Abstract
Nanomaterials have been developed for many biomedical applications, including medical imaging, drug-delivery and antimicrobial coatings. Intriguingly, nanoparticles can display ‘enzyme-like’ activity and have been explored as alternatives to natural enzymes in several industrial and energy-related applications. Recently, these catalytic nanomaterials with enzyme mimetic properties have found new biomedical applications, from biofilm disruption to neurodegeneration protection and tumor prevention. In this review article, we focus on recent in vivo studies demonstrating potential therapeutic uses of catalytic nanomaterials. We also provide insights about the relationship between catalytic activity, therapeutic efficacy and biocompatibility that are critical towards clinical translatability. Finally, we discuss current challenges and future directions of using these nanomaterials as novel platforms for the development of sustainable, affordable and safe therapeutics.
Keywords: nanoparticles, catalysis, enzyme mimetics, cancer, biofilm, imaging
Intrinsic enzyme-like properties of nanomaterials
Inorganic and organic materials have been extensively used to synthesize a variety of nanostructures such as nanoparticles (see Glossary) and nanocoatings. These include iron oxides, gold, silver, ceria, cadmium selenide, silica, graphene, carbon nanotubes and many others.[1–7] Nanoparticles have drawn significant attention over the past couple of decades due to their applications in a wide array of fields, such as devices, energy generation, additives to lubricants, chemical assays, therapeutics and medical imaging, among others.[8–13] Intriguingly, some nanomaterials have intrinsic enzyme mimetic properties under physiological conditions. For example, iron oxide nanoparticles exhibit high catalytic activity that is similar to natural enzymes such as catalase or peroxidase, and thereby have been termed nanozymes.[14] Over the past decade, these catalytic nanomaterials have been explored as low-cost alternatives to natural enzymes.[15] Attention in this area was initially focused on industrial applications and in vitro assays, such as chemical synthesis, biomolecule detection and fuel treatment or pollutant removal.[16–18] However, therapeutic applications have recently started to emerge for nanomaterials with enzyme-like properties, including biofilm disruption, anti-oxidation, tissue regeneration and prevention of tumors or infections (Box 1).[19–24] One of the first nanoparticles to be approved by the Food and Drug Administration (FDA) for clinical use was Feridex, an iron oxide-based contrast agent for magnetic resonance imaging (MRI).[11] Although the catalytic properties of nanoparticles have been explored in parallel to their development for biomedical applications, the potential for catalytic action has been largely ignored by the biomedical community or assumed to be absent due to passivating coatings. However, recent work has demonstrated that inorganic nanoparticles can maintain catalytic activity, even when formulated for medical use,[25] and provide therapeutic activity in vivo. For example, ceria-based nanoparticles can mimic superoxide dismutase (SOD) and exhibit neuroprotective and anti-inflammatory activities.[25, 26] Recently, iron oxides with peroxidase-like activity were reported to disrupt biofilms with exceptional efficacy and suppress the severity of an infectious oral disease while sparing normal tissues.[19] Furthermore, ferumoxytol, an FDA-approved iron-oxide nanoparticle iron deficiency treatment, inhibited tumor growth in mice.[22] These discoveries and others like them have opened the door for a new set of applications for inorganic materials with catalytic activity, whereby their multi-functionality can be exploited for innovative biomedical applications to treat biofilm infections, locally activate drugs, act as enzyme replacements, produce targeted cancer imaging and therapy or prevent oxidative stress.
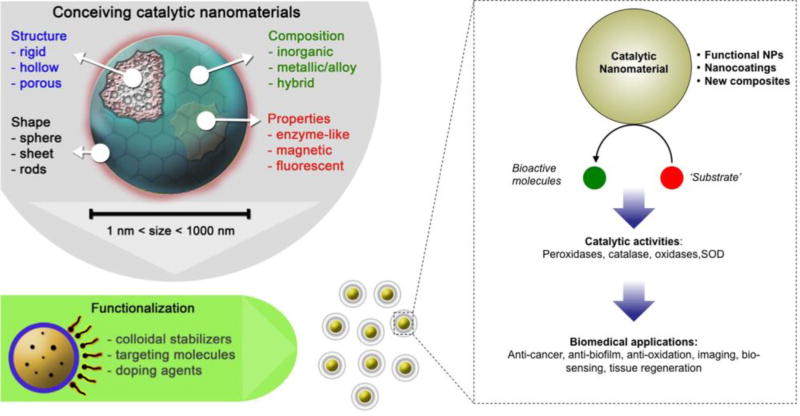
Box 1. Nanomaterials as enzyme mimetics: versatility and functionality of catalytic nanoparticles.
Nanoparticle enzyme mimetics can be made of many types of materials, take many forms and display various catalytic activities (see Figure I, Key Figure). The catalytic core can be formed from iron oxide, vanadium oxide, cerium oxide, manganese oxide, gold, gold-silver alloys, iridium, molybdenum sulfide, zeolites, platinum, carbon nanotubes, graphene and other materials. Enzymes that can be mimicked include peroxidases, haloperoxidase, glucose oxidase, sulfite oxidase, DNAses, glutathione peroxidase (GPx), catalase (CAT), SOD. The core type dictates the type of enzyme activity that can be mimicked, but other factors also affect catalytic activity. The morphologies of the cores can be spherical, rods, stars or other shapes. Morphology can affect activity via changing the surface area to volume ratio or by changing the ratios of different types of surfaces or vertexes in the cores, which can have differing activity towards the substrate.[81] The core size of some formulations can be precisely tuned, which is important since nanoparticle size has often been reported to affect catalytic activity, with smaller cores having higher activity than larger cores, likely due to higher surface area to volume ratios.[14] The cores can be used as colloidal suspensions, be integrated into larger nanoparticles or other biomaterials such as catheters or joint replacements. Furthermore, two or more of these materials can be combined or these materials can be integrated with natural enzymes, creating artificial-natural hybrid systems. Such combinations of catalytic centers form multi-enzyme complexes that can catalyze multi-step processes. The combinations of uricase and PtNPs or GPx-SOD-CAT nanozyme complexes for synergistic breakdown of ROS (see ‘Other therapeutic uses’ section) exemplify this promising approach. Therefore, these nanomaterials can be used as ‘nanosized building blocks’ to generate new nanocomposites or nanostructures or be used to coat existing surfaces (e.g. teeth) and exogenously introduced surfaces (restorative or implant materials). Furthermore, these functional NPs can be conceived as stimuli-triggered nanoparticles for localized and controlled activation in response to pathological environments (e.g. acidic pH) found in biofilms and tumors. Applications of catalytic nanoparticles include treatments for biofilm infections, wound disinfection, cancer, stroke and other diseases, in bio-sensing or imaging and as radio-protective agents.
Figure I, Key Figure.
Schematic depicting the properties and actions of enzyme-mimicking nanoparticles.
In this article we have defined nanomaterials as materials that have one of more dimensions within the nanometer size range, i.e. 1–1000 nm. Examples of such materials include nanoparticles such as dextran coated iron oxides (nanosized iron oxide cores that have a polysaccharide coating), or cerium oxide nanoparticles that are embedded into a polymeric matrix.[21] Carbon nanotubes may be more than 1000 nm long, but are still considered nanomaterials due to their nano-sized diameters. Furthermore, we have focused on materials whose catalytic activity arises from the nanomaterial itself (also termed nanozymes) rather than nanoparticles whose catalytic activity is due to enzymes absorbed on the nanoparticle surface.[27] Other excellent in-depth reviews on the nanozyme concept and broad applications are available elsewhere.[6, 14, 28, 29] Here, we focus on emerging biomedical applications of catalytic nanomaterials, and their progress towards clinical use based on recent findings using in vivo models.
Compared to previous generations of artificial enzymes that are based on organic molecules, nanozymes possess alternative routes for tuning activity via nanoscale engineering strategies. Given the number of known elements, there are myriad potential compositions of a nanomaterial. Well-studied examples include elemental nanoparticles such as gold, silver, copper and palladium;[4, 30–33] alloys such as gold-silver, gold-copper and iron-platinum;[34–36] various allotropes of carbon such as nanotubes, graphene and fullerenes;[37–40] and compounds such as iron oxides, cerium oxide, tantalum oxide, cadmium selenide and silica, to mention just a few.[3, 6, 41–43]
More importantly, nanozymes can be flexibly engineered to modify their properties and enhance catalytic activity. Methods are available to synthesize different types of nanoparticles or nanostructures in a wide range of sizes, shapes and composition as well as doping, surface modification and functionalization that modulate catalytic activities, biological functions and biocompatibility. For example, gold nanoparticles can be synthesized as spheres in the 1–200 nm size range or in a variety of morphologies such as rods, cubes, stars, shells, cages, sheets, wires and so forth.[4, 8, 14, 44] This is noteworthy, since the activities of nanozymes frequently depend on nanomaterial size and shape (Box 1). Furthermore, coatings can be applied during the nanomaterial synthesis process or in subsequent reaction steps.[11] Coatings may be needed for colloidal stability or biocompatibility and can be small molecules, lipids, proteins, polymers, additional nanomaterials (e.g. silica) and other substances.[11] Further reactions can be performed on coated nanoparticles in order to introduce additional functionalities such as the attachment of antibodies or peptides for targeting in vivo or drug loading, leading to multi-pronged effects for both therapeutics and diagnostics.
A nanozyme may have more than one type of catalytic activity. For example, iron oxide nanoparticles display peroxidase-like and catalase-like activities under acidic pH and neutral pH respectively, which provides flexibility for different applications depending on the target microenvironment.[45] In addition, different nanozymes can be assembled into one unit to achieve dual or multiple catalytic activities, which could mimic multi-enzyme complexes to accomplish sequential biochemical processes.[46] Finally, catalytic nanomaterials can be integrated with natural enzymes to form hybrid materials or multi-functional devices.[47] For example, a recent integrated nanozyme device successfully monitored neurochemicals in living brains using a rodent model by glucose oxidase converting glucose to gluconic acid and hydrogen peroxide, which then reacts with a dye at a second catalytic center to result in a colorimetric readout of the glucose levels.[48] These features make catalytic nanomaterials suitable as ‘building blocks’ to produce nanoparticles, nanocoatings or new hybrid materials as therapeutic platforms with multiple biomedical applications (Box 1). Currently available engineering approaches allow precise synthesis at low cost and large scale. Other advantages of nanoparticles include synthetic techniques that allow facile integration of multiple properties in the same platform, unique optical or magnetic properties, and high payload to targeting ligand ratios.[49] Nanomaterials can be used as dispersions in a solvent, be coated onto substrates, be embedded into other materials such as polymers or be formed into composites. In this context, the production of these ‘enzyme-mimetic materials’ can be scaled up to conceive new nanostructures with biological functionalities and therapeutic properties. Recent biomedical applications in vivo augur well for the therapeutic potential of catalytic nanomaterials.
In this review, we focus on recent developments of nanomaterials for catalytic biomedical applications such as diagnostics and therapeutics. We provide insights on factors affecting biocompatibility, catalytic activity and therapeutic efficacy. Limitations and expected directions for the clinical translatability of this technology into feasible products are also discussed.
New biomedical applications and in vivo studies
Nanoparticles possess many unique properties that can be exploited for use in biomedical applications. Owing to their small sizes, which are one or more orders of magnitude smaller than cells, nanoparticles can be taken up by cells or penetrate into tissues by passing in between cells and diffusing through the extracellular matrix.[50] Notable examples of the utility of this property in biomedical applications include accumulation in tumors, atherosclerotic plaques and biofilms (Table 1).[19, 51, 52] The emergence of the nanozyme concept has created new and exciting therapeutic opportunities for these nanoparticles beyond the traditional biomedical applications as carriers or for their optical and magnetic properties. These nanoparticles can perform catalytic processes under physiological conditions found in the human body (i.e., mild temperatures, pH 4–8 and in aqueous buffer). Therefore, nanozymes can be used as alternatives to natural enzymes to treat enzyme-deficiency related conditions in addition to the other roles nanoparticles can play in medicine. In this case, their intrinsic enzyme-like activities can be tailored to provide specific biological functions in vivo, a notion that has been supported by proof-of-concept in vitro studies. Furthermore, nanozymes are typically much more stable and also cheaper than natural enzymes, which would facilitate their future applications and product development.
Table 1.
Some examples of the biomedical applications of enzyme-like catalytic nanomaterials.
| Application | Nanozyme | Enzyme mimicked | References |
|---|---|---|---|
| Glucose detection | Iron oxide | Peroxidase | [54, 55] |
| HepB ELISA | Iron oxide | Peroxidase | [17] |
| Tumor staining in histology | Iron oxide | Peroxidase | [56] |
| Cancer cell detection | Silver halides | Peroxidase | [57] |
| Ebola virus detection | Iron oxide | Peroxidase | [58] |
| Anti-biofilm | Vanadium oxide | Haloperoxidases | [61–63] |
| Anti-biofilm | Iron oxide | Peroxidase | [19, 64] |
| Anti-biofilm | Gold-cerium | DNAse | [66] |
| Anti-biofilm/wound healing | Gold-graphitic carbon nitride | Peroxidase | [65] |
| Wound healing | Graphene oxide | Peroxidase | [24] |
| Anti-tumor | Iron oxide | Peroxidase | [22] |
| Retinal degeneration | Cerium oxide | SOD | |
| Stent coating | Cerium oxide | SOD | [70] |
| Radiation protection | Molybdenum sulfide | SOD | [39] |
| Anti-ischemia | Iron oxide | Peroxidase | [20] |
| Anti-ischemia | Cerium oxide | SOD and catalase | [71] |
| Anti-inflammation | Vanadium pentoxide-manganese oxide | Glutathione peroxidase and SOD | [73] |
| Hyperuricemia | Platinum | Catalase | [74] |
Imaging, diagnostics and biomarker monitoring
The use of catalytic nanoparticles in analyte detection is one of their most well-researched applications. One of the earliest examples was the detection of ammonia by LaCoO3 nanoparticles.[53] These nanoparticles catalyzed the oxidation of ammonia to NOx, which could be detected by light emission via reaction with luminol. Many types of nanoparticles possess peroxidase-like properties and can convert hydrogen peroxide into hydroxyl radicals. Consequently, there are numerous reports of hydrogen peroxide detection via use of peroxidase mimicking nanoparticles combined with a reporter substrate such as luminol or 3,3,5,5-tetramethylbenzidine (TMB).[54, 55] This process has been co-opted to detect other analytes of biological interest such as glucose by using co-factors such as glucose oxidase that converts the analyte to hydrogen peroxide, which is detected as described above.[54]
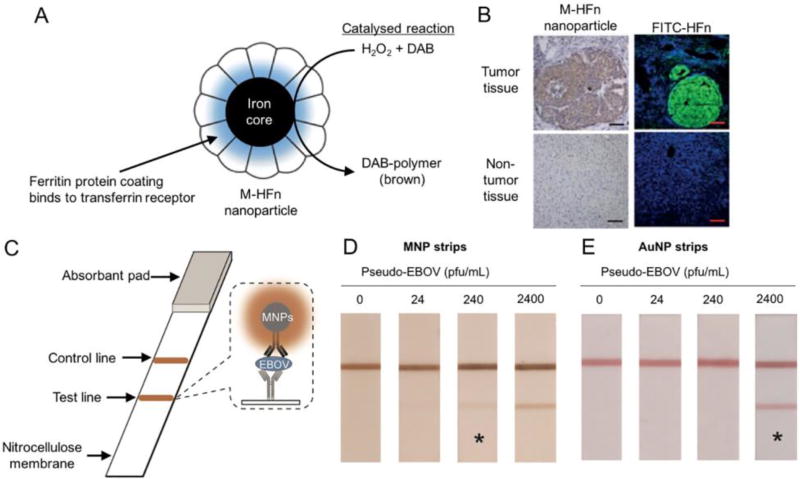
Nanozymes have subsequently been adapted for use in immunoassays such as enzyme-linked immunosorbent assays (ELISAs) or capture-detection assays via conjugation of antibodies or other targeting ligands. Gao and colleagues demonstrated that iron oxide nanoparticles could be used in an ELISA to detect hepatitis B virus surface antigen in a colorimetric fashion via a reaction between TMB and hydrogen peroxide.[17] The iron oxide nanoparticles replaced horseradish peroxidase in this assay. The same group subsequently used iron oxide nanoparticles encapsulated in ferritin (M-HFn) to detect transferrin receptor positive tumors in tissue sections (Fig. 1).[56] These nanoparticles were specific for the transferrin receptor (which is over expressed in many tumors) due to their ferritin-based coating. The iron cores served as catalysts for the reaction between hydrogen peroxide and 3,3’-diaminbenzidine (DAB) to produce DAB-polymer, which is brown in color. The authors showed that this staining process could be used to identify many types of cancer in clinical tissue section, with brown staining indicating tumor cells (Fig. 1B). Fluorescent staining using fluorescein isothiocyanate (FITC) labeled ferritin (FITC-HFn) confirmed the targeting of the nanoparticles to tumor cells. This method distinguished cancerous cells from normal cells with high sensitivity and specificity.
Figure 1. Catalytic nanoparticles for diagnostics.
(A) Magnetoferritin nanoparticles for targeting and visualizing tumor tissues. (B) Histology staining using M-HFn, hydrogen peroxide and DAB identified tumor tissue via brown staining (left) as confirmed by FITC-HFn fluorescence microscopy. (C) Nanozyme-strip for rapid local diagnosis of Ebola. Detection of Ebola virus in human serum at various concentrations using immunochromatographic strips based on (D) MNPs or (E) AuNP. The asterisk indicates the limit of visual detection of the test. Figure adapted with permission from references [56, 58].
Such approaches have been used to detect cancer cells,[57] Ebola virus,[58] single-nucleotide polymorphisms[59] and other targets. In the Ebola virus example, Duan and colleagues sought to create a cheap and simple to use immunochromatographic strip assay based on catalytic iron oxide nanoparticles (MNPs). In this assay, antibodies for the Ebola virus were dispensed onto membrane strips in a line, along with an IgG positive control (Fig. 1C). The strips were incubated with samples and MNPs that had been conjugated to another Ebola antibody. Absorbance of the MNPs on the lines and reaction with DAB and hydrogen peroxide as shown above resulted in a brown color. Comparison of the assay with a gold nanoparticle (AuNP) based assay proved the efficacy of the approach, with MNPs providing ten-fold better sensitivity on samples in human serum (Fig. 1D,E).
Biofilms and tumor prevention/disruption
Biofilms are clusters of bacterial cells that are firmly adherent to a surface and enmeshed in a protective extracellular matrix of polymeric substances, making them challenging to eradicate. Biofilms are the cause of numerous infectious diseases in humans.[60] Andre and colleagues studied the antibacterial activities of vanadium pentoxide nanowires.[61] They found that these nanowires have similar activity to haloperoxidases in that they catalyze the formation of hypobromous acid from hydrogen peroxide and bromide ions. The nanowires were shown to inhibit bacterial growth via laboratory incubations, but also prevented biofilms on the hull of a ship in the Atlantic Ocean.[62, 63] Subsequently, Gao and colleagues tested the combination of hydrogen peroxide and iron oxide nanoparticles (acting as a peroxidase mimetic) as an anti-biofilm agent.[64] The enhanced rate of hydrogen peroxide breakdown and release of reactive molecules caused by the nanoparticles resulted in the degradation of biofilm matrix components such as polysaccharides and killed the embedded bacteria.
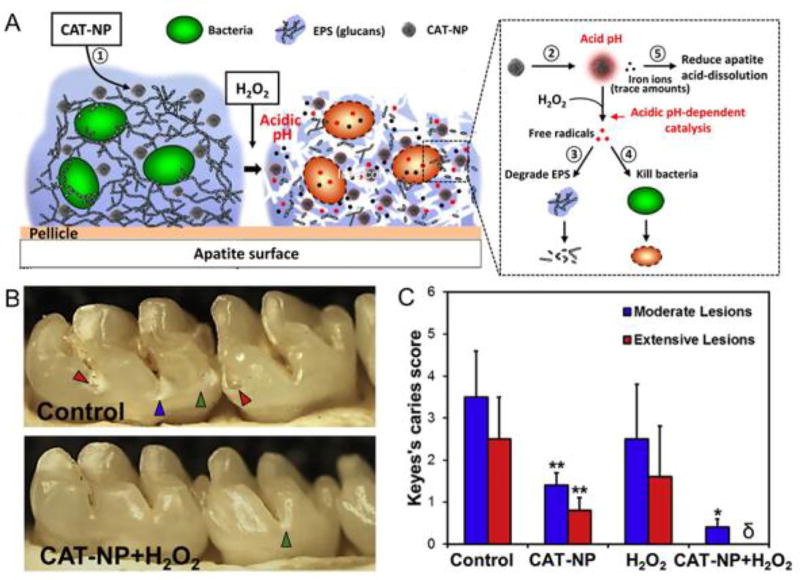
The anti-biofilm effect of the iron oxide-hydrogen peroxide combination was probed in greater detail in a follow-up study.[19] This study found that the nanoparticles (termed CAT-NPs) bind to biofilms and are catalytically active in situ. The peroxidase-like functionality was pH-dependent with greater catalytic activity at acidic pH (4.5) but minimal activity at neutral pH. Under the acidic pH found within pathogenic biofilms (Fig. 2A), CAT-NPs activated free-radical generation from hydrogen peroxide, which simultaneously degraded matrix components and resulted in almost complete eradication of bacteria within the biofilm. In vivo treatment using a rodent biofilm model revealed that daily topical applications, as used clinically, effectively reduced the development of tooth decay, pointing towards a new type of treatment for a costly biofilm-induced oral disease (Fig. 2B,C).[19] Furthermore, the pH-dependent functionality prevents catalytic reactions at physiological (neutral) pH and unmitigated free-radical production, providing biocompatibility in vivo.[19] Gold nanoparticles integrated with graphitic carbon nitride (g-C3N4) also have strong peroxidase-like activity with bactericidal effects against drug-resistant Escherichia coli and Staphylococcus aureus, when combined with hydrogen peroxide.[65] This hybrid nanozyme has anti-biofilm properties in vitro, and reduced bacterial infections while accelerating wound healing rate in vivo. DNAse-like enzymatic activity was observed in gold nanoparticles with multiple Ce(IV) centers that were confined on the surfaces of Fe3O4/SiO2 core/shell nanoparticles. These nanoparticles were capable of DNA cleavage, which inhibited the formation of biofilms and disrupted pre-formed biofilms in vitro.[66]
Figure 2. Nanocatalysis promotes biofilm disruption and prevention of tooth decay in vivo.
(A) Schematic depiction of the anti-biofilm effects of CAT-NP. (B) Images of teeth from a rat model, treated as noted. Arrowheads indicate severity of tooth decay caused by biofilms (green: initial lesions, blue: moderate lesions, red: severe lesions with cavitation). (C) Quantification of lesions in the different treatment groups (Keyes scoring is a method for simultaneous classification and quantification of the number and severity of caries lesions). Figure adapted with permission from reference [19].
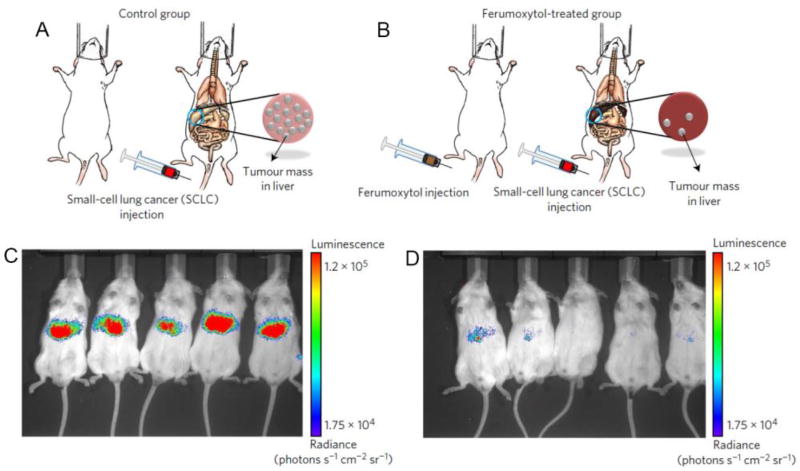
Besides anti-biofilm treatments, the catalytic properties of iron oxide nanoparticles have also found use as anti-tumor treatments. Zanganeh and colleagues observed that co-incubation of cancer cells with macrophage cells and an iron oxide nanoparticle known as ferumoxytol resulted in increased apoptosis of the cancer cells versus incubations with either macrophages or ferumoxytol alone.[22] This phenomenon was due to increased polarization of the macrophages towards a pro-inflammatory phenotype, leading them to attack the cancer cells. The cause of the change in polarization was an increase in reactive oxygen species generated in the macrophages, whose formation was catalyzed by the iron oxide nanoparticles. The authors observed reductions in tumor growth when cancer cells were injected before, after or at the same time as ferumoxytol. For example, as Fig. 3 shows, injection of ferumoxytol administered four days before tumor cell injection suppressed tumor growth, as assessed by luciferase imaging.
Figure 3. Iron oxide nanoparticles inhibit tumor growth by inducing pro-inflammatory macrophage polarization in tumor tissues.
Mice were either injected with cancer cells alone (A) or injected with cancer cells four days after treatment with iron oxide nanoparticles (B). (C) and (D) In vivo optical imaging of the tumor cells (which express luciferase) indicate that treatment with ferumoxytol reduces tumor growth compared to control. Figure adapted with permission from reference [22].
Other therapeutic uses
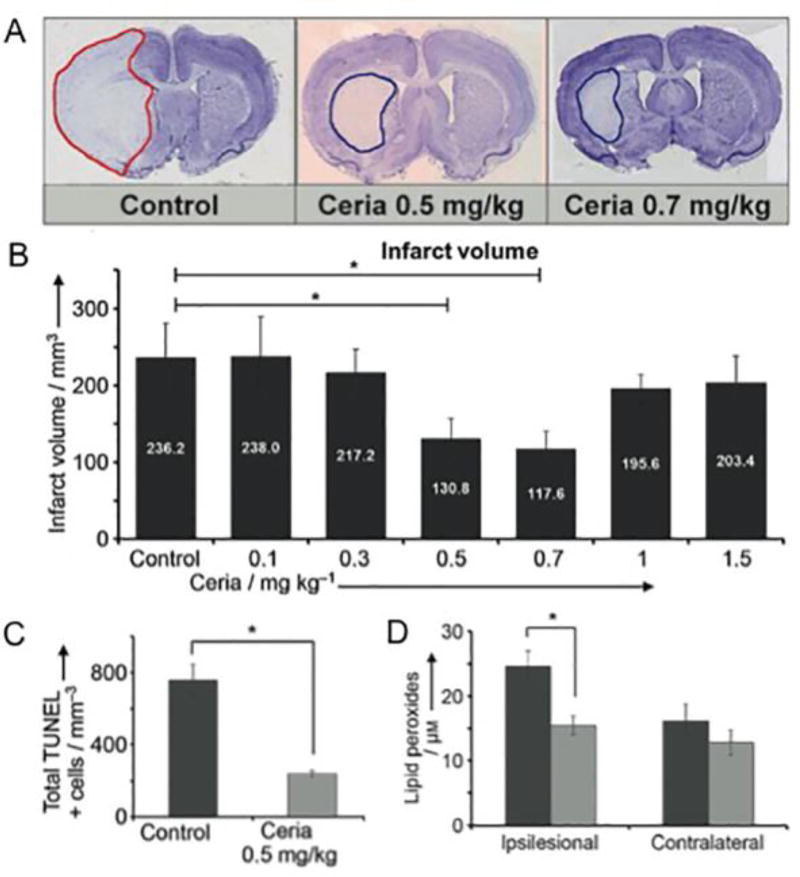
Cerium oxide-based nanoparticles have received significant attention for their catalytic properties: they have been shown to exhibit activity similar to SOD and catalase[6]. The result of such catalytic activity is potent anti-oxidative effects, which have been exploited in vitro to protect neurons and cardiac progenitor cells from oxidative stress.[67, 68] These results encouraged in vivo applications of cerium oxide nanoparticles to prevent retinal degeneration,[69] in stent coatings[70] and in reducing ischemia in stroke.[71] In the latter study, cerium oxide nanoparticles, or ceria, were synthesized and coated with phospholipid-polyethylene glycol. In vitro analyses confirmed the SOD and catalase-like properties of these nanoparticles. Various doses of these nanoparticles were injected into rats shortly after induction of stroke in these animals. As Fig. 4A/B shows, the infarct size was markedly smaller when the animals were given ceria and cell death within the infarct was reduced (Fig. 4C).[71] Importantly, the amount of lipid peroxides was found to be lower within the infarct zone when treated with ceria (Fig. 4D), indicating that the therapeutic effect was mediated by suppression of oxidative stress.
Figure 4. Cerium oxide (ceria) nanoparticles reduce injury caused by stroke.
(A) Histology sections from a rat model of stroke where ceria were administered after the stroke. (B) Quantification of stroke volumes from the different treatment groups. (C) Ceria treatment reduced TUNEL staining, a marker of cell death. (D) Ceria treatment (light gray) reduced lipid peroxide content in the stroke area compared to control (dark gray). Figure adapted with permission from reference [71].
A number of other in vivo applications of catalytic nanoparticles have been reported. Molybdenum sulfide nanodots have been shown to provide protection against ionizing radiation via their anti-oxidative catalytic properties.[39] Bandages that include graphene quantum dots result in improved wound healing due to an antibacterial effect arising from graphene’s peroxidase-like property.[24] Iron oxide nanoparticles have been shown to have cardioprotective and anti-aging benefits.[20, 72] Xiong and colleagues found that iron oxide nanoparticles can protect hearts from ischemic damage in a model of ischemia and reperfusion.[20] The mechanism is unclear, but it is likely due in part to the catalytic action of the nanoparticles to reduce the intracellular ROS and relieve injury caused by peroxidation. Song and coworkers showed that dietary Fe3O4 NPs could enhance the climbing ability of aged Drosophila and prolong their life span by reducing in vivo ROS levels, showing the ability to alleviate neurodegeneration and increase longevity in a Drosophila model of Alzheimer’s disease.[72]
Furthermore, multi-nanozyme complexes can provide additional types of therapeutic activities. For instance, a novel nanocomposite containing V2O5 nanowire (for glutathione peroxidase) and MnO2 nanoparticle (superoxide dismutase and catalase) provided a synergistic antioxidant effect for effective removal of ROS both in vitro and in vivo using a rodent inflammation model.[73] Recently, a novel hybrid artificial metalloenzyme was developed for hyperuricemia treatment by co-encapsulating uricase and platinum nanoparticles (PtNPs) within mesoporous silica nanoparticles.[74] This system can provide catalytic activity in tandem where uricase converts uric acid and oxygen into allantoin and H2O2 and the catalase-like activity of PtNPs converts the generated H2O2 into water and oxygen, reducing toxicity and providing a reagent for uricase. In vivo experiments showed a therapeutic activity without significant toxic effects during treatment of hyperuricemia mice.
Challenges and clinical translation
The complex relationship between catalytic activity, therapeutic efficacy and biocompatibility is a critical factor when assessing the translatability of nanozymes into clinical applications. The field of nanoparticle enzyme mimetics is in its infancy. Their catalytic activity is governed by intrinsic properties (e.g. size, shape and morphology, surface coating and composition) and extrinsic factors, such as pH, substrate availability and temperature, similar to natural enzymes.[14] Smaller nanoparticles often display greater activity than larger nanoparticles possibly due to a higher surface area to volume ratio. Nanospheres or nanowires appear to exhibit the highest activities compared to other shapes (e.g. sheets, triangular plates or octahedra), again due to higher surface area to volume ratio.[14] Differences in activity have also been found to be due to differing surface arrangement of atoms such as between triangular plates and octahedra. Access of the substrate to the nanoparticle surface affects activity, since Gao and colleagues showed that the peroxidase-like activity of iron oxide nanoparticles was affected by different types of coatings.[17] For some types of nanoparticles this will likely introduce significant challenges for biomedical applications. Pathologies such as tumors or atherosclerotic plaques are often targeted by using coatings that provide long circulation times and by attachment of targeting ligands.[49, 75]. Furthermore, robust coatings are needed to provide stability and biocompatibility in biological media for gold nanoparticles and others, which may limit their usefulness as catalytically active nanoparticles in biological systems, since access to their surface can be cut off by the coating. However, polysaccharide-coated iron oxide nanoparticles are clinically approved and have high catalytic activity,[17, 76] so for some nanoparticle types it is certainly possible to maintain both catalytic activity and be suitable for biomedical applications. Zhang and colleagues showed that alloying gold nanoparticles with other elements such as silver or copper can result in substantial increases in glucose oxidase-like activity, i.e. conversion of glucose to gluconic acid and hydrogen peroxide.[77, 78] This effect has been reported to be due to charge transfer from silver atoms to gold atoms, which results in greater electron availability at the gold atom reactive sites.[78] However, knowledge about what factors affect nanoparticle catalytic activity and long-term effects in biological systems is limited, and more in-depth research in this area will be valuable to further assess potential for clinical efficacy.
An interesting and potentially useful method to control the activation and duration of nanoparticle enzyme-like activity would be one whose activity could be triggered to turn on by an external physical or biological stimulus such as light or pH. For example, the catalytic activity can be turned on by the low pH of the intratumoral environment, which in turn could be used to locally convert a drug into its active form and thereby create tumor-specific toxicity.[48] Similar pH-triggered activity has been demonstrated by iron-oxide nanoparticles within the pathological acidic biofilm microenvironment.[19] A mechanism where the environment caused a change in the nanoparticle coating, which allowed access of the substrate to the nanoparticle surface, might be another way to achieve such a goal. For example, the catalytic activity of graphene quantum dots is modulated by the presence of carbonyl, ester and alcohol groups,[79] therefore an environmental condition that could change the ratio of these groups, such as cleaving a protecting group, could trigger the activity of such a nanoparticle. The destination of the nanoparticle and surrounding environment may determine its role and effects. By controlling catalytic activities within cellular compartments or pathological microenvironments, a desired efficacy with high biocompatibility could be achieved. Iron oxide nanoparticles may have peroxidase-like activity in lysosomes due to the acidic environment,[47] but they may display catalase-like activity in the cytoplasm of cells. On the other hand, the presence of ATP can allow iron oxides to have peroxidase-like activity even at neutral pH via stabilization of reaction intermediates[80] providing means to control nanozyme activity. Therefore, both stimuli-responsive and targeting capabilities would be attractive traits in therapeutic catalytic nanomaterials.
For the field to make real progress, an increase in the use of appropriate in vivo models and treatment regimens (e.g. frequency, administration routes and dosage) are critical for evaluation of both therapeutic efficacy and biocompatibility under clinically relevant experimental conditions and presence of biological fluids. Despite an increase in the number of in vivo studies in recent years, most of the work using catalytic nanomaterials has been performed in vitro, without taking into consideration the complexity of biological systems (Box 2). The challenge facing clinical translation of catalytic materials will be to design highly biocompatible materials that have the desired catalytic activity in the target site, which leads to therapeutic efficacy.
Box 2. Challenges and requirements for clinical translation of catalytic nanomaterials.
Clinical translation needs to be considered early on, in order to encourage nanoparticle designs and product development that are practical and efficacious in humans. The use of nanoparticle enzyme mimetics for diagnostics/bioassays may be valuable,[17, 58] but the greater translational challenge will be to use these agents therapeutically as topical or injectable solutions or as components of implants. Injected nanoparticles must be capable of being excreted and produce minimal side effects, in addition to producing therapeutic or other medically useful outcomes. Nanoparticle excretion may occur due to metabolism[76] or renal/fecal excretion.[35] Iron oxide nanoparticles, for example, are thought to be degraded in vivo and their components metabolized.[76] However, the body does not possess mechanisms to process heavier elements such as cerium and some nanoparticles, such as gold, cannot be degraded in vivo. These nanoparticles would need to be excreted, which occurs rapidly via the urine in the case for nanoparticles smaller than 6 nm[82] and can occur via the feces for larger nanoparticles.[35] Despite being hundreds of nanometers long in one dimension, carbon nanotubes can be renally excreted since they are less than 6 nm in two other dimensions.[83] Furthermore, the interactions of nanoparticles with the biological fluids (ranging from saliva in the mouth to blood and interstitial fluids) are important aspects for both topical and systemic use.[84] In-depth characterization of the effects of biological fluids on catalytic activity using laboratory models that mimic the physiological environments could help to predict the in vivo outcomes. In vitro or in silico models to correlate the physicochemical nanoparticle properties (size, surface area, doping type/concentration and chemistries) may help predict bioactivity against target cells or tissues. Furthermore, organ/tissue-on-chip technologies can provide more realistic and in vivo-like environments to assess bioactivity.[85, 86]
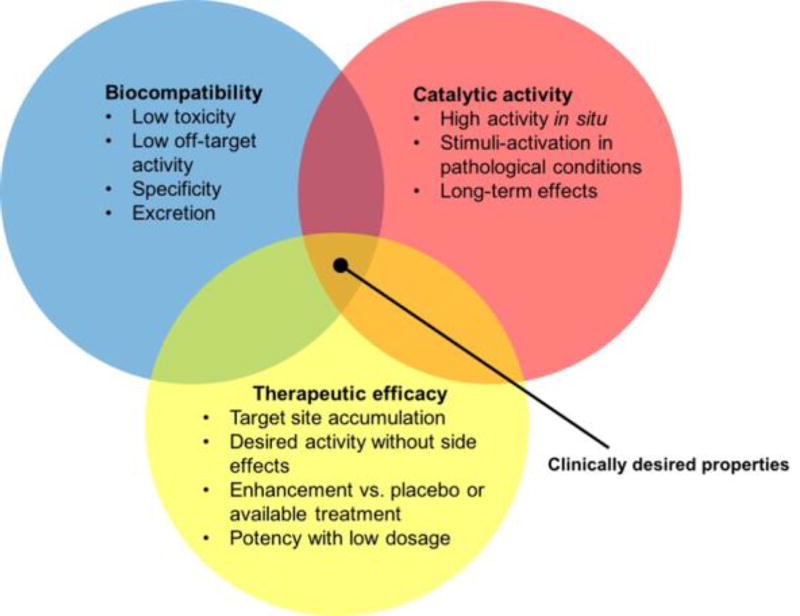
Another important issue is the biodistribution of catalytic nanoparticles, as well as the level of tissue or biofilm penetration depth. Nanoparticles face a series of complex biological barriers, and interactions with biological fluids and cellular components that can limit site-specific targeting or cause toxic side effects. Nevertheless, there has been a significant expansion of clinically-validated and safe nanomedicine-based drugs and biologics, and the number of FDA-approved therapeutic and imaging nano-sized agents are steadily increasing (51 FDA approved nanomedicines and 77 products in clinical trials).[84] The currently clinically approved nanoparticles are based on iron oxides (e.g. Feridex, Feraheme), lipids (e.g. Doxil or Marqibo) or proteins (Abraxane), although lipids and proteins have not been used as sources of catalytic activity. The comparatively simple designs of these previously approved agents may provide a template for initial attempts to translate catalytic nanomaterials. However, the translation of more complicated structures, such as multi-center nanozyme complexes, will likely be more challenging due to issues of control over the sequential reactions, multi-substrate availability, optimal substrate access and cost and complexity for synthesis. A number of gold nanoparticle formulations have been in clinical trials as anti-cancer therapeutics and they have a good safety profile,[87] although they need to be either small or specially engineered for degradation for excretion to occur.[88, 89] Overall, the prior approval of other nanoparticles for clinical use points towards a good likelihood of the translation of catalytic nanomaterials to patients. Further research on understanding and optimizing the relationship between catalytic activity, therapeutic efficacy and safety (see Figure I) will accelerate product development for clinical trials.
Figure I.
Diagram indicating the requirements for a clinically translatable catalytic nanoparticle agent.
Conclusions and outlook
Several nanomaterials can display enzyme-like (catalytic) properties that have been used in many industrial and laboratory applications, while showing potential for therapeutic use in vitro. Recently, promising biomedical applications of catalytic nanomaterials, ranging from diagnostics, anti-biofilm and anti-tumor properties to anti-oxidant and prevention of tissue degeneration have emerged, which have been supported by in vivo studies. These materials are often low cost and can be synthesized on large scales, while their flexible chemistries allow facile size and shape modification as well as bioconjugation or multi-unit hybrid systems for tandem catalysis, providing an exciting therapeutic platform for multiple therapeutic purposes.
There exist many opportunities for development of novel therapeutics using catalytic nanomaterials due to similarity to previously FDA-approved nanoparticles (Box 2). However, there are also hurdles and bottlenecks to overcome in order to achieve optimal therapeutic efficacy, minimal toxicity and long-term catalytic activity in vivo (see Outstanding Questions). For example, nanoparticles can display distinct enzymatic properties and kinetics depending on their microenvironment[19] and are challenging to control. The presence of biological fluids that change surface chemistries poses yet another challenge. Accessibility to biological targets either via topical or systemic application without cytotoxicity or potential for de novo generation of drug resistance should be carefully designed. Nevertheless, rapid advances in materials science and bioengineering should accelerate development of improved materials with enhanced targeting specificity and catalytic activity.
Outstanding Questions.
Can activity be increased by the use of coatings? Will certain types of coatings attract certain substrates to the nanoparticle or will the chemistry of the bonds of the coating to the nanoparticle surface play a role in activity? The effect of coatings and ligands on activity is currently poorly understood. Further investigations using in vivo models could help elucidate the role of coatings in targeting and catalysis, and its relationship with therapeutic effects.
Will formation of protein coronas alter reactivity or selectivity? Will the nanoparticle surface react with biological components to become inactivated or be inactivated by degradation? For a given nanoparticle formulation, it will be important to establish whether its activity is retained after long exposures to serum and other biological media or will diminish with time.
Can selectivity be achieved and is it necessary? Nanoceria, for example, has been shown to mimic SOD, catalase, oxidase and peroxidase. The off-target activities could prove counter-productive to the main desired activity. Nevertheless, nanoceria has proven to be therapeutically beneficial for certain conditions such as stroke. Therefore, off-target activities may not be problematic, as long as the targeted activity is in effect.
Can targeting precision be enhanced by controlling the localization of catalytic nanoparticles at the diseased sites and/or on-demand activation mechanisms by an external stimulus (e.g. light or administration of a pro-drug) for maximal efficacy during acute phase of a pathological process; e.g. anti-oxidant activity following stroke or antibacterial activity during active infection stage?
In addition, many of the catalytic materials can be activated via external-stimuli associated with disease (such as pH or even metabolic triggers), providing a controlled in situ production of bioactive agents or removal of toxic byproducts. Such stimuli-triggering functionality would allow catalytic activation during pathological processes but not at normal physiological conditions, providing targeted and precision medicines. However, further validation of proof-of-concept work using clinically relevant animal models as well as clinical trials are needed for rigorous evaluation. Future directions should focus on achieving maximal efficacy and specificity with minimal toxicity and long-term therapeutic effects as well as developing cost-effective formulations, which would be critical for clinical translatability. Given the unique features of nanocatalysis-based technology, further research could lead to a novel multi-functional platform for the development of sustainable, affordable and safe therapeutics for human use.
Trends box.
In the field of nanozymes, in vivo explorations of their effectiveness as therapies for many types of diseases, such as biofilm infections, stroke, myocardial ischemia, cancer, hyperuricemia, neurodegeneration and others, are on the rise.
Translational issues such as clearance, metabolism, degradability, biocompatibility and side effects are being assessed, as nanoparticles have the potential for long term retention in organs such as the liver and spleen.
Nanozymes are starting to be designed to have specificity of function or stimuli-triggering activity (some nanoparticles can exhibit specific enzymatic activity depending on their microenvironment).
There is a trend towards the use of iron oxide-based nanozymes for in vivo studies, since several iron oxide nanoparticles have been approved by the FDA for clinical use, such as MRI contrast agents and iron replacement therapy.
Acknowledgments
This work was supported by funding from NIH grants R01 DE025848 and R01 DE018023. We are also grateful for support from the University of Pennsylvania Research Foundation.
Glossary
- Biofilm
Structured microbial communities that are embedded in an extracellular matrix composed of polymeric substances such as proteins, polysaccharides and DNA, which are associated with many infectious diseases in humans as well as environmental and industrial contamination.
- Catalase
an enzyme that catalyzes the decomposition of hydrogen peroxide into water and oxygen.
- Nanoparticle
a particle that has one or more dimensions in the nano-size range, i.e. 1–1000 nm.
- Nanozyme
a nanoparticle-based enzyme mimetics These nanoparticles can be formed from many materials (e.g. gold, iron oxide, ceria, graphene and others, see Box 1 for more details). They catalyze the reactions of substrates in a similar fashion to natural enzymes such as peroxidases, catalase, superoxide dismutase and others.
- Peroxidase
an enzyme that catalyzes the oxidation of substrates by hydrogen peroxide.
- Reporter substrate
a substrate that, when it reacts, changes color, emits light or otherwise has an easily measurable property that can be used as a proxy measure of reaction rate. Examples of reporter substrates include luminol, TMB and diazoaminobenzene.
- Superoxide dismutase
an enzyme that alternately catalyzes superoxide (O2−) into molecular oxygen (O2) or hydrogen peroxide.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vivero-Escoto JL, et al. Silica-based nanoprobes for biomedical imaging and theranostic applications. Chem. Soc. Rev. 2012;41:2673–2685. doi: 10.1039/c2cs15229k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee N, Hyeon T. Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem. Soc. Rev. 2012;41:2575–2589. doi: 10.1039/c1cs15248c. [DOI] [PubMed] [Google Scholar]
- 3.Mal J, et al. Metal chalcogenide quantum dots: biotechnological synthesis and applications. RSC Adv. 2016;6:41477–41495. [Google Scholar]
- 4.Li N, et al. Anisotropic gold nanoparticles: synthesis, properties, applications, and toxicity. Angew. Chem. 2014;53:1756–1789. doi: 10.1002/anie.201300441. [DOI] [PubMed] [Google Scholar]
- 5.Dai L, et al. Carbon nanomaterials for advanced energy conversion and storage. Small. 2012;8:1130–1166. doi: 10.1002/smll.201101594. [DOI] [PubMed] [Google Scholar]
- 6.Walkey C, et al. Catalytic properties and biomedical applications of cerium oxide nanoparticles. Environ. Sci. Nano. 2015;2:33–53. doi: 10.1039/C4EN00138A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ravindran A, et al. Biofunctionalized silver nanoparticles: Advances and prospects. Colloids Surf., B. 2013;105:342–352. doi: 10.1016/j.colsurfb.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 8.Mieszawska AJ, et al. Multifunctional gold nanoparticles for diagnosis and therapy of disease. Mol. Pharmaceutics. 2013;10:831–847. doi: 10.1021/mp3005885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shahnazar S, et al. Enhancing lubricant properties by nanoparticle additives. Int. J. Hydrogen Energy. 2016;41:3153–3170. [Google Scholar]
- 10.Xu ZH, et al. Development of functional nanostructures and their applications in catalysis and solar cells. Coord. Chem. Rev. 2016;320:153–180. [Google Scholar]
- 11.Cormode DP, et al. Inorganic nanocrystals as contrast agents in MRI: synthesis, coating and introducing multifunctionality. NMR. Biomed. 2013;26:766–780. doi: 10.1002/nbm.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petryayeva E, Krull UJ. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing-A review. Anal. Chim. Acta. 2011;706:8–24. doi: 10.1016/j.aca.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Yang J, et al. Designed assembly and integration of colloidal nanocrystals for device applications. Adv. Mater. 2016;28:1176–1207. doi: 10.1002/adma.201502851. [DOI] [PubMed] [Google Scholar]
- 14.Wei H, Wang E. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem. Soc. Rev. 2013;42:6060–6093. doi: 10.1039/c3cs35486e. [DOI] [PubMed] [Google Scholar]
- 15.Lin Y, et al. Catalytically active nanomaterials: A promising candidate for artificial enzymes. Acc. Chem. Res. 2014;47:1097–1105. doi: 10.1021/ar400250z. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, et al. Decomposing phenol by the hidden talent of ferromagnetic nanoparticles. Chemosphere. 2008;73:1524–1528. doi: 10.1016/j.chemosphere.2008.05.050. [DOI] [PubMed] [Google Scholar]
- 17.Gao L, et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotech. 2007;2:577–583. doi: 10.1038/nnano.2007.260. [DOI] [PubMed] [Google Scholar]
- 18.Zhang LH, et al. Sensing H2O2 with layer-by-layer assembled Fe3O4-PDDA nanocomposite film. Electrochem. Commun. 2008;10:1524–1526. [Google Scholar]
- 19.Gao L, et al. Nanocatalysts promote Streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials. 2016;101:272–284. doi: 10.1016/j.biomaterials.2016.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiong F, et al. Cardioprotective activity of iron oxide nanoparticles. Sci. Rep. 2015;5:8579. doi: 10.1038/srep08579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandoli C, et al. Stem cell aligned growth induced by CeO2 nanoparticles in PLGA scaffolds with improved bioactivity for regenerative medicine. Adv. Funct. Mater. 2010;20:1617–1624. [Google Scholar]
- 22.Zanganeh S, et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechol. 2016;11:986–994. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu CP, et al. Tailoring enzyme-like activities of gold nanoclusters by polymeric tertiary amines for protecting neurons against oxidative stress. Small. 2016;12:4127–4135. doi: 10.1002/smll.201503919. [DOI] [PubMed] [Google Scholar]
- 24.Sun H, et al. Graphene quantum dots-band-aids used for wound disinfection. ACS Nano. 2014;8:6202–6210. doi: 10.1021/nn501640q. [DOI] [PubMed] [Google Scholar]
- 25.Karakoti AS, et al. PEGylated nanoceria as radical scavenger with tunable redox chemistry. J. Am. Chem. Soc. 2009;131:14144–14145. doi: 10.1021/ja9051087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guan Y, et al. Ceria/POMs hybrid nanoparticles as a mimicking metallopeptidase for treatment of neurotoxicity of amyloid-β peptide. Biomaterials. 2016;98:92–102. doi: 10.1016/j.biomaterials.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Yuan XF, et al. Structure and activity assay of nanozymes prepared by the coimmobilization of practically useful enzymes and hydrophilic block copolymers on gold nanoparticles. Langmuir. 2008;24:6903–6909. doi: 10.1021/la7039288. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, et al. Nanozymes in bionanotechnology: from sensing to therapeutics and beyond. Inorg. Chem. Front. 2016;3:41–60. [Google Scholar]
- 29.Ragg R, et al. Solids go bio: inorganic nanoparticles as enzyme mimics. Eur. J. Inorg. Chem. 2016:1906–1915. [Google Scholar]
- 30.Rycenga M, et al. Controlling the synthesis and assembly of silver nanostructures for plasmonic applications. Chem. Rev. 2011;111:3669–3712. doi: 10.1021/cr100275d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H, et al. Shape-controlled synthesis of Pd nanocrystals and their catalytic applications. Acc. Chem. Res. 2013;46:1783–1794. doi: 10.1021/ar300209w. [DOI] [PubMed] [Google Scholar]
- 32.Luo J, et al. A novel non-enzymatic glucose sensor based on Cu nanoparticle modified graphene sheets electrode. Anal. Chim. Acta. 2012;709:47–53. doi: 10.1016/j.aca.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 33.Lin Y, et al. Nano-gold as artificial enzymes: Hidden talents. Adv. Mater. 2014;26:4200–4217. doi: 10.1002/adma.201400238. [DOI] [PubMed] [Google Scholar]
- 34.Ferrando R, et al. Nanoalloys: from theory to applications of alloy clusters and nanoparticles. Chem. Rev. 2008;108:845–910. doi: 10.1021/cr040090g. [DOI] [PubMed] [Google Scholar]
- 35.Naha PC, et al. Gold silver alloy nanoparticles (GSAN): an imaging probe for breast cancer screening with dual-energy mammography or computed tomography. Nanoscale. 2016;8:13740–13754. doi: 10.1039/c6nr02618d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chou S-W, et al. In vitro and in vivo studies of FePt nanoparticles for dual modal CT/MRI molecular imaging. J. Am. Chem. Soc. 2010;132:13270–13278. doi: 10.1021/ja1035013. [DOI] [PubMed] [Google Scholar]
- 37.Yang K, et al. Nano-graphene in biomedicine: theranostic applications. Chem. Soc. Rev. 2013;42:530–547. doi: 10.1039/c2cs35342c. [DOI] [PubMed] [Google Scholar]
- 38.Heister E, et al. Are carbon nanotubes a natural solution? Applications in biology and medicine. ACS Appl. Mater. Interfaces. 2013;5:1870–1891. doi: 10.1021/am302902d. [DOI] [PubMed] [Google Scholar]
- 39.Zhang XD, et al. Highly catalytic nanodots with renal clearance for radiation protection. ACS Nano. 2016;10:4511–4519. doi: 10.1021/acsnano.6b00321. [DOI] [PubMed] [Google Scholar]
- 40.Song Y, et al. Graphene oxide: Intrinsic peroxidase catalytic activity and its application to glucose detection. Adv. Mater. 2010;22:2206–2210. doi: 10.1002/adma.200903783. [DOI] [PubMed] [Google Scholar]
- 41.Bonitatibus PJ, et al. Preclinical assessment of a zwitterionic tantalum oxide nanoparticle X-ray contrast agent. ACS Nano. 2012;6:6650–6658. doi: 10.1021/nn300928g. [DOI] [PubMed] [Google Scholar]
- 42.Tarn D, et al. Mesoporous silica nanoparticle nanocarriers: Biofunctionality and biocompatibility. Acc. Chem. Res. 2013;46:792–801. doi: 10.1021/ar3000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reddy LH, et al. Magnetic nanoparticles: design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012;112:5818–5878. doi: 10.1021/cr300068p. [DOI] [PubMed] [Google Scholar]
- 44.Chhour P, et al. Effect of gold nanoparticle size and coating on labeling monocytes for CT tracking. Bioconjugate Chem. 2017;28:260–269. doi: 10.1021/acs.bioconjchem.6b00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Z, et al. Dual enzyme-like activities of iron oxide nanoparticles and their implication for diminishing cytotoxicity. ACS Nano. 2012;6:4001–4012. doi: 10.1021/nn300291r. [DOI] [PubMed] [Google Scholar]
- 46.Lee Y, et al. Synthetic tuning of the catalytic properties of Au-Fe3O4 nanoparticles. Angew. Chem. 2010;49:1271–1274. doi: 10.1002/anie.200906130. [DOI] [PubMed] [Google Scholar]
- 47.Lee JW, et al. Superparamagnetic Fe3O4 nanoparticles-carbon nitride nanotube hybrids for highly efficient peroxidase mimetic catalysts. Chem. Commun. 2012;48:422–424. doi: 10.1039/c1cc15725f. [DOI] [PubMed] [Google Scholar]
- 48.Cheng H, et al. Integrated nanozymes with nanoscale proximity for in vivo neurochemical monitoring in living brains. Anal. Chem. 2016;88:5489–5497. doi: 10.1021/acs.analchem.6b00975. [DOI] [PubMed] [Google Scholar]
- 49.Cormode DP, et al. Nanotechnology in medical imaging: probe design and applications. Arterioscler. Thromb. Vasc. Biol. 2009;29:992–1000. doi: 10.1161/ATVBAHA.108.165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benoit D, Koo H. Targeted, triggered drug delivery to tumor and biofilm microenvironments. Nanomedicine. 2016;11:873–879. doi: 10.2217/nnm-2016-0014. [DOI] [PubMed] [Google Scholar]
- 51.Lobatto ME, et al. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat. Rev. Drug Discov. 2011;10:835–852. doi: 10.1038/nrd3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allen TM, Cullis PR. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 53.Shi J, et al. Determination of NH3 gas by combination of nanosized LaCoO3 converter with chemiluminescence detector. Talanta. 2003;61:157–164. doi: 10.1016/S0039-9140(03)00240-6. [DOI] [PubMed] [Google Scholar]
- 54.Chaichi MJ, Ehsani M. A novel glucose sensor based on immobilization of glucose oxidase on the chitosan-coated Fe3O4 nanoparticles and the luminol-H2O2-gold nanoparticle chemiluminescence detection system. Sens Actuators B Chem. 2016;223:713–722. [Google Scholar]
- 55.Wang YH, et al. Colorimetric detection of hydrogen peroxide and glucose using the magnetic mesoporous silica nanoparticles. Talanta. 2015;134:712–717. doi: 10.1016/j.talanta.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 56.Fan K, et al. Magnetoferritin nanoparticles for targeting and visualizing tumour tissues. Nat. Nanotech. 2012;7:459–464. doi: 10.1038/nnano.2012.90. [DOI] [PubMed] [Google Scholar]
- 57.Wang G-L, et al. Dual responsive enzyme mimicking activity of AgX (X = Cl, Br, I) nanoparticles and its application for cancer cell detection. ACS Appl. Mater. Interfaces. 2014;6:6434–6442. doi: 10.1021/am501830v. [DOI] [PubMed] [Google Scholar]
- 58.Duan D, et al. Nanozyme-strip for rapid local diagnosis of Ebola. Biosens. Bioelectron. 2015;74:134–141. doi: 10.1016/j.bios.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 59.Song Y, et al. Label-free colorimetric detection of single nucleotide polymorphism by using single-walled carbon nanotube intrinsic peroxidase-like activity. Chem. Eur. J. 2010;16:3617–3621. doi: 10.1002/chem.200902643. [DOI] [PubMed] [Google Scholar]
- 60.Koo H, et al. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017 doi: 10.1038/nrmicro.2017.99. online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andre R, et al. V2O5 nanowires with an intrinsic peroxidase-like activity. Adv. Funct. Mater. 2011;21:501–509. [Google Scholar]
- 62.Natalio F, et al. Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation. Nat. Nanotechnol. 2012;7:530–535. doi: 10.1038/nnano.2012.91. [DOI] [PubMed] [Google Scholar]
- 63.Herget K, et al. Haloperoxidase mimicry by CeO2−x nanorods combats biofouling. Adv. Mater. 2017;29:1603823. doi: 10.1002/adma.201603823. [DOI] [PubMed] [Google Scholar]
- 64.Gao LZ, et al. Ferromagnetic nanoparticles with peroxidase- like activity enhance the cleavage of biological macromolecules for biofilm elimination. Nanoscale. 2014;6:2588–2593. doi: 10.1039/c3nr05422e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Z, et al. Activation of biologically relevant levels of reactive oxygen species by Au/g-C3N4 hybrid nanozyme for bacteria killing and wound disinfection. Biomaterials. 2017;113:145–157. doi: 10.1016/j.biomaterials.2016.10.041. [DOI] [PubMed] [Google Scholar]
- 66.Chen Z, et al. A multinuclear metal complex based DNase-mimetic artificial enzyme: matrix cleavage for combating bacterial biofilms. Angew. Chem. 2016;55:10732–10736. doi: 10.1002/anie.201605296. [DOI] [PubMed] [Google Scholar]
- 67.Pagliari F, et al. Cerium oxide nanoparticles protect cardiac progenitor cells from oxidative stress. ACS Nano. 2012;6:3767–3775. doi: 10.1021/nn2048069. [DOI] [PubMed] [Google Scholar]
- 68.Dowding JM, et al. Cerium oxide nanoparticles protect against A beta-induced mitochondrial fragmentation and neuronal cell death. Cell Death Differ. 2014;21:1622–1632. doi: 10.1038/cdd.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen JP, et al. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat. Nanotechol. 2006;1:142–150. doi: 10.1038/nnano.2006.91. [DOI] [PubMed] [Google Scholar]
- 70.Son D, et al. Bioresorbable electronic stent integrated with therapeutic nanoparticles for endovascular diseases. ACS Nano. 2015;9:5937–5946. doi: 10.1021/acsnano.5b00651. [DOI] [PubMed] [Google Scholar]
- 71.Kim CK, et al. Ceria nanoparticles that can protect against ischemic stroke. Angew. Chem. 2012;51:11039–11043. doi: 10.1002/anie.201203780. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Y, et al. Dietary iron oxide nanoparticles delay aging and ameliorate neurodegeneration in drosophila. Adv. Mater. 2016;28:1387–1993. doi: 10.1002/adma.201503893. [DOI] [PubMed] [Google Scholar]
- 73.Huang Y, et al. Self-assembly of multi-nanozymes to mimic an intracellular antioxidant defense system. Angew. Chem. 2016;55:6646–6650. doi: 10.1002/anie.201600868. [DOI] [PubMed] [Google Scholar]
- 74.Liu X, et al. Artificial metalloenzyme-based enzyme replacement therapy for the treatment of hyperuricemia. Adv. Funct. Mater. 2016;26:7921–7928. [Google Scholar]
- 75.Pattni BS, et al. New developments in liposomal drug delivery. Chem. Rev. 2015;115:10938–10966. doi: 10.1021/acs.chemrev.5b00046. [DOI] [PubMed] [Google Scholar]
- 76.Corot C, et al. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv. Drug Deliv. Rev. 2006;58:1471–1504. doi: 10.1016/j.addr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 77.Zhang HJ, Toshima N. Glucose oxidation using Au-containing bimetallic and trimetallic nanoparticles. Catal. Sci. Tech. 2013;3:268–278. [Google Scholar]
- 78.Zhang HJ, et al. One-pot synthesis of Ag-Au bimetallic nanoparticles with Au shell and their high catalytic activity for aerobic glucose oxidation. J. Colloid Interface Sci. 2011;354:131–138. doi: 10.1016/j.jcis.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 79.Sun H, et al. Deciphering a nanocarbon-based artificial peroxidase: chemical identification of the catalytically active and substrate-binding sites on graphene quantum dots. Angew. Chem. 2015;54:7176–7180. doi: 10.1002/anie.201500626. [DOI] [PubMed] [Google Scholar]
- 80.Vallabani NVS, et al. ATP-mediated intrinsic peroxidase-like activity of Fe3O4-based nanozyme: One step detection of blood glucose at physiological pH. Colloids Surf. B Biointerfaces. 2017;153:52–60. doi: 10.1016/j.colsurfb.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 81.Liu S, et al. Structural effects of Fe3O4 nanocrystals on peroxidase-like activity. Chem. Eur. J. 2011;17:620–625. doi: 10.1002/chem.201001789. [DOI] [PubMed] [Google Scholar]
- 82.Choi HS, et al. Renal clearance of quantum dots. Nat. Biotech. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ruggiero A, et al. Paradoxical glomerular filtration of carbon nanotubes. Proc. Natl. Acad. Sci. USA. 2010;107:12369–12374. doi: 10.1073/pnas.0913667107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caracciolo G, et al. Biological identity of nanoparticles in vivo: Clinical implications of the protein corona. Trends Biotechnol. 2017;35:257–264. doi: 10.1016/j.tibtech.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 85.Koo H, Yamada KM. Dynamic cell–matrix interactions modulate microbial biofilm and tissue 3D microenvironments. Curr. Opin. Cell Biol. 2016;42:102–112. doi: 10.1016/j.ceb.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Esch EW, et al. Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov. 2015;14:248–260. doi: 10.1038/nrd4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Libutti SK, et al. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin. Cancer Res. 2010;16:6139–6149. doi: 10.1158/1078-0432.CCR-10-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hainfeld JF, et al. Gold nanoparticles: a new X-ray contrast agent. Brit. J. Radiol. 2006;79:248–253. doi: 10.1259/bjr/13169882. [DOI] [PubMed] [Google Scholar]
- 89.Cheheltani R, et al. Tunable, biodegradable gold nanoparticles as contrast agents for computed tomography and photoacoustic imaging. Biomaterials. 2016;102:87–97. doi: 10.1016/j.biomaterials.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]