Google Scholar]
- 27.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 28.Kannel WB, Wolf PA, Castelli WP, D’Agostino RB. Fibrinogen and risk of cardiovascular disease. The Framingham Study. JAMA. 1987;258:1183–1186. doi: 10.1001/jama.1987.03400090067035. [DOI] [PubMed] [Google Scholar]
- 29.Festa A, D’Agostino R, Jr, Tracy RP, Haffner SM, Insulin Resistance Atherosclerosis Study Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51:1131–1137. doi: 10.2337/diabetes.51.4.1131. [DOI] [PubMed] [Google Scholar]
- 30.Kamath S, Lip GYH. Fibrinogen: biochemistry, epidemiology and determinants. QJM. 2003;96:711–729. doi: 10.1093/qjmed/hcg129. [DOI] [PubMed] [Google Scholar]
- 31.Cistola DP, Robinson MD. Compact NMR relaxometry of human blood and blood components. Trends Analyt Chem. 2016;83:53–64. doi: 10.1016/j.trac.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hulley SB, Cummings SR, Browner WS. Designing clinical research: an epidemiologic approach. Philadelphia: Williams & Wilkins; 1988. [Google Scholar]
- 33.Kohn M. Sample size calculators for designing clinical research, UCSF Clinical & Translational Science Institute. http://www.sample-size.net/correlation-sample-size/. Accessed 24 June 2017.
- 34.O’Bryant SE, Johnson L, Reisch J, Edwards M, Hall J, Barber R, Devous MDS, Royall D, Singh M. Risk factors for mild cognitive impairment among Mexican Americans. Alzheimers Dement. 2013;9(622–631):e1. doi: 10.1016/j.jalz.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson MD, Cistola DP. Nanofluidity of fatty acid hydrocarbon chains as monitored by benchtop time-domain nuclear magnetic resonance. Biochemistry. 2014;53:7515–7522. doi: 10.1021/bi5011859. [DOI] [PubMed] [Google Scholar]
- 36.Motulsky H. Intuitive biostatistics: a nonmathematical guide to statistical thinking. New York: Oxford University Press; 2014. [Google Scholar]
- 37.Huber PJ, Ronchetti EM. Robust statistics. New Jersey: Wiley; 2009. [Google Scholar]
- 38.Klingenberg CP. Regression. MorphoJ User’s Guide. http://www.flywings.org.uk/MorphoJ_guide/frameset.htm?index.htm. Accessed 22 Jan 2015.
- 39.Wilcox RR, Keselman HJ. Modern regression methods that can substantially increase power and provide a more accurate understanding of associations. Eur J Pers. 2012;26:165–174. doi: 10.1002/per.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.American Diabetes Association 2. Classification and diagnosis of diabetes. Diabetes Care. 2017;40:S11–S24. doi: 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- 41.Huber PJ. Robust estimation of a location parameter. Ann Math Statist. 1964;35:73–101. doi: 10.1214/aoms/1177703732. [DOI] [Google Scholar]
- 42.Taylor EN, Forman JP, Farwell WR. Serum anion gap and blood pressure in the national health and nutrition examination survey. Hypertension. 2007;50:320–324. doi: 10.1161/HYPERTENSIONAHA.107.092643. [DOI] [PubMed] [Google Scholar]
- 43.Farwell WR, Taylor EN. Serum bicarbonate, anion gap and insulin resistance in the National Health and Nutrition Examination Survey. Diabet Med. 2008;25:798–804. doi: 10.1111/j.1464-5491.2008.02471.x. [DOI] [PubMed] [Google Scholar]
- 44.Farwell WR, Taylor EN. Serum anion gap, bicarbonate and biomarkers of inflammation in healthy individuals in a national survey. CMAJ. 2010;182:137–141. doi: 10.1503/cmaj.090329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lundblad R. Considerations for the use of blood plasma and serum for proteomic analysis. Int J Genomics Proteomics. 2003;1(2):58. [Google Scholar]
- 46.Robinson MD. Novel diagnostic and analytical applications of benchtop time-domain NMR. Greenville: East Carolina University; 2015. [Google Scholar]
- 47.Parker R. Variable clustering in JMP. https://community.jmp.com/t5/JMP-Blog/Variable-clustering-in-JMP/ba-p/30261. Accessed 19 June 2017.
- 48.Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med. 2013;30:803–817. doi: 10.1111/dme.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kahn SE, Leonetti DL, Prigeon RL, Boyko EJ, Bergstrom RW, Fujimoto WY. Proinsulin as a marker for the development of NIDDM in Japanese–American men. Diabetes. 1995;44:173–179. doi: 10.2337/diab.44.2.173. [DOI] [PubMed] [Google Scholar]
- 50.Mykkanen L, Haffner SM, Kuusisto J, Pyorala K, Hales CN, Laakso M. Serum proinsulin levels are disproportionately increased in elderly prediabetic subjects. Diabetologia. 1995;38:1176–1182. doi: 10.1007/BF00422366. [DOI] [PubMed] [Google Scholar]
- 51.Hanley AJ, D’Agostino R, Jr, Wagenknecht LE, Saad MF, Savage PJ, Bergman R, Haffner SM, Insluin Resistance Atrherosclerosis Study Increased proinsulin levels and decreased acute insulin response independently predict the incidence of type 2 diabetes in the insulin resistance atherosclerosis study. Diabetes. 2002;51:1263–1270. doi: 10.2337/diabetes.51.4.1263. [DOI] [PubMed] [Google Scholar]
- 52.Pfutzner A, Kunt T, Hohberg C, Mondok A, Pahler S, Konrad T, Lubben G, Forst T. Fasting intact proinsulin is a highly specific predictor of insulin resistance in type 2 diabetes. Diabetes Care. 2004;27:682–687. doi: 10.2337/diacare.27.3.682. [DOI] [PubMed] [Google Scholar]
- 53.Pfutzner A, Forst T. Elevated intact proinsulin levels are indicative of beta-cell dysfunction, insulin resistance, and cardiovascular risk: impact of the antidiabetic agent pioglitazone. J Diabetes Sci Technol. 2011;5:784–793. doi: 10.1177/193229681100500333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schuhmacher JH, Conrad D, Manke HG, Clorius JH, Matys ER, Hauser H, Zuna I, Maier-Borst W, Hull WE. Investigations concerning the potential for using 1H NMR relaxometry or high-resolution spectroscopy of plasma as a screening test for malignant lung disease. Magn Reson Med. 1990;13:103–132. doi: 10.1002/mrm.1910130111. [DOI] [PubMed] [Google Scholar]
- 55.Engstrom G, Hedblad B, Eriksson KF, Janzon L, Lindgarde F. Complement C3 is a risk factor for the development of diabetes: a population-based cohort study. Diabetes. 2005;54:570–575. doi: 10.2337/diabetes.54.2.570. [DOI] [PubMed] [Google Scholar]
- 56.Ginsberg HN, Zhang Y, Hernandez-Ono A. Metabolic syndrome: focus on dyslipidemia. Obesity. 2006;14:41S–49S. doi: 10.1038/oby.2006.281. [DOI] [PubMed] [Google Scholar]
- 57.Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32:1754–1759. doi: 10.1161/ATVBAHA.111.241885. [DOI] [PubMed] [Google Scholar]
- 58.Gilbert R, Logan S, Moyer VA, Elliott EJ. Assessing diagnostic and screening tests: part 1. Concepts. West J Med. 2001;174:405–409. doi: 10.1136/ewjm.174.6.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Felson DT. Screening for disease. A Boston University School of Public Health Web Module. http://sphweb.bumc.bu.edu/otlt/mph-modules/ep/ep713_screening/EP713_Screening_print.html. Accessed 23 June 2017.
- 60.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 61.Biessels GJ. Capitalising on modifiable risk factors for Alzheimer’s disease. Lancet Neurol. 2015;13:752–753. doi: 10.1016/S1474-4422(14)70154-1. [DOI] [PubMed] [Google Scholar]
- 62.De Felice FG, Ferreira ST. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes. 2014;63:2262–2272. doi: 10.2337/db13-1954. [DOI] [PubMed] [Google Scholar]
- 63.Willette AA, Bendlin BB, Starks EJ, et al. Association of insulin resistance with cerebral glucose uptake in late middle-aged adults at risk for alzheimer disease. JAMA Neurol. 2015;72:1013–1020. doi: 10.1001/jamaneurol.2015.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li TC, Yang CP, Tseng ST, Li CI, Liu CS, Lin WY, Hwang KL, Yang SY, Chiang JH, Lin CC. Visit-to-visit variations in fasting plasma glucose and HbA1c associated with an increased risk of Alzhemier disease: Taiwan diabetes study. Diabetes Care. 2017;40(9):1210–1217. doi: 10.2337/dc16-2238. [DOI] [PubMed] [Google Scholar]
- 65.Blumich B, Haber-Pohlmeier S, Wasif Z. Compact NMR. Boston: De Gruyter; 2014. [Google Scholar]
- 66.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 67.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 68.Duncan MH, Singh BM, Wise PH, Carter G, Alaghband-Zadeh J. A simple measure of insulin resistance. Lancet. 1995;346:120–121. doi: 10.1016/S0140-6736(95)92143-5. [DOI] [PubMed] [Google Scholar]
- 69.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 70.Legro RS, Finegood D, Dunaif A. A fasting glucose to insulin ratio is a useful measure of insulin sensitivity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1998;83:2694–2698. doi: 10.1210/jcem.83.8.5054. [DOI] [PubMed] [Google Scholar]
- 71.Chung BH, Segrest JP, Ray MJ, Brunzell JD, Hokanson JE, Krauss RM, Beaudrie K, Cone JT. Single vertical spin density gradient ultracentrifugation. Methods Enzymol. 1986;128:181–209. doi: 10.1016/0076-6879(86)28068-4. [DOI] [PubMed] [Google Scholar]


