Abstract
BACKGROUND:
Reducing the use of antibiotics for upper respiratory tract infections is needed to limit the global threat of antibiotic resistance. We estimated the effectiveness of probiotics and xylitol for the management of pharyngitis.
METHODS:
In this parallel-group factorial randomized controlled trial, participants in primary care (aged 3 years or older) with pharyngitis underwent randomization by nurses who provided sequential intervention packs. Pack contents for 3 kinds of material and advice were previously determined by computer-generated random numbers: no chewing gum, xylitol-based chewing gum (15% xylitol; 5 pieces daily) and sorbitol gum (5 pieces daily). Half of each group were also randomly assigned to receive either probiotic capsules (containing 24 × 109 colony-forming units of lactobacilli and bifidobacteria) or placebo. The primary outcome was mean self-reported severity of sore throat and difficulty swallowing (scale 0–6) in the first 3 days. We used multiple imputation to avoid the assumption that data were missing completely at random.
RESULTS:
A total of 1009 individuals consented, 934 completed the baseline assessment, and 689 provided complete data for the primary outcome. Probiotics were not effective in reducing the severity of symptoms: mean severity scores 2.75 with no probiotic and 2.78 with probiotic (adjusted difference −0.001, 95% confidence interval [CI] −0.24 to 0.24). Chewing gum was also ineffective: mean severity scores 2.73 without gum, 2.72 with sorbitol gum (adjusted difference 0.07, 95% CI −0.23 to 0.37) and 2.73 with xylitol gum (adjusted difference 0.01, 95% CI −0.29 to 0.30). None of the secondary outcomes differed significantly between groups, and no harms were reported.
INTERPRETATION:
Neither probiotics nor advice to chew xylitol-based chewing gum was effective for managing pharyngitis. Trial registration: ISRCTN, no. ISRCTN51472596
There are 800 consultations per 10 000 patients annually in the United States for pharyngotonsillitis.1 Although most cases are viral, a substantial proportion are caused by pathogenic streptococci.2–4 Despite the risk of antibiotic resistance from the prescribing of antibiotics in primary care, most patients who present with pharyngitis still receive these drugs.5–9 The risk of antibiotic resistance increases with the use of broader-spectrum antibiotics, an approach that has been advocated because of the waning effectiveness of penicillin V.10,11
Both patients and health professionals are concerned about complications of infections,12 but symptom control is actually patients’ main concern;13 therefore, finding alternatives to immediate antibiotics to help control symptoms is a priority. A major economic argument for using antibiotics is the assumption that individuals who are ill and the parents of children with illness will take more time off work.14 However, if simple treatments could limit the effects of both bacterial and viral infections and help patients to manage symptoms, enabling a quicker return to work, the societal arguments to use antibiotics would be weaker.
Xylitol is a birch sugar that causes local “bacterial interference” by inhibiting bacterial growth and adherence to the pharyngeal wall,15–18 which should reduce the inflammation and the severity of symptoms caused by bacterial infections. Although sorbitol has no such effect, chewing gum could plausibly help both bacterial and viral throat infections by generating more saliva. Probiotics are benign, nonpathogenic bacteria that may act through both local “interference” and the immune system — thus affecting both viral and bacterial infections — including local activation of immunoglobulin A and T cells.19,20 Cochrane reviews have suggested that probiotics can prevent recurrence of upper respiratory tract infections21 (although the quality of the evidence is limited) and that xylitol can also reduce recurrence.22
It is plausible that both probiotics and xylitol could limit the severity of pharyngeal infections and help with symptom control, but there is no direct evidence to support this supposition. Our aim was to estimate the efficacy of probiotics and xylitol chewing gum in the symptomatic management of pharyngitis.
Methods
Design and setting
This parallel-group, individually randomized controlled trial had an equal allocation ratio. We invited practices around the study centre in Southampton, England, to participate. There were no exclusion criteria for practices.
Participant recruitment and eligibility
We used a variety of recruitment mechanisms. We sent letters to individuals who had previously consulted with pharyngitis, inviting them to participate should a new episode develop. Participants were also identified opportunistically when presenting to their general practitioner. Participants were assessed by either the physician or a practice nurse to determine eligibility for the study.
We included previously well people, aged 3 years or older, with an acute illness (≤ 21 d), with sore throat as the main symptom and abnormal results on throat examination.
We excluded individuals with a history of peritonsillar abscess, rheumatic fever or glomerulonephritis; those reporting allergy to any constituents of the gum; and those with serious chronic disorders mandating antibiotics (e.g., cystic fibrosis). We also excluded individuals with suspected pregnancy or immune deficiency.
Intervention
We used a 3 × 2 factorial design, based on a xylitol factor and a probiotic factor.
The xylitol factor had 3 alternatives: xylitol gum (Wrigley Orbit gum with 15% xylitol), sorbitol gum (Wrigley Extra gum with no xylitol [i.e., no active ingredient]) or advice not to chew gum. Patients in the 2 gum groups were advised to chew 5 sticks per day for 3 months, based on a similar regimen in prior studies,16–18,22 and were provided with supplies of chewing gum. We included the nonchewing group to ensure that chewing itself was not important.
Each patient in these 3 groups was then randomly assigned to receive probiotic capsules or placebo capsules (all capsules supplied by Cultech), to be taken daily, with milk, for 3 months. Each active probiotic capsule contained a mixture of lactobacilli and bifidobacteria species (Lactobacillus acidophilus CUL60 [NCIMB 30157], Lactobacillus acidophilus CUL21 [NCIMB 30156], Bifidobacterium bifidum CUL20 [NCIMB 30153], Bifidobacterium animalis ssp. lactis CUL34 [NCIMB 30172]), which provided, in combination, 24 × 109 colony-forming units.
All study participants had access to usual care. Prescription of medication or referral was at the physicians’ discretion, according to their usual practices (i.e., not standardized).
Randomization
Randomization was carried out by nurses, who gave an intervention pack to each eligible patient, according to a predefined sequential order. The contents of each pack were previously determined by a University of Southampton statistician (independent of the main study team), who used computer-generated random numbers to determine 3 kinds of material and advice for each pack: no offer of chewing gum, advice to use xylitol-based chewing gum or advice to use sorbitol-based chewing gum. The patients in each group were also randomly assigned to receive either probiotic capsules or placebo probiotic capsules, as described above. Participants were blinded as to whether they were receiving probiotics; they could not be blinded as to whether they chewed gum or not, but were blinded to the hypothesis that xylitol could help.
We used sequential packs for 2 reasons. The complex factorial design made group differentiation more difficult to guarantee, so use of the packs facilitated immediate access to the correct structured materials and advice sheets for each group, ensuring robust group differentiation and greater logistic simplicity for recruiters. In addition, with its attention to equipoise, this method has resulted in robust randomization in several previous studies.23–26 In the current study, there was no evidence of selective use of numbered packs or of meaningful differences in group characteristics.
Baseline clinical data
We recorded the following baseline symptoms and signs, included in previous clinical scores:2,27–29 inflammation of the pharynx, presence of cough, temperature (using Tempa-DOT thermometers, 3M), pus on the tonsils, cervical nodes and duration of prior infection. The number of episodes of sore throat in the previous 3 months was also recorded, as was past tonsillectomy and smoking status. We did not take throat samples for culture: this approach is not recommended routinely in the United Kingdom and would have increased barriers to study entry.
Outcome measurement
Each participant was given a symptom diary, with instructions to complete the diary at the end of each day, for up to 14 days, documenting the severity of sore throat, difficulty swallowing, feeling unwell, fever and sleep disturbance. For very young children who were unable to complete the diary, parents were asked to supply the information. The format of the symptom diaries has been validated for use for both sore throat and other respiratory infections, each symptom being scored from 0 (no problem) to 6 (as bad as it could be).2,23,30,31 If a patient did not return the symptom diary, we sent a brief questionnaire to document the key outcomes; we have previously shown the reliability of this questionnaire.23 If the patient did not respond to the questionnaire, we made telephone calls to collect the same data.
The primary outcome was the severity of sore throat and difficulty swallowing on days 2–5. Before day 2, interventions are unlikely to affect symptoms, and after day 5 the symptoms usually present little or no problem. Data for several days (i.e., days 2–5) provide a more reliable estimate of symptomatic burden than data for a single day. We chose the 2-item score (sore throat and difficulty swallowing) as the main outcome because it is more reliable than either item alone and is internally very reliable (Cronbach α = 0.92).2
We also collected data for the following additional outcomes: time to complete resolution of symptoms and time to resolution of symptoms rated as moderately bad or worse in the symptom diary (reported as median number of days), time to return to work or normal activities, and reported episodes of sore throat in the previous 3 months. We also reviewed medical records for up to 6 months to document complications, recurrence of pharyngitis, new consultations and referrals. These assessments were blinded as to treatment group, an approach that has been shown to be reliable and unbiased.7
Changes to the protocol
Before the study began, our initial plan was to use a xylitol spray; however, the manufacturer of the spray declined to participate, so xylitol chewing gum was used instead, and a no-gum group was included to assess whether chewing itself was helpful. We originally allowed for a response rate of 80% and initially included only adults. Following commencement of the study, the Trial Steering Committee recommended that the sample size be increased, for 2 reasons: to include children as a subgroup and because of a lower-than-expected response rate (about 70%) for the primary outcome. This lower response rate also meant that our original plan for the primary analysis of complete cases was changed to an analysis using multiple imputed data (since we could not assume that missing data were missing completely at random). Nevertheless, for comparison, we also present here the complete case analysis for the primary outcome. We originally planned to measure patient satisfaction with the consultation using a measure developed specifically for a trial of management of acute pharyngitis;23 however, given the context of a longer treatment course and blinding of one of the interventions, this item was judged inappropriate and was dropped from the study.
Statistical analysis
We performed an intention-to-treat analysis using regression models (Cox regression for time to resolution of symptoms; negative binomial regression for number of sore throats and number of days to return to work; and multivariable binomial regression for reconsultation), controlling for variables that were judged on clinical grounds to be potential confounders, including antibiotics prescribed (as listed at the bottom of each table). We estimated the main effects of intervention group separately, as well as comparing the xylitol and no-xylitol groups and determining the interaction between xylitol and probiotics. We performed multiple imputation in Stata software (StataCorp LLC), using a chained equation model, including participants’ baseline characteristics, the outcome variables and the randomization group.
We performed the following subgroup analyses: children, patients with 3 or more of the criteria of Centor and colleagues27 and patients with higher temperature (> 37.5°C).23
We did not allow for multiple comparisons; rather, each group was treated as an experimental group in its own right, for comparison with control, following Freidlin and colleagues.32 The trial was originally powered a priori for adults for the 3 xylitol factor groups: to detect a standardized effect of 0.36 (0.5 points in the sore throat score, which would represent 1 person in 2 rating sore throat as a little problem instead of a moderately bad problem), assuming a 5% 2-sided significance level, required 123 (80% power) or 164 (90% power) participants per group. We initially allowed for 20% loss to follow-up, but with lower response rates, we revised the total sample size to 528 (80% power) and 705 (90% power), allowing for 30% loss to follow-up.
For children, we estimated that to detect a standardized effect size of 0.5 between groups, assuming a 5% 2-sided significance level, would require 64 children per group (80% power) or a total sample size of 276 children, allowing for 30% loss to follow-up.
Ethics approval
The study was approved by the Southampton Research Ethics Committee for Southampton and Southwest Hampshire (ref. no. 05/Q1702/11).
Results
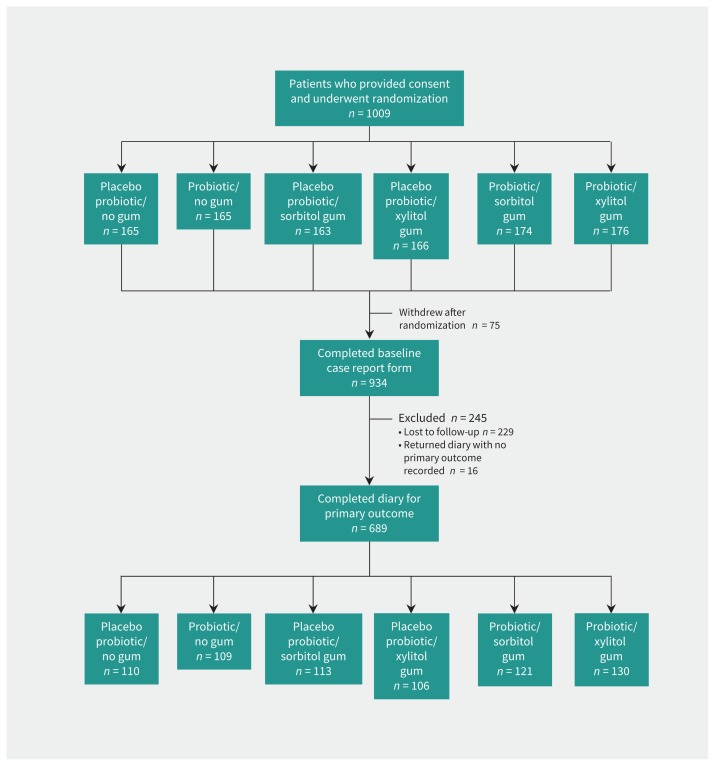
Participants were recruited between June 25, 2010, and June 30, 2014, with 1009 patients undergoing initial randomization. Of these, 934 had complete baseline data, and the primary outcome was recorded for 689 (73.8%) of these participants with baseline data (Figure 1). Because of consultation pressures during winter, practices kept poor records for eligible participants who were not recruited to the study. The main reason reported for not recruiting participants was being too busy, so physicians were also too busy to document data for those not recruited. Forty-nine people who did not want to participate gave reasons: 16 did not wish to chew gum, 24 did not have enough time, 6 were unwell, and 3 were pregnant or trying to become pregnant.
Figure 1:
Participant flow diagram. A total of 304 patients did not return the diary or brief questionnaire; this group consisted of the 75 patients who withdrew and the 229 who were lost to follow-up.
Participants recorded in their diaries the numbers of probiotic capsules and pieces of gum used each day. We agreed that participants who used 75% of more of their study medication would be considered “compliant.” According to that definition, 95.6% (281/294) of the probiotic group and 85.9% (317/369) of the gum group (84.4% [157/186] of the sorbitol group and 87.4% [160/183] of the xylitol group) were compliant in the first 14 days.
Patient characteristics were well balanced for the factorial groups (probiotic v. no probiotic; sorbitol or xylitol gum v. no gum) and also for the comparison between no xylitol (i.e., sorbitol and no-gum groups combined) and xylitol (Table 1). The participants had problems with recurrent infections, with more than 50% having had 2 or more episodes of sore throat during the 3 months before the study; in addition, about 10% of participants had a prior tonsillectomy.
Table 1:
Baseline demographic and clinical characteristics
| Characteristic | Treatment group; no. (%) of participants | ||||
|---|---|---|---|---|---|
| No probiotic n = 494 |
Probiotic n = 515 |
No gum n = 330 |
Sorbitol gum n = 337 |
Xylitol gum n = 342 |
|
| Age group | |||||
| Adults (≥ 15 yr) | 381/482 (79.0) | 408/504 (81.0) | 265/325 (81.5) | 268/334 (80.2) | 256/327 (78.3) |
| Children (3–15 yr) | 101/482 (21.0) | 96/504 (19.0) | 60/325 (18.5) | 66/334 (19.8) | 71/327 (21.7) |
| No. of episodes of sore throat in 3 mo before study | |||||
| 0 | 86/479 (18.0) | 107/507 (21.1) | 69/325 (21.2) | 55/336 (16.4) | 69/325 (21.2) |
| 1 | 117/479 (24.4) | 124/507 (24.5) | 93/325 (28.6) | 78/336 (23.2) | 70/325 (21.5) |
| 2 | 120/479 (25.1) | 119/507 (23.5) | 61/325 (18.8) | 92/336 (27.4) | 86/325 (26.5) |
| ≥ 3 | 156/479 (32.6) | 157/507 (31.0) | 102/325 (31.4) | 111/336 (33.0) | 100/325 (30.8) |
| Past tonsillectomy | 51/494 (10.3) | 55/515 (10.7) | 37/330 (11.2) | 36/337 (10.7) | 33/342 (9.6) |
| Ever smoked | 176/473 (37.2) | 186/490 (38.0) | 113/316 (35.8) | 130/329 (39.5) | 119/318 (37.4) |
| Clinical signs and symptoms at first consultation | |||||
| Substantial pharyngeal inflammation | 321/482 (66.6) | 342/503 (68.0) | 214/323 (66.2) | 237/333 (71.2) | 212/329 (64.4) |
| Cough | 258/486 (53.1) | 274/505 (54.2) | 185/326 (56.7) | 167/335 (49.9) | 180/330 (54.5) |
| Temperature > 37.5°C | 39/487 (8.0) | 25/506 (4.9) | 22/327 (6.7) | 23/334 (6.9) | 19/332 (5.7) |
| Pus on tonsils | 158/479 (33.0) | 151/502 (30.1) | 93/320 (29.1) | 105/333 (31.5) | 111/328 (33.8) |
| Cervical nodes | 260/471 (55.2) | 292/494 (59.1) | 168/316 (53.2) | 200/329 (60.8) | 184/320 (57.5) |
| Duration of illness > 7 d (before consultation) | 107/481 (22.2) | 109/505 (21.6) | 73/323 (22.6) | 73/333 (21.9) | 70/330 (21.2) |
There was no evidence of an interaction between xylitol and probiotics for the primary outcome, the mean score for sore throat and difficulty swallowing on days 2–5 after the consultation (interaction term −0.08, 95% confidence interval [CI] −0.57 to 0.41). Table 2 documents the primary outcome: there were no significant differences between groups for both the xylitol and the probiotic groups, which suggests that neither intervention helped in controlling acute symptoms. The results were similar for the complete case analysis (Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.170599/-/DC1).
Table 2:
Mean symptom score for sore throat and difficulty swallowing, based on the imputed data set (primary outcome)
| Treatment | Symptom score on days 2–5, mean ± SD | Difference (95% CI) | |
|---|---|---|---|
| Univariable | Multivariable* | ||
| Probiotic comparison | |||
| No probiotic | 2.75 ± 1.57 | Reference | Reference |
| Probiotic | 2.78 ± 1.60 | 0.03 (−0.21 to 0.26) | −0.001 (−0.24 to 0.24) |
| Gum comparison | |||
| No chewing gum | 2.73 ± 1.54 | Reference | Reference |
| Sorbitol gum | 2.72 ± 1.57 | −0.01 (−0.37 to 0.39) | 0.07 (−0.23 to 0.37) |
| Xylitol gum | 2.73 ± 1.64 | 0.003 (−0.29 to 0.29) | 0.01 (−0.29 to 0.30) |
| Xylitol comparison | |||
| No xylitol | 2.78 ± 1.56 | Reference | Reference |
| Xylitol | 2.73 ± 1.64 | −0.05 (−0.29 to 0.20) | −0.03 (−0.28 to 0.22) |
Note: CI = confidence interval, SD = standard deviation.
Based on multiple linear regression, controlling for age, duration of current sore throat, number of sore throat episodes in the past 3 months, prior tonsillectomy, inflammation of pharynx, cough, temperature > 37.5°, pus on tonsils, cervical nodes, ever smoked and antibiotics prescribed (none, immediate, delayed).
There was no evidence of an important effect in any of the predefined subgroups: children, participants with 3 or more of the Centor criteria and those with temperature above 37.5°C (Table 3). For all comparisons, there was no significant difference between groups in terms of symptom resolution (Table 4), recurrence of sore throat (Appendix 2, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.170599/-/DC1) or reconsultations (Appendix 3, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.170599/-/DC1). The only comparison of borderline significance was a modest reduction in the number of sore throat episodes for those using xylitol relative to the other groups (adjusted risk ratio [RR] 0.85, 95% CI 0.72 to 1.01); this result was likely due to chance.
Table 3:
Estimates for predefined subgroups, based on the imputed data set
| Subgroup and treatment | Symptom score on days 2–5, mean ± SD | Difference (95% CI) | |
|---|---|---|---|
| Univariable | Multivariable* | ||
| Children | |||
| Probiotic comparison | |||
| No probiotic | 2.68 ± 1.61 | Reference | Reference |
| Probiotic | 2.52 ± 1.47 | −0.16 (−0.73 to 0.41) | −0.27 (−0.90 to 0.36) |
| Gum comparison | |||
| No chewing gum | 2.60 ± 1.50 | Reference | Reference |
| Sorbitol gum | 2.65 ± 1.53 | 0.06 (−0.61 to 0.73) | 0.11 (−0.59 to 0.82) |
| Xylitol gum | 2.55 ± 1.60 | −0.05 (−0.75 to 0.66) | −0.09 (−0.83 to 0.66) |
| Xylitol comparison | |||
| No xylitol | 2.63 ± 1.52 | Reference | Reference |
| Xylitol | 2.55 ± 1.60 | −0.08 (−0.66 to 0.50) | −0.15 (−0.76 to 0.47) |
| Temperature > 37.5°C | |||
| Probiotic comparison | |||
| No probiotic | 2.80 ± 1.74 | Reference | Reference |
| Probiotic | 2.52 ± 1.18 | −0.28 (−1.21 to 0.65) | −0.14 (−1.37 to 1.08) |
| Gum comparison | |||
| No chewing gum | 2.48 ± 1.40 | Reference | Reference |
| Sorbitol gum | 2.87 ± 1.61 | 0.39 (−0.66 to 1.45) | 0.01 (−1.27 to 1.30) |
| Xylitol gum | 2.69 ± 1.58 | 0.21 (−1.02 to 1.45) | 0.08 (−1.45 to 1.60) |
| Xylitol comparison | |||
| No xylitol | 2.69 ± 1.53 | Reference | Reference |
| Xylitol | 2.69 ± 1.58 | 0.01 (−1.06 to 1.08) | 0.07 (−1.15 to 1.28) |
| Centor score ≥ 3 | |||
| Probiotic comparison | |||
| No probiotic | 2.86 ± 1.60 | Reference | Reference |
| Probiotic | 2.87 ± 1.61 | 0.003 (−0.36 to 0.37) | −0.04 (−0.42 to 0.34) |
| Gum comparison | |||
| No chewing gum | 2.92 ± 1.54 | Reference | Reference |
| Sorbitol gum | 2.74 ± 1.59 | −0.18 (−0.60 to 0.24) | −0.14 (−0.56 to 0.20) |
| Xylitol gum | 2.95 ± 1.66 | 0.02 (−0.42 to 0.47) | 0.06 (−0.40 to 0.52) |
| Xylitol comparison | |||
| No xylitol | 2.83 ± 1.57 | Reference | Reference |
| Xylitol | 2.95 ± 1.66 | 0.12 (−0.26 to 0.50) | 0.13 (−0.26 to 0.53) |
Note: CI = confidence interval, SD = standard deviation.
Based on multiple linear regression, controlling for age, duration of current sore throat, number of sore throat episodes in the past 3 months, prior tonsillectomy, inflammation of pharynx, cough, temperature > 37.5°C, pus on tonsils, cervical nodes, ever smoked and antibiotics prescribed (none, immediate, delayed).
Table 4:
Hazard ratios for symptom resolution, based on the imputed data set
| Treatment | Time to complete symptom resolution, d, median (IQR) | HR (95% CI) | Time to resolution of mod bad/worse symptoms, d, median (IQR) | HR (95% CI) | ||
|---|---|---|---|---|---|---|
| Univariable | Multivariable* | Univariable | Multivariable* | |||
| Probiotic comparison | ||||||
| No probiotic | 9 (6 to 13) | 1.00 (ref) | 1.00 (ref) | 5 (3 to 8) | 1.00 (ref) | 1.00 (ref) |
| Probiotic | 9 (6 to 13) | 1.03 (0.86 to 1.24) | 1.02 (0.84 to 1.24) | 4 (2 to 7) | 1.09 (0.94 to 1.28) | 1.08 (0.91 to 1.28) |
| Gum comparison | ||||||
| No chewing gum | 9 (6 to 13) | 1.00 (ref) | 1.00 (ref) | 4 (2 to 7) | 1.00 (ref) | 1.00 (ref) |
| Sorbitol gum | 9 (7 to 14) | 0.90 (0.72 to 1.14) | 0.88 (0.69 to 1.12) | 5 (3 to 8) | 0.83 (0.69 to 1.01) | 0.85 (0.69 to 1.04) |
| Xylitol gum | 8 (6 to 12) | 1.08 (0.87 to 1.35) | 1.09 (0.86 to 1.39) | 4 (2 to 7) | 0.93 (0.77 to 1.12) | 0.95 (0.77 to 1.17) |
| Xylitol comparison | ||||||
| No xylitol | 9 (6 to 13) | 1.00 (ref) | 1.00 (ref) | 4 (2 to 7) | 1.00 (ref) | 1.00 (ref) |
| Xylitol | 8 (6 to 12) | 1.14 (0.94 to 1.38) | 1.16 (0.95 to 1.43) | 4 (2 to 7) | 1.02 (0.87 to 1.20) | 1.03 (0.87 to 1.24) |
Note: CI = confidence interval, HR = hazard ratio, IQR = interquartile range, “mod bad/worse” = moderately bad or worse.
Using Cox regression controlling for age, duration of current sore throat, number of sore throat episodes in the past 3 months, prior tonsillectomy, inflamed pharynx, cough, temperature > 37.5°C, pus on tonsils, cervical nodes, ever smoked, antibiotics prescribed (none, immediate, delayed).
There were no differences between groups in terms of work-related absences, and number of days to return to work or normal activities was not significantly different between groups (RR for probiotic group 1.02 [95% CI 0.70 to 1.50] and for xylitol group 1.22 [95% CI 0.82 to 1.81]). No harms were reported.
Interpretation
This is one of the few studies to address the effectiveness of 2 promising over-the-counter remedies for acute pharyngitis. The results show that neither probiotics nor xylitol is likely to have a meaningful effect.
Previous studies have not addressed short-term symptom control. Although prior evidence suggested that probiotics and xylitol may prevent recurrence,21,22 we documented only a nonsignificant 15% reduction in recurrence with xylitol.
Limitations
Follow-up was lower than in our previous trials,2,23 but the imputed estimates were similar to the complete data, and we assume that participants with follow-up were also more likely to comply with study recommendations, which makes the null results even more convincing. The extent of attrition may reflect suboptimal engagement of practices in the consent process, problems with the recruitment mechanism or participants’ distaste for chewing gum; the latter explanation seems less likely, given similar follow-up for all groups for the xylitol factor. The symptomatic outcomes were all self-reported, but self-reported symptom diaries are known to be reliable, valid and sensitive to change.23,25,30,33 Despite an open design for the xylitol factor, there was no evidence of any placebo effect that would have biased the outcome assessment. The follow-up period was short, so the effect of treatments on recurrence of pharyngitis could not be reliably assessed. Practices’ records for patients who were not recruited were poor, because the main reason for not recruiting was being too busy. The clinical characteristics were similar to those of recent cohorts,7 except for more frequent sore throats, more participants having had tonsillectomy and a longer duration of illness (10 d v. mean of 5 d seen previously23); as such, we may have underestimated the effect of the interventions in a less severely affected group. The borderline significant finding for xylitol in reducing the number of recurrences is likely to represent type I error, given the number of outcomes assessed. We did not have sufficient power to assess the subgroup of children separately, so its negative findings must be interpreted with caution.
Conclusion
Neither probiotics nor xylitol chewing gum was effective in controlling the symptoms of pharyngitis. As such, there is no reason for clinicians to advise patients to use either of these treatments for the symptomatic management of pharyngitis.
Acknowledgements
The authors are grateful to Cultech Ltd. (Port Talbot, West Glamorgan, UK) for supplying the probiotic capsules, and in particular to Sue Plummer (for advice and support related to the products and packs) and Iveta Garaiova (for preparing the recruitment packs and providing relevant advice and support). The authors are also thankful to the local general practice champions who promoted the study and all the doctors, practices and patients who agreed to participate. Finally, the authors thank Pat Allexant for administrative support on the study.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Paul Little contributed to developing the original protocol, led the funding application, supervised the running of the study, contributed to the analysis and led the drafting of the paper. Beth Stuart developed the analysis protocol, led the quantitative analysis and helped to draft the paper. Zoe Wingrove conceived the study and developed the initial protocol, contributed to managing the study and commented on drafts of the paper. Mark Mullee contributed to developing the analysis protocol, supervised the quantitative analysis and contributed to drafting the paper. Tammy Thomas contributed to developing the protocol; provided overall day-to-day management of the study; coordinated recruitment, follow-up and data entry; and commented on drafts of the paper. Sophie Johnson contributed to developing the protocol; provided day-to-day administrative support to the study, follow-up and data entry; and commented on drafts of the paper. Gerry Leydon contributed to developing the protocol and supervising the study and commented on drafts of the paper. Samantha Richards-Hall helped to develop the protocol, commented on the study materials, participated in regular management meetings overseeing the research program and contributed to drafting the paper. Ian Williamson and Michael Moore contributed to developing the protocol, managing the study and drafting the paper. Lily Yao and Shihua Zhu helped to develop the protocol for analysis, led the review of data from medical records and contributed to drafting the paper. All of the authors gave final approval of the version to be publishd and agreed to be accountable for all aspects of the work.
Funding: This article presents independent research funded by the UK National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (reference no. RP-PG-0407-10098). The views expressed are those of the authors and not necessarily those of the UK National Health Service, the NIHR or the UK Department of Health. The University of Southampton was the sponsor, but neither it nor the funder had any role in the running of the study, the analysis, the write-up or the interpretation of the results.
Data sharing: The data set used for this analysis is available to others upon request to Paul Little with details of the analysis proposed. Such requests will be reviewed by the authors and access granted if the request is deemed scientifically justified.
References
- 1.Sims Sanyahumbi A, Colquhoun S, Wyber R, et al. Global disease burden of group A Streptococcus. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: basic biology to clinical manifestations. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2016. Available: https://www.ncbi.nlm.nih.gov/books/NBK333415 (accessed 2017 Nov. 17). [PubMed] [Google Scholar]
- 2.Little P, Hobbs FD, Moore M, et al. ; PRISM investigators. PRImary care Streptococcal Management (PRISM) study: in vitro study, diagnostic cohorts and a pragmatic adaptive randomised controlled trial with nested qualitative study and cost-effectiveness study. Health Technol Assess 2014;18:vii–xxv, 1–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis 2012;55:1279–82. [DOI] [PubMed] [Google Scholar]
- 4.Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med 2016;164:425–34. [DOI] [PubMed] [Google Scholar]
- 5.Petersen I, Johnson A, Islam A, et al. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ 2007; 335:982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gulliford M, Latinovic R, Charlton J, et al. Selective decrease in consultations and antibiotic prescribing for acute respiratory tract infections in UK primary care up to 2006. J Public Health (Oxf) 2009;31:512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Little P, Stuart B, Hobbs FD, et al. ; DESCARTE investigators. Antibiotic prescription strategies for acute sore throat: a prospective observational cohort study. Lancet Infect Dis 2014;14:213–9. [DOI] [PubMed] [Google Scholar]
- 8.Gulliford MC, Dregan A, Moore MV, et al. Continued high rates of antibiotic prescribing to adults with respiratory tract infection: survey of 568 UK general practices. BMJ Open 2014;4:e006245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goossens H, Ferech M, Vander Stichele R, et al. ; ESAC Project Group. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 2005;365:579–87. [DOI] [PubMed] [Google Scholar]
- 10.Casey JR, Pichichero ME. Meta-analysis of cephalosporin versus penicillin treatment of group A streptococcal tonsillopharyngitis in children. Pediatrics 2004;113:866–82. [DOI] [PubMed] [Google Scholar]
- 11.van Driel ML, De Sutter AI, Habraken H, et al. Different antibiotic treatments for group A streptococcal pharyngitis. Cochrane Database Syst Rev 2016; 9: CD004406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar S, Little P, Britten N. Why do general practitioners prescribe antibiotics for sore throat? Grounded theory interview study. BMJ 2003;326:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Driel ML, De Sutter A, Deveugele M, et al. Are sore throat patients who hope for antibiotics actually asking for pain relief? Ann Fam Med 2006; 4: 494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Howe RS, Kusnier LP., 2ndDiagnosis and management of pharyngitis in a pediatric population based on cost-effectiveness and projected health outcomes. Pediatrics 2006;117:609–19. [DOI] [PubMed] [Google Scholar]
- 15.Kontiokari T, Laitinen J, Järvi L, et al. Dietary factors protecting women from urinary tract infection. Am J Clin Nutr 2003;77:600–4. [DOI] [PubMed] [Google Scholar]
- 16.Tapiainen T, Luotonen L, Kontiokari T, et al. Xylitol administered only during respiratory infections failed to prevent acute otitis media. Pediatrics 2002; 109:E19. [DOI] [PubMed] [Google Scholar]
- 17.Uhari M, Kontiokari T, Koskelika M, et al. Xylitol chewing gum in the prevention of acute otitis media: a double blind randomised trial. BMJ 1996; 313: 1180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uhari M, Kontiokari T, Niemelä M. A novel use of xylitol sugar in preventing acute otitis media. Pediatrics 1998;102:879–84. [DOI] [PubMed] [Google Scholar]
- 19.Hatakka K, Savilahti E, Pönkä A, et al. Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial. BMJ 2001;322:1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wanke CA. Do probiotics prevent childhood illnesses? BMJ 2001;322: 1318–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hao Q, Dong BR, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev 2015;(2):CD006895. [DOI] [PubMed] [Google Scholar]
- 22.Azarpazhooh A, Limeback H, Lawrence HP, et al. Xylitol for preventing acute otitis media in children up to 12 years of age. Cochrane Database Syst Rev 2011; (11):CD007095. [DOI] [PubMed] [Google Scholar]
- 23.Little P, Williamson I, Warner G, et al. An open randomised trial of prescribing strategies for sore throat. BMJ 1997;314:722–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Little P, Gould C, Williamson I, et al. A pragmatic randomised controlled trial of two prescribing strategies for acute otitis media. BMJ 2001;322:336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little P, Rumsby K, Kelly J, et al. Information leaflet and antibiotic prescribing strategies for acute lower respiratory tract infection: a randomised controlled trial. JAMA 2005;293:3029–35. [DOI] [PubMed] [Google Scholar]
- 26.Little P, Moore M, Kelly J, et al. ; PIPS Investigators. Ibuprofen, paracetamol, and steam for patients with respiratory tract infections in primary care: pragmatic randomised factorial trial. BMJ 2013;347:g6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centor RM, Witherspoon JM, Dalton HP. The diagnosis of strep throat in the emergency room. Med Decis Making 1981;1:239–46. [DOI] [PubMed] [Google Scholar]
- 28.Dobbs F. A scoring system for predicting group A streptococcal throat infection. Br J Gen Pract 1996;46:461–4. [PMC free article] [PubMed] [Google Scholar]
- 29.Breese BB. A simple scorecard for the tentative diagnosis of streptococcal pharyngitis. Am J Dis Child 1977;131:514–7. [DOI] [PubMed] [Google Scholar]
- 30.Watson L, Little P, Williamson I, et al. Validation study of a diary for use in acute lower respiratory tract infection. Fam Pract 2001;18:553–4. [DOI] [PubMed] [Google Scholar]
- 31.Little P, Turner S, Rumsby K, et al. Dipsticks and diagnostic algorithms in urinary tract infection: development and validation, randomised trial, economic analysis, observational cohort and qualitative study. Health Technol Assess 2009;13(9):iii–iv, ix,–xi, 1–73. [DOI] [PubMed] [Google Scholar]
- 32.Freidlin B, Korn E, Gray R, et al. Multi-arm clinical trials of new agents: some design considerations. Clin Cancer Res 2008;14:4368–71. [DOI] [PubMed] [Google Scholar]
- 33.Watson L, Little P, Williamson I, et al. Validation study of a diary for use in acute lower respiratory tract infection. Fam Pract 2001;18:553–4. [DOI] [PubMed] [Google Scholar]