Abstract
Introduction
Glioblastomas (GBM) may originate de novo (primary), or following transformation from a lower grade glioma (secondary), and it has been postulated that these tumors may have different biological behaviors.
Methods
We performed a correlative analysis involving 204 patients with glioma treated prospectively on NCCTG clinical trials. Central pathology review of tumor tissues taken at the time of initial diagnosis and at recurrence were performed in all patients.
Results
Tumors progressed from low (WHO grade 2) to high (grade 3–4) at recurrence in 45% low grade oligodendroglioma patients, in 70% with low grade oligoastrocytoma, and 74% with low grade astrocytoma (p=0.031). Median overall survival (OS) from initial diagnosis varied by histology: oligodendroglioma, 8.8 years; (95% CI 5.7–10.2); oligoastrocytoma, 4.4 years (95% CI 3.5–5.6); astrocytoma grade 2 3.1 years (astrocytoma grade 2–4, 2.1 years) (95% CI 1.7–2.5, p<0.001). Mean time to recurrence (TTR) also varied between patients with de novo GBM, those secondary GBM, and those that remained non-GBM at recurrence (1.1 ± 1.1 years vs. 2.9 ± 1.8 vs. 4.0 ± 2.9, respectively, p < 0.001). Median OS from time of recurrence also varied between these three categories (0.7 years, 95% CI: 0.5–1.1 vs.0.6 years, CI: 0.5–1.0 vs. 1.4 years, 95% CI: 1.1–2.0, respectively) (p <0.001).
Conclusions
At time of relapse, transformation to higher grade is frequent in low grade pure and mixed astrocytomas, but is observed in less than half of those with low grade oligodendroglioma. From time of recurrence, OS was not significantly different for those with primary versus secondary GBM, and it may thus be reasonable include patients with secondary GBM in clinical therapeutic trials for recurrent disease.
Keywords: Glioblastoma, Low Grade Glioma, Survival, de-differentiation
Introduction
Glioblastomas may present de novo (primary), or secondarily, following transformation from a prior lower grade glioma (LGG). It has been postulated that the outcome of secondary GBM patients is more favorable. However, it is not clear whether survival of secondary GBM patients varies as a function of initial histology, or whether survival also differs from the time of relapse for patients with de novo vs. secondary GBM.
To better define these associations, we performed a subset analysis involving patients who, during the process of evaluation of eligibility for NCCTG clinical trials, had central pathology review of tumor tissues obtained at both the time of original diagnosis and from time of recurrence. As all patients were subsequently treated on at least one NCCTG clinical trial, accurate and prospectively-obtained demographic and survival data were available for analysis. The goals of this study were to correlate GBM status (primary vs. secondary) with survival outcome from time of first relapse, and also to determine the frequency of transformation of initially low grade tumors as a function of histologic subtype.
Methods
The NCCTG database utilized for this analysis was comprised of 2421 glioma patients treated on at least one NCCTG prospective Phase II or III trial either for newly diagnosed (N=1893) or recurrent disease (N=528), during the time period from 1985–2005. From this group, we focused the analysis on the subset of patients (N=224) in whom tumor tissues from both the time of initial surgical diagnosis and from time of surgery for first relapse were available for central pathology review by an officially designated NCCTG neuropathologist (B.S or C.G.). Patients with surgery for recurrence < 90 days from the initial surgical diagnosis were excluded (n=15), as were patients with Grade 1 gliomas (N=5), leaving a subset for the final analysis of 204 patients. The distribution of patients with paired specimens as a function of the disease status (newly diagnosed or recurrence) at time of enrollment on an NCCTG clinical trial is depicted in Table 1, and the specific NCCTG Clinical Trials (14 trials for newly diagnosed disease, 14 for recurrent disease) on which the patients in this subset were enrolled is delineated in Table 2.
TABLE 1.
Disease status at time of NCCTG clinical trial enrollment; glioma patients with paired tumor specimens from initial diagnosis and recurrence
| Disease Status at Enrollment | N |
|---|---|
|
| |
| Newly Diagnosed Disease | 85 |
| Recurrent Disease | 87 |
| Both Newly Diagnosed and Recurrence* | 36 |
|
| |
| Total | 208 |
enrolled on 2 sequential NCCTG trials
TABLE 2.
NCCTG clinical trial distribution among glioma patients with paired tumor specimens from initial diagnosis and recurrence
| NCCTG trial, Newly Diagnosed Glioma | N | Glioma Grade(s) | Study Phase | Study Treatment |
|---|---|---|---|---|
| 857251 | 6 | HGG | III | PCNU vs. BCNU |
| 860351 | 2 | HGG | Pilot | AHRT+BCNU |
| 867251 | 50 | LGG | III | Low vs High Dose RT |
| 887202 | 2 | HGG | II | RT+BCNU+Interferon |
| 887252 | 15 | HGG | III | RT+BCNU +/− Interferon |
| 907201 | 1 | HGG | I–II | RT+ SRS+BCNU |
| 917201 | 2 | HGG | Pilot | BCNU+CDDP+VP16 |
| 927203 | 3 | HGG | I | BCNU+CDDP+VP16 |
| 937202 | 8 | LGG | II | PCV |
| 937252 | 24 | GBM | III | RT vs AHRT, and BCNU vs BCNU+CDDP |
| 987251 | 1 | AA | II | Pre-RT BCNU+CDDP+VP16 |
| 987252 | 5 | GBM | II | Pre-RT BCNU+CDDP+VP16 |
| N0074 | 1 | GBM | II | RT+ Gefinitib |
| N0177 | 1 | GBM | I–II | RT+TMZ+Erlotinib |
| Total | 121 |
| NCCTG Trial, Recurrent Glioma | N | Glioma Grades | Study Phase | Study Treatment |
|---|---|---|---|---|
| 847251 | 1 | All | II | fludarabine |
| 867202 | 14 | All | II | interferon+DFMO |
| 867253 | 6 | All | II | interferon+BCNU |
| 887251 | 3 | All | II | ifosfamide+mesna |
| 897251 | 6 | All | II | 5-fluorouracil+citrovorum |
| 897252 | 10 | All | II | amonafide |
| 917251 | 18 | All | II | MOP |
| 927251 | 7 | All | II | topotecan |
| 937251 | 2 | All | II | cladribine |
| 957253 | 10 | All | II | DTIC |
| 967251 | 25 | All | II | Irinotecan |
| 987254 | 8 | GBM | II | pyrazoloacridine+CBDCA |
| N0272 | 5 | AO | II | imatinib |
| N997B | 8 | GBM | II | temsirolimus |
| Total | 123 |
GBM=glioblastoma; HGG = high grade glioma ( WHO grade 3 or 4); AG= anaplastic glioma (WHO Grade 3); AA= anaplastic astrocytoma (WHO grade 3); LGG=low grade glioma (WHO grade 2); AO= anaplastic oligodendroglioma (WHO grade 3); AHRT= accelerated hyperfractionated radiotherapy; PCNU=chloroethyldioxypiperidylnitrosourea; BCNU+bischlorethylnitrosurea; CDDP=cis-platinum; VP16=etoposide; PCV=procarbazine, CCNU and vincristine; DFMO=difluoromethylornithine; MOP=nitrogen mustard, vincristine and procarbazine; DTIC=dacarbazine; CBDCA=carboplatinum
From the database, we identified the subset of 204 patients who had paired tumor samples from time of both initial diagnosis and recurrence in two ways. In the newly diagnosed patients (N=121), tumor tissues were reviewed as a eligibility requirement for an NCCTG trial; subsequently, the tumor tissues from time of recurrence were either obtained when the patient enrolled on a subsequent NCCTG trial (N=36) or were otherwise reviewed by our NCCTG neuropathologists at the time of surgery for recurrence at the Mayo Clinic Rochester (N=85). The remaining patients in the dataset had all undergone surgery for relapse, just prior to enrollment on an NCCTG trial for recurrent disease (N=123). In this latter group, tumor tissues from the time of recurrence, and also comparative tissues from the time of initial surgical diagnosis, were centrally reviewed in the process of determination of eligibility for the NCCTG recurrent disease trial. As expected, not all patients in the NCCTG database overall had tissues available from both time points for central review.
All patients provided informed written consent for the specific NCCTG clinical trial on which they were treated, which included evaluation of available brain tumor tissues from all prior resections or biopsies. All protocols were approved by the individual Institutional Review Boards at the NCCTG investigational sites.
The NCCTG is a NCI Clinical Cooperative Group comprised of multiple participating community-based treatment sites, and the Mayo Clinic sites, which serves as the reseach base. The NCCTG neuro-oncology database involving previous and ongoing clinical trials is maintained at he Mayo Clinic in Rochester, MN. This database has evolved over the time of this analysis, but in general collects baseline demographic data and eligibility information, pre-registration central pathology review reports; and specific on-treatment and post-treatment information, including but not limited to: treatment, toxicity and outcome data (1).
Statistical methods
Variables in the analysis included histologic subtype, tumor grade, extent of resection, overall survival from time of initial diagnosis, overall survival from time of recurrence, time to recurrence from initial diagnosis, and time to second relapse from time of initial recurrence. Extent of resection was accepted as determined by the individual NCCTG site investigators, and was based on the composite of operative reports and post-resection imaging factors. The chi-square test of analysis of variance was used to compare categorical and continuous variables across the selected groups. Cumulative survival probabilities were estimated using the Kaplan-Meier method. The log-rank test was used to compare survivals of groups. In addition, in survival comparisons, proportional hazards regression models were fit, and age was included as a covariate. In all cases, two-sided p-values <0.05 were considered statistically significant.
Results
At initial diagnosis, the extent of surgical resection was known for 175 of the 204 (86%) patients, and included: biopsy only (N=58, 33%); subtotal resection (N=79, 45%), and gross total resection (N=38, 22%). The extent of resection at time of recurrence was known for 64 of the 204 (31%) patients, and included biopsy only (N=17, 27%), subtotal resection (N=32, 50%), and gross total resection (N=15, 23%).
Of those with pure grade 2 astrocytoma, 12/15 (80%) had biopsy as their only initial surgery, as did 31/71 (44%) of those with pure grade 2 oligo or mixed oligoastrocytoma (p=0.021). In comparison, 15/89 (17%) of grade 3 glioma patients in whom extent of resection was verified at time of initial surgery had biopsy only.
Based on pathologic review of tumor tissues from both the time of initial diagnosis and recurrence, there were a total of 99 patients who initally had low grade (i.e. grade 2) tumors. We identified evidence of transformation (i.e., grade 2 to grade 3–4) in a total of 60 of these patients: 14 of 19 (74%) astrocytomas, 28 of 40 (70%) oligoastrocytomas (OA), and 18 of 40 (45%) oligodendrogliomas, respectively (p=0.031) (Table 3).
TABLE 3.
COMPARISON OF HISTOLOGIC DIAGNOSIS INITIAL SURGERY AND AT RECURRENCE
| Histology at Initial Diagnosis | Histology at Recurrence | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Astro 2 | Astro 3 | GBM | OA 2 | OA 3–4 | Oligo 2 | Oligo 3–4 | |
| Astro 2 | 19 | 2 | 6 | 6 | 2 | 2 | 1 | 0 |
| Astro 3 | 19 | 0 | 8 | 10 | 0 | 1 | 0 | 0 |
| GBM | 71 | 0 | 0 | 71 | 0 | 0 | 0 | 0 |
| OA 2 | 40 | 1 | 4 | 9 | 10 | 13 | 1 | 2 |
| OA 3–4 | 11 | 0 | 1 | 2 | 0 | 8 | 0 | 0 |
| Oligo 2 | 40 | 1 | 1 | 0 | 3 | 8 | 18 | 9 |
| Oligo 3–4 | 4 | 0 | 0 | 0 | 0 | 2 | 0 | 2 |
| Total | 204 | 4 | 20 | 98 | 15 | 34 | 20 | 13 |
Astro = astrocytoma; OA = oligoastrocytoma; GBM = glioblastoma; Oligo = oligodendroglioma. Bolded numbers indicate the patients who transformed from a low-grade to high-grade tumor.
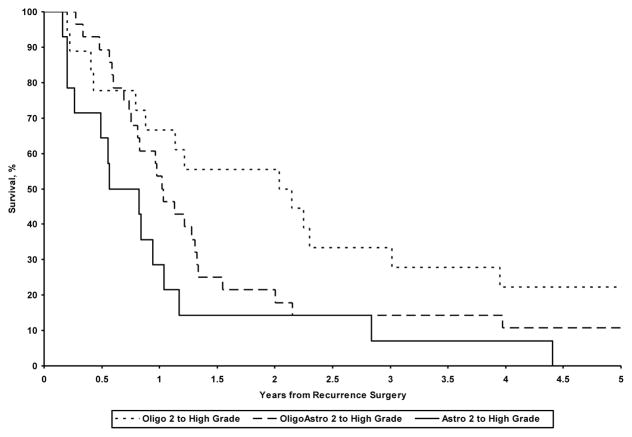
The median overall survival (OS) from the time of initial diagnosis differed as a function of histologic subtype: astrocytoma grade 2, 3.1 years (95% CI: 1.8, 5.1); oligoastrocytoma grade 2, 4.5 years (95% CI: 4.0, 5.9); oligodendroglioma grade 2, 8.8 years (95% CI: 5.8, 12.6), astrocytoma grade 3, 4.1 years (95% CI: 1.9, 5.0), glioblastoma, 1.7 years (95%CI: 1.5, 2.2), oligoastrocytoma grade 3–4 3.2 (95% CI: 2.0, 5.9), oligodendroglioma grade 3–4, 6.1 years (95% CI: 2.9, 9.9) (p<0.001). Median survival from time of recurrence also differed as a function of histologic subtype for patients who transformed from a low-grade to a high-grade glioma (Figure 1, p=0.026): astrocytoma grade 3–4, 0.7 years (95% CI: 0.3, 1.0); oligoastrocytoma grade 3–4, 1.0 years (95% CI: 0.8, 1.3); and oligodendroglioma grade 3, 2.1 yrs (95% CI: 0.9, 3.0).
Figure 1.
Comparison of overall survival from the time of recurrence as a function of initial histology: low grade tumors transforming to high grade at recurrence
The mean ± SD time to recurrence also differed between patients with de novo GBM, those with non-GBM at initial surgery and GBM at recurrence, and those with non-GBM at both time points: 1.1±1.1 years, 2.9 ± 1.8 years, and 4.0 ± 2.9 years , respectively (p<0.001).
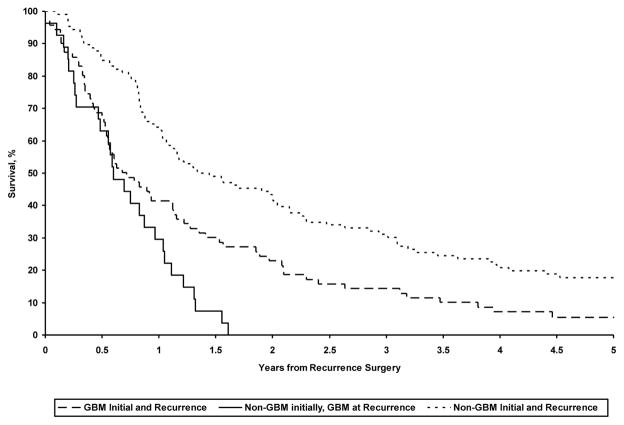
Survival from the time of recurrence also differed as a function of tumor grade. Median overall survival, measured from the time of recurrence, was 0.7 years (95% CI: 0.5, 1.1) in patients presenting with GBM at initial diagnosis and at recurrence; median survival those with low grade glioma at initial diagnosis, but GBM at recurrence was 0.6 years (95% CI: 0.5, 1.0); median survival for those with non-GBM initially and at recurrence was 1.4 years (95% CI: 1.1, 2.0) (p <0.001). (Figure 2) In direct comparisons of patients presenting with GBM initially, versus those who were found to have GBM only at recurrence, OS differed from the time of recurrence, although the magnitude of the difference was small (0.7 vs 0.6 yrs. P=0.01).
Figure 2.
Comparison of overall survival from the time of recurrence for primary and secondary GBM patients
Discussion
Glioblastomas may present de novo (primary), or develop, presumably by transformation, from a lower grade (secondary) glioma. In general, the diagnosis of secondary GBM is made when there is histologic evidence of GBM at time of recurrence, but a history of a prior low grade glioma; or, when there is pathologic evidence of both Grade 2–3 glioma, as well as GBM in the tissue specimen removed at the time of recurrence. Secondary GBMs are relatively rare, constituting only 5% of cases (2, 3). It is logical to assume that patients with a history of a lower grade tumor who subsequently develop GBM might have better overall outcome, but this remains unproven. The median overall survival of secondary GBM patients appears longer than de novo GBM patients (7.8 vs. 4.7 mos., p=0.003), although this difference does not remain significant after adjusting for age (4, 5).
Certain clinical factors have been associated with poorer outcome in LGG patients, presumably reflecting earlier transformation and/or more aggressive growth characteristics. The European Organisation for Research and Treatment of Cancer (EORTC) developed a scoring system which correlated with poorer outcome, based on the number of less favorable prognostic indicators, which included age ≥ 40 years, presence of an astrocytic component, tumor size ≥ 6 cm, midline involvement, and presence of neurologic deficit before surgery. Low risk patients (score 0–2) had a median OS of 7.7 years, vs. 3.2 years for high risk (score 3–5) patients (6). A UCSF scoring system, based on pre-operative status, also predicted poorer outcome (PFS and OS) for patients with a larger number of less favorable variables, which included age > 50, Karnofsky Performance Status ≤ 80, tumor > 4 cm, or location in ‘eloquent’ areas of brain. (7). In addition to size and midline involvement, there is some evidence that high baseline relative cerebral blood volume (rCBV) in LGG as determined by perfusion MRI may be more common in tumors likely to progress early or undergo transformation (8). Recently, evidence of growth of LGG at 6 months on MRI was a better independent predictor of growth and patient survival that was baseline tumor volume, rCBV or diffusion coefficient determinations (9). Also, LGG with areas of hypermetabolism as determined by 2-FDG PET imaging appear to have a shorter time to progression (10).
In our current study, progression from an initially low grade glioma to high grade at time of recurrence was observed in over 60% of patients, and the frequency varied as a function of initial histology. Transformation was less frequent in pure oligodendroglioma (approximately 45%), but common for either pure low grade astrocytoma or low grade mixed oligoastrocytoma (approximately 70% for both). The frequency of transformation of mixed oligoastrocytoma was as frequent as with pure astrocytoma. The reason for this is unclear, but presumably the transforming element is the astrocytic component. Although not ascertained in the current study, it is known that the frequency of co-deletion in mixed oligoastrocytoma (26%) and pure astrocytoma (8%) is lower than with pure oligodendroglioma (44%). This theoretically could explain this difference, as co-deletion has been associated with longer survival. In our study, the survival from original diagnosis and also from recurrence did vary significantly as a function of histology, and as expected was longer in patients with pure oligodendrogliomas initially. In a prior report, the frequency of 1p19q co-deletion did not appear to differ between newly diagnosed and recurrent patients (for those with oligodendroglioma, 39 vs 56%; those with mixed glioma, 21 vs 33%; and those with pure astrocytoma 8 vs 6%, respectively) (11). However, a potential shortcoming of that analysis was that tissues were not paired from the same patients.
A recent NCCTG study showed that a shorter time interval from initial diagnosis to recurrence was an independent prognostic factor favoring longer survival for patients with recurrent malignant (Grade 3–4) glioma (1). Our current study extends that observation, in that our secondary GBM patients, with longer intervals since original diagnosis as compared to primary GBM patients, experienced similar (actually slightly shorter) survival as compared to primary GBM patients, from the time of recurrence. One explanation might be that some de novo GBM patients progressed or died early, or otherwise were not candidates for a second surgery, which might have enriched our study population for the ‘longer living’ de novo GBM patients. However, it is also conceivable that ‘secondary’ glioblastomas may be more aggressive following transformation at recurrence than de novo GBMs. In fact, stem cells derived from recurrent glial tumors which have undergone malignant progression from lower grade have been reported as more aggressive than stem cells derived from de novo GBM (12).
In our analysis, slightly more than one-quarter of our patients had biopsies, as opposed to more extensive resections, at either initial diagnosis or recurrence. With biopsy, there is always the possibility of pathology sampling error, and theoretically, if a large group of patients in the study cohort have sample error, outcome data may be affected in the analysis. This problem technically could be an explanation for the relatively short survival (3.1 years) of the population of grade 2 astrocytoma patients, although this would be difficult to prove. It is not entirely clear whether patients with LGG who undergo biopsy have shorter OS than those with more extensive subtotal resections. Some investigators have reported longer survival with greater extent of resection (13), and decreased relapse rates have been observed in patients with postoperative residual tumor of < 1 cm as opposed to > 1 cm (14). Although it appears that more extensive resection affects time to progression and relapse rate, the effect on overall survival is still a matter of debate. In one large series which specifically evaluated prognostic factors in LGG, extensive resection (>90%) was associated with a lower hazard ratio as compared with <90% resection in the univariate analysis, but this difference did not remain significant in the multivariable analysis. (15). Extent of resection is not one of the four prognostic factors (eloquent location; Karnofsky performance status less than 90; age > 50 years, and tumor diameter > 4 cm) utilized in the UCSF Scoring System for low grade gliomas, which has been validated in a multi-institutional analysis. (16)
What is more compelling as a potential explanation for the relatively short survival observed in our low grade glioma patients is that the inherent tumor biology may have been less favorable. Our analysis was based on a study population collected over 20 years, in studies conducted prior to our current understanding of the importance of tumor biomarkers. During this time, not only has the conventional histologic classifications of glioma evolved, but the field has also seen early implementation of prognostic molecular markers (such as 1p/19q co-deletion status, and the presence of IDH1 mutations) in clinical trials and practice. Although 1p/19q status was performed on some of the patients in our database, this data was not prospectively collected on most of the studies, and the available numbers were too small for meaningful analyses. Assays for IDH1 mutations were not performed in any of the studies, which predated this finding. Other factors are likely important, including amplification of EGFR, and gene promotor hypermethylation profile. (17, 18)
In future clinical trials, several biomarkers which measure tumor intracellular signal processing, receptor status, gene, transcriptome, and protein expression profiles will likely play an increasing role in determining prognosis and possibly, prediction of response to specific therapies. It is quite possible that tumor profiling may render the ‘primary’ and ‘secondary’ designations obsolete in the near future. Already, unsupervised global genomic analysis has identified a specific genomic subclass for de novo GBM, and two distinctly different genomic subclasses for secondary GBM (19). De novo GBMs typically have mutations in EGFR, PTEN and INK4A/ARF/CDKN2A, while secondary GBMs usually display PDGF and TP53 alterations (20). Increased EGFR and CDK4 amplification, and fewer p53 alterations have been reported more frequently with de novo GBM (21). Survivin, which is associated with anti-apoptotic activity, has been more frequently observed in de novo GBMs as compared with secondary GBMs (83 vs. 46%, p<0.001); additionally, the time to progression from low grade glioma to secondary GBM was shorter in survivin-positive as compared to survivin-negative cases (mean 16 vs. 24 mos., P<0.05) (22). Upregulation of ASCL1 and inhibition of Notch signaling pathway may also characterize secondary GBM (23). Her-2 expression has been reported to be higher in de novo GBM and low in secondary GBM; high expression correlated with poorer survival (24). Recently, the presence of an IDH-1 mutation was reported to correlate with longer survival (27.1 vs 11.3 mos., p<0.0001) and was more frequent in secondary GBM than de novo GBM (73% vs 3.7%, p < 0.0001) (25). In our patient subset, we were not able to perform these intriguing correlative studies, due to lack of sufficient tissue. However, it is likely that some of these and other biomarkers might be utilized as stratification factors, or even for determination of eligibility in future clinical therapeutic trials.
The high rate of transformation observed in this analysis (70% in both the grade 2 pure astrocytoma and mixed astrocytoma subsets) adds support for the argument that ‘low grade’ gliomas are not necessarily benign.. This data may strengthen the argument for early intervention as opposed to ‘‘watchful waiting”. Both PFS and OS of low grade glioma patients have been reported to be longer following earlier resection. (26)
Another important observation from our study is that the survival of secondary GBM patients was not longer than that of de novo GBM patients, as measured from the time of recurrence. With the exception of patients with a history of low grade oligodendroglioma, our data suggests that in clinical therapeutic trials for recurrent GBM which utilize a survival endpoint, it is reasonable to consider both primary and secondary GBM patients as eligible for inclusion.
Footnotes
Disclosures: The authors have no relevant conflicts of interest to disclose
References
- 1.Wu W, Lamborn KR, Buckner JC, Novotny P, Chang SM, O’Fallon JR, Galanis E, Jaeckle KA, Prados MD. Joint NCCTG and NABTC prognostic factors analysis for high grade recurrent glioma. Neuro Oncol. 2010;12(2):164–72. doi: 10.1093/neuonc/nop019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohgaki H, Kleihues Genetic pathways to primary and secondary glioblastoma. Am J Path. 2007;170:1445–53. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dropcho EJ, Soong SJ. The prognositic impact of prior low grade histology in patients with anaplastic gliomas: a case-control study. Neurology. 1996;47:684–90. doi: 10.1212/wnl.47.3.684. [DOI] [PubMed] [Google Scholar]
- 4.Ohgaki H, Dessen P, Jourde B, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64:6892–99. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- 5.Ohgaki H, Kleihues Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64:479–89. doi: 10.1093/jnen/64.6.479. [DOI] [PubMed] [Google Scholar]
- 6.Pignatti F, van den Bent M, Curran D, et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20:2076–2084. doi: 10.1200/JCO.2002.08.121. [DOI] [PubMed] [Google Scholar]
- 7.Chang EF, Smith JS, Chang SM, et al. Preoperative prognosic classification system for hemispheric low-grade gliomas in adults. J Neurosurg. 2008;109:817–24. doi: 10.3171/JNS/2008/109/11/0817. [DOI] [PubMed] [Google Scholar]
- 8.Law M, Oh S, Johnson G, et al. Perfusion magnetic resonance imaging predicts patient outcome as an adjunct to histopathology: a second reference standard in the surgical and nonsurgical treatment of low-grade gliomas. Neurosurgery. 2006;58:1099–107. doi: 10.1227/01.NEU.0000215944.81730.18. [DOI] [PubMed] [Google Scholar]
- 9.Caseiras B, Ciccarelli O, Altmann DR, et al. Low-grade gliomas: six-month tumor growth predicts patient outcome better than admission tumor volume, relative cerebral blood volume, and apparent diffusion coefficient. Radiology. 2009;253:505–12. doi: 10.1148/radiol.2532081623. [DOI] [PubMed] [Google Scholar]
- 10.Kruer MC, Kaplan AM, Etzl MM, Jr, et al. The value of positron emission tomography and proliferation index in predicting progression in low-grade astrocytomas of childhood. J Neurooncol. 2009;95:239–45. doi: 10.1007/s11060-009-9922-4. [DOI] [PubMed] [Google Scholar]
- 11.Smith JS, Perry A, Borell TJ, et al. Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas and mixed oligoastrocytomas. J Clin Oncol. 2000;18:636–45. doi: 10.1200/JCO.2000.18.3.636. [DOI] [PubMed] [Google Scholar]
- 12.Huang Q, Zhang QB, Dong J, et al. Glioma stem cells are more aggressive in recurrent tumors with malignant progression than in the de novo tumor, and both can be maintained long-term in vitro. BMC Cancer. 2008;8:304. doi: 10.1186/1471-2407-8-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanai N, Berger MS. Operative techniques for gliomas and the value of extent of resection. Neurotherapeutics. 2009;6:478–86. doi: 10.1016/j.nurt.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw EG, Berkey B, Coons SW, et al. Recurrence following neurosurgeon-determined gross-total resection of adult supratentorial low-grade glioma: results of a prospective clinical trial. J Neurosurg. 2008;109:835–41. doi: 10.3171/JNS/2008/109/11/0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pignatti F, van den Bent M, Curran D, et al. European Organization for Research and Treatment of Cancer Radiotherapy Cooperative Group. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20:2076–84. doi: 10.1200/JCO.2002.08.121. [DOI] [PubMed] [Google Scholar]
- 16.Chang EF, Clark A, Jensen RL, et al. Multiinstitutional validation of the University of California at San Francisco Low-Grade Glioma Prognostic Scoring System. Clinical article. J Neurosurg. 2009;111:203–10. doi: 10.3171/2009.2.JNS081101. [DOI] [PubMed] [Google Scholar]
- 17.Martinez R, Rohde V, Schackert G. Different molecular patterns in glioblastoma multiforme subtypes upon recurrence. J Neurooncol. 2009 Jul 31; doi: 10.1007/s11060-009-9967-4. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez R, Setien F, Voelter C, et al. CpG island promoter hypermethylation of the pro-apoptotic gene caspase-8 is a common hallmark of relapsed glioblastoma multiforme. Carcinogenesis. 2007;28:1264–8. doi: 10.1093/carcin/bgm014. [DOI] [PubMed] [Google Scholar]
- 19.Maher EA, Brennan C, Wen PY, et al. Marked genomic differences characterize de novo and secondary glioblastoma subtypes and identify two distinct molecular and clinical secondary glioblastoma entities. Cancer Res. 2006;66:11502–13. doi: 10.1158/0008-5472.CAN-06-2072. [DOI] [PubMed] [Google Scholar]
- 20.Zheng H, Ying H, Yan H, Kimmelman AC, et al. p53 and PTEN control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 2008;455:1129–33. doi: 10.1038/nature07443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruano Y, Ribalta T, de Lope AR, et al. Worse outcome in de novo glioblastoma multiforme with concurrent epidermal growth factor receptor and p53 alteration. Am J Clin Pathol. 2009;131:257–63. doi: 10.1309/AJCP64YBDVCTIRWV. [DOI] [PubMed] [Google Scholar]
- 22.Xie D, Zeng YX, Wang HJ, et al. Expression of cytoplasmic and nuclear survivin in de novo and secondary human glioblastoma. Br J Cancer. 2006;94:108–14. doi: 10.1038/sj.bjc.6602904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Somasundaram K, Reddy SP, Vinnakota K, et al. Upregulation of ASCL1 and inhibition of Notch signaling pathway characterize progressive astrocytoma. Oncogene. 2005;24:7073–83. doi: 10.1038/sj.onc.1208865. [DOI] [PubMed] [Google Scholar]
- 24.Mineo JF, Bordron A, Baroncini M, et al. Low HER2-expressing glioblastomas are more often secondary to anaplastic transformation of low-grade glioma. J Neurooncol. 2007;85:281–7. doi: 10.1007/s11060-007-9424-1. [DOI] [PubMed] [Google Scholar]
- 25.Nobusawa S, Watanabe T, Kleihues P, et al. IDH1 mutations as a molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res. 2009;15:6002–7. doi: 10.1158/1078-0432.CCR-09-0715. [DOI] [PubMed] [Google Scholar]
- 26.Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26:1338–45. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]