Abstract
Background
Fever is strongly associated with poor outcome after traumatic brain injury (TBI). We hypothesized that early fever is a direct result of brain injury and thus would be more common in TBI than in patients without brain injury, and associated with inflammation.
Methods
We prospectively enrolled patients with major trauma with and without TBI from a busy level I trauma center ICU. Patients were assigned to one of four groups based on their presenting Head Abbreviated Injury Severity Scale scores (HAIS): Polytrauma: Head AIS score >2, one other region>2, Isolated Head: Head AIS score>2, all other regions <3, Isolated Body: One region >2, excluding Head/Face, Minor Injury: No region with AIS>2. Early fever was defined as at least one recorded temperature of >38.3°C in the first 48 hours after admission. Outcome measures included neurologic deterioration, length of stay in the ICU, hospital mortality, discharge Glasgow Outcome Scale-Extended (GOSE), and plasma levels of 7 key cytokines at admission and 24 hours (exploratory).
Results
Two hundred and sixty-eight patients were enrolled; including subjects with Polytrauma (n=59), Isolated Head (n=97), Isolated Body (n=100) and Minor Trauma (n=12). The incidence of fever was similar in all groups irrespective of injury (11–24%). In all groups, there was a significant association between the presence of early fever and death in the hospital (6–18% v. 0–3%), as well as longer median ICU stays (3–7 days v. 2–3 days). Fever was significantly associated with elevated IL-6 at admission (50.7pg/dL v. 16.9pg/dL, P=0.0067) and at 24 hours (83.1pg/dL v. 17.1pg/dL, P=0.0025) in the isolated head injury group.
Conclusion
Contrary to our hypothesis, early fever was not more common in patients with brain injury, though fever was associated with longer ICU stays and death in all groups. Additionally, fever was associated with elevated IL-6 levels in isolated head injury.
Keywords: Traumatic Brain Injury, Fever, Polytrauma, Inflammation
Introduction
Traumatic brain injury (TBI) is a common, burdensome disease in the United States (US), as an estimated 5.3 million Americans live with disability incurred from TBI.1 Secondary brain injury, which stems from the complex sequence of events beginning at the initial insult and continuing into the acute hospitalization, represents a potential target for intervention to limit disability after TBI. One possible cause of secondary brain injury is fever. Fever is associated with worse outcome in TBI patients,2 as well as in experimental models of TBI.3 In a study of over 7,000 patients with TBI, those who had high fever (>39.0°C) within 72 hours of the injury had six times the mortality of afebrile patients.2 Even low-grade fever (38 – 39°C) was associated with increased mortality in this study. Moreover, fever burden, particularly early after TBI is associated with poor prognosis.4 However, observational studies in TBI linking fever with poor outcome often lack a control group with trauma but without head injury. The incidence of early fever in major trauma without head injury is not well known, and it is unclear if fever is associated with poor outcome in the same fashion. There is evidence to suggest that early fever is associated with higher injury severity scores (ISS) but not mortality in non-TBI trauma patients, leading the authors to impugn systemic inflammation as a possible cause of early fever.5 In contrast, in TBI, it is assumed that early fever reflects injury to the brain structures responsible for homeostatic control of temperature, namely the anterior hypothalamus. We hypothesized that early fever is a direct result of brain injury, and thus would be more common in TBI than in major trauma without brain injury. As an exploratory aim, we measured 7 key cytokines (Interleukin 1beta, Interleukin 2, Interleukin 4, Interleukin 6, Interleukin 8, Interleukin 10, and Tumor necrosis factor alpha) at 2 time points (admission, 24 hours after trauma) to assess the systemic inflammatory responses in febrile and afebrile patients.
Methods
Patients, Protocols
We prospectively enrolled major trauma patients with and without TBI from a busy level I trauma center ICU from October 2013 through June 2015. Major trauma was defined as injuries sufficient to warrant ICU admission. All patients meeting this criterion were approached for informed consent. Patients refusing consent, that could not be consented, or who were not admitted to the ICU were excluded. For patients with severe brain injury who were unable to provide informed consent themselves, consent was obtained from the patient’s legally authorized representative (LAR). If and when they regained the ability to provide consent during the hospitalization, the patient was approached to reaffirm consent. Per ICU protocol, temperatures were recorded at least every 4 hours (and as frequently as hourly) for the first 48 hours after admission. Early fever was defined as at least one recorded temperature of >38.3°C in the first 48 hours after admission. Temperatures were measured with an oral probe in the majority of measurements. A bladder catheter thermistor or rarely, an axillary probe were used if the oral route was inaccessible. If the temperature threshold of 38.3°C was met or exceeded, cultures of blood and urine, as well as sputum for intubated patients, were routinely obtained, unless the patient was immediately post-operative (<24 hours). Additionally, febrile patients received interventions in a tiered fashion, starting with acetaminophen, followed by external cooling measures such as ice packs or cooling blankets if medication alone was ineffective after the first hour. If these measures were ineffective, an adhesive cooling device was applied. The Institutional Review Boards approved the study prior to screening of any patients.
Definitions, Variables, Outcome Measures
Information regarding the hospital course was abstracted from the electronic medical record, and included: Intensive Care Unit (ICU) length of stay (LOS), hospital LOS, death during the admission, Glasgow Coma Score (GCS) at time of discharge from ICU, Glasgow Outcome Scale-Extended (GOSE) at time of hospital discharge. Abbreviated Injury Scale (AIS) scores for each body region, and overall Injury Severity Scores (ISS) were abstracted from the trauma registry. Patients were assigned to one of four groups based on their presenting Head Abbreviated Injury Severity Scale score (HAIS):
Polytrauma: Head AIS score >2, one other region>2
Isolated Head: Head AIS score>2, all other regions <3
Isolated Body: One region >2, excluding Head/Face
Minor Injury: No region with AIS>2
Hypotension was defined as systolic blood pressure (SBP) <90 mm Hg and hypoxia was defined as oxygenation saturation <90% measured in the field or on admission to the emergency department. Shock was recorded by measuring base deficit in mEq/L. Culture verified infection was determined if a culture drawn within 48 hours of admission grew a pathogenic organism not felt to be a contaminant.
Cytokine Measurement
Plasma samples were obtained on admission (<8 hours from the trauma) and at 24 hours after injury, and banked at −80°C for subsequent analysis. Plasma was analyzed using a Luminex human analyte platform that screens secreted proteins using multiplex fluorescent immunoassay; samples were run in duplicate for confirmation, and the medians [interquartile range] in pg/mL reported. To control data quality, if more than 20% of measurements were below the threshold of detection, the cytokine value was not included for further analysis. Interleukin is abbreviated as “IL”.
Statistical Methods
Descriptive statistics (median and interquartile range for continuous variables; frequencies and percentages for categorical variables) were used to describe clinical data by group. Group comparisons for categorical variables were performed using Fisher’s exact tests and ordinal comparisons were made with the Wilcoxon-Mann-Whitney test for two group comparisons, and the Kruskal-Wallis H test for three group comparisons. Non-parametric methods were used for ordinal data and continuous data that were highly skewed. A total sample size of 268 patients was determined in order to detect a 15% difference in the incidence of fever between patients with and without brain injury, using a two-sample comparison of proportions with alpha=0.05 and power=0.80. Statistical analysis was performed using Stata software (Stata 12, StataCorp, College Station, TX). Significance was set at p ≤0.05.
Results
Subject Characteristics by Trauma Group
Over 500 patients who sustained trauma were screened (n=510), and 268 patients were enrolled; including subjects with Polytrauma (n=59), Isolated Head (n=97), Isolated Body (n=100) and Minor Trauma (n=12) (Figure 1). Patients with Minor Injury were excluded from further analysis, as these subjects did not qualify as having major trauma (no body region with AIS>2). Subjects in the polytrauma group were more seriously injured than the other two groups, having higher median injury severity scores, lower GCS on admission and higher median base deficit. Patients with isolated head injury were older than those patients with isolated body or polytrauma (n<0.01). Likewise, rates of admission hypoxia, and admission hypotension were uncommon and similar between all three groups. (Table 1). The incidence of fever was similar in all three groups (P=0.126), ranging from 11%–24%. About half (52%) of patients were treated with acetaminophen in the first 48 hours for the indication of fever, and 11% required a cooling blanket for fever control. No patients required an adhesive cooling device for refractory fever.
Figure 1.
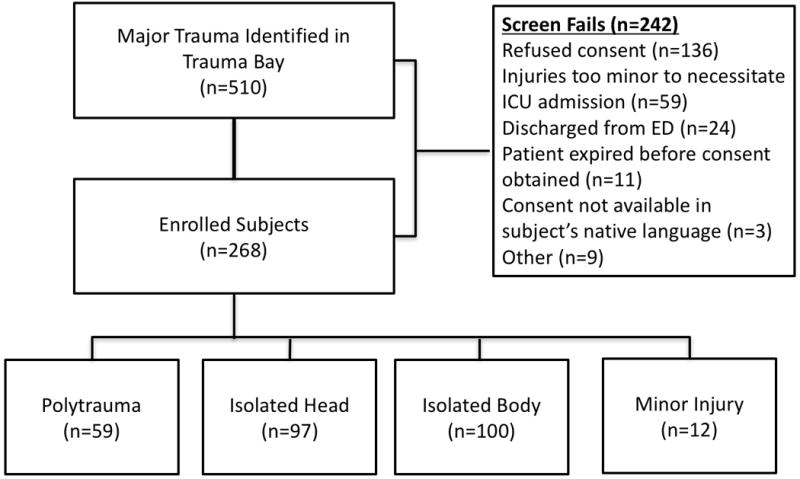
Flow diagram of enrollment
Table 1.
Subject Characteristics by Trauma Group. Asterisks denotes significant difference.
| Polytrauma (n=59) | Isolated Head (n=97) | Isolated Body (n=100) | P | |
|---|---|---|---|---|
| Median Age [IQR] | 54 [36–63] | 64.5 [46–74.5] | 52 [35–64] | 0.003 |
| Median ISS [IQR] | 29 [26–38] | 17 [11–21] | 19 [14–25] | <0.001 |
| Median Admission GCS [IQR] | 14 [6–15] | 15 [13–15] | 15 [15–15] | <0.001 |
| Median Base Deficit [IQR] | 1 [0–3.5] | 0 [0–1] | 0 [0–3] | 0.002 |
| Early Fever | 24% (14) | 11% (11) | 17% (17) | 0.126 |
| O2 Sat<90%* | 29% (17) | 26% (25) | 25% (25) | 0.864 |
| SBP<90 mm Hg* | 29% (17) | 18% (17) | 23% (23) | 0.253 |
Measured in the field or on presentation to the Emergency Department
Early Fever Status, Injury Severity, and Inflammation by Trauma Group
More severe injury was not associated with fever status, except in the isolated head injury group. In the isolated head injury group, febrile patients were sicker than afebrile patients, specifically with febrile patients having significantly higher ISS (26 [19–27] v. 17 [10–19], P=0.0015), lower admission GCS (14 [4–15] v. 15 [14–15], P=0.0008) and higher Head AIS (5 [4–5] v. 4 [3–4], P=0.0023). There was no association between fever and injury severity (ISS) in the polytrauma or isolated body injury groups. Culture verified infection rates were similar between febrile and afebrile patients in all 3 groups. (Table 2) There were no differences in hypoxia or hypotension on admission by fever status in any of the three groups.
Table 2.
Subject Characteristics and Outcome by Fever Status. Asterisks denotes significant difference
| Polytrauma (n=59) |
Isolated Head (n=97) |
Isolated Body (n=100) |
||||
|---|---|---|---|---|---|---|
| Fever (n=14) |
No Fever (n=45) |
Fever (n=11) |
No Fever (n=86) |
Fever (n=17) |
No Fever (n=83) |
|
| Median ISS [IQR] | 34 [22–43] | 29 [26–34] | 26 [19–27]* | 17 [10–19] | 17 [14–21] | 21 [11–26] |
| Median Admission GCS [IQR] | 7 [3–15] | 14 [12–15] | 14 [4–15]* | 15 [14–15] | 15 [14–15] | 15 [15–15] |
| Median Head AIS [IQR] | 4 [3–5] | 3 [3–4] | 5 [4–5]* | 4 [3–4] | 0 [0–0] | 0 [0–0] |
| Culture Verified Infection | 21%(3) | 7%(3) | 9%(1) | 7%(6) | 6%(1) | 5%(4) |
| Neuro Deterioration %(n) | 42% (6) | 24% (11) | 36% (4)* | 9% (8) | n/a | n/a |
| Death in Hospital | 14%(2)* | 0%(0) | 18%(2)* | 3%(3) | 6%(1)* | 0%(0) |
| Median Length of stay in ICU in days [IQR] | 7 [4–13]* | 3 [2–7] | 5 [2–8]* | 2 [2–3] | 3 [2–10]* | 2 [2–4] |
| Median Length of stay in hospital in days [IQR] | 18 [12–27]* | 9 [5–14] | 11 [3–17] | 4 [3–6] | 9 [5–22] | 7 [5–11] |
| Median Discharge GOSE [IQR] | 3 [3–5]* | 5 [3–8] | 3 [2–5]* | 7 [4–8] | n/a | n/a |
Inflammation and Outcome by Fever Status
In all three groups, there was a significant association between fever and poor outcome, specifically death in the hospital, as well as longer ICU stays. Fever was also associated with worse median discharge GOSE in both the polytrauma and isolated head injury groups. Patients in the polytrauma group with fever had a longer hospital stay than those without fever. (Table 2)
Patients in all three injury groups had similar admission and 24-hour values of the cytokines we examined including IL-1beta, IL-4, and TNF-alpha, regardless of fever status. (Table 3) A notable exception was IL-6, which was significantly elevated in patients with fever in the isolated head injury group on admission, which persisted at 24 hours. In addition, median IL-6 rose in the febrile patients with isolated body injury at the 24-hour mark. We also observed elevations of IL-2 in the non-febrile polytrauma patients at 24 hours, and elevations in IL-10 in the febrile isolated head injured patients at 24 hours. (Table 3) IL-8 was excluded from further analysis as >20% of the values we obtained were below the detection threshold.
Table 3.
Cytokine Values by Fever Status. Median values listed in pg/mL.
| Admission | Polytrauma (n=59) | Isolated Head (n=97) | Isolated Body (n=100) | |||
|---|---|---|---|---|---|---|
| Fever (n=14) | No Fever (n=45) | Fever (n=11) | No Fever (n=86) | Fever (n=17) | No Fever (n=83) | |
| Median IL-1 beta | 1.3 [0.11–4.2] | 3.1 [1.2–5.5] P=0.08 | 2.34 [1.3–4.1] | 2.9 [0.8–5.1] P=0.63 | 2.5 [0.5–4.1] | 3 [0.78–4.7] P=0.82 |
| Median IL-2 | 31.0 [11.4–48.4] | 44.1 [24.21–58.1] P=0.06 | 34.7 [29.8–41.8] | 39.4 [24.7–48.8] P=0.68 | 34.8 [23.1–42.1] | 41.4 [28.2–52.7] P=0.22 |
| Median IL-4 | 19.4 [4.1–50.2] | 31.6 [6.8–84.7] P=0.35 | 34.6 [21.4–81.7] | 36.0 [10.9–70.7] P=0.45 | 26.3 [8.9–78.5] | 44.92 [11.8–79.8] P=0.65 |
| Median IL-6 | 27.8 [13.7–62.68] | 40.7 [9.2–115.1] P=0.45 | 50.7 [19.6–102.8] | 16.9 [5.1–30.62] P=0.0067 | 31.0 [10.5–85.6] | 31.8 [5.7–48.6] P=0.36 |
| Median IL-10 | 8.4 [5.8–17.3] | 15.5 [5.7–28.1] P=0.11 | 12.78 [7.0–19.0] | 8.5 [2.5–13.4] P=0.16 | 12.0 [5.3–22.8] | 11.1 [4.3–30.7] P=0.94 |
| Median TNF-a | 53.1 [34.9–111.9] | 72.1 [46.9–91.0] P=0.46 | 79.9 [45.4–94.2] | 77.1 [41.0–96.0] P=0.88 | 69.2 [45.9–131.61] | 76.8 [49.1–114.3] P=0.93 |
| 24 Hours | Polytrauma (n=42) | Isolated Head (n=70) | Isolated Body (n=100) | |||
| Fever (n=14) | No Fever (n=32) | Fever (n=11) | No Fever (n=59) | Fever (n=17) | No Fever (n=83) | |
| Median IL-1 beta | 0.3 [0–1.6] | 1.91 [0.2–5.1] P=0.11 | 1.6 [0.2–3.0] | 1.1 [0.2–3.4] P=0.91 | 1.7 [0.4–5.7] | 1.7 [0.5–3.8] P=0.57 |
| Median IL-2 | 16.6 [11.9–32.7] | 35.1 [18.3–55.4] P=0.03 | 34.9 [20.0–43.3] | 28.2 [15.6–41.3] P=0.50 | 34.3 [18.8–58.2] | 31.9 [22.6–46.5] P=0.89 |
| Median IL-4 | 6.1 [1.1–41.3] | 29.1 [4.7–87.1] P=0.18 | 44.2 [27.6–73.7] | 31.7 [4.0–71.6] P=0.30 | 37.5 [8.0–89.1] | 36.1 [10.9–70.5] P=0.98 |
| Median IL-6 | 38.7 [6.2–94.9] | 28.1 [14.6–134.1] P=0.72 | 83.1 [37.9–126.0] | 17.1 [2.5–44.0] P=0.0025 | 113.9 [46.8–223.7] | 40.8 [18.3–72.3] P=0.0057 |
| Median IL-10 | 2.2 [0–15.8] | 5.3 [1.3–15.1] P=0.28 | 12.1 [3.6–33.8] | 3.7 [0.9–8.2] P=0.019 | 7.9 [3.1–22.2] | 8.6 [2.3–15.5] P=0.55 |
| Median TNF-a | 37.3 [34.1–99.4] | 62.2 [39.7–100.8] P=0.17 | 77.3 [38.1–109.5] | 57.1 [36.8–99.1] P=0.38 | 73.2 [48.1–167.1] | 71.1 [40.7–114.5] P=0.50 |
Discussion
Contrary to our original hypothesis, the incidence of early fever in trauma patients appears to be similar, regardless of the presence of brain injury, though the pattern of inflammation might be unique in isolated TBI with regard to IL-6 levels. Our study is the largest prospective cohort study to examine fever in trauma patients with and without TBI. Our estimate of the incidence of fever is in line with prior studies of early fever in neurologically injured patients,6 and echoes a smaller study of fever in critically ill trauma patients where no difference in incidence of fever was observed between patients with TBI and without TBI.5 That study by Bengualid et. al. was a retrospective report of 162 trauma patients with and without TBI. We observed a lesser incidence of early fever than in the Bengualid study (16% v. 40%), possibly because we chose a more stringent definition of fever (>38.3°C vs >38.0°C). The more stringent temperature demarcation is in line with previous studies of fever in neurologically injured patients,7 as well as a position statement from the American College of Critical Care Medicine recommending defining fever as any temperature above 38.2°C in intensive care unit patients.8 Our observations contrast with a retrospective study of critically ill patients, which found fever to be more common in neurologically injured subjects.9 However, this study included few trauma patients (<10%) and defined fever as any temperature ≥37.5°C during the first 24 hours of hospitalization.
Fever was associated with in-hospital mortality, similar to other observational studies of neurologically injured patients,2,6,9 but in opposition to the Bengualid study.5 Caution should be applied in the interpretation of our findings, however, as mortality was a relatively rare event in our study. Fever was associated with prolonged ICU stay in all of the groups. Unfortunately, we do not have data on how fever impacted outcome in the non-neurologically injured isolated body trauma group beyond hospital stay. Other authors have found that early elevations in temperature in a mixed trauma population might be protective, and that lack of fever on days 1–2 was associated with higher rates of infection and mortality.10 Notably, this study excluded trauma patients with severe head injury. Similar to these investigations, we observed a low rate of documented infection to accompany early fever.
Early fever is consistently associated with poorer outcomes in patients with brain injury. In a large, retrospective study of Chinese head trauma patients, both the degree and duration of early (<72 hours post-trauma) fever were strongly related with the outcomes of patients with acute TBI.2 The intensity-dependency suggests that fever might contribute to secondary brain injury. Experimentally induced hyperthermia exacerbates pathophysiologic processes like excitotoxicity, apoptosis, and inflammation.11 However, efforts to eliminate fever, including hypothermia, have not been shown to improve outcomes clinically.12,13 These initial results might reflect our incomplete understanding of the relationship between fever and poor outcome in brain-injured patients.
Investigators have speculated that the association between fever and lower GCS on presentation is related to the severity of the neuronal injury,14 particularly diffuse axonal injury and frontal lobe injury.15 Hypothalamic injury with loss of temperature regulation is often cited as the mechanism underlying non-infectious or neurogenic fever.7,16–18 It is possible that the relationship is broader and might not require hypothalamic injury. Tissue trauma, and the subsequent cascade of aseptic inflammation may lead to the generation of fever similar to the response to exogenous pyrogens. In classic models of infectious fever, endogenous pyrogenic cytokines (IL-1, IL-6, and TNF-alpha) are released into the bloodstream, stimulate production of prostaglandins in the central nervous system, resulting in thermogenesis mediated by the anterior hypothalamus,19 though this is only one of several pathways resulting in temperature elevation.20 Our study suggests a persistent association between early fever and elevated levels of IL-6 peripherally in isolated head injury, though it is impossible to infer cause or effect. Elevated peripheral levels of IL-6 after TBI have been associated with elevated ICP21 and poor outcome22,23 in some studies, while other authors observed no relationship with outcome.24 It is unclear why the association between elevated IL-6 and fever was not observed in patients with polytrauma who also have brain injury. The later rise of IL-6 at 24 hours in the isolated body injury group might be related to operative repair of orthopedic injuries that often occurs on day 1 after injury, but this hypothesis would need to be directly tested. In TBI, rises in IL-10 peripherally have been associated with elevated intracranial pressure and sympathetic nervous system activation,25 which might help to explain the association between fever and elevated IL-10 we observed at 24 hours in our isolated head injury group. Interleukin 2 production peaks at 24 hours after injury, and can be suppressed by more severe injury,26 which may explain the reduction in the IL-2 level in the febrile polytrauma patients at 24 hours. Future research might interrogate the association between IL-6 and fever by altering plasma IL-6 levels and measuring the impact on fever burden and outcomes in patients with brain injury.
Limitations
Our study has several important limitations. Temperatures were not uniformly measured in all patients, and ranged from as frequently as hourly to once every four hours, potentially leading to missing data. It is possible that the pattern of missing data was non-random, introducing bias. Transient temperature elevations might have been missed, underestimating the incidence of fever. Temperatures were measured from different sources, including oral (66%), bladder (20%) and axillary (14%), which may have introduced additional variability, though oral temperatures are thought to represent core temperatures relatively well, even in the critically ill.27 Axillary measurements may underestimate core temperatures (by 0.33±0.55°C in one study of critically ill adults),28 potentially underestimating the incidence of fever. Additionally, we did not measure the efficacy of fever therapy, and we do not know if fever therapy altered the outcome of our febrile patients. Despite the protocolled, tiered approach to fever outlined in the methods, only about half of febrile patients received antipyretic medication. This is perhaps due to the short duration of the temperature elevation. Another important limitation was that not all infections are culture positive, and thus occult infections may have been missed. We selected the first 48 hours as the window for early fever in order to capture non-infectious fever, as other investigators have found that fever documented within 72 hours of admission predicts negative evaluation for infection.29 It is unclear what role, if any, fever or brain trauma played in the decision to convert to comfort care in the 8 patients who died in hospital. Future studies should account for withdrawal of life sustaining measures in order to better address the relationship between mortality and fever. Finally, a few of our subjects were missing 24-hour cytokine levels. It is possible that the pattern was non-random introducing bias, though most subjects did have 24-hour values (83%, 212/256).
Conclusion
Early fever is common in trauma, with or without brain injury. Early fever may be a consequence of tissue trauma with resultant aseptic inflammation rather than hypothalamic damage. It remains to be shown whether altering or preventing early fever improves outcomes after trauma. Future studies should investigate the relationship between fever and aseptic inflammation, utilizing consistent temperature measurements with a view to intervention.
Acknowledgments
The project described was supported in part by Award Number 5K12HL108974-03 from the National Heart, Lung, and Blood Institute. This publication was supported by Oregon Clinical and Translational Research Institute (OCTRI), grant number (UL1TR000128) from the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. Dr. Hinson has consulted for Remedy Pharmaceuticals about a clinical study (CHARM), which Remedy funds. Payment for this consulting is made directly to the OHSU Foundation. Dr. Hinson may benefit from these funds by using them to fund her OHSU research.
Footnotes
Level of Evidence: III; Prognostic and Epidemiological study
Author Contributions
Hinson: study design, data collection, data analysis, data interpretation, writing
Rowell: data collection, data interpretation, revision
Lin: data analysis, data interpretation
Morris: study design, revision
Schreiber: study design, critical revision
Disclosure
The authors report no conflicts of interest.
This study was presented at the 30th annual meeting of the EAST Annual Scientific Assembly, January 10–14th, 2017, in Hollywood, FL.
References
- 1.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Jiang J. Chinese Head Trauma Data Bank: effect of hyperthermia on the outcome of acute head trauma patients. J Neurotrauma. 2012;29(1):96–100. doi: 10.1089/neu.2011.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dietrich WD, Alonso O, Halley M, Busto R. Delayed posttraumatic brain hyperthermia worsens outcome after fluid percussion brain injury: a light and electron microscopic study in rats. Neurosurgery. 1996;38(3):533–541. doi: 10.1097/00006123-199603000-00023. discussion 541. [DOI] [PubMed] [Google Scholar]
- 4.Bao L, Chen D, Ding L, Ling W, Xu F. Fever Burden Is an Independent Predictor for Prognosis of Traumatic Brain Injury. In: Elder JB, editor. PLoS ONE. 3. Vol. 9. 2014. p. e90956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bengualid V, Talari G, Rubin D, Albaeni A, Ciubotaru RL, Berger J. Fever in trauma patients: evaluation of risk factors, including traumatic brain injury. Am J Crit Care Off Publ Am Assoc Crit-Care Nurses. 2015;24(2):e1–5. doi: 10.4037/ajcc2015856. [DOI] [PubMed] [Google Scholar]
- 6.Oh HS, Jeong HS, Seo WS. Non-infectious hyperthermia in acute brain injury patients: relationships to mortality, blood pressure, intracranial pressure and cerebral perfusion pressure. Int J Nurs Pract. 2012;18(3):295–302. doi: 10.1111/j.1440-172X.2012.02039.x. [DOI] [PubMed] [Google Scholar]
- 7.Badjatia N. Hyperthermia and fever control in brain injury. Crit Care Med. 2009;37(Supplement):S250–S257. doi: 10.1097/CCM.0b013e3181aa5e8d. [DOI] [PubMed] [Google Scholar]
- 8.O’Grady NP, Barie PS, Bartlett JG, Bleck T, Carroll K, Kalil AC, Linden P, Maki DG, Nierman D, Pasculle W, et al. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med. 2008;36(4):1330–1349. doi: 10.1097/CCM.0b013e318169eda9. [DOI] [PubMed] [Google Scholar]
- 9.Rincon F, Patel U, Schorr C, Lee E, Ross S, Dellinger RP, Zanotti-Cavazzoni S. Brain injury as a risk factor for fever upon admission to the intensive care unit and association with in-hospital case fatality: a matched cohort study. J Intensive Care Med. 2015;30(2):107–114. doi: 10.1177/0885066613508266. [DOI] [PubMed] [Google Scholar]
- 10.Mizushima Y, Ueno M, Idoguchi K, Ishikawa K, Matsuoka T. Fever in trauma patients: friend or foe? J Trauma. 2009;67(5):1062–1065. doi: 10.1097/TA.0b013e3181b848fc. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich WD, Bramlett HM. Hyperthermia and central nervous system injury. Prog Brain Res. 2007;162:201–217. doi: 10.1016/S0079-6123(06)62011-6.. [DOI] [PubMed] [Google Scholar]
- 12.Clifton GL, Valadka A, Zygun D, Coffey CS, Drever P, Fourwinds S, Janis LS, Wilde E, Taylor P, Harshman K, et al. Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study: Hypothermia II): a randomised trial. Lancet Neurol. 2011;10(2):131–139. doi: 10.1016/S1474-4422(10)70300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broessner G, Beer R, Lackner P, Helbok R, Fischer M, Pfausier B, Rhorer J, Kuppers-Tiedt L, Schneider D, Schmutzhard E. Prophylactic, endovascularly based, long-term normothermia in ICU patients with severe cerebrovascular disease: bicenter prospective, randomized trial. Stroke J Cereb Circ. 2009;40(12):e657–665. doi: 10.1161/STROKEAHA.109.557652. [DOI] [PubMed] [Google Scholar]
- 14.Natale JE, Joseph JG, Helfaer MA, Shaffner DH. Early hyperthermia after traumatic brain injury in children: risk factors, influence on length of stay, and effect on short-term neurologic status. Crit Care Med. 2000;28(7):2608–2615. doi: 10.1097/00003246-200007000-00071. [DOI] [PubMed] [Google Scholar]
- 15.Thompson HJ, Pinto-Martin J, Bullock MR. Neurogenic fever after traumatic brain injury: an epidemiological study. J Neurol Neurosurg Psychiatry. 2003;74(5):614–619. doi: 10.1136/jnnp.74.5.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sazbon L, Groswasser Z. Outcome in 134 patients with prolonged posttraumatic unawareness. Part 1: Parameters determining late recovery of consciousness. J Neurosurg. 1990;72(1):75–80. doi: 10.3171/jns.1990.72.1.0075. [DOI] [PubMed] [Google Scholar]
- 17.Thompson HJ, Tkacs NC, Saatman KE, Raghupathi R, McIntosh TK. Hyperthermia following traumatic brain injury: a critical evaluation. Neurobiol Dis. 2003;12(3):163–173. doi: 10.1016/s0969-9961(02)00030-x. [DOI] [PubMed] [Google Scholar]
- 18.Meier K, Lee K. Neurogenic Fever: Review of Pathophysiology, Evaluation, and Management. J Intensive Care Med. 2016 Jan; doi: 10.1177/0885066615625194. [DOI] [PubMed] [Google Scholar]
- 19.Netea MG, Kullberg BJ, Van der Meer JWM. Circulating Cytokines as Mediators of Fever. Clin Infect Dis. 2000;31(Supplement 5):S178–S184. doi: 10.1086/317513. [DOI] [PubMed] [Google Scholar]
- 20.Niven DJ, Leger C, Stelfox HT, Laupland KB. Fever in the Critically Ill: A Review of Epidemiology, Immunology, and Management. J Intensive Care Med. 2012;27(5):290–297. doi: 10.1177/0885066611402463. [DOI] [PubMed] [Google Scholar]
- 21.Hergenroeder GW, Moore AN, McCoy JP, Jr, Samsel L, Ward NH, Clifton GL, Dash PK. Serum IL-6: a candidate biomarker for intracranial pressure elevation following isolated traumatic brain injury. J Neuroinflammation. 2010;7:19. doi: 10.1186/1742-2094-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venetsanou K, Vlachos K, Moles A, Fragakis G, Fildissis G, Baltopoulos G. Hypolipoproteinemia and hyperinflammatory cytokines in serum of severe and moderate traumatic brain injury (TBI) patients. Eur Cytokine Netw. 2007;18(4):206–209. doi: 10.1684/ecn.2007.0112. [DOI] [PubMed] [Google Scholar]
- 23.Woiciechowsky C, Schöning B, Cobanov J, Lanksch WR, Volk H-D, Döcke W-D. Early IL-6 plasma concentrations correlate with severity of brain injury and pneumonia in brain-injured patients. J Trauma. 2002;52(2):339–345. doi: 10.1097/00005373-200202000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Kalabalikis P, Papazoglou K, Gouriotis D, et al. Correlation between serum IL-6 and CRP levels and severity of head injury in children. Intensive Care Med. 1999;25(3):288–292. doi: 10.1007/s001340050837. [DOI] [PubMed] [Google Scholar]
- 25.Woiciechowsky C, Volk HD. Increased intracranial pressure induces a rapid systemic interleukin-10 release through activation of the sympathetic nervous system. Acta Neurochir Suppl. 2005;95:373–376. doi: 10.1007/3-211-32318-x_76. [DOI] [PubMed] [Google Scholar]
- 26.Abraham E. The Effects of Hemorrhage and Trauma on Interleukin 2 Production. Arch Surg. 1985;120(12):1341. doi: 10.1001/archsurg.1985.01390360007002. [DOI] [PubMed] [Google Scholar]
- 27.Jefferies S, Weatherall M, Young P, Beasley R. A systematic review of the accuracy of peripheral thermometry in estimating core temperatures among febrile critically ill patients. Crit Care Resusc J Australas Acad Crit Care Med. 2011;13(3):194–199. [PubMed] [Google Scholar]
- 28.Nonose Y, Sato Y, Kabayama H, Arisawa A, Onodera M, Imanaka H, Nishimura M. Accuracy of recorded body temperature of critically ill patients related to measurement site: a prospective observational study. Anaesth Intensive Care. 2012;40(5):820–824. doi: 10.1177/0310057X1204000510. [DOI] [PubMed] [Google Scholar]
- 29.Rabinstein AA, Sandhu K. Non-infectious fever in the neurological intensive care unit: incidence, causes and predictors. J Neurol Neurosurg Psychiatry. 2007;78(11):1278–1280. doi: 10.1136/jnnp.2006.112730. [DOI] [PMC free article] [PubMed] [Google Scholar]


