Abstract
Purpose
Non-adherence to the antiretroviral (ARV) regimen is a critical factor in determining efficacy of ARV drugs for pre-exposure prophylaxis (PrEP). A long-acting parenteral formulation may be an effective alternative to daily oral dosing. A pharmacokinetic and tissue distribution study of drug-loaded nanoparticle (NP) was performed in female humanized CD34+-NSG mice.
Methods
Mice received 200 mg/kg each of tenofovir alafenamide (TAF) and elvitegravir (EVG) as free drugs (TAF+EVG solution) or as drug loaded NP (TAF+EVG NP) formulation by subcutaneous (SubQ) administration. Plasma and tissue were collected to determine tenofovir (TFV) and EVG concentrations using LC-MS/MS. Non-compartmental analysis was performed using WinNonlin.
Results
SubQ administration of TAF+EVG NP formulation resulted in long residence time and exposure for both drugs. The AUC(0–72h) of TFV and EVG was 14.1±2.0, 7.2±1.8 μg×hr/mL from drugs in solution (free) and the AUC(0–14day) for the same drugs was 23.1±4.4, 39.7±6.7 μg×hr/mL from NPs. The observed elimination half-life (t1/2) for TFV of free and NPs were 14.2 h, 5.1 days and for EVG 10.8 h, 3.3 days, respectively.
Conclusion
This study documents that a TAF+EVG NP provides sustained release, which can overcome patient non-adherence to dosing and may facilitate prediction of appropriate protective drug concentration for HIV prophylaxis.
Keywords: Pharmacokinetics, tissue distribution profile, nanoformulation, tenofovir alafenamide, elvitegravir
Introduction
Combination antiretroviral (ARV) therapy reduces HIV-1 plasma viral load and improves patient life expectancy when used as treatment (1). But the rate of HIV-1 transmission remains high, with 2.1 million new infections occurring worldwide in 2015 alone (2). Based on the efficacy in several pre-exposure prophylaxis (PrEP) trials, US Food and Drug Administration (FDA) has approved daily oral tenofovir disoproxil fumurate (TDF) in combination with emtricitabine (FTC) (Truvada™, Gilead Sciences Inc., USA) for PrEP to prevent HIV-1 infection in men and women (3). PrEP trials also highlighted the importance of adherence to daily oral regimen to maintain quantifiable tenofovir in plasma, which increased efficacy.
Long-acting parenteral ARV nanoformulations can significantly reduce the dosing frequency (4) and thus improve patient adherence and effectiveness for HIV-1 prevention (5). Long-term release of antiretroviral drugs is feasible using intravaginal devices (i.e. rings), implants, and injectable formulations (6, 7). Each of these techniques has drawbacks for the administration of the product. Among these, injectable formulations would offer ease of administration and hence would be advantageous over daily oral dose for prophylaxis. Besides the patient adherence to therapy, a major factor that contributes to protection against HIV-1 is the proper drug concentration in the target mucosal tissues, such as vaginal and rectal tissue (8). Tissue drug concentrations are affected by many variables such as dose, frequency, mode of administration, tissue permeability, protein binding, and elimination half-life. The effective tenofovir (TFV) plasma concentration to prevent HIV-1 infection has been estimated to be 90–110 ng/mL (9). However, the concentration at the site of infection (vaginal or rectal) and in the other tissues, has not consistently been reported. Consequently, pharmacokinetic (PK) studies that focus on the mucosal compartments are critical for the future success of PrEP strategies.
Several studies and clinical trials have proved that TDF, an ester prodrug of TFV is effective for prevention of HIV-1 (10, 11, 12). Presently, tenofovir alafenamide (TAF), a FDA-approved nucleotide reverse transcriptase inhibitor prodrug is preferred over TDF due to its comparatively improved side effect profile (less kidney and bone toxicity) for HIV-1 treatment (13). A long acting formulation containing TAF would be highly desirable. While FTC has proven to be effective for prevention, we hypothesized elvitegravir (EVG), a FDA-approved integrase strand transfer inhibitor, could be a novel drug choice for prevention based on its drug class and clinical pharmacokinetics.
We have previously reported the formulation and characterization of polymeric nanoparticles (NPs) with TAF and EVG entrapped within the polymer (14). In the present study, we report the pharmacokinetic profile and tissue distribution of TAF and EVG drugs after subcutaneous (SubQ) administration in humanized mice as TAF+EVG NPs and compared with TAF+EVG free drugs (in solution).
Materials and methods
Chemicals
Phosphate buffered saline was purchased from Sigma-Aldrich (St. Louis, MO, USA). TFV reference standard (>99%) was purchased from United States Pharmacopeia, (Rockville, MD). EVG reference standard (>99%) was purchased from Sequoia, (Pangbourne, United Kingdom). Internal standards (TFV-d6 and EVG-d6) were purchased from Toronto Research Chemicals Inc., Canada. Milli-Q water was obtained from in-house Milli-Q water purification system, Millipore, USA. LC-MS grade methanol, acetonitrile, formic acid and trifluoroacetic acid were purchased from Fisher Scientific, USA.
ARV loaded NP preparation and characterization
NP preparation and characterization was carried according to our previously reported publication (14). Briefly, to formulate TAF+EVG NPs, we followed oil-in-water (o-w) emulsion methodology. The organic phase (dichloromethane), containing PLGA and PF-127 at 1:1 ratio along with TAF and EVG at 50 mg/100 mg PLGA, was emulsified with aqueous phase of 1 % PVA solution, followed by sonication. The organic phase was evaporated and the emulsion was clarified using a dialysis membrane (Slide-A-lyzer, 10K, Pierce, Inc.) and then freeze-dried. The physicochemical characteristics (size, poly dispersity index-PDI and surface charge) of the TAF+EVG NPs were analyzed using a Zeta-sizer Instruments and the percentage drug entrapment efficiency (%EE) was analyzed by HPLC following slight modification of previously published procedure (15). (Mobile phase: 25mM potassium dihydrogen phosphate: acetonitrile (45:55 v/v); TAF and EVG: absorbance maximum was at 260 and 313 nm respectively; retention time 4 and 20 min respectively). Intra-day and inter-day variability was < 15%.
To evaluate stability of TAF+EVG NPs over time (1 year), 5 mg of freeze dried TAF+EVG NPs were dispersed in 1 mL 40% DMSO solvent at time 0 (freshly prepared and freeze dried) and at time 1 year (same batches that were used in this study, 1 year old freeze dried NPs stored at 4°C). The size, PDI and % EE were evaluated as stated above. Mean ± standard error (SE) of mean obtained from four different batches of TAF+EVG NPs was calculated and statistical significance test was performed to evaluate stability.
Pharmacokinetic study design
Female humanized CD34+ NOD.Cg-PrkdcscidIL2rgtm1Wjl/Szj (NOD/SCID/IL2rgnull, CD34+-NSG) mice were purchased from Jackson Laboratory (Bar Harbor, MA) and allowed to acclimate to the animal facility for seven days. Mice were divided into two groups, one group of 15 mice (3 mice/time point) were injected SubQ with 200 mg/kg (each TAF and EVG) as TAF+EVG NP in 1 mL of 5% dextrose. Another group of 15 mice (3 mice/time point) were injected SubQ with 200 mg/kg (each TAF and EVG) of free drug in 1 mL of 5% dextrose (TAF+EVG solution). Two SubQ injections of 0.5 mL were given with a gap of 4 h, to avoid dose volume stress on mice. At 1, 4, 7, 10 and 14 days after injection of TAF+EVG NP and at 4, 8, 24, 48 and 72 h after injection of free TAF+EVG in solution, mice were sacrificed by carbon dioxide inhalation and cervical dislocation. Institutional Animal Care and Use Committee approved protocol (#0989) and procedures were followed.
Blood and tissue collection
Whole blood was collected in EDTA containing blood collection tubes, centrifuged at 2000 RPM for 10 minutes and plasma harvested. The tissues included liver, kidney, spleen, lymph node, female reproductive tract (FRT, consisting ovaries and fallopian tube), vagina, colon, and injection site were harvested, placed into tubes and frozen at −80°C until analysis by LC-MS/MS.
Determination of drug concentration in plasma and tissue
Plasma and tissues samples were analyzed for drug concentration by following our previously reported method (16). Briefly, 100 μL aliquot of plasma or tissue homogenate (tissues were homogenized in deionized [1:1 wt/vol] using beads) was mixed with 25 μL of internal standard spiking solution followed by 100 μL of 1 % trifluoroacetic acid and samples were vortexed for 30 sec. The discovery C18 solid phase extraction cartridges were equilibrated with 1mL of methanol followed by water and samples were loaded. Cartridges were washed with water followed by 5% methanol and eluted with 1 mL of methanol. Eluent was evaporated to dryness under the stream of nitrogen at 40°C, reconstituted with 100 μL of 50 % acetonitrile in water and 5 μL was injected into the LC-MS/MS instrument. The lower limit of quantification (LLOQ) for TFV and EVG was 10 and 5 ng/mL respectively in both plasma and tissue. If the observed concentrations were < these LLOQ values, the results were reported as half the LLOQ.
An Agilent 1200 HPLC system (Agilent Technologies, CA, USA) coupled with AB Sciex API 3200 Q Trap with an electrospray ionization source (Applied Biosystems, Foster City, CA, USA) was used. Chromatographic separation was carried out on Restek Pinnacle DB Biph (2.1mm × 50mm, 5μm) column with isocratic mobile phase consisting of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B) (48:52 v/v) at a flow rate of 0.250 mL/min. The dynamic range of the validated assay was 10 to 4000 ng/mL for TFV and 5 to 2000 ng/mL for EVG.
Determination of pharmacokinetic parameters of drug loaded NPs and free drug
The measured concentrations of TFV and EVG were subjected to noncompartmental pharmacokinetic analysis using Phoenix WinNonlin software version 6.4 (Certera Inc., Princeton, N.J.). The following pharmacokinetic parameters were calculated from the plasma and tissue concentration-time data: maximum drug concentration (Cmax), time at which Cmax was observed (tmax), area under the concentration time data from 0 to last concentration measured (AUC(0-t)), half-life of elimination (t1/2), volume of distribution (V/F) and total body clearance (CL/F).
Results
Characterization of NPs
The NPs physicochemical characterization has been reported in our earlier article (14). Briefly, the o-w emulsion methodology resulted in uniform well-defined TAF+EVG NPs having 221.6 ± 6.1 nm size, with PDI; a measure of the NP size variability of 0.107 ± 0.01 (less than < 0.2, i.e. uniform size-distribution), and surface charge of −19.2 ± 1.7 (n=5). The %EE of TAF and EVG into the NPs were 57.28± 4.0% and 49.5 ± 4.3%, respectively (n=4). The statistical significance data shows, freeze-dried TAF+EVG NPs are stable over 1 year at 4°C (Table III).
Table III.
Mean ± standard error (SE) stability data of TAF+EVG NPs over time.
| Parameter | At time ‘0’ | At time ‘1 year’ | Statistical significance |
|---|---|---|---|
| Size (nm) | 221.6 ± 6.09 | 210.05 ± 10.27 | ns |
| PDI | 0.107 ± 0.012 | 0.148 ± 0.007 | ns |
| % EE-TAF | 57.28 ± 3.96 | 55.15 ± 5.27 | ns |
| % EE-EVG | 49.46 ± 4.26 | 46.33 ± 4.87 | ns |
ns: Statistically not significant
Pharmacokinetic profile and tissue distribution profile of ARV NPs and free drug
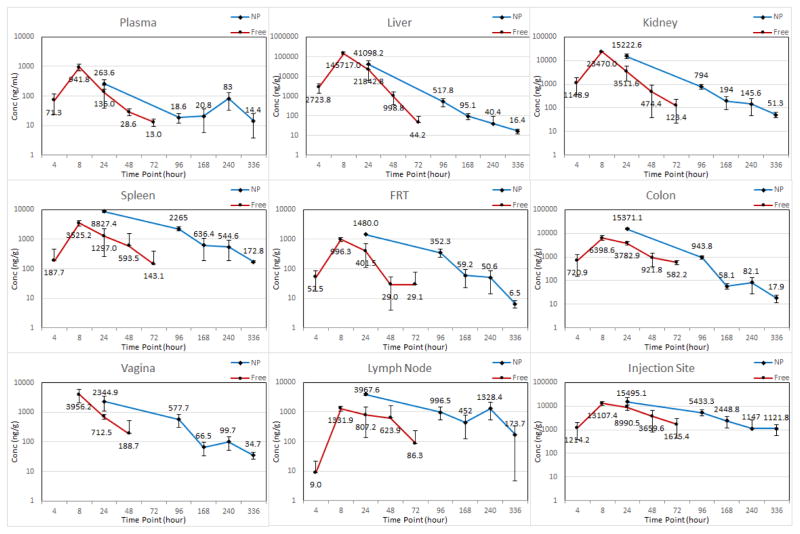
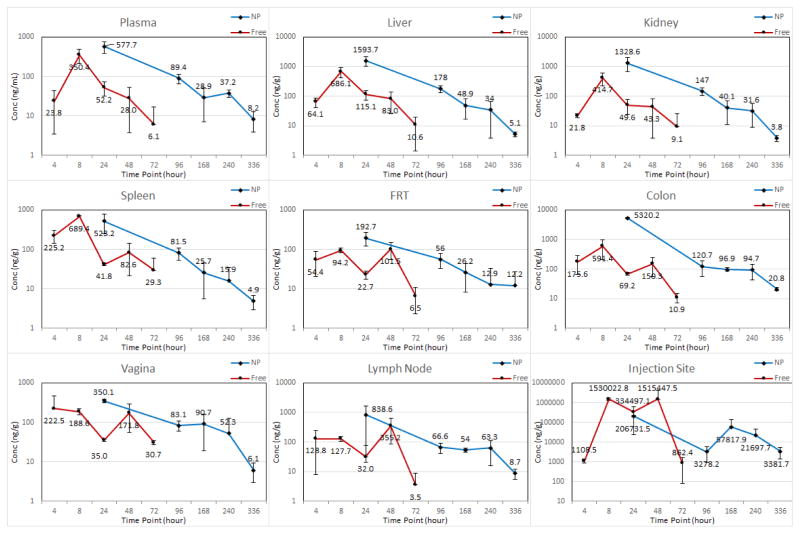
The sustainability and extent of permeability of ARV drug loaded NPs into tissues was evaluated by comparing the plasma and tissue concentrations of free ARV drugs with ARV drugs in the nanoformulation over time. The mean plasma and tissue concentration versus time profiles of free and nanoformulation after SubQ injection are shown in Figures 1 and 2. The mean plasma pharmacokinetic parameters of free and nanoformulation are presented in Table I. The calculated mean plasma Cmax for TFV (263.6 ng/mL) obtained from the nanoformulation was > 110 ng/mL, which has been theorized to be the effective plasma TFV concentration to prevent HIV-1 infection (9). The mean plasma Cmax of EVG (577.7 ng/mL) was greater than that achieved with free drug (350.4 ng/mL). The t1/2 of the nanoformulation was 5.1 and 3.3 days for TFV and EVG, which is approximately 8- and 5-fold higher compared to free TAF and EVG respectively (Table I), suggesting long residence time of both drugs in biological fluids. The lower clearance (CL/F) and higher AUC(0-t) for ARV in nanoformulation indicates the NP formulation prolongs ARV residence time compared to the same dose of free drugs.
Figure 1.
Plasma and tissue concentrations of TFV after subcutaneous administration of 200 mg/kg dose of free and NP formulations to mice. Error bars represent standard deviation of three mice measurements by LC-MS/MS. In graph legend, “Conc” represent concentration, “NP” represents TAF+EVG NP and “Free” represents TAF+EVG in solution.
Figure 2.
Plasma and tissue concentrations of EVG after subcutaneous administration of 200 mg/kg dose of free and NP formulations to mice. Error bars represent standard deviation of three mice measurements by LC-MS/MS. In graph legend, “Conc” represent concentration, “NP” represents TAF+EVG NP and “Free” represents TAF+EVG in solution.
Table I.
Mean ± standard error (SE) plasma pharmacokinetic parameters of free and nanoformulated TAF and EVG.
| Parameter | Units | Tenofovir | Elvitegravir | ||
|---|---|---|---|---|---|
| Free | NP | Free | NP | ||
| Cmax | ng/mL | 941.8±137.1 | 263.6±67.6 | 350.4±79.9 | 577.7±136.0 |
| tmax | Hour (h)/Day | 8 h | 1 Day | 8 h | 1 Day |
| AUC(0-t) | μg×hr/mL | 14.1±2.0 | 23.1±4.4 | 7.2±1.8 | 39.7± 6.7 |
| t1/2 | Hour (h)/Day | 14.2 h | 5.1 Day | 10.8 h | 3.3 Day |
| V/F | L/kg | 285.3 | 1369.9 | 427.7 | 553.4 |
| CL/F | L/h/kg | 14.0 | 7.8 | 27.6 | 4.9 |
The mean pharmacokinetic parameters of TFV and EVG for all tissues are presented in Table II. The tissue concentrations especially at the anatomic sites of infection (vagina and colon) demonstrated a detectable TFV and EVG concentrations even on day 14 when administrated as nanoformulation. Overall, in majority of the tissues studied, nano-encapsulated ARV demonstrated drug concentrations on day 10 equal to or higher than the 72 h concentration of the drugs when administered as free. In all tissues, the AUC(0-t) of ARV in nanoformulation was always higher than the free drugs. From the Cmax, AUC(0-t) and t1/2 of the tissues, it is evident that the drug exposure with nanoformulation is efficient and prolonged. Also, the tissue drug concentration vs time profile for both TFV and EVG at the SubQ injection site, indicates that nanoformulation prolongs the diffusion from the administration site. Some of the tissue concentrations from nanoformulation were found to be statistically significant compared to drugs in solution. The less significance can be correlated to staggered approach and scarce sample size.
Table II.
Mean ± standard error (SE) tissue pharmacokinetic parameters of free and nanoformulated TAF and EVG.
| Tissue | Formulation | Cmax (ng/g) | AUC(0-t) (μg×hr/g) | t1/2 |
|---|---|---|---|---|
| Spleen | TFV-Free | 3,525.2±389.0 | 83.8±19.1 | 15.1 h |
| TFV-NP | 8,827.4±504.7* | 686.6±35.0* | 2.9 Day | |
| EVG-Free | 689.4±14.3 | 11.6±1.1 | 16.8 h | |
| EVG-NP | 523.2±194.5 | 34.4±9.5 | 2.6 Day | |
| Lymph Node | TFV-Free | 1,331.9±154.7 | 48.4±16.7 | 14.9 h |
| TFV-NP | 3,967.6±214.1* | 414.7±64.2 | 3.7 Day | |
| EVG-Free | 355.2±157.0 | 11.0±3.8 | 9.3 h | |
| EVG-NP | 838.6±472.2 | 54.7±23.1 | 2.4 Day | |
| Colon | TFV-Free | 6,398.6±850.1 | 175.9±13.9 | 17.8 h |
| TFV-NP | 15,371.1±782.7* | 817.7±38.5* | 1.4 Day | |
| EVG-Free | 591.4±219.8 | 13.3±3.0 | 12.5 h | |
| EVG-NP | 5,320.2±207.8* | 280.0±11.2* | 4.2 Day | |
| Vagina | TFV-Free | 3,956.2±1,066.2 | 62.6±12.4 | 9.3 h |
| TFV-NP | 2,344.9±874.0 | 169.0±43.6 | 2.2 Day | |
| EVG-Free | 222.5±141.8 | 8.8±1.9 | 30.8 h | |
| EVG-NP | 350.1±28.0 | 34.0±4.7 | 1.8 Day | |
| FRT | TFV-Free | 996.3±100.6 | 27.5±7.4 | 12.7 h |
| TFV-NP | 1,480.0±2.6 | 105.2±5.3* | 1.9 Day | |
| EVG-Free | 101.5±26.4 | 4.6±0.8 | 20.1 h | |
| EVG-NP | 192.7±41.7 | 16.8±2.5 | 4.5 Day | |
| Liver | TFV-Free | 145,717.0±13,183.4 | 2,215.3±362.1 | 5.4 h |
| TFV-NP | 41,098.2±13,098.0 | 2,021.0±628.8 | 2.8 Day | |
| EVG-Free | 686.1±151.0 | 15.3±3.6 | 10.3 h | |
| EVG-NP | 1,593.7±403.3 | 95.9±19.5 | 2.1 Day | |
| Kidney | TFV-Free | 23,470.0±1,201.1 | 383.2±67.1 | 9.9 h |
| TFV-NP | 15,222.6±2,436.0 | 816.5±117.3 | 2.7 Day | |
| EVG-Free | 414.7±114.7 | 8.5±2.2 | 12.3 h | |
| EVG-NP | 1,328.6±467.3 | 80.1±22.5 | 2.0 Day | |
| SubQ site | TFV-Free | 13,107.4±1,558.2 | 445.1±55.2 | 19.8 h |
| TFV-NP | 15,495.1±5,025.0 | 1,461.5±255.4 | 4.3 Day | |
| EVG-Free | 1,530,022.8±129,742.5 | 60,772.1±36,075.5 | 7.0 h | |
| EVG-NP | 206,731.5±15,157.8* | 25,726.9±9,858.3 | 1.2 Day |
indicates p<0.05. FRT: female reproductive tract; SubQ site: injection site of ARV administration
No adverse events or mortality was observed and mice were healthy after injecting the formulation through completion of PK study. While collecting mice injection site samples for analysis of drug concentration on different days (1, 4, 7, 10 and 14) after sacrificing mice suggests that a patch was formed and lasted till day 10, which was disappeared in Day 14 mice.
Discussion
The primary objective of this study was to evaluate and compare pharmacokinetic profiles and biodistribution of drug loaded NPs versus free drug with emphasis on HIV-1 prevention. All the HIV-1 prevention trials with TDF/FTC demonstrated greater efficacy with measurable TFV concentration in plasma (10, 11, 12, 17). For PrEP, plasma TFV and PBMC active metabolite (TFV-diphosphate) concentrations are acceptable scale for estimating protective drug levels (18). The Partners-in-PrEP clinical trial reported plasma TFV levels > 40 ng/mL was significantly protective (19). The accurate protective concentration at the site of infection necessary for full protection against HIV-1 has not been established. Therefore, protective drug concentration at site of infection must be defined. The present study showed that the NP formulation achieved the reported protective plasma concentration (~100 ng/mL). The tissue exposure was efficient in all the tissues studied with NP formulation compared to free drugs in solution (TFV AUC(0-t) was 62.6±12.4, 169.0±43.6, EVG AUC(0-t) was 8.8±1.9, 34.0±4.7 μg×hr/g for free drugs and NP formulation respectively at vagina). A comparison of vaginal AUC values of both drugs from the present study indicate prolonged exposure of ARV drugs. Indeed, the vaginal AUC from the NP formulation was approximately 2.7–3.9 times higher for both TFV and EVG compared to the free drugs in solution administered to the mice. The better tissue exposure is important for the PrEP agents where the initial virologic events are localized (20).
Previous studies on pharmacokinetic parameters of TFV have been investigated in humanized mice. Female mice received 61.5 mg/kg TDF orally had a peak TFV level of 1990 ng/mL, AUC of 11,251 ng×h/mL and t1/2 of 17h (8). In our study TAF+EVG NP formulation demonstrated plasma TFV AUC of 23,134 ng×h/mL and t1/2 of 5.1 day for mice receiving 200 mg/kg SubQ (Table I). The overall drug exposure demonstrated by the tissue: plasma AUC ratios (T:P ratio) for TFV were 7.3 and 35.3 for vaginal and colon tissues respectively. This observed differential accumulation in various tissues may be due to distinct cellular uptake of nanoparticles. Rhesus macaques administered 50 mg/kg TAF/FTC/EVG/COB orally demonstrated peak TFV levels of 1,326 ng/mL and AUC of 9,934 ng×h/mL (21). The plasma AUC for EVG in macaques (Rhesus, 50 mg/kg) was reported as 4,012 ng×h/mL (22). In contrast, the plasma TFV AUC and EVG AUC in the CD34+-NSG mice in this study were approximately 2.3 and 9.9-fold higher than AUC observed in macaques with equivalent dosing.
The use of single drug may not be adequate to confer full protection, hence combination of ARV drugs with different mechanism of action were selected. We have previously published the addition of EVG in vitro is synergistic to TFV for HIV-1 protection (14). However, further trials are appropriate in larger animal models for the ARV NPs to prove a link between tissue drug concentration and efficacy.
Although the goal of this project was to evaluate and compare the pharmacokinetic and tissue distribution profile of free and nanoformulated ARV drugs for HIV-1 prevention, it is conceivable that this formulation could be used in both post-exposure prophylaxis as a one-time administration, as well as a treatment regimen. The back-up systemic drug and biodistribution of drug to sanctuary sites (lymph node, spleen and brain etc.) is an effective strategy to prevent and eradicate virus within the biological system (23, 24), which was accomplished in this study. Sanctuary sites include areas within the body with reduced ARV penetration compared to plasma (23). Future studies will be aimed at analyzing metabolite (TFV-diphosphate) concentration in PBMC and tissue CD4 cells to study cellular uptake and pharmacokinetics of ARV drugs via the nanoformulation.
In conclusion, most of the prevention clinical trials conducted has claimed non-adherence is a critical variable for protection from HIV-1 infection (25). These experiments provide evidence for sustained release of drugs from our nanoformulation, which could potentially reduce dosing frequency for prevention of HIV-1 infection. However further studies are necessary in larger animal model with multiple dosing to confirm these results.
Acknowledgments
This work was supported by NIH grant RO1 AI117740-01 to C.J.D. The Animal Research Facility is supported by Grant Number G20RR024001 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. We thank Gilead Sciences, Inc. for donation of the tenofovir and elvitegravir drug powders.
Abbreviations
- %EE
Percentage entrapment efficiency
- ARV
Antiretroviral
- AUC
Area under the curve
- COB
Cobicistat
- EDTA
Ethylenediaminetetraacetic acid
- EVG
Elvitegravir
- FRT
Female reproductive tract
- FTC
Emtricitabine
- HPLC
High performance liquid chromatography
- LC-MS/MS
Liquid chromatography-tandem mass spectrometry
- LLOQ
Lower limit of quantification
- NP
Nanoparticle
- PBMC
Peripheral blood mononuclear cell
- PDI
Polydispersity index
- PK
Pharmacokinetic
- PLGA
Poly(lactic-co-glycolic acid)
- PrEP
Preexposure prophylaxis
- PVA
Poly(vinyl alcohol)
- RPM
Revolutions per minute
- SubQ
Subcutaneous
- TAF
Tenofovir alafenamide
- TDF
Tenofovir disoproxil fumurate
- TFV
Tenofovir
References
- 1.Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services; 2016. Jul 14, Available from: http://aidsinfo.nih.gov/guidelines. [Google Scholar]
- 2.Global HIV Statistics. UNAIDS; Nov, 2016. Fact Sheet. Available from: http://www.unaids.org/en/resources/fact-sheet. [Google Scholar]
- 3.FDA approves first drug for reducing the risk of sexually acquired HIV infection. Available from: https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm311821.htm.
- 4.Destache CJ, Belgum T, Goede M, Shibata A, Belshan MA. Antiretroviral release from poly(DL-lactide-co-glycolide) nanoparticles in mice. J Antimicrob Chemother. 2010;65(10):2183–2187. doi: 10.1093/jac/dkq318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spreen WR, Margolis DA, Pottage JC., Jr Long-acting injectable antiretrovirals for HIV treatment and prevention. Current opinion in HIV and AIDS. 2013;8(6):565–571. doi: 10.1097/COH.0000000000000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friend DR, Clark JT, Kiser PF, Clark MR. Multipurpose prevention technologies: products in development. Antiviral Res. 2013;100(Suppl):S39–47. doi: 10.1016/j.antiviral.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 7.Gunawardana M, Remedios-Chan M, Miller CS, Fanter R, Yang F, Marzinke MA, Hendrix CW, Beliveau M, Moss JA, Smith TJ, Bauma MM. Y Pharmacokinetics of Long-Acting Tenofovir Alafenamide (GS-7340) Subdermal Implant for HIV Prophylaxis. Antimicrob Agents Ch. 2015;59(7):3913–3919. doi: 10.1128/AAC.00656-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veselinovic M, Yang KH, LeCureux J, Sykes C, Remling-Mulder L, Kashuba AD, Akkina R. HIV pre-exposure prophylaxis: mucosal tissue drug distribution of RT inhibitor Tenofovir and entry inhibitor Maraviroc in a humanized mouse model. Virology. 2014;464–465:253–263. doi: 10.1016/j.virol.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hendrix CW. Exploring concentration response in HIV pre-exposure prophylaxis to optimize clinical care and trial design. Cell. 2013;155(3):515–518. doi: 10.1016/j.cell.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 10.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science (New York, NY) 2010;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapia M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernandez T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallas EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang F, McConnell JJ, Zheng JH, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. The New England journal of medicine. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, Ronald A, Tumwesigye E, Were E, Fife KH, Kiarie J, Farquhar C, John-Stewart G, Kakia A, Odoyo J, Mucunguzi A, Nakku-Joloba E, Twesigye R, Ngure K, Apaka C, Tamooh H, Gabona F, Mujugira A, Panteleeff D, Thomas KK, Kidoguchi L, Krows M, Revall J, Morrison S, Haugen H, Emmanuel-Ogier M, Ondrejcek L, Coombs RW, Frenkel L, Hendrix C, Bumpus NN, Bangsberg D, Haberer JE, Stevens WS, Lingappa JR, Celum C. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. The New England journal of medicine. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sax PE, Zolopa A, Brar I, Elion R, Ortiz R, Post F, Wang H, Callebaut C, Martin H, Fordyce MW, McCallister S. Tenofovir alafenamide vs. tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr. 2014;67(1):52–58. doi: 10.1097/QAI.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 14.Mandal S, Prathipati PK, Kang G, Zhou Y, Yuan Z, Fan W, Li Q, Destache CJ. Tenofovir alafenamide and elvitegravir loaded nanoparticles for long-acting prevention of HIV-1 vaginal transmission. AIDS. 2017;31(4):469–476. doi: 10.1097/QAD.0000000000001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandal S, Zhou Y, Shibata A, Destache CJ. Confocal fluorescence microscopy: An ultra-sensitive tool used to evaluate intracellular antiretroviral nano-drug delivery in HeLa cells. AIP Adv. 2015;5(8):084803. doi: 10.1063/1.4926584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prathipati PK, Mandal S, Destache CJ. Simultaneous quantification of tenofovir, emtricitabine, rilpivirine, elvitegravir and dolutegravir in mouse biological matrices by LC-MS/MS and its application to a pharmacokinetic study. Journal of pharmaceutical and biomedical analysis. 2016;129:473–481. doi: 10.1016/j.jpba.2016.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, Henderson FL, Pathak SR, Soud FA, Chillag KL, Mutanhaurwa R, Chirwa LI, Kasonde M, Abebe D, Buliva E, Gvetadze RJ, Johnson S, Sukalac T, Thomas VT, Hart C, Johnson JA, Malotte CK, Hendrix CW, Brooks JT. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. The New England journal of medicine. 2012;367(5):423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 18.Patterson KB, Prince HA, Kraft E, Jenkins AJ, Shaheen NJ, Rooney JF, Cohen MS, Kashuba AD. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Science translational medicine. 2011;3(112):112re114. doi: 10.1126/scitranslmed.3003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donnell D, Baeten JM, Bumpus NN, Brantley J, Bangsberg DR, Haberer JE, Mujugira A, Mugo N, Ndase P, Hendrix C, Celum C. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr. 2014;66(3):340–348. doi: 10.1097/QAI.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson CG, Cohen MS, Kashuba AD. Antiretroviral pharmacology in mucosal tissues. J Acquir Immune Defic Syndr. 2013;63(Suppl 2):S240–247. doi: 10.1097/QAI.0b013e3182986ff8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tenofovir alafenamide pharmacology reviews. Centre for Drug Evaluation and Research, Department of Health and Human Services; Application Number: 207561. Available from: www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207561Orig1s000PharmR.pdf. [Google Scholar]
- 22.Massud I, Martin A, Dinh C, Mitchell J, Jenkins L, Heneine W, Pau CP, Garcia-Lerma JG. Pharmacokinetic profile of raltegravir, elvitegravir and dolutegravir in plasma and mucosal secretions in rhesus macaques. J Antimicrob Chemother. 2015;70(5):1473–1481. doi: 10.1093/jac/dku556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cory TJ, Schacker TW, Stevenson M, Fletcher CV. Overcoming pharmacologic sanctuaries. Current opinion in HIV and AIDS. 2013;8(3):190–195. doi: 10.1097/COH.0b013e32835fc68a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson PL, Garcia-Lerma JG, Heneine W. Nondaily preexposure prophylaxis for HIV prevention. Current opinion in HIV and AIDS. 2016;11(1):94–101. doi: 10.1097/COH.0000000000000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, Malahleha M, Owino F, Manongi R, Onyango J, Temu L, Monedi MC, Mak’Oketch P, Makanda M, Reblin I, Makatu SE, Saylor L, Kiernan H, Kirkendale S, Wong C, Grant R, Kashuba A, Nanda K, Mandala J, Fransen K, Deese J, Crucitti T, Mastro TD, Taylor D. Preexposure prophylaxis for HIV infection among African women. The New England journal of medicine. 2012;367(5):411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]