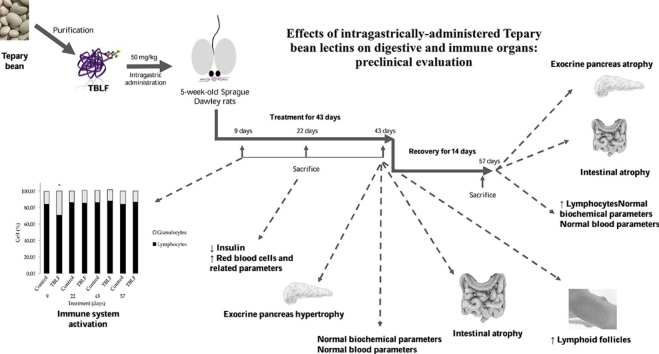
Graphical abstract
Keywords: Digestive tract, Immune system, Lectins, Phaseolus acutifolius, Tepary bean
Highlights
-
•
TBLF activates immune system through an increase of granulocytes and CD4+ cells.
-
•
TBLF provoked activation of Peyer’s patches lymphoid follicles.
-
•
TBLF provoked intestinal atrophy with no recovery after two-weeks without treatment.
-
•
TBLF provoked exocrine pancreas hypertrophy with a partial recovery.
-
•
Spleen did not show significant changes after TBLF treatment.
Abstract
Previous work showed that Tepary bean (Phaseolus acutifolius) lectins exhibit differential cytotoxic effects on cancer cell lines by apoptosis induction. In vivo studies using a Tepary bean lectin fraction (TBLF, 50 mg/kg of body weight) after colon cancer induction in rats showed that TBLF inhibited early precancerous lesions without systemic toxicity however, loss of body weight gain and activation of immune cells were observed. In order to know more about the possible adverse effects, we evaluated the administration of TBLF on digestive and immune organs. Sprague Dawley rats were administered TBLF for six weeks and allowed to recover for two weeks. Immune activation was observed through an increased lymphocyte-granulocyte ratio, an increased number of lymphoid follicles in intestinal Peyer’s patches and a slight expansion of the splenic white pulp. Atrophy was observed in small intestine villi and crypt foci of the colon without normalization after the recovery period. Pancreas histopathology showed hypertrophy after the six-week administration period, particularly vacuolation and trabecular widening; but after the two-week recovery period atrophy was observed, suggesting a partial compensatory type process. Our results show that TBLF activates the immune system and affects digestive organs through direct interaction with intestinal epithelium, and indirectly by producing pancreatic hyperfunction. Further work will focus in longer recuperation periods after TBLF treatment.
1. Introduction
Lectins are proteins or glycoproteins of that have the ability to recognise and specifically bind to free carbohydrates or cell membrane glycoconjugates. Specially plant lectins have shown anticancer properties, mainly due to glycosylation changes on cancer cell membranes [1,2]. Leguminous lectins exhibit selective cytotoxic effects on cancer cells through several mechanisms such as apoptosis or autophagy [[3], [4], [5], [6], [7], [8], [9]] however, the observed effects are lectin-dependent and some of them have shown to be either cytotoxic or mitogenic such as on lymphocytes [10,4]. Particularly, plant lectins exhibit important effects against several digestive system cancers, showing significant potential as therapeutic agents [2]. Some lectins such as European Mistletoe lectin are used as complementary therapy [4,11,12] however, among the aspects to be taken into account in assessing the potential of plant lectins as anticancer drugs are those related to their toxicity.
Lectins are anti-nutritional factors that produce systemic and/or toxic effects, depending on their source, their concentration and the route of administration [[13], [14], [15], [16]]. Leguminous lectins are able to resist gastric and intestinal digestion and they can remain intact throughout the digestive tract for several days [[16], [17], [18], [19]]. They can bind to surface glycoproteins or glycolipids of intestinal epithelium, affecting nutrient absorption and in turn digestive organ function can be compromised [20,21]. Furthermore, lectins are considered antigenic molecules and they can activate immune response in an immunomodulatory-like manner [22].
Previous studies have shown that a Tepary bean lectin fraction (TBLF), obtained by molecular weight exclusion chromatography, exhibit cytotoxic effects on cancer cells [7]. When TBLF (50 mg/body weight kg) was tested in rats, an inhibitory effect on early precancerous lesions was observed through apoptosis induction (data not published). However, a loss in weight gain of 10% was provoked without apparent systemic toxic effects measured through blood markers for hepatic, pancreatic renal and nutritional status, while exhibiting immunomodulatory properties [16]. Compensatory effects on body weight and food intake were observed at the end of the treatment, but the origin of such effects remains to be elucidated. In order to continue with the evaluation of the potential of TBLF as a therapeutic agent against colon cancer, it is necessary to further understand its adverse effects. Therefore, this study evaluated the systemic effects of the intragastric administration of TBLF in rats, with emphasis on adverse effects on the digestive tract and immune system.
2. Materials and methods
2.1. Tepary bean lectin fraction extraction
Tepary bean seeds were obtained from a local market at Hermosillo, Sonora, Mexico, and a sample was deposited and identified at Dr. Jerzy Rzedowski Herbarium of the Department of Natural Sciences, Autonomous University of Querétaro. TBLF was obtained as previously described [7]. Briefly, aqueous extract was obtained from ground bean seeds. A sequential selective precipitation to 40% and to 70% with ammonium sulphate was performed. The protein was dialysed and separated by molecular weight exclusion chromatography using a Sephadex G-75 column. TBLF was identified by agglutination test using A+ type erythrocytes.
2.2. In vivo assays
Assays were performed using 5-week-old Sprague Dawley rats which were purchased from the Institute of Neurobiology of the National Autonomous University of Mexico (INB-UNAM). The animals were placed in individual boxes with water and rodent chow food ad libitum (Rodent Laboratory Chow 5001, Saint Louis, MO, USA) with a circadian cycle adjusted to 12 h light/12 h dark, at 21–23 °C and a relative humidity of 30%. The animals were left for one week of acclimatisation before the experiments were commenced. The experimental protocols were based on Official Mexican Standards [23] and approved by the Bioethics Committee of the Natural Sciences Department of the Autonomous University of Querétaro.
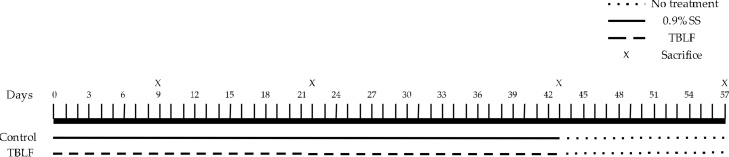
After acclimatisation, the rats were randomised to the control and the TBLF-treated groups (n = 62 per group). Treated rats received 50 mg/kg of TBLF (5000 agglutination units/mg protein) in saline solution by intragastric administration every third day for 43 days (6 weeks) and were allowed to stay for 14 days more without treatment. Control rats were only administered saline solution (Fig. 1). Food intake was determined by weighing the food twice per week throughout the treatment, and mean daily intake was calculated. The weight and length of the rats were measured using a rat scale (Ohaus triple beam balance) and a measuring tape (fiberglass 152 cm). Rats were sacrificed by decapitation on days 9 (n = 12 per group), 22 (n = 12 per group), 43 (n = 22 per group) (administration period) and after 14 days more (Day 57, no treatment period, n = 16 per group), in order to collect the blood and organs for further testing.
Fig. 1.
Experimental design. After one week of acclimatisation rats were treated for 43 days with TBLF (50 mg/kg of body weight) or saline solution for control animals. Sacrifice was done at days 9, 22 43 and after two weeks without any treatment (Day 57).
EDTAK2 vacutainer tubes were used to collect blood for the determination of the complete blood count (CBC) (CellDyn® 1600), and serum was obtained by collecting blood in vacutainer tubes with separator gel, followed by centrifugation at 1500g for 15 min. CD4+ and CD8+ lymphocytes were determined by flow cytometry (FACS Arias®), where CD4+ antibody was marked with FITC (Biolegend 201505) and CD8+ antibody with PerCP (Biolegend 201712). Serum was stored at −80 °C until use for biochemical markers determination such as pancreatic markers (α-amylase, Spinreact, Catalogue No. 341-10 and insulin, Merck, Ref. EZRMI–13 K, E.U.), nutritional markers (glucose, Spinreact, Catalogue No. MD-410-11; total protein, Spinreact, Catalogue No. 200-55; serum albumin, SL Elitech, Catalogue No. ALBU-0600), renal function (serum creatinine, Spinreact, Catalogue No. 221-30 and urea, Spinreact, Catalogue No. 283-17) and hepatic function (alanine aminotransferase (ALT), Elitech, Catalogue No. 318-10).
Dissection of liver, heart, kidneys, pancreas, small intestine, colon, thymus and spleen was performed after sacrifice. Organs were weighed on an analytical balance (300 g ± 0.0001 g). Small intestine and colon length were determined using a fiberglass tape measure (Dry Mark). Organ weight and length were adjusted to body weight individually and normalised with respect to the control group mean. For the histopathological evaluation, pancreas, intestines and spleen were fixed in 10% formaldehyde and stained with haematoxylin-eosin for subsequent histological analysis by microscopy (Axiovert Alternate Microscope A1 Zeiss), and interpreted using IMAGEJ image analyser software where 40 slices per group from at least three different animals were analysed. Pancreatic lipid presence was determined using oily red staining. Briefly, oily red dye powder was added to 99% isopropanol until a sediment was formed which was no longer dissolved. The working solution was prepared one hour before use and was made by mixing 3 parts of the saturated solution of oily red with two parts of distilled water and filter just before use. Freeze-dried pancreas samples were immersed in 60% isopropanol and stained for 15 min in the oily red working solution, then they were revealed in 60% isopropanol until a sample of lipid control appeared colored. Pancreas samples were washed in water and then the nuclei were contrasted with Gill hematoxylin for 2–3 min and then washed thoroughly under running water. Samples were rinsed in distilled water and assembled into gelatin-glycerin (7 g gelatin dissolved slowly in 42 mL of water without forming lumps, 50 mL of glycerin was added, heated it in a water bath and 1 g phenol was added, finally it was filtered still hot). This solution can be refrigerated and at the time of use it is necessary to heat slightly to liquefy the gelatin.
The immunohistochemical analyses were performed using samples of Peyer’s. Briefly, paraffin was removed from the samples at 54 °C for 45 min and hydrated in xylene, 100% alcohol, 90% alcohol, 80% alcohol and distilled water for 20 min. The slides were then incubated with 3% peroxide in absolute methanol (1: 9 v/v) for 30 min to remove endogenous peroxidase activity. Non-specific binding sites were blocked using defatted milk for 30 min at room temperature. Tissues were incubated for 24 h at 4° C with the polyclonal antibodies: anti-IL-6 (Santa Cruz, CA, USA), NFKb (Invitrogen, Thermo Fisher Sci. Inc. USA) and anti TNFα (Santa Cruz, CA, USA) in a diluted to 1:2000. The tissues were incubated with the corresponding biotinylated secondary antibody goat anti rabbit or rabbit anti goat (Santa Cruz, CA, USA) for one h at room temperature and then incubated with the avidin-peroxidase complex for one h at room temperature. The reaction was run using a solution of 6 mg of diaminobenzidine in 10 mL of 0.05 M Tris-HCl, pH 7.4 and 10 μL of 30% H2O2 for 10 min. This reaction produced a sepia-colored precipitate in the immunoreactive cells. Samples were contrasted with Harris hematoxylin and samples were dehydrated using 96% alcohol, absolute alcohol, absolute alcohol/xylene (vol/vol) and absolute xylene and mounted with resin. The samples were analyzed under a microscope (10×) and photographs were obtained.
Differences between treatments were analysed using three types of statistical tests using STATA software version 12. The Student’s-t test was performed for independent samples in order to compare control vs treated rats. The multivariate Hotelling’s T2 test was used to explain the effect of TBLF on all explanatory variables in a single model. Finally, multilevel regressions were performed for variables grouped and/or nested per subject over time.
3. Results
3.1. Effects of TBLF on nutritional, hematopoietic and biochemical markers
TBLF was administered for 6 weeks (43 days) every third day, followed by two weeks without treatment as a recovery period (from Day 44 to Day 57). TBLF administration did not produce changes in the rats’ development. Body weight gain was 54.4% and 51.5% for control and treated rats, respectively (multilevel regression p = 0.478, data not shown), and body length gain was 59.5% and 61.4% for control and treated rats, respectively (multilevel regression p = 0.126, data not shown). Food intake was not different between groups (multilevel regression p = 0.611, data not shown). Nutritional parameters were not affected after TBLF administration in contrast to previous studies using older rats, in which TBLF produced a loss of body weight gain [16]. In this study, rats in accelerated development (5-week-old rats) were treated and no effects were observed, compared to 15-week-old rats, which were in the final phase of growth that showed a decrease of 10% in body weight gain. Our results suggest that detrimental effects of TBLF depend on animals’ age and growth stage [24].
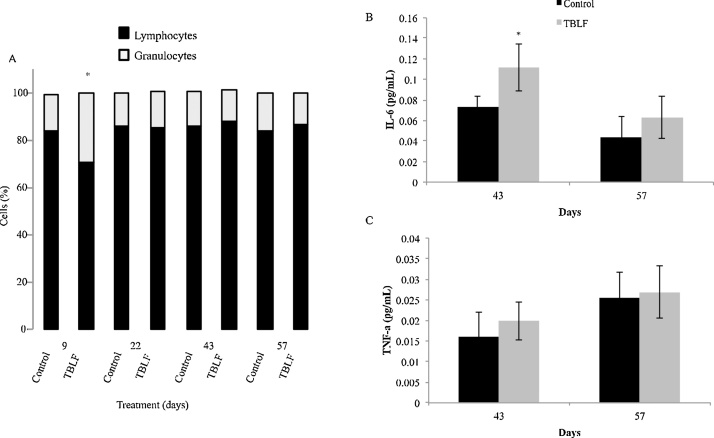
Complete blood count (Table 1) showed a significant increase in lymphocyte percent on Day 9 of treatment, after which no differences were observed; then, after the recovery period an increase was observed once again. CD4+ and CD8+ lymphocytes were quantified, showing an increase of CD4+ on Day 9 but a decrease of CD8+ after that, no differences were observed through the treatment; finally, a decrease of CD4+ was observed after the recovery period. One significant change was seen in the lymphocyte-granulocyte ratio, from 90:10 in control rats versus 70:30 in treated rats on Day 9 of treatment (Fig. 2). These findings suggest that TBLF affects the immune system response. Additionally, on Day 22 it was observed an increase in red blood cells and related parameters, where haemoglobin was the only parameter that showed an increase after the recovery period, on Day 57. Platelets showed an increase at the end of the treatment with no other changes. Lectins are considered antigenic molecules [2,4,6,16,22]; thus, once they reach the digestive tract, they can trigger an immune activation response; such as the increase in total and CD4+ lymphocytes and a decrease in CD8+ lymphocytes, as well as the lymphocyte-granulocyte ratio. After the recovery period, it was observed a decrease in CD4+ lymphocytes, may be related with a compensatory effect. Serum proinfamatory cytokines were determined in days 43 and 57. Only IL-6 showed a significative increase on Day 43 but after the two-weeks recovery period no changes respect to control rats were observed while TNF-α did not show changes. IL-6 increase suggests immune activation effect of TBLF, this cytokine is a modullator of CD4 T cells [25].
Table 1.
Complete blood count after TBLF administration.
| Blood parameter | Control | TBLF | p | |
|---|---|---|---|---|
| Day 9 | White blood cells (10^3/μL) | 6.06 ± 0.55 | 6.42 ± 2.4 | 0.926 |
| Red blood cells (10^6/μL) | 6.11 ± 0.43 | 6.19 ± 0.51 | 0.583 | |
| Haemoglobin (g/dL) | 15.68 ± 0.94 | 15.60 ± 0.73 | 0.376 | |
| Haematocrit (%) | 39.80 ± 2.5 | 39.13 ± 2.0 | 0.719 | |
| Mean corpuscular volume (fL) | 65.16 ± 1.3 | 63.35 ± 2.0 | 0.515 | |
| Mean corpuscular haemoglobin (pg) | 25.68 ± 0.34 | 25.27 ± 0.98 | 0.948 | |
| Mean corpuscular haemoglobin concentration (pg) | 39.41 ± 0.81 | 39.75 ± 0.10 | 0.482 | |
| Platelets (10^3/μL) | 901.25 ± 125.5 | 889.17 ± 87.88 | 0.352 | |
| Lymphocytes (%) | 81.73 ± 2.3 | 86.78 ± 3.0* | 0.024* | |
| CD4+ (%) | 61.24 ± 3.75 | 67.03 ± 2.29 | 0.031* | |
| CD8+ (%) | 36.08 ± 3.18 | 22.85 ± 5.02 | 0.004* | |
| Day 22 | White blood cells (10^3/μL) | 7.9 ± 1.9 | 8.6 ± 2.3 | 0.583 |
| Red blood cells (10^6/μL) | 6.4 ± 0.59 | 7.3 ± 0.39 | 0.001* | |
| Haemoglobin (g/dL) | 15.45 ± 3.0 | 16.88 ± 0.41 | 0.001* | |
| Haematocrit (%) | 44.09 ± 9.9 | 43.6 ± 1.0 | 0.911 | |
| Mean corpuscular volume (fL) | 61.14 ± 0.47 | 61.30 ± 1.0 | 0.029* | |
| Mean corpuscular haemoglobin (pg) | 24.05 ± 1.04 | 22.97 ± 1.01 | 0.039* | |
| Mean corpuscular haemoglobin concentration (pg) | 38.1 ± 1.6 | 38.7 ± 0.57 | 0.455 | |
| Platelets (10^3/μL) | 984.9 ± 172.18 | 860.5 ± 172.8 | 0.111 | |
| Lymphocytes (%) | 74.37 ± 14.9 | 84.5 ± 2.29 | 0.058 | |
| CD4+ (%) | 63.92 ± 1.81 | 65.47 ± 4.82 | 0.574 | |
| CD8+ (%) | 32.27 ± 1.50 | 32.10 ± 4.99 | 0.959 | |
| Day 43 | White blood cells (10^3/μL) | 8.06 ± 1.23 | 8.8 ± 2.57 | 0.298 |
| Red blood cells (10^6/μL) | 7.8 ± 0.4 | 8.0 ± 0.35 | 0.327 | |
| Haemoglobin (g/dL) | 17.38 ± 1.45 | 17.7 ± 0.43 | 0.521 | |
| Haematocrit (%) | 44.5 ± 1.9 | 45.38 ± 1.3 | 0.218 | |
| Mean corpuscular volume (fL) | 56.5 ± 0.96 | 56.7 ± 1.35 | 0.643 | |
| Mean corpuscular haemoglobin (pg) | 22.1 ± 1.81 | 22.1 ± 2.11 | 0.984 | |
| Mean corpuscular haemoglobin concentration (pg) | 39.11 ± 2.9 | 38.98 ± 3.31 | 0.918 | |
| Platelets (10^3/μL) | 817.15 ± 93.7 | 904.7 ± 49.18 | 0.011* | |
| Lymphocytes (%) | 85.46 ± 3.7 | 81.88 ± 8.7 | 0.193 | |
| CD4+ (%) | 58.50 ± 3.79 | 60.00 ± 2.37 | 0.359 | |
| CD8+ (%) | 36.52 ± 3.34 | 34.64 ± 2.55 | 0.225 | |
| Day 57 | White blood cells (10^3/μL) | 8.3 ± 2.26 | 8.61 ± 1.57 | 0.803 |
| Red blood cells (10^6/μL) | 8.1 ± 0.3 | 8,28 ± 0.49 | 0.302 | |
| Haemoglobin (g/dL) | 17.21 ± 0.35 | 18.28 ± 0.67 | 0.050* | |
| Haematocrit (%) | 42.5 ± 1.59 | 44.99 ± 2.21 | 0.072 | |
| Mean corpuscular volume (fL) | 53.16 ± 1.24 | 54.34 ±1.24 | 0.151 | |
| Mean corpuscular haemoglobin (pg) | 21.9 ± 1.24 | 22.10 ± 0.83 | 0.671 | |
| Mean corpuscular haemoglobin concentration (pg) | 41.2 ± 1.09 | 40.66 ± 0.78 | 0.372 | |
| Platelets (10^3/μL) | 916.2 ± 170.4 | 817.58 ± 94.44 | 0.254 | |
| Lymphocytes (%) | 83.94 ± 1.54 | 86.54 ± 1.22 | 0.012* | |
| CD4+ (%) | 60.62 ± 5.63 | 46.33 ± 7.62 | 0.013* | |
| CD8+ (%) | 40.70 ± 5.78 | 43.08 ± 3.36 | 0.414 |
Asterisks represent a significant difference between values (Student’s-t, p < 0.05). ND, not detected.
Fig. 2.
TBLF effect on lymphocyte-granulocyte ratio and serum cytokines. Rats were treated with TBLF (50 mf/kg) for six weeks every third day (43 days) and were without treatment for a further two weeks (Days 57). A) Determination of lymphocyte-granulocyte ratio by CBC. B) IL-6 and C) TNF-α determination on Days 43 and 57. Asterisks represent a significant difference between control and treated rats at each measurement day (Student’s-t, p ≤ 0.05).
Biochemical markers did not change on Day 9; on Day 22 the only data that showed significant difference was a decrease in insulin. At the end of the treatment period (Day 43) a decrease was observed in serum albumin and glucose, and after the two-week recovery period (Day 57), a decrease was observed in serum albumin and urea (Student’s-t, p < 0.05) (Table 2). These changes did not show a time-dependent tendency and could be related with individual sensitivity; however, it will be necessary to evaluate the effects of TBLF on digestibility and some other parameters of nutritional status [17,[26], [27], [28], [29]]. Some authors had shown detrimental effects of phytohaemagglutinin on insulin levels [30,31]; however, the only change observed with TBLF was on Day 22, which was not consistent through the treatment. At the end of the treatment and the recovery periods, no changes insulin levels were obseved, suggesting no physiologically relevant effects of TBLF on pancreatic production or secretion of insulin.
Table 2.
Biochemical markers after TBLF administration.
| Blood marker | Control | TBLF | p | |
|---|---|---|---|---|
| Day 9 | Albumin (mg/dL) | 3.67 ± 1.38 | 3.39 ± 1.87 | 0.13 |
| Total protein (mg/dL) | 4.4 ± 0.63 | 5.11 ± 0.73 | 0.22 | |
| α-Amylase (U/L) | 896.3 ± 216.5 | 886.7 ± 256.5 | 0.46 | |
| Urea (mmol/L) | 29.2 ± 3.7 | 31.4 ± 2.15 | 0.40 | |
| Serum creatine (mg/dL) | 0.59 ± 0.16 | 0.52 ± 0.03 | 0.49 | |
| GPT (U/L) | 60.6 ± 3.8 | 61.3 ± 13.8 | 0.90 | |
| Glucose (mg/dL) | 139.4 ± 8.4 | 128.9 ± 12.5 | 0.37 | |
| Insulin (μIU/mL) | 16.29 ± 3.29 | 15.53 ± 1.13 | 0.75 | |
| Day 22 | Albumin (mg/dL) | 3.83 ± 1.68 | 3.72 ± 1.9 | 0.95 |
| Total protein (mg/dL) | 5.05 ± 1.11 | 5.02 ± 1.02 | 0.34 | |
| α-Amylase (U/L) | 1219.2 ± 537.43 | 992.46 ± 183.7 | 0.65 | |
| Urea (mmol/L) | 33.35 ± 6.3 | 37.6 ± 6.5 | 0.38 | |
| Serum creatine (mg/dL) | 0.59 ± 0.08 | 0.6 ± 0.03 | 0.85 | |
| GPT (U/L) | 42.3 ± 8.5 | 60.25 ± 16.9 | 0.21 | |
| Glucose (mg/dL) | 133.5 ± 0.1 | 131.9 ± 4.3 | 0.69 | |
| Insulin (μIU/mL) | 20.23 ± 3.41 | 14.53 ± 1.00 | 0.04* | |
| Day 43 | Albumin (mg/dL) | 3.92 ± 1.65 | 3.89 ± 2.01 | 0.02* |
| Total protein (mg/dL) | 4.6 ± 1.57 | 5.22 ± 1.25 | 0.07 | |
| α-Amylase (U/L) | 1047.57 ± 187.15 | 712.35 ± 222.1 | 0.33 | |
| Urea (mmol/L) | 36.1 ± 8.5 | 42.4 ± 6.6 | 0.11 | |
| Serum creatine (mg/dL) | 0.57 ± 0.16 | 0.5± 0.10 | 0.28 | |
| GPT (U/L) | 46.0 ± 4.7 | 44.1 ± 7.5 | 0.56 | |
| Glucose (mg/dL) | 136.4 ± 13.1 | 121.2 ± 12.6 | 0.04* | |
| Insulin (μIU/mL) | 18.27 ± 5.05 | 16.50 ± 2.85 | 0.41 | |
| Day 57 | Albumin (mg/dL) | 5.04 ± 0.8 | 3.77 ± 1.01 | 0.01* |
| Total protein (mg/dL) | 4.6 ± 1.6 | 4.55 ± 1.74 | 0.95 | |
| α-Amylase (U/L) | 933.08 ± 245.53 | 848.5 ± 209.5 | 0.44 | |
| Urea (mmol/L) | 42.69 ± 9.7 | 33.7 ± 5.89 | 0.02* | |
| Serum creatine (mg/dL) | 0.54 ± 0.06 | 0.59 ± 0.12 | 0.26 | |
| GPT (U/L) | 44.6 ± 7.6 | 49.7 ± 12.5 | 0.46 | |
| Glucose (mg/dL) | 147.9 ± 14.1 | 144.4 ± 39.8 | 0.86 | |
| Insulin (μIU/mL) | 17.88 ± 3.05 | 16.80 ± 2.10 | 0.44 |
Asterisks represent a significant difference between values (Student’s-t, p < 0.05). ND, not detected.
3.2. Effects of TBLF on target organs
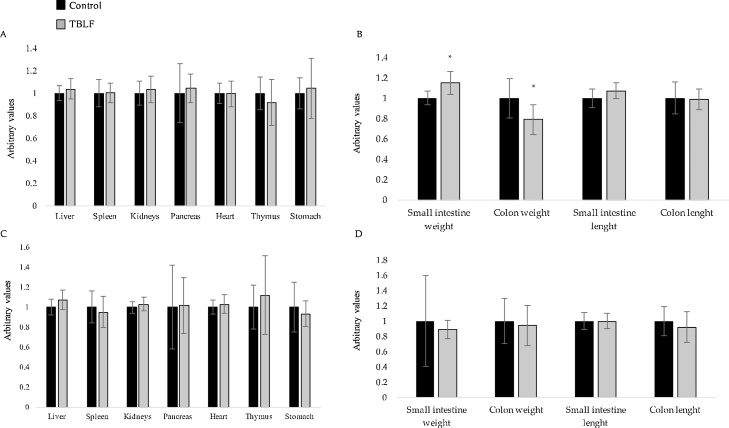
Organ weight and length for the complete treatment and the recovery period are shown in Fig. 3. Only small intestine and colon weights were affected after six weeks of TBLF administration. Small intestine weight of treated rats was 14% higher than that of control rats (Student’s-t, p = 0.02; Hotelling’s, p = 0.328). In contrast, colon weight in treated rats was 20% lower than in control rats (Student’s-t, p = 0.02).
Fig. 3.
Organ weight and length after TBLF administration. A) Organ weight after 43 days of treatment. B) Intestine weight and length after 43 days of treatment. C) Organ weight after two weeks of recovery. D) Intestine weight and length after two weeks of recovery. Organ weight and length were adjusted to body weight individually and normalised with respect to the control group mean (arbitrary units). Asterisks represent a significant difference between values (Student’s-t, p < 0.05).
Spleen weight was not affected by TBLF treatment; however, a non-significant increase of the white pulp was evident on Days 43 and 57 (Fig. 4), suggesting immune system activation. Germinal centres (GCs) are structures in which the maturation of the antibodies’ affinity takes place during the primary response. In this study, GCs did not show changes.
Fig. 4.
Spleen histopathology after TBLF treatment. A) 5 x Microphotographs of spleen after 43 days of treatment with TBLF and then a 14-day recovery period. a. White pulp, b. Red pulp, c. Germinal centres, d. Follicles. B) White pulp area. No significant differences between control and treated rats were observed (Student’s-t, p < 0.05). There were analysed 40 slices per group from at least 3 different animals.
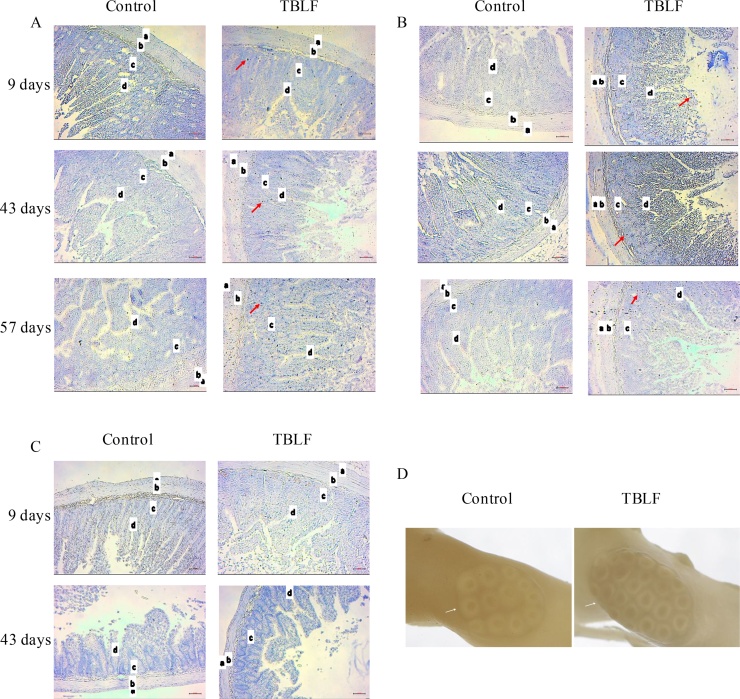
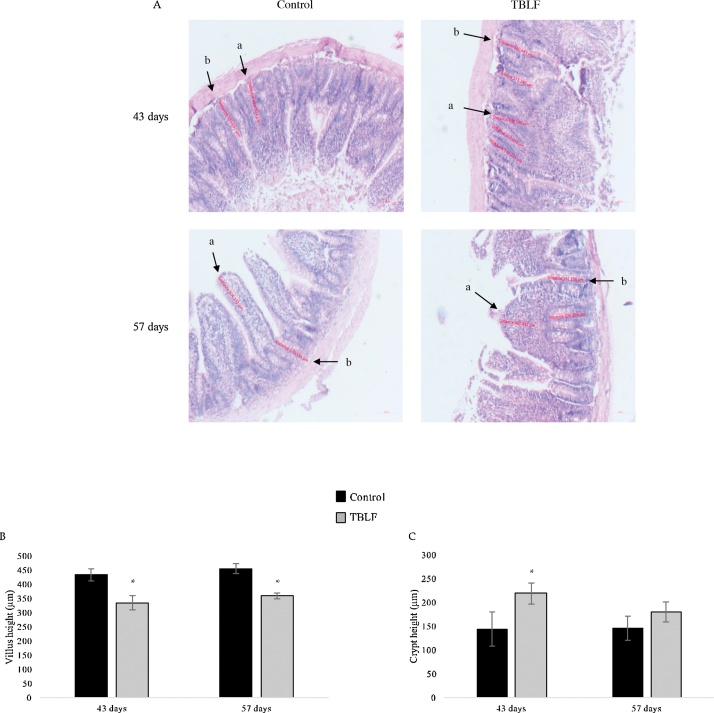
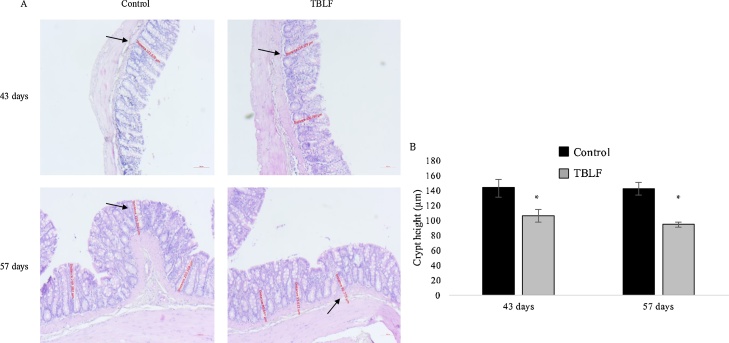
Macroscopic observation of intestines allowed us to perceive that TBLF treatment had produced thinning of intestinal tissue. Fig. 5 shows a photograph of control and treated duodenum in which it is possible to observe that Peyer’s patches in the TBLF-treated SD rats showed an increased number of lymphoid follicles, suggesting a hyperactivation of immune response. In the treated rats, thinning of the small intestine was apparent, exhibiting a translucent appearance. Immune cell activation was determined in Peyer’s patches by measuring the presence of IL-6, TNF-α and Nfκ-B. An increase of IL-6 and Nfκ-B was observed while no changes were evident for TNF-α. These results, together with the observed changes in lymphocyte-granulocyte ratio and the slight increase in the splenic white pulp at the end of the treatment suggest immune response activation [[32], [33], [34],22,25]. Upon evaluation of the histological sections of the ileum, the villi in treated intestines were found to display atrophy with respect to control intestines. It was possible to observe structural changes in which the villi tended to be broader at the base and thinner toward the apex, which gave them a triangular appearance as a result of a fusion of the villi, causing the crypt depth to decrease or disappear. After 43 days, the villi height decreased approximately 100 μm in the treated group with respect to the control group. After the 14-day recovery period, the treated intestines exhibited decreased villi height of approximately 75 μm with respect to the control group. The small intestine crypts’ depth increased 55 μm on average, with respect to control, throughout the treatment (Fig. 6). Fig. 7 shows the effect of TBLF treatment on colon epithelium. After the 43 days of treatment a significant decrease in crypt height was observed suggesting also colonic atrophy.
Fig. 5.
Immunohistochemical analyses for cytokines in duodenum Peyer’s patches after TBLF treatment. A) IL-6 analysis, B) Nfκ-B analysis, C) TNF-α analysis. All immunohistochemical analyses were performed at 10X resolution. Red arrows show positive signals; a) smooth muscle, b) primary follicle, c) lamina propria, d) M cells. D) The Peyer’s patches in the treated rats showed more lymphoid follicles (white arrows) and a thinner appearance than in control intestines. Photographs were taken using a Carl Zeiss macro 100 lens with extension tubes. There were analysed 40 slices per group from at least 3 different animals.
Fig. 6.
Histopathology analysis of ileum after TBLF treatment. A) 5 x microphotographs of ileum show that treated rats displayed atrophic villi (a) and an increase of crypt height (b) after 43 days of treatment that did not recover after the 14-day recovery period. B) Quantitative analysis of villi height. C) Quantitative analysis of crypt height. Asterisks represent a significant difference between the control and treated rats (Student’s-t, p < 0.05). There were analysed 40 slices per group from at least 3 different animals.
Fig. 7.
Histopathology analysis of colon after TBLF treatment. A) 5 x microphotographs of colon show crypt atrophy (black arrows) after 43 days of treatment that did not recover after the 14-day recovery period. B) Quantitative analysis of crypt height. Asterisks represent a significant difference between the control and treated rats (Student’s-t, p < 0.05). There were analysed 40 slices per group from at least 3 different animals.
TBLF resulted in intestinal atrophy. It has been reported that oral administration of other lectins can affect intestinal epithelium through binding to membrane carbohydrates, mainly in duodenum and jejunum, without a loss of biological functions [16]. Such effects can damage the intestinal wall which can compromise nutrient absorption and assimilation. This is the most significant anti-nutritional effect of lectins. Alterations have also been reported in microvilli functions (brush border), in addition to a decrease in bowel villi height and increased crypt depth as a function of lectin dose, administration route and exposure time [35,36]. Small intestine showed an increase in weight but not in length. Intestine growth is considered a compensatory effect of the interaction between lectins and intestinal villi with the purpose of increasing the absorption surface for nutrients. In contrast, colon weight was reduced by 20% with respect to control rats. This finding differs from that reported for peanut agglutinin, where an increase of up to 166% in cell proliferation in the proximal colon was observed [29]. However, it is well known that each lectin exhibits specific effects on cells [2].
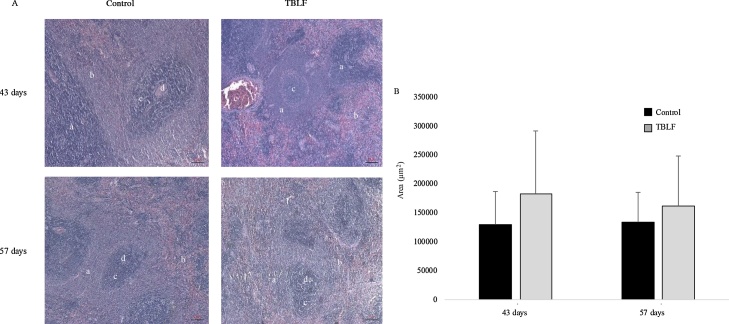
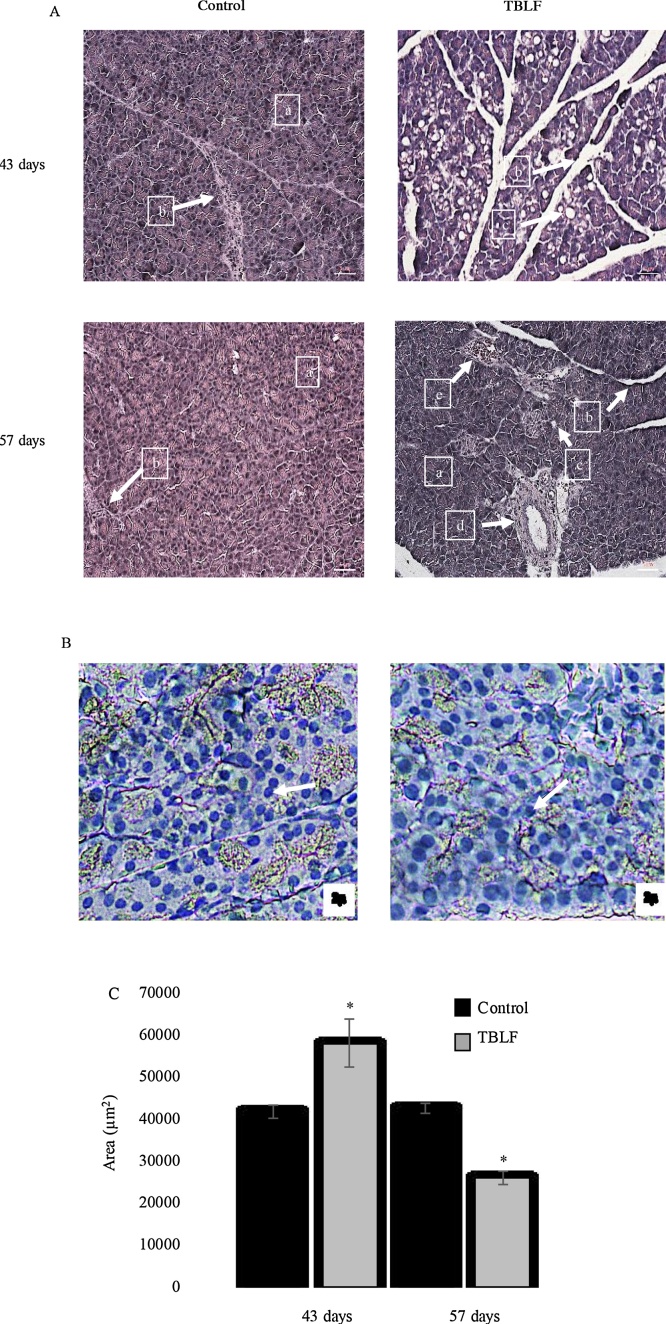
Normal nuclei of pancreatic acini are located in a central position, as in pancreatic acinar cells of the control rats, compared to the nuclei of acini of the treated rats, which were in the periphery. Pancreas histopathology showed an increase in pancreatic acini size in the treated rats at the end of treatment; the mean size of pancreatic acini was 41.5 × 103 ± 9.4 × 103 μm2 and 68.3 × 103 ± 31.9 × 103 μm2 for the control and treated groups, respectively, showing a significant difference (Student’s t, p = 0.011). This finding suggests pancreatic hypertrophy (Fig. 8). Also, the presence of vacuoles in the exocrine acinar cells were observed with a mean area of 849.6 ± 316.3 μm2, while the control group did not show them, lipid vacuoles were discarded by staining with oily red. The presence of vacuoles and the granulated content of the acini suggest hyperactivity. The morphology of normal pancreatic acinar cells is alveolar, whereas in the treated rats the cells were round, having lost their characteristic morphology. No inflammation or necrosis was observed in the pancreas nor was there any change in the organ’s endocrine gland. After two weeks of recovery, mean pancreatic acini area was 42.4 × 103 ± 6.3 × 103 μm2 and 26.5 × 103 ± 8.8 × 103 μm2 for the control and treated groups, respectively, with a significant difference (Student’s t, p = 0.0001). The decrease in acini area suggests a regression of the tissue, possibly as a compensatory process but in this case, it could be considered atrophy.
Fig. 8.
Exocrine pancreas histopathology after TBLF treatment. A) 10 x microphotographs of exocrine pancreas after 43 days of treatment with TBLF and then a 14-day recovery period. (a) Pancreatic acini, (b) Trabeculae (connective tissue), (c) Vacuoles, (d) Duct, (e) Blood vessel. B) 20 x microphotographs of exocrine pancreas after 43 days of treatment with TBLF stained with oily red for lipid detection. White arrows show haematoxylin-stained nuclei. C) Pancreatic acini area. Asterisks represent a significant difference between the control and treated rats (Student’s-t, p < 0.05). There were analysed 40 slices per group from at least 3 different animals.
Detrimental effects on small intestine, in consequence, affects other digestive organs such as the pancreas. We found exocrine pancreatic acini hypertrophy, where the digestive enzymes are secreted. As lipid drops were discarded by staining with oily red, the presence of vacuoles may be related to an exacerbated increase in pancreatic enzyme production, in order to compensate for the intestine atrophy and the digestion problems associated with TBLF administration. Small intestine maintains a very close communication with pancreas through intestinal hormones such as cholecystokinin and secretin. The down-regulation feedback of cholecystokinin secretion results in an increased pancreatic secretion of luminous enzymes (trypsin, chymotrypsin and amylase) in a compensatory manner [35,[37], [38], [39]]. Secretin has been observed as a trophic factor for pancreas growth in rats [40]. Serotonin is another important molecule secreted by the small intestine. It has been observed that piglets administered red kidney bean lectin showed increased serotonin-immunoreactive enterochromaffin cells in duodenum, suggesting a stimulating effect of gut maturation [41]. Therefore, it is necessary to study the effect of TBLF on cholecystokinin, secretin and serotonin secretion as well as other pancreatic enzymes. After the 14-day recovery period, atrophy of exocrine pancreas was observed, possibly as a regression by a compensatory process. Pancreas weight changes have been observed after administration with some lectins, such as with common bean and soybean lectins [42]. This is attributed to the low digestibility of the protein and dietary lipids in the intestine, due to a villous atrophy and disrupted permeability [43], which affects rats’ growth. Additionally, piglets treated with kidney bean lectins showed an increase in pancreatic acini area but no increase in enzyme content or pancreatic weight [36]; therefore, it is necessary to explore changes in pancreatic enzyme secretion following TBLF administration.
In summary, TBLF provoked atrophy in intestines that did not recover after 14 days of treatment rest. Exocrine pancreas showed hypertrophy, suggesting a compensatory effect with the purpose of compensation regarding digestive function, and after 14 days of treatment rest, pancreas showed atrophy, possibly in a recovery process. Neither biochemical markers nor body weight showed physiological changes. An immune activation was observed since the Peyer’s patches and the lymphocyte-granulocyte ratio showed reactivity.
4. Conclusions
TBLF has shown cytotoxic properties against cancer cells and an inhibitory effect of early precancerous lesions in rat colon. In order to proceed with the study of the anticancer properties of TBLF, it is necessary to determine the adverse effects of its administration in a pharmacological administration scheme. Previous works had shown that intragastric administration of TBLF at a dose of 50 mg/kg for six weeks, every third day, is enough to inhibit early precancerous lesion in colon, with no apparent systemic toxicity in rats; however, a loss in body weight gain was observed in addition to immune activation. Here we confirm that TBLF is able to stimulate the immune system, mainly because the lymphocyte-granulocyte ratio was affected at Day 9 of treatment and due to the increased lymphoid follicles in the Peyer’s patches. The major adverse effect of TBLF administration was intestinal atrophy, which did not recover to a normal state after the 14-day recovery period. Exocrine pancreas showed hypertrophy throughout the treatment, suggesting pancreatic overwork as compensation for intestinal digestion dysfunction. However, after 14 days of treatment rest, pancreas acini showed a possible atrophy, suggesting a compensatory regression. Our results demonstrate that, after the TBLF administration scheme used, 14 days of treatment rest are not sufficient to allow for complete recovery of affected organs. Further work will focus on the observed adverse effects and the recovery of normal function, mainly with regard to the digestive system, in order to contribute to a complete understanding of the undesirable effects.
Conflict of interest
The authors declare no conflicts of interest. The founding sponsors had no role in the design of the study, nor in the collection, analyses or interpretation of data, neither in the writing of the manuscript, nor in the decision to publish the results.
Acknowledgements
We thank CONACYT Ciencia Básica (CB-2014-01-241181), PFCE-2017, FOFI-UAQ 2015 for their financial support, José Leonardo Cardoso Villegas for his assistance in Peyer’s patch photography and Verónica Patricia Andrade Portillo for her veterinary assistance in the care, handling and sacrificing of animals. We also thank Elizabeth Mendiola-Olaya for her technical assistance, and Patrick Weill for his editing of the English language.
Contributor Information
Alejandro Blanco-Labra, Email: alejandroblancolabra@gmail.com.
Teresa García-Gasca, Email: tggasca@uaq.edu.mx.
References
- 1.Sharon N., Lis H. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology. 2004;14(11):53–62. doi: 10.1093/glycob/cwh122. [DOI] [PubMed] [Google Scholar]
- 2.Estrada-Martínez L.A., Moreno-Celis U., Cervantes-Jiménez R., Ferriz-Martínez R.A., Blanco-Labra A., García-Gasca T. Plant lectins as medical tools against digestive system cancers. Int. J. Mol. Sci. 2017;18:1403. doi: 10.3390/ijms18071403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pellegrina C.D., Rizzi C., Mosconi S., Zoccatelli G., Peruffo A., Chignola R. Plant lectin as carriers for oral drugs: is wheat germ agglutinin a suitable candidate? Toxicol. Appl. Pharmcol. 2005;207(2):170–178. doi: 10.1016/j.taap.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Gong F., Ma Y., Ma A., Yu Q., Zhang J., Nie H., Chen X., Shen B., Li N., Zhang D. A lectin from Chinese mistletoe increases gammadelta T cell mediated cytotoxicity through induction of caspase-dependent apoptosis. Acta Biochim. Biophys. Sin. 2007;39(6):445–452. doi: 10.1111/j.1745-7270.2007.00300.x. [DOI] [PubMed] [Google Scholar]
- 5.Petrossian K., Banner L., Oppenheimer S. Lectin binding and effects in culture on human cancer and non- cancer cell lines: examination of issues of interest in drug design strategies. Acta Histochem. 2007;109(6):491–500. doi: 10.1016/j.acthis.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferríz-Martínez R.A., Torres-Arteaga I.C., Blanco-Labra A., García-Gasca T. The New Approaches in the Treatment of Cancer. Nova Science Publishers; 2010. The role of plant lectins in cancer treatment; pp. 71–90. [Google Scholar]
- 7.García-Gasca T., García-Cruz M., Hernandez-Rivera E., López-Matínez J., Castañeda-Cuevas A.L., Yllescas-Gasca L., Rodríguez-Méndez A.J., Mendiola-Olaya E., Castro-Guillén J.L., Blanco-Labra A. Effects of Tepary bean (Phaseolus acutifolius) protease inhibitor and semipure lectin fractions on cancer cells. Nutr. Cancer. 2012;64:1269–1278. doi: 10.1080/01635581.2012.722246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang Q.L., Zhang S., Tian M., Zhang S.Y., Xie T., Chen D.Y., Chen Y.J., He J., Liu J., Ouyang L., Jiang X. Plant lectins, from ancient sugar-binding proteins to emerging anti-cancer drugs in apoptosis and autophagy. Cell Prolif. 2015;48(1):17–28. doi: 10.1111/cpr.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yau T., Dan X., Ng C.C., Ng T.B. Lectins with potential for anti-cancer therapy. Molecules. 2015;20(3):3791–3810. doi: 10.3390/molecules20033791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen L.W., Svenson R.H., Yachnin S. Purification of mitogenic proteins derived from Phaseolus vulgaris: isolation of potent and weak phytohemagglutinins possessing mitogenic activity. Proc. Natl. Acad. Sci. U. S. A. 1969;63(2):334–341. doi: 10.1073/pnas.63.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bock P.R., Friedel W.E., Hanisch J., Karasmann M., Schneider B. Efficacy and safety of long-term complementary treatment with standardized european mistletoe extract (Viscum album L.) in addition to the conventional adjuvant oncological therapy in patients with primary non-metastasized mammary carcinoma. results of a multi-center, comparative, epidemiological cohort study in Germany and Switzerland. Arzneimittelforschung. 2004;54(8):456–466. doi: 10.1055/s-0031-1296999. [DOI] [PubMed] [Google Scholar]
- 12.Kienle G.S., Kiene H. Review article: influence of Viscum album l (European mistletoe) extracts on quality of life in cancer patients: a systematic review of controlled clinical studies. Integr. Cancer Ther. 2010;9(2):142–157. doi: 10.1177/1534735410369673. [DOI] [PubMed] [Google Scholar]
- 13.Liener I.E. Antinutritional factors in legume seeds: state of the art. Recent advances of research in antinutritional factors in legume seeds. Proceedings of the First International Workshop on Antinutritional Factors (ANF) in Legume Seeds; Wageningen, The Netherlands; 1989. pp. 6–13. [Google Scholar]
- 14.Reynoso Camacho R., González de Mejía E., Loarca Piña G. Purification and acute toxicity of a lectin extracted from Tepary bean (Phaseolus acutifolius) Food Chem. Toxicol. 2003;41(1):21–27. doi: 10.1016/s0278-6915(02)00215-6. [DOI] [PubMed] [Google Scholar]
- 15.Osman M., Reid P., Weber Ch. The effect of feeding Tepary Bean (Phaseolus acutifolius) proteinase inhibitors on the growth a pancreas of young mice. Pak. J. Nutr. 2003;2(3):111–115. [Google Scholar]
- 16.Ferriz-Martínez R., García-García K., Torres-Arteaga I., Rodriguez-Mendez A.J., Guerrero-Carrillo M.J., Moreno-Celis U., Ángeles-Zaragoza M.V., Blanco-Labra A., Gallegos-Corona M.A., Robles-Álvarez J.P., Mendiola-Olaya E., Andrade-Montemayor H.M., Garcia O.P., García-Gasca T. Tolerability assessment of a lectin fraction from Tepary bean seeds (Phaseolus acutifolius) orally administered to rats. Toxicol. Rep. 2015;2:63–69. doi: 10.1016/j.toxrep.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pusztai A., Grant G., Duguid T., Brown D.S., Peumans W.J., Van Damme E.J., Bardocz S. Inhibition of starch digestion by a-amylase inhibitor reduces the efficiency of utilization of dietary proteins and lipids and retards the growth of rats. J. Nutr. 1995;125(6):1554–1562. doi: 10.1093/jn/125.6.1554. [DOI] [PubMed] [Google Scholar]
- 18.Rhodes J.M. Beans means lectins. Gut. 1999;44(5):593–594. doi: 10.1136/gut.44.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lajolo F., Genovese M. Nutritional significance of lectins and enzyme inhibitors from legumes. J. Agric. Food Chem. 2002;50(22):6592–6598. doi: 10.1021/jf020191k. [DOI] [PubMed] [Google Scholar]
- 20.González de Mejía E., Prisecaru V. Lectins as bioactive plant proteins: a potential in cancer treatment. Crit. Rev. Food Sci. Nutr. 2005;45:425–445. doi: 10.1080/10408390591034445. [DOI] [PubMed] [Google Scholar]
- 21.Campion B., Perrone D., Galasso I., Bollini R. Common bean (Phaseolus vulgaris L.) lines devoid of major lectin proteins. Plant Breed. 2009;128:199–204. [Google Scholar]
- 22.Pereira-da-Silva G., Carvalho F.C., Roque-Barreira M.C. Neutrophil activation induced by plant lectins: modulation of inflammatory processes. Inflamm. Allergy Drug Targets. 2012;11(6):433–441. doi: 10.2174/187152812803589985. [DOI] [PubMed] [Google Scholar]
- 23.NOM Norma Oficial Mexicana . Secretaría de Agricultura Ganadería, Desarrollo Rural, Pesca y Alimentación; 1999. Especificaciones técnicas Para La Producción, Cuidado Y Uso De Los Animales De Laboratorio. (NOM-062-ZOO-1999 http://www.ibt.unam.mx/computo/pdfs/bioterio.NOM-062.pdf) [Google Scholar]
- 24.Alemán C.L., Más R.M., Rodeiro I., Noa M., Hernández C., Menéndez R., Gámez R. Reference database of the main physiological parameters in Sprague-Dawley rats from 6 to 32 months. Lab. Anim. 1998;32(4):457–466. doi: 10.1258/002367798780599802. [DOI] [PubMed] [Google Scholar]
- 25.Dienz O., Rincon M. The effects of IL-6 on CD4 T cell responses. Clin. Immunol. 2009;130:27–33. doi: 10.1016/j.clim.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakata S., Kimura T. Effect of ingested toxic bean lectins on the gastrointestinal tract in the rat. J. Nutr. 1985;115(12):1621–1629. doi: 10.1093/jn/115.12.1621. [DOI] [PubMed] [Google Scholar]
- 27.Palmer R.M., Pusztai A., Bain P., Grant G. Changes in rates of tissue protein synthesis in rats induced in vivo by consumption of kidney bean lectins. Comp. Biochem. Physiol. 1987;88(1):179–183. [Google Scholar]
- 28.Pusztai A., Bardocz S. Biological effects of plant lectins on the gastrointestinal tract: metabolic consequences and applications. Trends Glycosci. Glicotechnol. 1996;8(41):149–165. [Google Scholar]
- 29.Jordinson M., Goodlad R.A., Brynes A., Bliss P., Ghatei M.A., Bloom S.R., Fitzgerald A., Grant G., Bardocz S., Pusztai A., Pignatelli M., Calam J. Gastrointestinal responses to a panel of lectins in rats maintained on total parenteral nutrition. Am. J. Physiol. 1999;276:1235–1242. doi: 10.1152/ajpgi.1999.276.5.G1235. [DOI] [PubMed] [Google Scholar]
- 30.Bardocz S., Grant G., Pusztai A. The effect of phytohaemagglutinin at different dietary concentrations on the growth, body composition and plasma insulin of the rat. Br. J. Nutr. 1996;76(4):613–626. doi: 10.1079/bjn19960067. [DOI] [PubMed] [Google Scholar]
- 31.Santidrián S., de Moya C.C., Grant G., Frühbeck G., Urdaneta E., García M., Marzo F. Local (gut) and systemic metabolism of rats is altered by consumption of raw bean (Phaseolus vulgaris L var Athropurpurea) Br. J. Nutr. 2003;89(3):311–319. doi: 10.1079/BJN2002777. [DOI] [PubMed] [Google Scholar]
- 32.Cesta M.F. Normal structure, function, and histology of the spleen. Toxicol. Pathol. 2006;34(5):455–465. doi: 10.1080/01926230600867743. [DOI] [PubMed] [Google Scholar]
- 33.Hayden M.S., West A.P., Ghosh S. NF-kappaB and the immune response. Oncogene. 2006;25:6758–6780. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 34.Pellegrina C.D., Perbellini O., Scupoli M.T., Tomelleri C., Zanetti C., Zoccatelli G., Fusi M., Peruffo A., Rizzi C., Chignola R. Effects of wheat germ agglutinin on human gastrointestinal epithelium: insights from an experimental model of immune/epithelial cell interaction. Toxicol. Appl. Pharmacol. 2009;237(2):146–153. doi: 10.1016/j.taap.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Hara T., Mukunoki Y., Tsukamoto Miyoshi M., Hasegawa K. Susceptibility of Kintoki bean lectin to digestive enzymes in vitro and its behavior in the digestive organs of mouse in vivo. J. Nutr. Sci. Vitaminol. (Tokio) 1984;30(4):381–394. doi: 10.3177/jnsv.30.381. [DOI] [PubMed] [Google Scholar]
- 36.Radberg K., Biernat M., Linderoth A., Zabielski R., Pierzynowski S.G., Westrom B.R. Enteral exposure to crude red kidney bean lectin induces maturation of the gut in suckling pigs. J. Anim. Sci. 2001;79(10):2669–2678. doi: 10.2527/2001.79102669x. [DOI] [PubMed] [Google Scholar]
- 37.Dillberger J.E. Age-related pancreatic islet changes in Sprague-Dawley rats. Toxicol. Pathol. 1994;22(1):48–55. doi: 10.1177/019262339402200107. [DOI] [PubMed] [Google Scholar]
- 38.Grant G., Edwards J.E., Ewan E.C., Murray S., Atkinson T., Farningham D.A., Pusztai A. Secretion of pancreatic digestive enzymes induced in rats by first-time oral exposure to kidney bean E2L2 lectin is mediated only in part by cholecystokinin (CCK) Pancreas. 1999;19(4):382–389. doi: 10.1097/00006676-199911000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Grant G., Alonso R., Edwards J.E., Murray S. Dietary soya beans and kidney beans stimulate secretion of cholecystokinin and pancreatic digestive enzymes in 400-day-old Hooded-Lister rats but only soya beans induce growth of the pancreas. Pancreas. 2000;20(3):305–312. doi: 10.1097/00006676-200004000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Morisset J. Seventy years of pancreatic physiology: take a look back. Pancreas. 2014;43(8):1174–1184. doi: 10.1097/MPA.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 41.Zacharko-Siembida A., Valverde Piedra J.L., Strzalka B., Arciszewski M.B. Red kidney bean (Phaseolus vulgaris) lectin stimulation increases the number of enterochromaffin cells in the small intestine of suckling piglets. Bull. Vet. Inst. Pulawy. 2014;58(2):289–294. [Google Scholar]
- 42.Kelsall A., Fitzgerald A.J., Howard C.V., Evans R.C., Singh R., Rhodes J.M., Goodlad R.A. Dietary lectins can stimulate pancreatic growth in the rat. Int. J. Exp. Pathol. 2002;83(4):203–208. doi: 10.1046/j.1365-2613.2002.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Y., Qin G., Sun Z., Che D., Bao N., Zhang X. Effects of soybean agglutinin on intestinal barrier permeability and tight junction protein expression in weaned piglets. Int. J. Mol. Sci. 2011;12(12):8502–8512. doi: 10.3390/ijms12128502. [DOI] [PMC free article] [PubMed] [Google Scholar]