Abstract
Introduction
A Brief Resolved Unexplained Event (BRUE) describes an event associated with a change in muscle tone, color, respiration and responsiveness that is unexplained after an appropriate examination. Some infants with higher risk BRUE may undergo endoscopy as part of their evaluation.
Objective
This retrospective study aimed to identify the endoscopic findings in infants who have experienced a higher risk BRUE. We also compared the characteristics, pre-natal, natal and post-natal risk factors between 23 infants who underwent endoscopic evaluation and 23 race/sex/term/preterm-matched infants who did not undergo endoscopic evaluation.
Results
Twenty-three infants with higher risk BRUE underwent an esophagogastroduodenoscopy and flexible sigmoidoscopy from a total 119 infants with BRUE. Apnea (87%) was the most common presentation of BRUE. Most were female (57%) with a mean age at BRUE presentation of 2.73 months. We found 10 (43.5%) term infants and 13 (56.5%) preterm infants in our study. There were no significant differences in characteristics, pre-natal, natal and post-natal risk factors between the infants who underwent endoscopy and those who did not undergo endoscopy. The most common abnormal endoscopic finding was lymphonodular hyperplasia (LNH) associated with eosinophilia in the rectosigmoid colon. The proportion of females in the LNH group was significantly higher than the non-LNH group.
Conclusion
Rectosigmoid LNH and eosinophilia, which are associated with milk soy protein intolerance (MSPI), were the most common findings on endoscopic evaluation. Although there is no proof of causation between MSPI and BRUE, MSPI should be considered in the differential diagnosis for higher risk BRUE.
Keywords: Brief Resolved Unexplained Events, Endoscopy, Histology, Lymphonodular Hyperplasia, Eosinophilia
Introduction
Brief Resolved Unexplained Events (BRUE) is a more precise term that applies to infants previously described as having Apparent Life-Threatening Events (ALTE). BRUE is defined as a sudden, brief, and now resolved, episode occurring in an infant younger than 1 year. It is frightening to the observer and the episode is characterized by 1) cyanosis or pallor, 2) absent, decreased or irregular breathing, 3) change in tone (hyper- or hypotonia), and 4) altered level of responsiveness. Higher risk BRUE is a diagnosis based on the history and physical examination that suggests the need for further investigation and management1. The incidence of BRUE was described as 0.6 to 2.46 per 1,000 live births and 0.6% to 0.8% of all emergency visits for infants younger than 1 year2. Gastroesophageal reflux (GER) is one of the possible etiologic explanations for symptoms experienced by patients undergoing evaluation for possible BRUE. Gastroesophageal reflux (GER) and milk soy protein intolerance (MSPI) are frequent disorders in infants. A possible association between GER and MSPI has been suggested3. Up to 40% of infants with GER are noted to have MSPI4.
Food allergies can be classified as immediate (IgE-mediated) and late-onset (non-IgE mediated, mixed IgE and cell-mediated) reactions that can also be life-threatening. Food allergies are distinguished from food intolerances. The food intolerances are nonspecific, non-allergic reactions which do not involve the immune system. Food intolerance is a delay in symptom onset, prolonged symptoms, and negative IgE serology20. Cow’s milk protein allergy (CMPA) is the most common non-IgE immune-mediated food allergy in infants with an incidence of 2% to 3% in the first year of life5. There are several factors that affect the CMPA immunological mechanism including genetics, dose of cow milk, frequency of consumption, age at first cow milk exposure and cow milk transmission via breast-milk6. There is not one manifestation that is specific for CMPA. Many of its symptoms are of the skin, but can also be of the gastrointestinal or respiratory tracts. Half of those diagnosed with CMPA have atopic eczema and 25% to 50% have some gastrointestinal symptoms7. CMPA rarely develops after 12 months of age and usually develops within 2 months after beginning cow’s milk or soy8. The ESPGHAN guidelines recommend to re-evaluate every 6 to 12 months to assess if patients have developed tolerance to cow’s milk and there was a resolution in 75% by 3 years, and > 90% by 6 years9.
There are some differences between cow's milk protein allergy (CMPA) and milk soy protein intolerance (MSPI). One of the currently available cow's milk substitutes for infants is soy protein formula. Soybeans have been cultivated in Eastern countries for many centuries and were first used to feed US babies with cow’s milk allergy in 192921. The general agreement is that a significant number of children with cow's milk protein intolerance develop soy protein intolerance when soy milk is used in dietary management. However, the American Academy of Pediatrics states that infants with IgE-associated symptoms of cow's milk allergy may benefit from a soy formula because the risk of cross-reactivity does not appear to be very high. The prevalence rate of soy protein allergy was reported to be 1.1%, compared with a 3.4% prevalence rate of cow's milk protein allergy21.
No single laboratory test is sensitive or specific enough to make a diagnosis of CMPA or MSPI. Esophagogastroduodenoscopy (EGD) and flexible sigmoidoscopy in conjunction with the evaluation of biopsy specimens may be considered for confirming a diagnosis of CMPA or MSPI. Some infants who experience a higher risk BRUE may undergo EGD and flexible sigmoidoscopy as part of their evaluation.
Objective
The objective of our study was to describe the clinical characteristics and endoscopic and histologic findings in infants with gastrointestinal symptoms who have experienced a higher risk BRUE.
Method
Study population
Our study was a retrospective review of records of infants younger than 12 months who presented to University of South Alabama Children’s and Women’s Hospital with an admission diagnosis of BRUE from October 2015 to February 2017. The Institutional Review Board of the University of South Alabama approved this study.
Data collection
Infants were identified from a query of medical records using the ICD-10 code for BRUE (R68.13). Initially, two investigators (C.J., M.G.) each reviewed the electronic medical charts to ensure consistent data. Patients who were diagnosed with BRUE and who also underwent an EGD and flexible sigmoidoscopy between October 2015 and February 2017 were included in our study.
We investigated demographics, gestational age, chronic condition defined as underlying diseases (including congenital heart disease, genetic diseases, bronchopulmonary dysplasia, and preexisting known GER or MSPI), initial presentation, admission history, formula feeding, weight/length at admission, duration of hospitalization and detailed information on the endoscopic and histologic findings. The definition of eosinophilia in duodenum and colon/rectum of our study was >10 eosinophils/high power field and >5 eosinophils/high power field if the infant had already been on a hypoallergenic formula.
We also chose a control group from the infants who did not undergo endoscopy by matching the age, race, sex, and term/preterm status at birth. We investigated these characteristics, pre-natal, natal and post-natal risk factors, and endoscopic findings.
Endoscopy
Indications for endoscopy included investigations to identify the source of fecal occult blood or evaluation of an infant with unexplained crying or distressed behavior following the pediatric GERD clinical practice guidelines. The patients were given nothing by mouth of formula or breast milk for 4 hours with clear Pedialyte given until 2 hours prior to the procedures (EGD and flexible sigmoidoscopy). Electrocardiogram, blood pressure and pulse oximetry were monitored through the procedures. Midazolam was given per rectum before the procedure for sedation. Cetacaine spray was administered topically to the pharynx prior to the procedures.
Esophagogastroduodenoscopy and flexible sigmoidoscopy with biopsy were performed using standard techniques. Biopsies were obtained from the second portion of the duodenum, antrum, esophagus at 3 & 6 cm above the lower esophageal sphincter, sigmoid colon, and rectum for histopathologic evaluation. The same pediatric pathologist performed histological analysis.
Statistical Analysis
All data collected were recorded in a clinical database. Software R was used for all statistical analyses. The quantitative data such as gestational age or weight were summarized using mean ± SD and categorical data such as gender or presence/absence of a condition were summarized using percentages. Due to small sample size in this study, Wilcoxon test was used to compare the distributions of quantitative outcomes for the two groups and Fisher’s exact test was used to compare the incidence rates of categorical variables in the two groups. Significance level of 0.05 was used to determine the significance of results.
Results
Initial data showed 23 (19.3%) infants presenting with higher risk BRUE also had undergone an EGD and flexible sigmoidoscopy from a total 119 infants with BRUE from October 2015 to February 2017. Most were female (57%) with a mean age at BRUE presentation of 2.73 ± 2.76 months. The manifestations of BRUE were apnea (20/23; 87%), cyanosis (10/23; 43.5%), choking (5/23; 21.7%), back arching (4/23; 17.4%) and gagging (1/23; 4.3%). The characteristics of our study are shown in Table 1. There were 10 (43.5%) term infants and 13 (56.5%) preterm infants. The duration between BRUE presentation and endoscopy (mean ± SD) was 37.74 ± 43.91 days, with prior to, during, and after BRUE presentation being 7/23 (30%), 7/23 (30%) and 9/23 (40%) respectively.
Table 1.
Characteristics of infants who presented with BRUE
| Characteristics | Endoscopy group (N = 23) |
Non-endoscopy group (N = 23) |
|---|---|---|
|
| ||
| Gender | ||
| - Female (%) | 13/23 (57%) | 13 (57%) |
| - Male (%) | 10/23 (43%) | 10 (43%) |
|
| ||
| Age of study in months (mean ± SD) | 2.73 ± 2.76 | 3.69 ± 2.68 |
|
| ||
| Ethnicity | ||
| - Caucasian | 18 (78.2%) | 19 (82.6%) |
| - African American | 4 (17.4%) | 4 (17.4%) |
| - Hispanic | 1 (4.4%) | 0 |
|
| ||
| Term at birth | 10 (43.5%) | 10 (43.5%) |
| Preterm at birth | 13 (56.5%) | 13 (56.5%) |
|
| ||
| Gestational age in weeks (mean ± SD) | 35.23 ± 2.94 | 34.23 ± 5.07 |
|
| ||
| Birth weight in kilograms (mean ± SD) | 2.43 ± 0.86 | 2.06 ± 0.89 |
|
| ||
| Weight at diagnosis in kilograms (mean ± SD) | 4.42 ± 2.02 | 5.02 ± 2.06 |
|
| ||
| Length at diagnosis in centimeters (mean ± SD) | 52.90 ± 13.59 | 55 ± 9.29 |
|
| ||
| Underlying diseases (%) | ||
| - Clinical diagnosis of GERD | 12/23 (52%) | 6/23 (25.09) |
| - Clinical diagnosis of MSPI | 6/23 (26%) | 1/23 (4.3%) |
| - ASD | 5/23(22%) | 0 |
| - Others | 4/23 (17.4%)* | 8/23 (34.78)** |
|
| ||
| Formula | ||
| - 100% amino acid-based formula | 8/23 (34.8%) | 0 |
| - Cow milk-based formula | 7/23 (30.4%) | 16 (70%) |
| - Extensively hydrolyzed formula | 3/23 (13%) | 4 (17%) |
| - Breast milk | 3/23 (13%) | 3 (13%) |
| - Others | 2/23 (8.8%)*** | 0 |
|
| ||
| Hospitalized duration in days (mean ± SD) | 4.39 ± 3.14 | 5.08 ± 7.05 |
|
| ||
| Stool occult blood test | ||
| Negative | 10/23 (43.5%) | 7 (30.4%) |
| Positive | 5/23 (21.7%) | 2 (8.7%) |
| Not done | 8/23 (34.8%) | 14 (60.9) |
SD, standard deviation; ASD, atrial septal defect
Ventricularseptal defect (1), Cri du chat (1), seizure disorder (1), apnea of prematurity (1)
Ventricular septal defect (1), seizure disorder (1), patent ductus arteriosus (1), bronchopulmonary dysplasia (2), apnea of prematurity (1), hydronephosis (1), thanatophoric dysplasia (1)
Isomil (1), regular (1)
In addition, the 96 infants who did not undergo endoscopy included male (49/56; 51%) with a mean age at BRUE presentation of 2.27 ± 2.38 months. Most were 54/96 (56%) Caucasian and 42/96 (44%) African American infants. The mean gestational age was 36.02 ± 3.85 weeks, which were 65/96 (67.7%) term and 31/96 (32.3%) preterm infants. We selected the race/sex/term/preterm-matched controls in our 23 control patients. The demographics of the 23 infants who did not undergo endoscopy (control group) were shown in Table 1 and 2. Moreover, there were no significant differences in age, gestational age, number of gestations, number of abortions, birth weight, weight, length and hospitalized duration between the two groups. We also compared the pre-natal, natal and post-natal risk factors between the endoscopic and non-endoscopic groups in Table 2.
Table 2.
Comparison of pre-natal, natal and post-natal risk factors between endoscopic group and non-endoscopic group
| Characteristics | Endoscopy group (N=23) |
Non- endoscopy group (N=23) |
P-value |
|---|---|---|---|
|
| |||
| Age of study in months (mean ± SD) | 2.73 ± 2.76 | 3.69 ± 2.68 | 0.113 |
|
| |||
| Gestational age in weeks (mean ± SD) | 35.30 ± 2.94 | 34.24 ± 5.07 | 0.833 |
|
| |||
| Number of gestation at pregnancy (mean ± SD) | 2.17 ± 1.23 | 2.96 ± 2.27 | 0.386 |
|
| |||
| Number of abortion (mean ± SD) | 0.35 ± 0.93 | 0.35 ± 1.11 | 0.738 |
|
| |||
| Birth weight in kilograms (mean ± SD) | 2.43 ± 0.86 | 2.06 ± 0.89 | 0.255 |
|
| |||
| Weight at diagnosis in kilograms (mean ± SD) | 4.42 ± 2.02 | 5.02 ± 2.06 | 0.199 |
|
| |||
| Length at diagnosis in centimeters (mean ± SD) | 52.90±13.59 | 55 ± 9.29 | 0.482 |
|
| |||
| Hospitalized duration in days (mean ± SD) | 4.39 ± 3.14 | 5.08 ± 7.05 | 0.592 |
|
| |||
| Complication during pregnancy (N) | 11 | 11 | 1.000 |
|
| |||
| Delivery method | |||
| - Cesarean section (N) | 12 | 11 | 1.000 |
| - Vaginal delivery (N) | 11 | 12 | 1.000 |
|
| |||
| Complication after delivery | |||
| - Respiratory distress (N) | 7 | 8 | 1.000 |
| - Suspected sepsis (N) | 6 | 7 | 0.749 |
|
| |||
| Respiratory support after delivery (N) | 7 | 8 | 1.000 |
|
| |||
| History of smoking exposure (N) | 9 | 9 | 1.000 |
|
| |||
| Numbers of investigations during admission (mean ± SD) | 2.39 ± 1.34 | 2.47 ± 1.44 | 0.833 |
|
| |||
| GERD medication prior discharge home | |||
| - Histamine 2 inhibitor (N) | 16 | 10 | 0.136 |
|
| |||
| Changing formula prior discharge home (N) | 10 | 6 | 0.353 |
SD, standard deviation
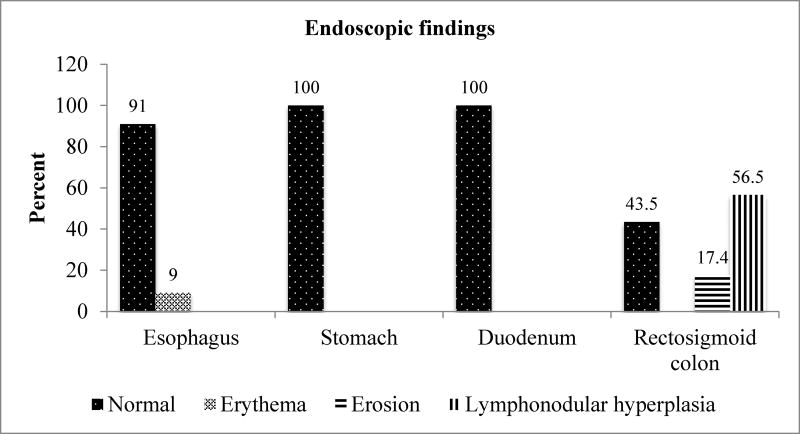
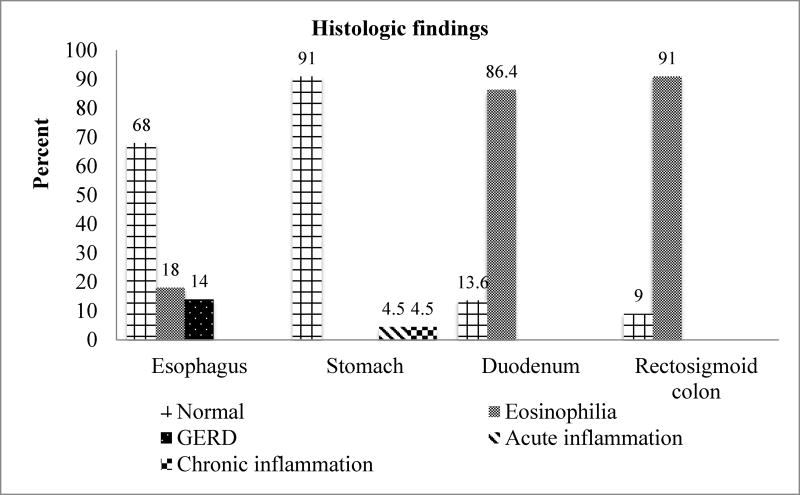
Endoscopic and histologic findings (Figure 1)
Figure 1.
Results of EGD and flexible sigmoidoscopic findings in BRUE infants
Lymphonodular hyperplasia (LNH, 13/23; 56.5%) and erosions in the rectosigmoid colon (4/23; 17.4%) were the two most common pathologic findings at endoscopy (Figure 1). Moreover, our study showed normal endoscopic findings in the stomach and duodenum. One of our subjects did not have biopsies taken during endoscopy. Therefore, we included 22 histologic subjects in our study. Eosinophilia in the rectosigmoid colon (20/22; 91%) and eosinophilia in the duodenum (19/22; 86.4%) were the two most common pathologic findings. Interestingly, our study showed normal endoscopic findings (100%) in the duodenum, however histologic findings revealed duodenal eosinophilia (19/22; 86.4%). Moreover, we found normal endoscopic findings (21/23; 91%) and erythema (2/23; 9%) in esophagus, however histologic findings showed normal histology (15/22; 68%), eosinophilia (4/22; 18%), and GERD (3/22; 14%) in esophagus.
We demonstrated the relationship between infants who were diagnosed with MSPI prior to the BRUE episode and the endoscopic finding of LNH was not significant. (P-value = 0.656) However, there was a significant correlation between infants who were diagnosed with MSPI prior to the BRUE episode and histologic findings of eosinophilia (P-value = 0.015).
We compared the difference in characteristics between lymphonodular hyperplasia (LNH) and non-lymphonodular hyperplasia (Table 3). We found the proportion of females in the LNH group was significantly higher than in non-LNH group. Otherwise, we did not find any significant differences in age, gestational age, birth weight, weight and length at BRUE presentation, hospitalization duration, preterm/term and hypoallergenic/non-hypoallergenic formula between LNH and non-LNH (Table 3). We also compared the differences in characteristics between eosinophilia and non-eosinophilia (Table 3) and found no significant differences in characteristics between the groups. Due to the fact that there were only two patients in the non-eosinophilia group, we might not have a large enough sample size to detect the potential differences. For example, in the non-eosinophilia group, 100% of the infants were preterm at birth while in the eosinophilia; the percentage is only 55%. Further studies with larger sample sizes are needed to verify these potential differences.
Table 3.
Comparison of the differences in characteristics between lymphonodular hyperplasia and non-lymphonodular hyperplasia and between eosinophilia and non-eosinophilia.
| Characteristics | Lymphonodular hyperplasia (13) |
Non- lymphonodular hyperplasia (10) |
P-value | Eosinophilia (20) |
Non- eosinophilia (2) |
P-value |
|---|---|---|---|---|---|---|
|
| ||||||
| Gender | ||||||
| - Male | 3 (23%) | 7 (70%) | 9 (45%) | 0 | ||
| - Female | 10 (77%) | 3 (30%) | 0.039 | 11 (55%) | 2 (100%) | 0.494 |
|
| ||||||
| Age of study in months (mean ± SD) | 1.90 ± 1.19 | 3.79 ± 3.85 | 0.257 | 2.34 ± 2.03 | 2.00 ± 1.41 | 1.000 |
|
| ||||||
| Gestational age in weeks (mean ± SD) | 35.33 ± 2.43 | 35.25 ± 3.77 | 1.000 | 35.49 ± 3.04 | 33.50 ± 0.71 | 0.337 |
|
| ||||||
| Birth weight in kg (mean ± SD) | 2.60 ± 0.87 | 2.11 ± 0.90 | 0.224 | 2.42 ±0.95 | 2.08 ± 0 | 0.933 |
|
| ||||||
| Weight at diagnosis in kg (mean ± SD) | 4.10 ± 1.50 | 4.83 ± 2.59 | 0.733 | 4.282±1.852 | 3.45 ± 0.92 | 0.776 |
|
| ||||||
| Length at diagnosis in cm (mean ± SD) | 50.43 ± 14.37 | 56.10 ± 12.47 | 0.901 | 53.67 ± 6.92 | 30.55 ± 35.99 | 0.607 |
|
| ||||||
| Hospitalized duration in days (mean ± SD) | 4.84 ± 3.65 | 3.80 ± 2.39 | 0.683 | 4.35 ± 3.15 | 5.50± 4.95 | 0.862 |
|
| ||||||
| Number of preterm at birth | 9 (70%) | 4 (40%) | 11 (55%) | 2 (100%) | ||
| Number of term at birth | 4 (30%) | 6 (60%) | 0.222 | 9 (45%) | 0 | 0.494 |
|
| ||||||
| Formula | ||||||
| -Hypoallergic formula | 7 (53.8%) | 4 (40%) | 10 (50%) | 1 (50%) | ||
| - Non-hypoallergic formula | 6 (46.2%) | 6 (60%) | 0.680 | 10 (50%) | 1 (50%) | 1.000 |
SD, standard deviation
Discussion
An association between GER and cow milk hypersensitivity was observed in infants. Nielsen RG et al reported that there was a GER and cow milk hypersensitivity association in 10 of 17 patients with severe GER.3
Endoscopic findings of the rectum and colon in food protein-induced enterocolitis syndrome include erythema, increased hyperplasia, and multiple erosions mimicking infectious colitis in severe cases10. One review article of allergic colitis in infants reported 225/314 (71.6%) showed friability and erosions of the colonic mucosa at colonoscopy or rectosigmoidoscopy and 236/264 (89.3%) showed eosinophil infiltrations in the colonic mucosa on biopsies11. LNH in the rectosigmoid colon has been described as a sign of food allergy in children. Kokkonen et al reported that duodenal LNH is a characteristic endoscopic finding associated with food allergy, as seen commonly in infants12. In contrast, our study showed LNH was more common in the rectosigmoid colon rather than the duodenum. Interestingly, patients with duodenal LNH mostly present a local reaction of delayed-type food hypersensitivity, which is not shown as atopic allergy by skin prick test or elevated serum IgE13. In addition, 55% with LNH of the colon showed concomitant LNH in the duodenal bulb.14 Thus, colonic LNH is a sensitive endoscopic finding of food hypersensitivity, most likely related to a cell-mediated immune response. However, our study showed no concomitant LNH of duodenum and rectosigmoid colon, even though eosinophilic infiltrates were found in intestinal biopsies from both regions. This histologic finding supports the importance of obtaining mucosal biopsies in the evaluation of these patients.
One South Korean study included 38 children with food protein-induced proctocolitis who underwent sigmoidoscopy for evaluation of rectal bleeding. Endoscopy showed LNH in 94.7% of patients and 97.4% had eosinophil counts ≥ 6/hpf in histologic assessment of the rectosigmoid mucosa15, which was similar to our histologic findings. One descriptive study of 116 children with suspected CMPA who underwent an EGD and flexible sigmoidoscopy demonstrated that the rectum had the largest numbers of eosinophils, followed by the duodenum16 as similar to our study. Interestingly, Austin et al reported no significant difference in eosinophil numbers between patients with CMPA and those in the control group17.
Moreover, Hwang et al reported exclusive breastmilk feeding in 94.7% of their subjects at the onset of symptoms of food protein-induced proctocolitis while only 5.3% of their patients were formula-fed or mixed-fed15, which differs from our study (only 13% breastfeeding). A significant proportion of our patient population was previously changed to a hypoallergenic formula prior to endoscopic evaluation (11/23; 47.8%).
One prospective study of 30 infants with MSPI and a history of weight loss and persistent allergic symptoms while on extensively hydrolyzed formula showed improved in weight gain after 12 weeks of amino acid-based formula feeding18. Moreover, extensively hydrolyzed formulas and amino acid-based formulas have been shown to improve gut barrier function19. Dietary management of cow’s milk protein intolerance usually starts with an extensively hydrolyzed infant formula. However, for infants with extremely severe or life-threatening symptoms, an amino acid-based formula may be used as the first management9.
There are no evidence-based guidelines for the diagnostic evaluation of infants with higher risk BRUE. The endoscopic and histologic findings alone are nonspecific and not helpful to diagnose underlying conditions associated with BRUE. These procedures should be interpreted in the context of medical history, physical examination and laboratory tests. The diagnostic yields of these endoscopic and histologic findings are higher for diagnoses other than MSPI. Clinical manifestations of food allergy, especially MSPI in BRUE infants, need to be known in order to avoid errors in diagnostic orientation and therapy, which may be responsible for worsened symptoms or recurrences. We speculate that MSPI may be associated with BRUE because infants with MSPI can have symptoms of vomiting and retching that disrupt the normal gastric motor activity and can lead to apnea or cyanosis.
There are limitations in our study. First, this was a retrospective study, which could be subject to bias and incomplete/missing data. Second, our study had a small subject pool including only 23 endoscopic subjects and 22 histologic subjects.
Conclusion
To our knowledge, this is the first study of the characteristics of endoscopic and histologic findings in infants with higher risk BRUE who subsequently underwent evaluation for gastrointestinal symptoms. The present study revealed that rectosigmoid lymphonodular hyperplasia and eosinophilia were the most common findings from endoscopic and histopathologic evaluations. Although there is no proof of association of higher risk BRUE and MSPI, inclusion of MSPI in the differential diagnosis for higher risk BRUE infants may be warranted.
Figure 2.
Results of histologic findings in BRUE infants
Acknowledgments
Funding source: Statistical analysis in this project was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001417.
Abbreviations
- BRUE
Brief Resolved Unexplained Events
- ALTE
Apparent Life-Threatening Events
- GERD
Gastroesophageal Reflux Disease
- GER
Gastroesophageal reflux
- MSPI
Milk Soy Protein Intolerance
- CMPA
Cow’s Milk Protein Allergy
- EGD
Esophagogastroduodenoscopy
- LNH
Lymphonodular Hyperplasia
Footnotes
Financial disclosure: No financial relationships relevant to this article to disclose.
Conflict of interest: The authors have no conflicts of interest to disclose.
References
- 1.Tieder JS, Bonkowsky JL, Etzel RA, Franklin WH, Gremse DA, Herman B, et al. Brief Resolved Unexplained Events (Formerly Apparent Life-Threatening Events) and Evaluation of Lower-Risk Infants: Executive Summary. Pediatrics. 2016;137(5) doi: 10.1542/peds.2016-0591. [DOI] [PubMed] [Google Scholar]
- 2.Fu LY, Moon RY. Apparent life-threatening events: an update. Pediatr Rev. 2012;33(8):361–8. doi: 10.1542/pir.33-8-361. quiz 368-9. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen RG, Bindslev-Jensen C, Kruse-Andersen S, Husby S. Severe gastroesophageal reflux disease and cow milk hypersensitivity in infants and children: disease association and evaluation of a new challenge procedure. J Pediatr Gastroenterol Nutr. 2004;39(4):383–91. doi: 10.1097/00005176-200410000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Iacono G, Carroccio A, Cavataio F, Montalto G, Kazmierska I, Lorello D, et al. Gastroesophageal reflux and cow’s milk allergy in infants: a prospective study. J. Allergy Clin Immunol. 1996;97:822–7. doi: 10.1016/s0091-6749(96)80160-6. [DOI] [PubMed] [Google Scholar]
- 5.Høst A. Frequency of cow's milk allergy in childhood. Ann Allergy Asthma Immunol. 2002;89(6 Suppl 1):33–7. doi: 10.1016/s1081-1206(10)62120-5. [DOI] [PubMed] [Google Scholar]
- 6.Fiocchi A, Brozek J, Schunemann H, Bahna SL, von Berg A, Beyer K, et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines. World Allergy Organ J. 2010;3:57–161. doi: 10.1097/WOX.0b013e3181defeb9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klemola T, Vanto T, Juntunen-Backman K, Kalimo K, Korpela R, Varjonen E, et al. Allergy to soy formula and to extensively hydrolyzed whey formula in infants with cow’s milk allergy: a prospective, randomized study with a follow-up to the age of 2 years. J Pediatr. 2002;140:219–24. doi: 10.1067/mpd.2002.121935. [DOI] [PubMed] [Google Scholar]
- 8.Vandenplas Y, De Greef E, Devreker T. Treatment of Cow's Milk Protein Allergy. Pediatr Gastroenterol Hepatol Nutr. 2014;17(1):1–5. doi: 10.5223/pghn.2014.17.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koletzko S, Niggemann B, Arato A, Dias JA, Heuschkel R, Husby S, et al. Diagnostic Approach and Management of Cow’s-Milk Protein Allergy in Infants and Children: ESPGHAN GI Committee Practical Guidelines. J Pediatr Gastroenterol Nutr. 2012;55(2):221–9. doi: 10.1097/MPG.0b013e31825c9482. [DOI] [PubMed] [Google Scholar]
- 10.Dupont C, Heyman M. Food protein-induced enterocolitis syndrome: laboratory perspectives. J Pediatr Gastroenterol Nutr. 2000;30(Suppl):S50–7. doi: 10.1097/00005176-200001001-00008. [DOI] [PubMed] [Google Scholar]
- 11.Lozinsky AC, Morais MB. Eosinophilic colitis in infants. J Pediatr (Rio J) 2014;90:16–21. doi: 10.1016/j.jped.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Kokkonen J. Lymphonodular hyperplasia on the duodenal bulb indicates food allergy in children. Endoscopy. 1999;31(6):464–7. doi: 10.1055/s-1999-51. [DOI] [PubMed] [Google Scholar]
- 13.Mansueto P, Iacono G, Seidita A, D'Alcamo A, Sprini D, Carroccio A. Review article: intestinal lymphoid nodular hyperplasia in children--the relationship to food hypersensitivity. Aliment Pharmacol Ther. 2012;35(9):1000–9. doi: 10.1111/j.1365-2036.2012.05062.x. [DOI] [PubMed] [Google Scholar]
- 14.Kokkonen J1, Karttunen TJ. Lymphonodular hyperplasia on the mucosa of the lower gastrointestinal tract in children: an indication of enhanced immune response? J Pediatr Gastroenterol Nutr. 2002;34(1):42–6. doi: 10.1097/00005176-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Hwang JB, Park MH, Kang YN, Kim SP, Suh SI, Kam S. Advanced Criteria for Clinicopathological Diagnosis of Food Protein-induced Proctocolitis. J Korean Med Sci. 2007;22(2):213–7. doi: 10.3346/jkms.2007.22.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cervantes-Bustamante R, Pedrero-Olivares I, Toro-Monjaraz EM, Murillo-Márquez P, Ramírez-Mayans JA, Montijo-Barrios E, et al. Histopathologic findings in children diagnosed with cow's milk protein allergy. Rev Gastroenterol Mex. 2015;80(2):130–4. doi: 10.1016/j.rgmx.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Augustin MT, Kokkonen J, Karttunen TJ. Evidence for increased apoptosis of duodenal intraepithelial lymphocytes in cow's milk sensitive enteropathy. J Pediatr Gastroenterol Nutr. 2005;40(3):352–8. doi: 10.1097/01.mpg.0000151748.07469.bf. [DOI] [PubMed] [Google Scholar]
- 18.Vanderhoof J, Moore N, de Boissieu D. Evaluation of an Amino Acid-Based Formula in Infants Not Responding to Extensively Hydrolyzed Protein Formula. J Pediatr Gastroenterol Nutr. 2016;63(5):531–533. doi: 10.1097/MPG.0000000000001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arvola T, Moilanen E, Vuento R, Isolauri E. Weaning to hypoallergenic formula improves gut barrier function in breast-fed infants with atopic eczema. J Pediatr Gastroenterol Nutr. 2004;38:92–6. doi: 10.1097/00005176-200401000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Turnbull JL, Adams HN, Gorard DA. Review article: the diagnosis and management of food allergy and food intolerances. Aliment Pharmacol Ther. 2015;41(1):3–25. doi: 10.1111/apt.12984. [DOI] [PubMed] [Google Scholar]
- 21.American Academy of Pediatrics. Committee on Nutrition. American Academy of Pediatrics. Committee on Nutrition. Soy Protein-based Formulas: Recommendations for Use in Infant Feeding. Pediatrics. 1998;101(1 Pt 1):148–53. [PubMed] [Google Scholar]