Abstract
Small animal models, particularly mouse models, of human diseases are becoming an indispensable tool for biomedical research. Studies in animal models have provided important insights into the etiology of diseases and accelerated the development of therapeutic strategies. Detailed phenotypic characterization is essential, both for the development of such animal models and mechanistic studies into disease pathogenesis and testing the efficacy of experimental therapeutics. Magnetic Resonance Imaging (MRI) is a versatile and non-invasive imaging modality with excellent penetration depth, tissue coverage, and soft tissue contrast. MRI, being a multi-modal imaging modality, together with proven imaging protocols and availability of good contrast agents, is ideally suited for phenotyping mutant mouse models. Here we describe the applications of MRI for phenotyping structural birth defects involving the brain, heart, and kidney in mice. The versatility of MRI and its ease of use are well suited to meet the rapidly increasing demands for mouse phenotyping in the coming age of functional genomics.
INTRODUCTION
Small animal models, particularly mouse models, of human diseases are becoming an indispensable tool for biomedical research. Studies in animal models have provided important insights into the etiology of diseases and accelerated the development of therapeutic strategies. Mice and human share similar physiology and anatomy, including the 4-chambered hearts, vascular systems, and similar structure and function of visceral organs. More importantly, many genes, gene modifiers, as well as biological processes and molecular pathways, are conserved between mice and humans (Georgi et al., 2013; Nadeau, 2001; Nguyen and Xu, 2008), despite some species-specific differences (Mestas and Hughes, 2004). The advancement of modern transgenic technology, including CRISPR-Cas9 gene targeting, availability of the Mouse Genome Database (MGD) (Blake et al., 2014; Bult et al., 2008), and systematic production of knockout mice in the Knockout Mouse Project, and in the international collaborations comprising EMMA (European Mouse Mutant Archive) and IPAD-MD (Infrastructure for Phenotyping, Arching and Distribution of Mouse Diseases Models), an unprecedented number of mutant mice are now available for interrogating and modeling human diseases. Both NIH-funded KOMP2 (Knockout Mouse Phenotyping Program) and international collaboratives such as the IMPC (International Mouse Phenotyping Consortium), are pursuing efforts for detailed phenotypic characterization to gain mechanistic insights into gene function. Some of these mutant mouse models will undoubtedly prove useful not only for studying human disease pathogenesis, but also for testing the efficacy of experimental therapeutics.
While many imaging modalities are available for phenotyping mice, magnetic resonance imaging (MRI) has unique advantages in allowing non-invasive imaging, and can be used to obtain functional and structural information, as well as metabolic status in the living animals. Moreover, with MRI, it is possible to achieve much higher resolution than either ultrasound imaging or computed tomography (CT). Hence, MRI is an important imaging modality that should be included in any phenotypic characterization of mutant mice or other small animal models. MRI, which is based on the detection of protons, is appropriate for animal and human imaging, because the body is comprised of more than 70% water. It is non-invasive, utilizing low-energy radio-frequency (RF) waves in magnetic fields to form images without ionizing radiation, such as is used in CT. MRI offers complete coverage of the whole body or organ volume, without limitation in depth penetration as in other imaging modalities, such as optical or ultrasound imaging. Hence, with MRI, high-resolution three-dimensional (3D) imaging over the entire intact body can be achieved in vivo or postmortem, without autopsy or laborious histological sectioning. Live MRI imaging also avoids the confounding problem of artifacts from tissue shrinkage or distortion from tissue fixation and embedding. Hence, using MRI data obtained from living animals, volumetric and morphometric data can be obtained reliably, and longitudinal imaging over time can be used to track physiological changes or examine developmental progression and emergence of birth defects.
MRI has excellent intrinsic soft tissue contrast because the relaxation characteristics of water protons are governed by the micro-environment of each tissue. The modern MRI platforms have made available a wide variety of pulse sequences to sensitize MRI acquisition to many different conditions, such as T1-weighting, T2-weighting, proton-density weighting, diffusion-weighting, flow-encoding, displacement-encoding, tissue perfusion, and tissue oxygenation sensitive, as well as fast imaging for mechanical motions. In addition, various classes of MRI contrast agents are available for blood pool, infarction, cellular, and molecular imaging. Choosing specific combinations of relaxation and contrast mechanisms has broadened the diagnostic capabilities of MRI in both the clinical and preclinical settings (De Leon-Rodriguez et al., 2015). MRI is extremely versatile and has become in itself a multi-modal imaging tool for structural and functional imaging to phenotype animal models with a wide spectrum of birth defects. In this overview, we discuss the application of MRI for phenotyping birth defects in the brain, heart, and kidney in mice.
Brain MRI
MRI is an ideal imaging modality for assessment of brain anatomy because white matter, gray matter, and cerebrospinal fluid (CSF) of the brain possess different proton relaxation characteristics in a magnetic field, thus yielding excellent intrinsic MRI contrast without the need for exogenous contrast agents. When placed in the magnetic field, the nuclear magnetic moments of the protons in the body align with the field, producing a net equilibrium magnetization directed along the external magnetic field. The pulsed radio frequency waves directed at the object perturb this equilibrium magnetization, and as the protons relax back to the equilibrium state, the processing proton nuclear magnetic spins emit radio frequency waves which can induce electric current in the receiving coil to generate oscillating signals with specific frequencies and phases. These electric signals are then Fourier transformed and reconstructed into images of the target object. The amplitude of the signals is governed by the proton density and the relaxation time constants of the tissue.
There are two main types of relaxation mechanisms of excited magnetic moments. The longitudinal or spin-lattice relaxation is the process that the excited spin system recovers back to the thermal equilibrium to realign with the external magnetic field. The time constant for this relaxation process is T1. The transverse or the spin-spin relaxation is the process that the tipped spins de-phase on the transverse plane. The time constant for this relaxation process is T2. The T2 decay is caused by exchange of energy from one nucleus to another, as a result of intrinsic magnetic fields of nuclei interlacing with each other. This spin-spin energy transfer results in loss of coherence or dephasing of the spin system. Both T1 and T2 occur at different rates in different tissues, such as white matter, gray matter, and CSF (Just and Thelen, 1988; Mazaheri et al., 2006; Wansapura et al., 1999; Wright et al., 2008). At 3-Tesla, T1 are 830 msec, 1330 msec, and 3000 msec, and T2 are 80 msec, 110 msec, and 300 msec for white matter, gray matter, and CSF, respectively. By choosing different acquisition conditions with different echo time (TE) and recovery time (TR), MRI acquisition can be sensitized to T1-weighting or T2-weighting to highlight different types of brain tissues and conditions. Table 1 shows typical contrast appearance in the structural brain MRI. Figure 1 shows T2-weighted anatomical MRI of live mouse brains. In this T2-weighted condition, CSF appears to be bright, the corpus callosum appears to be dark, and the gray matter appears to be gray. Figure 2 shows 3D maximum intensity projection (MIP) of this T2-weighted MRI, highlighting the ventricular system of the mouse brain.
Table 1.
Typical contrast appearance in the structural brain MRI
| Tissue condition | T1-weighted MRI | T2-weighted MRI |
|---|---|---|
| Cortex | Gray | Light gray |
| White matter | Light | Dark gray |
| CSF | Dark | Bright/white |
| Infection | Dark | Bright |
| Inflammation | Dark | Bright |
| Subdural collection | Dark | Bright |
| Edema | Dark | Bright |
| Tumor | Dark | Bright |
| Hemorrhage/blood | Bright | Dark |
| Fat | Bright | Light gray |
| Bone | Dark | Dark |
Figure 1.
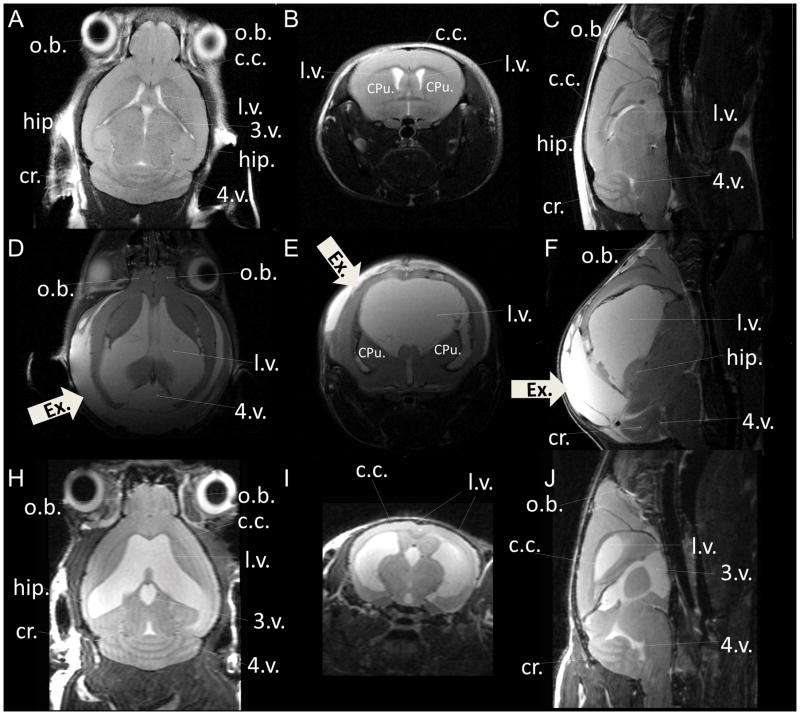
In vivo T2-weighted anatomical MRI of live mouse brain. (A–C) wild-type control mouse at 8 months of age; (D–F) homozygous mutant mouse with a single motile cilia gene mutation at 21 days of age; (H–J) homozygous mutant mouse with the same single motile cilia gene mutation at 13 months of age, showing a coronal view (A, D, H), an axial view (B, E, I), and a sagittal view (C, F, J) from the 3D volume stacks. The thick white arrowheads labeled with “Ex.” point to accumulation of extra-axial fluid in the hydrocephalus mutant. Images were acquired at 7-Tesla with in-plane resolution 66 μm and slice thickness 0.5 mm. o.b.= olfactory bulb; c.c.= corpus callosum; hip= hippocampus; cr.= cerebellum; l.v.= lateral ventricle; 3.v. = 3rd ventricle; 4.v. = 4th ventricle; CPu = caudate putamen.
Figure 2.
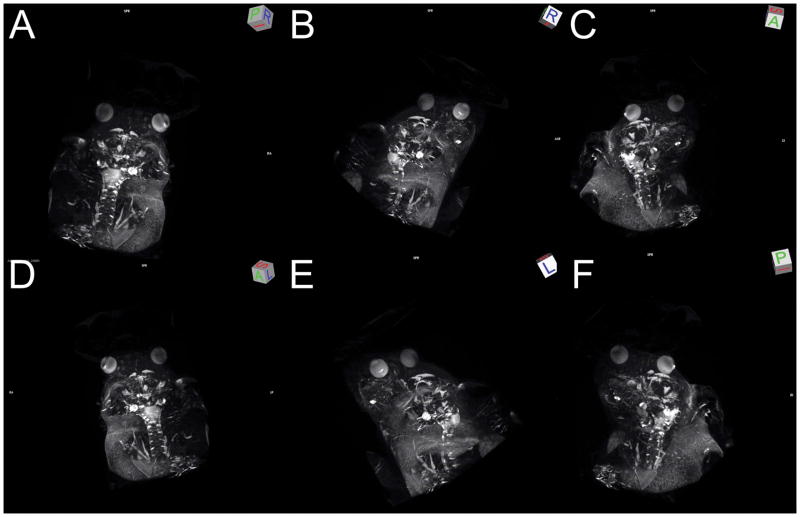
3D maximum-intensity projection reconstruction of the in vivo 3D T2-weighted MRI, highlighting the ventricular systems and CSF in a WT control mouse. The 3D T2-weighted MRI was acquired at 7T with the voxel size: 98 μm x 98 μm x 156 μm. (A–F) Still shots of 3D rendering at different viewing angles, indicated by the 3D viewing orientation on the upper right corner of each panel. L: left; R: right; A: anterior; P: posterior; S: sagittal.
Anatomical Analysis with MRI Based Virtual Histology
MR-based “virtual histology” (Cleary et al., 2011; Petiet et al., 2008) is gaining importance in phenotyping and characterizing mouse central nervous system (CNS), complementing conventional histology. Many MRI protocols have been established for imaging brain in both normal and mutant animals (Ahrens et al., 1998; Badea et al., 2012; Benveniste et al., 2007; Bowden et al., 2011; Choi et al., 2003; Choi et al., 2007; Jack et al., 2007; Martinez-Martinez et al., 2014; Petiet et al., 2008; Vajda et al., 2004). Mice are typically MR scanned after administration of inhalable anesthetic agents, such as isoflurane via a nose cone. The imaging procedure is simple and robust and can be done in neonatal and postnatal mice, as well as young and old adult mice. Moreover, the imaging can be repeated many times, allowing longitudinal tracking of development or disease progression. Many brain regions, such as hippocampus, cerebellum, and olfactory bulbs, can be easily identified given their excellent intrinsic contrast. We note increasing availability of a growing number of web-based MRI rodent and murine brain atlas (Badea et al., 2012; Bowden et al., 2011; Cleary et al., 2011) for different types of MRI contrast. Variability of brain anatomy of different inbred mouse strains also has been documented (Scholz et al., 2016), facilitating analysis of mutant mouse models generated in different strain background.
MRI Analysis of Mouse Models of Congenital Hydrocephalus
One example of an area where brain MRI has been well utilized is in the analysis of hydrocephalus, which is characterized by excessive accumulation of cerebrospinal fluid (CSF) in the brain, usually accompanied by enlarged brain ventricles. It can be observed congenitally at birth, and is a common neurological disorder seen with an estimated incidence of 1–3 per 1000 live births (Carter et al., 2012). It is often fatal if left untreated, and as yet the molecular etiology for congenital hydrocephalus is largely unknown. Using in vivo MRI, the development of excessive CSF and enlargement of the brain ventricles can be carefully tracked in mutant mouse models of congenital hydrocephalus. Changes in the gray matter and white matter, also can be evaluated non-invasively by in vivo MRI. Figure 1 shows in vivo T2-weighted anatomical brain MRI of a wild-type (WT) control mouse at 8 months of age (Fig. 1A–C), and homozygous mutant mice with a single gene mutation at 21 days of age (Fig. 1D–F), and at 13 months of age (Fig. 1H–J). CSF is bight whereas the white matter is dark and the gray matter appears in between, or gray in this T2-weighted anatomical brain MRI.
Contrast Agent Enhanced MRI
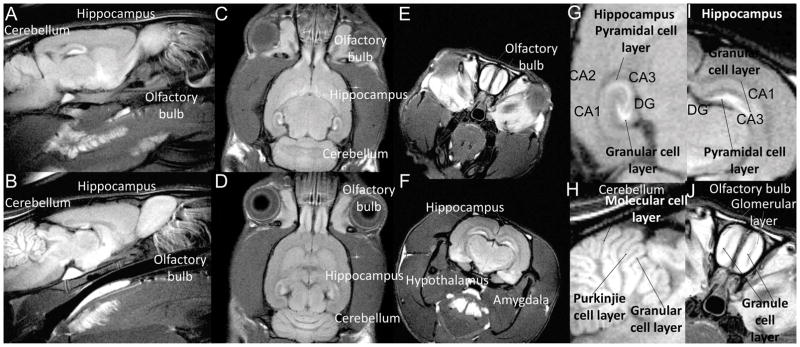
While intrinsic contrast can be generated in MR imaging using different pulse sequences and imaging conditions, exogenous contrast agents can be used to selectively enhance different regions or cellular layers in tissues of interest. Gadolinium-based contrast agents, such as Gd-DOPA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid), have been used for characterization of the developing CNS (Martinez-Martinez et al., 2014). Divalent manganese (Mn2+) cations are MRI T1 contrast agents, also acting biologically as a Ca2+ analogue, that can enter cells through voltage-gated calcium channels. Taking advantage of the dual property of manganese, we have directly imaged neuronal activation in the brain (Lin and Koretsky, 1997b) and examined neuroarchitecture (Aoki et al., 2004; Silva et al., 2004) in vivo. Shown in Figure 3 is an animal with Mn2+ administered systemically, which allowed the delineation of specific brain areas, including olfactory bulbs, hippocampus, cerebellum, amygdala, hypothalamus, and pituitary gland. Also note, Mn2+ labels certain cellular layers more intensely in these brain structures, such as the granular cell layer in the dentate gyrus (D.G.) and pyramidal cell layers in the hippocampus (Fig. 3G, I), the Purkinjie cell layer, granular cell layer, and molecular cell layer in cerebellum (Fig. 3H), and glomerular layer and granule cell layer in the olfactory bulb (Fig. 3J). Hence, with manganese contrast, micro-architecture of the brain can be characterized in live animals, allowing the detailed characterization of altered brain architecture in mutant mouse models.
Figure 3.
In vivo T1-weighted MRI 2 days after systemic Mn2+ administration of a WT control animal. (A, B) sagittal view; (C, D) axial view; (E, F) coronal view. (G, H) Enlarged partial view to show hippocampus; (H) enlarged partial view to show cerebellum; (J) enlarged partial view to show olfactory bulb. DG=dentate gyrus. [Taken from Yijen Lin Wu Ph.D. dissertation (Lin and Koretsky, 1997a).]
Volumetric and Morphometric Analysis of Brain MRI
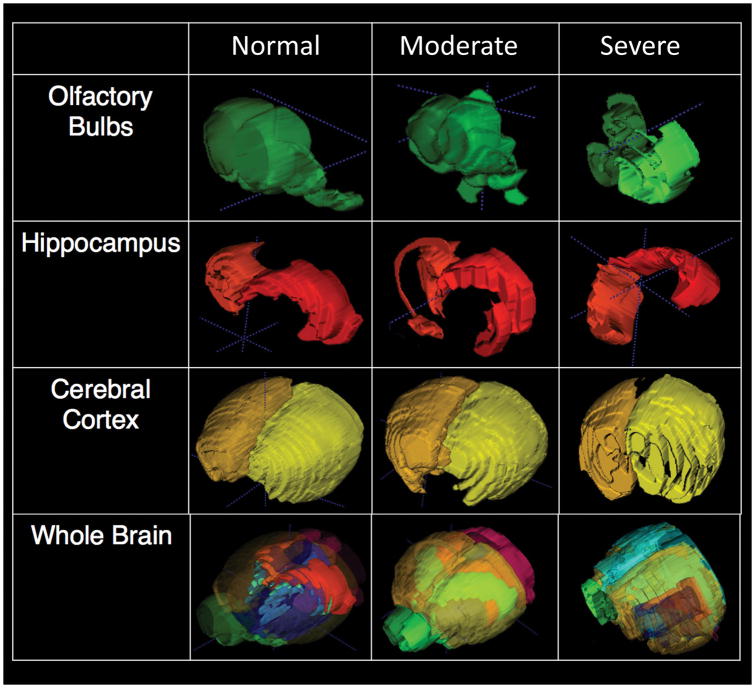
Regional and global brain volumes are known to correlate with cognitive functions, aging, and disease progression (Dall’Ara et al., 2016; Persson et al., 2014, 2016; Raz et al., 2013). From the in vivo brain MRI, with either intrinsic or exogenous contrast (as in Figs 1–3), volumetric and morphometric analysis can be obtained for each brain region of interest. Figure 4 shows segmentation and volumetric analysis for a normal (left panels) mouse and mutant mice exhibiting moderate (middle panels), or severe (right panels) hydrocephalus. The mutant mice not only exhibited enlarged brain ventricles with excessive CSF, but dysplasia was observed in various brain regions, such as in the olfactory bulbs, hippocampus, and cerebellum. This was indicated by the altered morphology and shapes of these brain structures relative to those of control mice. Morphometric analysis (Allan et al., 2016; Ghaznavi et al., 2013; Kim et al., 2016; Wang et al., 2011) of these brain regions can be used in conjunction with other studies to gain insights into the molecular mechanisms and developmental etiology of these brain abnormalities.
Figure 4.
Volumetric and morphometric analysis of the brain from the in vivo T2-weighted MRI. (left) normal WT mouse; (middle) homozygous mutant mouse with moderate hydrocephalus; (right) homozygous mutant mouse with very severe hydrocephalus. Examples of segmentation of different regions of interest (ROS) are shown for olfactory bulb, hippocampus, cerebral cortex, and the combined ROIs of the whole brain.
Diffusion Tensor Imaging
Diffusion tensor imaging (DTI), first described in 1994 (Basser et al., 1994; Beaulieu and Allen, 1994), utilizes MRI to probe anisotropic water diffusion in neuronal tracks to derive neuronal fiber organization in the brain (Alexander et al., 2007; Assaf and Pasternak, 2008), and to probe the characteristics of cellular structures (Ackerman and Neil, 2010). Diffusion of water molecules is caused by random thermal motions and is compartmentalized in the biological tissues. In biological tissue, water diffusion is restricted and usually anisotropic, occurring inside, outside, and around the cellular structures. On the other hand, water molecules within cells can only diffuse to the cell boundaries. Diffusion MRI leverages this anisotropic water diffusion in the biological tissue to generate MRI contrast. In neuronal fibers, water molecules can readily diffuse along the fiber, but are very limited perpendicular to the fiber orientation, allowing mapping of neuronal fibers. Under pathological conditions, such as with inflammation and edema, the diffusion of water molecules changes around the affected site, which can be quantified by diffusion MRI, allowing evaluation of physiological changes in disease states.
Diffusion MRI provides a non-invasive method to map white matter connections. DTI models water diffusion pattern in neuronal axons as a Gaussian distribution, and the axonal direction can be determined by the principal direction of the tensor (Basser et al., 1994; Basser and Pierpaoli, 1996; Pierpaoli et al., 1996). Based on this principal direction, the trajectories of the axonal connections can be tracked by diffusion fiber tractography, a computational approach which reveals the axonal connections between cortical areas (Alexander and Barker, 2005; Wiegell et al., 2000). Graph theoretical analysis (Bernhardt et al., 2015; Bernhardt et al., 2013; Chiang and Haneef, 2014; Sporns, 2013) views brain connections as a graph and applies graph-based measures to analyze it. A graph is defined as a set of nodes or vertices and the edges or lines between them. Its topology can be quantitatively described by a wide variety of measures, including network characteristic path length, clustering coefficient, global efficiency, and local efficiency. Using fiber tractography, 3D geometry of the axonal architecture can be delineated, allowing direct visualization of the brain’s neuronal networks. Figure 5 shows an example of DTI tractography (Fig. 5, A,B) that delineates the neural connectivity in a control and mutant mouse brain. Shown in Figure 5 are the neuronal networks observed in a WT mouse (Fig. 5A, C) and mutant mouse model (Fig. 5B, D). Network analysis revealed distinct alterations and changes in the organization of brain connections in the mutant mouse brain (Fig. 5D), compared to the WT mouse (Fig. 5C), indicating the mutation plays an essential role in the regulation of neurogenesis and organization of the neural network.
Figure 5.
Diffusion MRI tractography and network analysis of a WT control mouse (A, C) and a homozygous mutant mouse (B, D). (A, B) Diffusion MRI tractography showing neuronal tracks and projections. (C, D) The connectogram plots the overall connectivity to illustrates the connection strength. A total of 13 ROIs were manually assigned independently. The ROIs were used as the brain parcellation, and the connectivity matrix was calculated by using count of the connecting tracks. Brain regions: CTX -Cerebral cortex, STR –Striatum, PAL-Pallidum, BS-Brain stem, IB-Interbrain, TH-Thalamus, HY- Hypothalamus, MB-Midbrain, HB-Hindbrain, P-Pons, MY-Medulla, CB-Cerebellum.
Cardiac MRI
The field of cardiovascular MR (CMR), in both research and clinical arenas, has evolved rapidly over the past decade. CMR applications include assessment of cardiac anatomy, regional wall motion, myocardial perfusion, myocardial viability, cardiac function assessment, assessment of myocardial infarction, and myocardial injury (Vanhoutte et al., 2016). CMR is increasingly used to characterize genetic and pharmacological mouse models (Ebrahimi et al., 2013; Gotschy et al., 2013; Gotschy et al., 2017; Sosnovik et al., 2007). With cine MRI, heart is imaged at the same imaging plane many times throughout the cardiac cycle. Typically, 10 to 30 cardiac phases per cardiac cycle are captured to generate cine movie loops for describing cardiac motion. Multiple imaging planes are needed to cover the whole volume of the heart. Figure 6 shows cine CMR of a WT control (Fig. 6, A–D) and a homozygous situs inverses totalis (SIT) mutant (Fig. 6, E–H) at the end-diastole (ED, Fig. 6A, C, E, G) and at the end-systole (ES, Fig. 6B, D, F, H). The global systolic function, such as ejection fraction, stroke volume, fractional shortening, and cardiac output, can be derived from the cine MRI for normal and mutant mice.
Figure 6.
Cine cardiac MRI (A–D) a WT control mouse and (E–H) a situs inverses totalis homozygous mutant mouse at ED (A, C, E, G) and ES (B, D, F, H), showing long-axis view (A, B, E, F) and short-axis view (C, D, G, H). WT: wild type; SIT: situs inverses totalis mutant; ED: end-diastole; ES: end-systole; LV: left ventricle; RV: right ventricle. The L/R with the double arrowheads indicates the left (L) and right (R) side of the body.
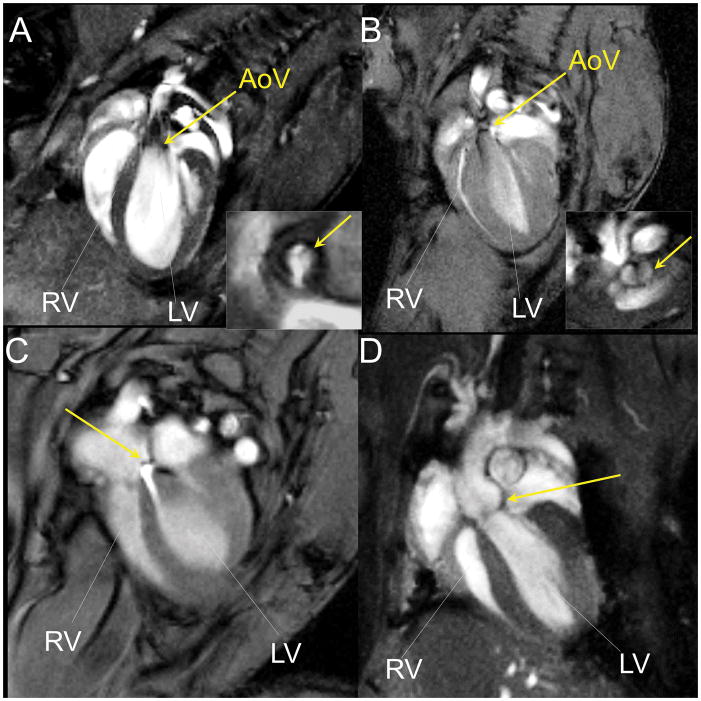
In vivo cardiac MRI can be used to phenotype cardiac function in mutant mice with a spectrum of congenital heart anomaly. The mouse in Fig. 7A showed normal tricuspid aortic valve (Fig. 7A insect) and normal aortic outflow, but the mutant mouse in Fig. 7B showed bicuspid aortic valve (Fig. 7B insect). This mutant exhibited aortic stenosis, as shown by the abnormal narrowed and turbulent aortic outflow (Fig. 7B). In another mutant shown in Fig. 7C, the atrial septal defect (ASD) phenotype was observed. This is associated with an extra jet of blood flowing from the left atrium to the right atrium via an abnormal opening in the atrial septal wall. The aortic root of the mutant mouse in Figure 7D was dilated.
Figure 7.
CMR of mutant mice with congenital heart anomaly. (A) A mouse with normal aortic outflow and tricuspid aortic valve (the insect). The yellow arrows point to the aortic valve. (B) A mutant mouse with aortic stenosis and bicuspid aortic valve (the insect). The yellow arrows point to the aortic valve. (C) A mutant mouse with atrial septal defect (ASD). The yellow arrow points to the jet of blood flowing from left atrium to the right atrium. (D) A mutant mouse with dilated aortic root. The yellow arrow points to the aortic root. Abbreviation: LV - left ventricle, RV - right ventricle, AoV - aortic valve.
Myocardial Tagging
In addition to cine MRI, regional wall motion can be quantified by tagging MRI followed by strain analysis. Myocardial tagging (Axel et al., 1992; McVeigh and Zerhouni, 1991; Shehata et al., 2009; Young et al., 1994; Zerhouni, 1993; Zerhouni et al., 1988) places stripes or grids on the heart by applying a series of short RF-saturation pulses to generate regional tags on the myocardium, in order to track regional myocardial motions in detail during the cardiac cycle. Figure 8 shows tagging MRI of a WT control mouse (Fig. 8A, B), with a short-axis view at the end-diastole (Fig. 8A) and the end-systole (Fig. 8B). The tagging pulses are usually applied at the R-wave. The tags appear to be straight at the diastolic phase (Fig. 8A). When the myocardium contracts, the tags will stretch/elongate or compress/shorten accordingly, and thus can be used to quantify the degrees of myocardial wall motion. The tags will fade over time as the saturated spins recover, according to the tissue T1 time constant. The native myocardial T1 is around 900 msec at 1.5-Tesla, 1100 msec at 3-Tesla, and 1900 msec at 7-Tesla. For humans, depending on the field strength, sometimes the diastolic phase cannot be fully evaluated with tagging due to the faded tags. Mice have much shorter cardiac cycles (around 150–200 ms). Thus, myocardial tagging can track the full systolic and diastolic phases in mouse hearts.
Figure 8.
Tagging MRI for regional wall motion and strain analysis of a mouse heart. (A) Short-axis tagging at the end diastole; (B) short-axis tagging at the end systole; (C) schematic drawing showing the directions of the radial strain (Err), circumferential strain (Ecc); and the longitudinal strain (Ell) on a short-axis view. (D) Temporal changes of mean Ecc of 18 animals with different degrees of wall motion capability. The X-axis is the time expressed in % of cardiac cycle. The y-axis is the mean Ecc values of the myocardium from each animal from the mid-level short-axis imaging plane. Each line with different color is from each individual animal. (E) American Heart Association 6-segment model of a short-axis view, dividing myocardium into 6 regions: R6, anterior; R5, anteriolateral; R4, lateral; R3, inferior; R2, inferioseptal; and R1, anterioseptal. LV: left ventricle; RV: left ventricle. (F) a bullseye view of Ecc values for 4 short-axis slices, each with 6 segments. The 4 circular rings represent 4 short-axis planes with the apical planes toward the middle and the basal planes on the outside. The black arrow indicates the intersecting point of RV and LV, the starting point of the septal wall. The 6 segment is divided as in E. The mean peak Ecc of each segment is shown, according to the color scales on the right.
Myocardial Strain Measurements
Strains (Castillo et al., 2005) are values that quantify the extent of ventricular deformation throughout cardiac phases: stretching/elongation or compression/shortening. Strains are categorized into two main classes in relation to the heart axes: normal strains are defined in relation to the short-axis planes, and principal strains are defined in relation to the direction of the myocardial fiber bundles. Figure 8C shows the directions of the normal strain. Three orthogonal strain-tensor sets define normal strains: the circumferential strain (Ecc), the radial strain (Err), and the longitudinal strain (Ell), in which strain-tensors are tangent to the epicardium surface, perpendicular to the epicardium surface towards the center of the LV, and perpendicular to the short-axis plane along the long-axis of the LV, respectively. Ventricular wall motion can be quantified by strain, commonly analyzed by the FDA-approved harmonic phase (HARP) method (Castillo et al., 2005; Osman and Prince, 2000; Shehata et al., 2009). Fig. 8D shows temporal changes of mean Ecc throughout cardiac phases of 18 animals with different degrees of ventricular wall motion capability. In addition to mean Ecc, regional wall motion can be quantified by regional Ecc mapping. Fig. 8E shows conventional American Heart Association 6-segment model of a short-axis view, dividing the myocardium into 6 regions: R6, anterior; R5, anteriolateral; R4, lateral; R3, inferior; R2, inferioseptal; and R1, anterioseptal. Fig. 8F shows a bullseye view of mean peak Ecc values for 4 short-axis imaging planes, each with 6 segments. Ecc values of the mid-wall layers are normally used for strain analysis, because the Multi-Ethnic Study of Atherosclerosis (MESA) clinical study showed that the mid-wall layer yields the best inter-observer and intra-observer consistency (Castillo et al., 2005).
DTI for Tracking Myocardial Fibers
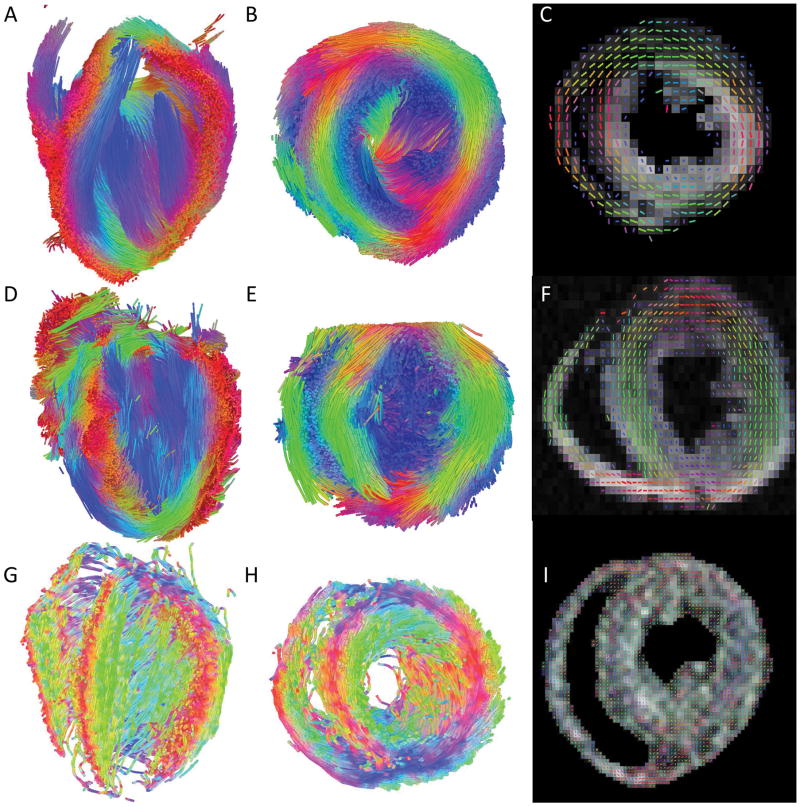
Similar to tracking neuronal fibers, DTI (Fig. 9) can be used to delineate myocardial fiber architecture. Figure 9 shows diffusion MRI tractography (Fig. 8A, B, D, E, G, H) and fiber orientation map (Fig. 9C, F, I) of a wild-type mouse (Fig. 9A–C), and two different mutant mice (Fig. 9D–I). The mutant mouse hearts showed abnormal myocardial fiber organization compared to the WT heart.
Figure 9.
DTI of a WT control heart (A–C) and 2 mutant (D–I) hearts. (A, B, D, E, G, H) Diffusion MRI tractography with the long-axis view (A, D, G) and a short-axis view (B, E, H) and (C, F, I) fiber orientation mapping.
Renal MRI
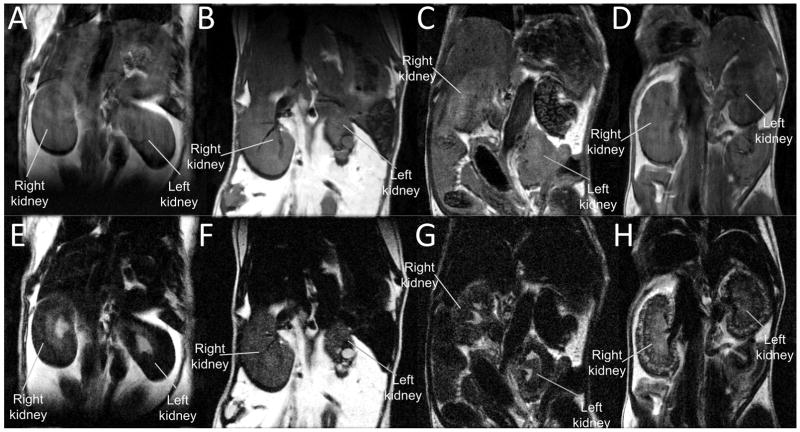
Renal anomalies also can be readily observed by in vivo MRI. Figure 10 shows T2-weighted in vivo MRI of a WT normal control animal (Fig. 10A, E), and 3 mutant mice (Fig. 10B–D, F–H). Shorter echo time with less T2-weighting (Fig. 10A–D, TE = 10 msec) shows the outline and gross anatomy of the kidneys. The heavier T2-weighting with longer echo time (Fig. 10E–H, TE = 84 msec) highlights areas with more water content, such as major calyx (Fig. 10E) in normal kidneys where urine is collected, and cysts (Fig. 10F–H). Normal WT control animal (Fig. 10E) displayed normal urine production and collection in the major calyx, which is lacking in the mutant kidneys (Fig. 10F–H). Various mutant mice with different mutations showed a wide spectrum of renal abnormalities, such as dysplastic kidney (Fig. 10B, F) with cysts; hydronephrosis (Fig 10C, G), and polycystic kidneys (Fig. 10D, H).
Figure 10.
In vivo T2-weighted MRI for kidneys. (A, E) WT control mouse; (B, F) mutant mouse with dysplastic kidneys; (C, G) mutant mouse with hydronephrosis; (D, H) mutant mouse with polycystic kidneys. (A–D) MRI with shorter echo time, TE = 10 msec; (E–H) MRI with longer echo time, TE = 84 msec, acquired at 7-Tesla.
Dynamic contrast-enhanced MRI (DCE-MRI) for Renal Perfusion
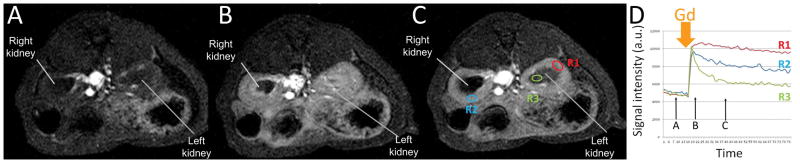
Dynamic contrast-enhanced MRI (DCE-MRI) (Gordon et al., 2014) with a single bolus of gadobenate dimeglumine (Gd)-based contrast agent administration has long been used for assessing organ perfusion. Figure 11 shows dynamic T1-weighted MRI before (Fig. 11A) and at two time points after a single bolus of intravenous Gd administration (Fig. 11B, C). Fig. 11D shows the time course of kinetic signal evolution at three different regions of interest (ROIs), qualitatively. The renal cortex and medulla have different blood flow wash-in and wash-out dynamics and it can be measured with DCE-MRI.
Figure 11.
Renal perfusion by dynamic contrast-enhanced MRI (DCE-MRI) in a WT control animal with temporal resolution 43 sec per frame. (A–C) T1-weighted MRI acquired at the time points indicated in D; (D) temporal evolution of signal obtained at 3 different regions of interest (ROI) as indicated in C. ROI 1(R1, red) is from the left cortex, ROI 2 (R2, blue) is from the right cortex; and ROI 3 (green R3) is from the left medulla. The Y axis is the mean signal intensity in each ROI with arbitrary unit (a.u.). The X axis is time in sec. The time for single intravenous Gd bolus injection (MultiHance, gadobenate dimeglumine, 0.2 mmol/kg body weight) is indicated by the thick orange arrow.
CONLCUSION
MRI is a versatile and non-invasive imaging modality with excellent penetration depth, tissue coverage, and soft tissue contrast. Many MR imaging protocols have been established for structural and functional imaging in small animal models, including fetal and neonatal mice. MRI is an ideal imaging modality for phenotyping mutant mouse models that will help to meet the rapidly increasing demands of the coming age of functional genomics.
Acknowledgments
This project is supported by NIH grants HL098180, HL132024, GM104412, and S10-OD010340.
References
- Ackerman JJH, Neil JJ. Biophysics of Diffusion in Cells. In: Jones DK, editor. Diffusion MRI: Theory, Methods and Applications. Oxford U. Press; Oxford: 2010. pp. 110–124. [Google Scholar]
- Ahrens ET, Laidlaw DH, Readhead C, Brosnan CF, Fraser SE, Jacobs RE. MR microscopy of transgenic mice that spontaneously acquire experimental allergic encephalomyelitis. Magnetic resonance in medicine. 1998;40:119–132. doi: 10.1002/mrm.1910400117. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DC, Barker GJ. Optimal imaging parameters for fiber-orientation estimation in diffusion MRI. NeuroImage. 2005;27:357–367. doi: 10.1016/j.neuroimage.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Allan TW, Besle J, Langers DR, Davies J, Hall DA, Palmer AR, Adjamian P. Neuroanatomical Alterations in Tinnitus Assessed with Magnetic Resonance Imaging. Front Aging Neurosci. 2016;8:221. doi: 10.3389/fnagi.2016.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki I, Wu YJ, Silva AC, Lynch RM, Koretsky AP. In vivo detection of neuroarchitecture in the rodent brain using manganese-enhanced MRI. NeuroImage. 2004;22:1046–1059. doi: 10.1016/j.neuroimage.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. Journal of molecular neuroscience : MN. 2008;34:51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- Axel L, Goncalves RC, Bloomgarden D. Regional heart wall motion: two-dimensional analysis and functional imaging with MR imaging. Radiology. 1992;183:745–750. doi: 10.1148/radiology.183.3.1584931. [DOI] [PubMed] [Google Scholar]
- Badea A, Gewalt S, Avants BB, Cook JJ, Johnson GA. Quantitative mouse brain phenotyping based on single and multispectral MR protocols. NeuroImage. 2012;63:1633–1645. doi: 10.1016/j.neuroimage.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Allen PS. Determinants of anisotropic water diffusion in nerves. Magnetic resonance in medicine. 1994;31:394–400. doi: 10.1002/mrm.1910310408. [DOI] [PubMed] [Google Scholar]
- Benveniste H, Ma Y, Dhawan J, Gifford A, Smith SD, Feinstein I, Du C, Grant SC, Hof PR. Anatomical and functional phenotyping of mice models of Alzheimer’s disease by MR microscopy. Ann N Y Acad Sci. 2007;1097:12–29. doi: 10.1196/annals.1379.006. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Bonilha L, Gross DW. Network analysis for a network disorder: The emerging role of graph theory in the study of epilepsy. Epilepsy Behav. 2015;50:162–170. doi: 10.1016/j.yebeh.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Hong S, Bernasconi A, Bernasconi N. Imaging structural and functional brain networks in temporal lobe epilepsy. Front Hum Neurosci. 2013;7:624. doi: 10.3389/fnhum.2013.00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake JA, Bult CJ, Eppig JT, Kadin JA, Richardson JE. The Mouse Genome Database: integration of and access to knowledge about the laboratory mouse. Nucleic acids research. 2014;42:D810–817. doi: 10.1093/nar/gkt1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden DM, Johnson GA, Zaborsky L, Green WD, Moore E, Badea A, Dubach MF, Bookstein FL. A symmetrical Waxholm canonical mouse brain for NeuroMaps. J Neurosci Methods. 2011;195:170–175. doi: 10.1016/j.jneumeth.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bult CJ, Eppig JT, Kadin JA, Richardson JE, Blake JA. The Mouse Genome Database (MGD): mouse biology and model systems. Nucleic acids research. 2008;36:D724–728. doi: 10.1093/nar/gkm961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Vogel TW, Zhang Q, Seo S, Swiderski RE, Moninger TO, Cassell MD, Thedens DR, Keppler-Noreuil KM, Nopoulos P, Nishimura DY, Searby CC, Bugge K, Sheffield VC. Abnormal development of NG2+PDGFR-alpha+ neural progenitor cells leads to neonatal hydrocephalus in a ciliopathy mouse model. Nat Med. 2012;18:1797–1804. doi: 10.1038/nm.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo E, Osman NF, Rosen BD, El-Shehaby I, Pan L, Jerosch-Herold M, Lai S, Bluemke DA, Lima JA. Quantitative assessment of regional myocardial function with MR-tagging in a multi-center study: interobserver and intraobserver agreement of fast strain analysis with Harmonic Phase (HARP) MRI. J Cardiovasc Magn Reson. 2005;7:783–791. doi: 10.1080/10976640500295417. [DOI] [PubMed] [Google Scholar]
- Chiang S, Haneef Z. Graph theory findings in the pathophysiology of temporal lobe epilepsy. Clin Neurophysiol. 2014;125:1295–1305. doi: 10.1016/j.clinph.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IY, Lee SP, Guilfoyle DN, Helpern JA. In vivo NMR studies of neurodegenerative diseases in transgenic and rodent models. Neurochem Res. 2003;28:987–1001. doi: 10.1023/a:1023370104289. [DOI] [PubMed] [Google Scholar]
- Choi JK, Dedeoglu A, Jenkins BG. Application of MRS to mouse models of neurodegenerative illness. NMR Biomed. 2007;20:216–237. doi: 10.1002/nbm.1145. [DOI] [PubMed] [Google Scholar]
- Cleary JO, Modat M, Norris FC, Price AN, Jayakody SA, Martinez-Barbera JP, Greene ND, Hawkes DJ, Ordidge RJ, Scambler PJ, Ourselin S, Lythgoe MF. Magnetic resonance virtual histology for embryos: 3D atlases for automated high-throughput phenotyping. NeuroImage. 2011;54:769–778. doi: 10.1016/j.neuroimage.2010.07.039. [DOI] [PubMed] [Google Scholar]
- Dall’Ara E, Boudiffa M, Taylor C, Schug D, Fiegle E, Kennerley AJ, Damianou C, Tozer GM, Kiessling F, Muller R. Longitudinal imaging of the ageing mouse. Mech Ageing Dev. 2016;160:93–116. doi: 10.1016/j.mad.2016.08.001. [DOI] [PubMed] [Google Scholar]
- De Leon-Rodriguez LM, Martins AF, Pinho MC, Rofsky NM, Sherry AD. Basic MR relaxation mechanisms and contrast agent design. Journal of magnetic resonance imaging : JMRI. 2015;42:545–565. doi: 10.1002/jmri.24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi B, Crane JA, Knudsen BE, Macura SI, Grande JP, Lerman LO. Evolution of cardiac and renal impairment detected by high-field cardiovascular magnetic resonance in mice with renal artery stenosis. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2013;15:98. doi: 10.1186/1532-429X-15-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgi B, Voight BF, Bucan M. From mouse to human: evolutionary genomics analysis of human orthologs of essential genes. PLoS genetics. 2013;9:e1003484. doi: 10.1371/journal.pgen.1003484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaznavi F, Evans A, Madabhushi A, Feldman M. Digital imaging in pathology: whole-slide imaging and beyond. Annu Rev Pathol. 2013;8:331–359. doi: 10.1146/annurev-pathol-011811-120902. [DOI] [PubMed] [Google Scholar]
- Gordon Y, Partovi S, Muller-Eschner M, Amarteifio E, Bauerle T, Weber MA, Kauczor HU, Rengier F. Dynamic contrast-enhanced magnetic resonance imaging: fundamentals and application to the evaluation of the peripheral perfusion. Cardiovasc Diagn Ther. 2014;4:147–164. doi: 10.3978/j.issn.2223-3652.2014.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschy A, Bauer E, Schrodt C, Lykowsky G, Ye YX, Rommel E, Jakob PM, Bauer WR, Herold V. Local arterial stiffening assessed by MRI precedes atherosclerotic plaque formation. Circ Cardiovasc Imaging. 2013;6:916–923. doi: 10.1161/CIRCIMAGING.113.000611. [DOI] [PubMed] [Google Scholar]
- Gotschy A, Bauer WR, Winter P, Nordbeck P, Rommel E, Jakob PM, Herold V. Local versus global aortic pulse wave velocity in early atherosclerosis: An animal study in ApoE-/--mice using ultrahigh field MRI. PLoS One. 2017;12:e0171603. doi: 10.1371/journal.pone.0171603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Marjanska M, Wengenack TM, Reyes DA, Curran GL, Lin J, Preboske GM, Poduslo JF, Garwood M. Magnetic resonance imaging of Alzheimer’s pathology in the brains of living transgenic mice: a new tool in Alzheimer’s disease research. Neuroscientist. 2007;13:38–48. doi: 10.1177/1073858406295610. [DOI] [PubMed] [Google Scholar]
- Just M, Thelen M. Tissue characterization with T1, T2, and proton density values: results in 160 patients with brain tumors. Radiology. 1988;169:779–785. doi: 10.1148/radiology.169.3.3187000. [DOI] [PubMed] [Google Scholar]
- Kim H, Kim JH, Possin KL, Winer J, Geschwind MD, Xu D, Hess CP. Surface-based morphometry reveals caudate subnuclear structural damage in patients with premotor Huntington disease. Brain Imaging Behav. 2016 doi: 10.1007/s11682-016-9616-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YJ, Koretsky AP. Doctoral Dissertation. Carnegie Mellon University; Pittsburgh, PA: 1997a. An Approach to Direct Imaging of Brain Activation with MRI by Activity-Induced Manganese Dependent, AIM, Contrast. [Google Scholar]
- Lin YJ, Koretsky AP. Manganese ion enhances T1-weighted MRI during brain activation: an approach to direct imaging of brain function. Magnetic resonance in medicine. 1997b;38:378–388. doi: 10.1002/mrm.1910380305. [DOI] [PubMed] [Google Scholar]
- Martinez-Martinez MA, Pacheco-Torres J, Borrell V, Canals S. Phenotyping the central nervous system of the embryonic mouse by magnetic resonance microscopy. NeuroImage. 2014;97:95–106. doi: 10.1016/j.neuroimage.2014.04.043. [DOI] [PubMed] [Google Scholar]
- Mazaheri Y, Biswal BB, Ward BD, Hyde JS. Measurements of tissue T1 spin-lattice relaxation time and discrimination of large draining veins using transient EPI data sets in BOLD-weighted fMRI acquisitions. NeuroImage. 2006;32:603–615. doi: 10.1016/j.neuroimage.2006.03.051. [DOI] [PubMed] [Google Scholar]
- McVeigh ER, Zerhouni EA. Noninvasive measurement of transmural gradients in myocardial strain with MR imaging. Radiology. 1991;180:677–683. doi: 10.1148/radiology.180.3.1871278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. Journal of immunology (Baltimore, Md: 1950) 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Nadeau JH. Modifier genes in mice and humans. Nature reviews. Genetics. 2001;2:165–174. doi: 10.1038/35056009. [DOI] [PubMed] [Google Scholar]
- Nguyen D, Xu T. The expanding role of mouse genetics for understanding human biology and disease. Disease models & mechanisms. 2008;1:56–66. doi: 10.1242/dmm.000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman NF, Prince JL. Visualizing myocardial function using HARP MRI. Phys Med Biol. 2000;45:1665–1682. doi: 10.1088/0031-9155/45/6/318. [DOI] [PubMed] [Google Scholar]
- Persson N, Ghisletta P, Dahle CL, Bender AR, Yang Y, Yuan P, Daugherty AM, Raz N. Regional brain shrinkage over two years: individual differences and effects of pro-inflammatory genetic polymorphisms. NeuroImage. 2014;103:334–348. doi: 10.1016/j.neuroimage.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson N, Ghisletta P, Dahle CL, Bender AR, Yang Y, Yuan P, Daugherty AM, Raz N. Regional brain shrinkage and change in cognitive performance over two years: The bidirectional influences of the brain and cognitive reserve factors. NeuroImage. 2016;126:15–26. doi: 10.1016/j.neuroimage.2015.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petiet AE, Kaufman MH, Goddeeris MM, Brandenburg J, Elmore SA, Johnson GA. High-resolution magnetic resonance histology of the embryonic and neonatal mouse: a 4D atlas and morphologic database. Proc Natl Acad Sci U S A. 2008;105:12331–12336. doi: 10.1073/pnas.0805747105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Raz N, Schmiedek F, Rodrigue KM, Kennedy KM, Lindenberger U, Lovden M. Differential brain shrinkage over 6 months shows limited association with cognitive practice. Brain Cogn. 2013;82:171–180. doi: 10.1016/j.bandc.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, LaLiberte C, van Eede M, Lerch JP, Henkelman M. Variability of brain anatomy for three common mouse strains. NeuroImage. 2016;142:656–662. doi: 10.1016/j.neuroimage.2016.03.069. [DOI] [PubMed] [Google Scholar]
- Shehata ML, Cheng S, Osman NF, Bluemke DA, Lima JA. Myocardial tissue tagging with cardiovascular magnetic resonance. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2009;11:55. doi: 10.1186/1532-429X-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AC, Lee JH, Aoki I, Koretsky AP. Manganese-enhanced magnetic resonance imaging (MEMRI): methodological and practical considerations. NMR Biomed. 2004;17:532–543. doi: 10.1002/nbm.945. [DOI] [PubMed] [Google Scholar]
- Sosnovik DE, Dai G, Nahrendorf M, Rosen BR, Seethamraju R. Cardiac MRI in mice at 9.4 Tesla with a transmit-receive surface coil and a cardiac-tailored intensity-correction algorithm. Journal of magnetic resonance imaging : JMRI. 2007;26:279–287. doi: 10.1002/jmri.20966. [DOI] [PubMed] [Google Scholar]
- Sporns O. Structure and function of complex brain networks. Dialogues Clin Neurosci. 2013;15:247–262. doi: 10.31887/DCNS.2013.15.3/osporns. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajda Z, Pedersen M, Doczi T, Sulyok E, Nielsen S. Studies of mdx mice. Neuroscience. 2004;129:993–998. doi: 10.1016/j.neuroscience.2004.08.055. [DOI] [PubMed] [Google Scholar]
- Vanhoutte L, Gerber BL, Gallez B, Po C, Magat J, Jean-Luc B, Feron O, Moniotte S. High field magnetic resonance imaging of rodents in cardiovascular research. Basic Res Cardiol. 2016;111:46. doi: 10.1007/s00395-016-0565-2. [DOI] [PubMed] [Google Scholar]
- Wang Y, Song Y, Rajagopalan P, An T, Liu K, Chou YY, Gutman B, Toga AW, Thompson PM. Surface-based TBM boosts power to detect disease effects on the brain: an N=804 ADNI study. NeuroImage. 2011;56:1993–2010. doi: 10.1016/j.neuroimage.2011.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansapura JP, Holland SK, Dunn RS, Ball WS., Jr NMR relaxation times in the human brain at 3.0 tesla. Journal of magnetic resonance imaging : JMRI. 1999;9:531–538. doi: 10.1002/(sici)1522-2586(199904)9:4<531::aid-jmri4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Wiegell MR, Larsson HB, Wedeen VJ. Fiber crossing in human brain depicted with diffusion tensor MR imaging. Radiology. 2000;217:897–903. doi: 10.1148/radiology.217.3.r00nv43897. [DOI] [PubMed] [Google Scholar]
- Wright PJ, Mougin OE, Totman JJ, Peters AM, Brookes MJ, Coxon R, Morris PE, Clemence M, Francis ST, Bowtell RW, Gowland PA. Water proton T1 measurements in brain tissue at 7, 3, and 1.5 T using IR-EPI, IR-TSE, and MPRAGE: results and optimization. Magma (New York, NY) 2008;21:121–130. doi: 10.1007/s10334-008-0104-8. [DOI] [PubMed] [Google Scholar]
- Young AA, Imai H, Chang CN, Axel L. Two-dimensional left ventricular deformation during systole using magnetic resonance imaging with spatial modulation of magnetization. Circulation. 1994;89:740–752. doi: 10.1161/01.cir.89.2.740. [DOI] [PubMed] [Google Scholar]
- Zerhouni EA. Myocardial tagging by magnetic resonance imaging. Coronary artery disease. 1993;4:334–339. doi: 10.1097/00019501-199304000-00004. [DOI] [PubMed] [Google Scholar]
- Zerhouni EA, Parish DM, Rogers WJ, Yang A, Shapiro EP. Human heart: tagging with MR imaging--a method for noninvasive assessment of myocardial motion. Radiology. 1988;169:59–63. doi: 10.1148/radiology.169.1.3420283. [DOI] [PubMed] [Google Scholar]