Abstract
Purpose
To present a novel multisource rotating shield brachytherapy (RSBT) apparatus for the simultaneous precise angular and linear positioning of partially-shielded 153Gd brachytherapy sources in interstitial needles for the treatment of locally-advanced prostate cancer. It is designed to lower the dose to nearby healthy tissues, the urethra in particular, relative to conventional high-dose-rate brachytherapy (HDR-BT) techniques.
Methods and Materials
Following needle implantation through the patient template, an angular drive mechanism is docked to the patient template. Each needle is coupled to a multisource afterloader catheter by a connector passing through a shaft. The shafts are rotated about their axes by translating a moving template between two stationary templates. Shafts’ surfaces and moving template holes are helically threaded with the same pattern such that translation of the moving template causes simultaneous rotation of the shafts. The rotation of each shaft is mechanically transmitted to the catheter/source/shield combination, inside the needles, via several key/keyway pairs. The catheter angles are simultaneously incremented throughout treatment, and only a single 360° rotation of all catheters is needed for a full treatment. For each rotation angle, source depth in each needle is controlled by a multisource afterloader, which is proposed as an array of belt-driven linear actuators, each of which drives a wire that controls catheter depth in a needle.
Results
Treatment plans demonstrated RSBT with the proposed apparatus reduced urethral D0.1cc below that of conventional HDR-BT by 31% for urethral dose gradient volume within 3 mm of the urethra surface. Treatment time to deliver 20 Gy with the proposed multisource RSBT apparatus using nineteen 62.4 GBq 153Gd sources is 122 min.
Conclusions
The proposed RSBT delivery apparatus enables a mechanically feasible urethra-sparing treatment technique for prostate cancer in a clinically reasonable timeframe.
1. INTRODUCTION
High-dose-rate brachytherapy (HDR-BT) for prostate cancer is an effective prostate cancer therapy that can be delivered as a monotherapy (Demanes et al 2014)1–6 or a boost to external beam radiotherapy.7–16 While achieving tumor control is paramount, prostate cancer patients may live with the side effects of their treatment for decades, and anticipated side effects play a strong role in treatment decisions.17,18 Urinary retention and urethral stricture have been shown to be experienced at higher rates by patients undergoing BT monotherapy or EBRT with HDR-BT boost than by patients undergoing EBRT alone.19,20 Urethral stricture, occurring mostly in the bulbomembranous urethra or apex/external sphincter region,21 is the most frequent late toxicity of combined EBRT and HDR-BT treatments.22 The reported urethral stricture rates (5.2%,23 6.6%,24 8%,22 and 10%20) are considerably greater than those reported for EBRT monotherapy (1.7%,23 2%,20 and 3%25,26). Urethral brachytherapy dose is likely the underlying cause of urethral stricture in combined EBRT and HDR-BT treatments.27–29
The target conformity and bladder, urethra, and rectal sparing capabilities of conventional HDR-BT is restricted based on the geometric constraints imposed by the position and shape of the catheters, as well as the radially symmetric radiation dose distributions emitted by conventional unshielded BT sources. Dose distribution conformity and sensitive healthy tissue avoidance can be considerably improved through the use of rotating shield brachytherapy (RSBT) in conjunction with a 153Gd radiation source that is amenable to partial shielding within an interstitial needle (192Ir is not).30–33 The sources rotate during delivery in an optimized fashion,34,35 directing dose away from sensitive structures and into the targeted tissue.
The purpose of the current work is to introduce a novel, multisource, prostate RSBT apparatus. In a previous study,31 a partially-shielded source/catheter/needle system was defined, based on the 153Gd isotope, and was shown by simulation to be capable of lowering the urethral dose up to 44% relative to conventional HDR-BT. An apparatus for mechanically delivering the dose through the inserted needles was not proposed, however, and the multisource RSBT apparatus presented here overcomes the technical barriers to implementation of the previously proposed approach.31 Since the dose rate per unit mass for 153Gd is substantially lower than that of 192Ir, multiple shielded 153Gd sources need to be used simultaneously in order to deliver RSBT dose distributions of up to 20 Gy in a clinically acceptable time of fewer than 2 hours. The apparatus presented in this work can control the emission angles and depths of up to 20 partially-shielded 153Gd sources simultaneously.
2. METHODS AND MATERIALS
2.A. Brachytherapy source
Enabling the multisource RSBT technique requires an appropriate radioisotope and a technically feasible rotating shield system that fits inside a 14-gauge needle used for prostate cancer brachytherapy. For this apparatus 153Gd is selected as the source isotope due to its reasonable dose rate, energy spectrum ranging from 40 keV to 105 keV (60.9 keV average), half-life of 242 days, and its potential for mass production via neutron irradiation of 151Eu or 152Gd.31 A nitinol (NiTi) RSBT needle containing a rotating catheter as well as a shielded 153Gd source was designed, and the dose rate distribution about the partially shielded source was calculated using the MCNP5 Monte Carlo code and a published 153Gd spectrum,36 with photons emitted in the intermediate range of 40–105 keV. The modeled source was a 7.41 g/cm3 Gd2O3 pellet containing 2,442 GBq of 153Gd per gram of Gd2O3, which could be generated by neutron irradiation of spent dual-photon absorptiometry sources containing about 87% 152Gd.37 The Monte Carlo dose calculation method is the same as that employed by.31
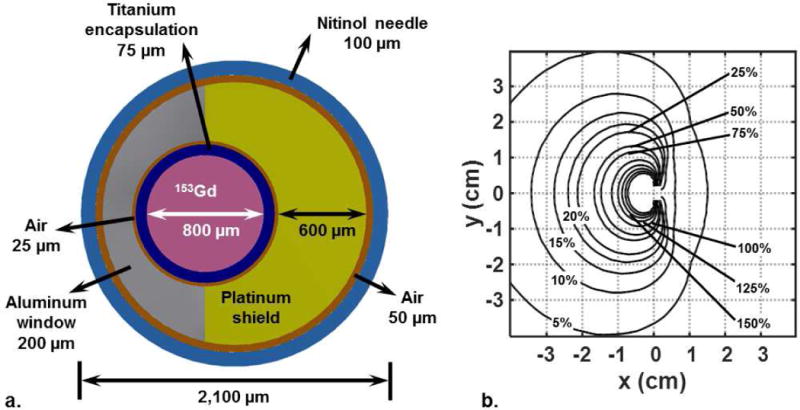
The shielded 153Gd source and its calculated relative dose rate distribution are shown in Figure 1(a) and 1(b), respectively. The source exhibits azimuthal anisotropy in its dose rate distribution due to the presence of platinum shielding on one side. In the proximal and distal directions, the platinum shield shown in Figure 1(a) has cylindrical platinum end cap welded to it that prevent the 153Gd source pellet from sliding out. The end caps function as receivers for the aluminum window, and, when they are welded to the platinum shield, the aluminum window is fixed in place. The dose rate on the platinum shielded side of the source at 1 cm off-axis was to about 7% of that on the unshielded side. The calculated dose rate of the RSBT source 1 cm from the geometric center of the catheter on the side with the aluminum window and along the axial plane passing through the geometric center of the active radiation source was 6.82×102 cGy h−1. The dose rate at 1 cm from the central axis of the 192Ir source was 4.246×104 cGy h−1 along the axial plane passing through the geometric center of the active source.
Figure 1.
(a) Needle/source system with a spatially-offset 153Gd source and a platinum shield used as the brachytherapy source in multisource RSBT apparatus. (b) Directionally biased dose rate distribution from the source/shield, normalized to 100% at 1 cm from the source, shown in a plane perpendicular to the source axis.
2.B. Multisource angular drive mechanism
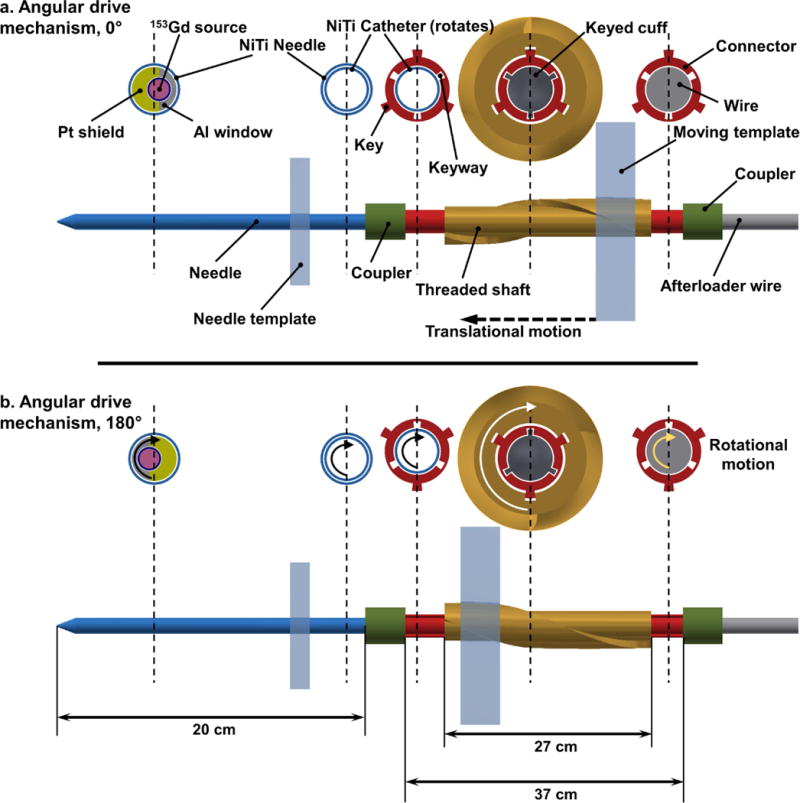
The RSBT system is equipped with an angular drive mechanism that controls the rotation of nitinol catheter-mounted source/shields for all implanted needles simultaneously. Following needle implantation, the angular drive mechanism is docked to the patient template in which the needles are all held at the same rotation angle at a given time while the catheters are rotated by translating a moving template between two stationary templates using redundant motors. A two-frame conceptual diagram of the angular drive mechanism is shown in Figure 2. It consists of the cross section of five points along a single inserted needle starting from the combination of the afterloader wire and the connector to the combination of needle/source/shield inside the prostate. Figure 2 shows how translational motion of the moving template causes a 180° rotation of a shielded source inside a needle. When the moving template is translated longitudinally the shafts rotate, as threaded holes of the moving template exert enough resistive force to the threaded exterior peripheral wall of the shaft which is fixed axially. The angular orientation of the shielded source is also fixed and known during treatment via a proximal keyed cuff that attaches the remote afterloader wire to the source catheter. Partial shields around the sources are oriented at a known angle by means of the keys and the keyways machined into various parts.
Figure 2.
Angular drive mechanism incorporating side views and cross sections of several points along the axis of a single needle. Translational motion of the moving template rotates the shaft, connector, and source/shield/catheter from (a) 0° to (b) 180° angular positions. Each needle, implanted through the patient template, is coupled to the catheter-mounted afterloader wire through a keyed connector (red), which passes through a rotating shaft. The catheter is rigidly attached to a proximal keyed cuff that enables the angular orientation of the shielded source to be fixed and known at all times during treatment. Items in the figure are not to scale.
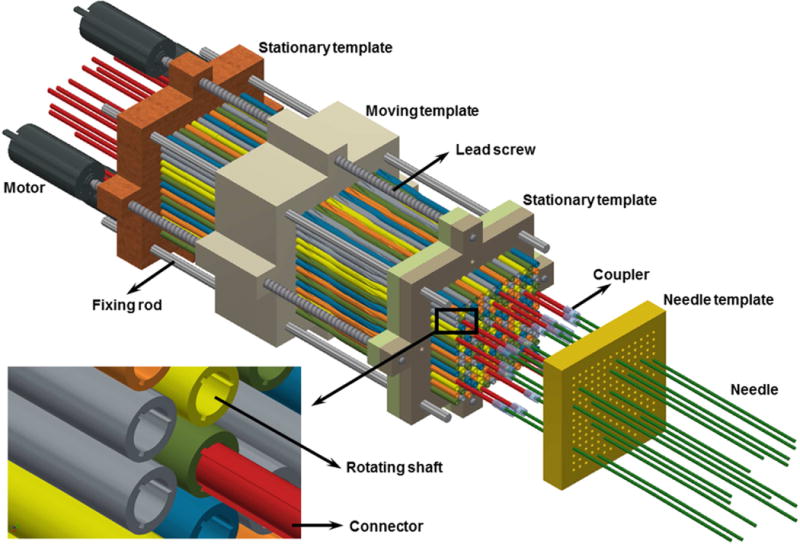
Figure 3 shows the whole multichannel angular drive mechanism with all the shafts and templates, which is docked to twenty inserted needles. As shown, the connector can move freely in the longitudinal direction in order to connect with needles that protrude from the patient at varying distances. The rotating shafts are threaded to provide a pitch with 10 cm translation per shaft rotation (1 mm per 3.6° rotation), which is sufficient to balance the tradeoffs between rotational accuracy, apparatus length, and resistive force exerted on the moving template by the shafts during motion. In order to create the desired translation of the moving template, four motors are arranged to rotate the lead screws contacting the moving template. When the lead screws rotate, the moving template translates longitudinally while the other two templates provide rigidity to the system.
Figure 3.
Multichannel angular drive system. All shafts are locked at the same angular orientation at a given time by the moving template, which, when translated, simultaneously rotates all of the shafts. The moving template is translated by redundant motors that are attached to lead screws, and the shaft angular positions are known based on the position of the template. A subset of the connectors (red) is shown in this figure.
2.C. Multisource remote afterloader
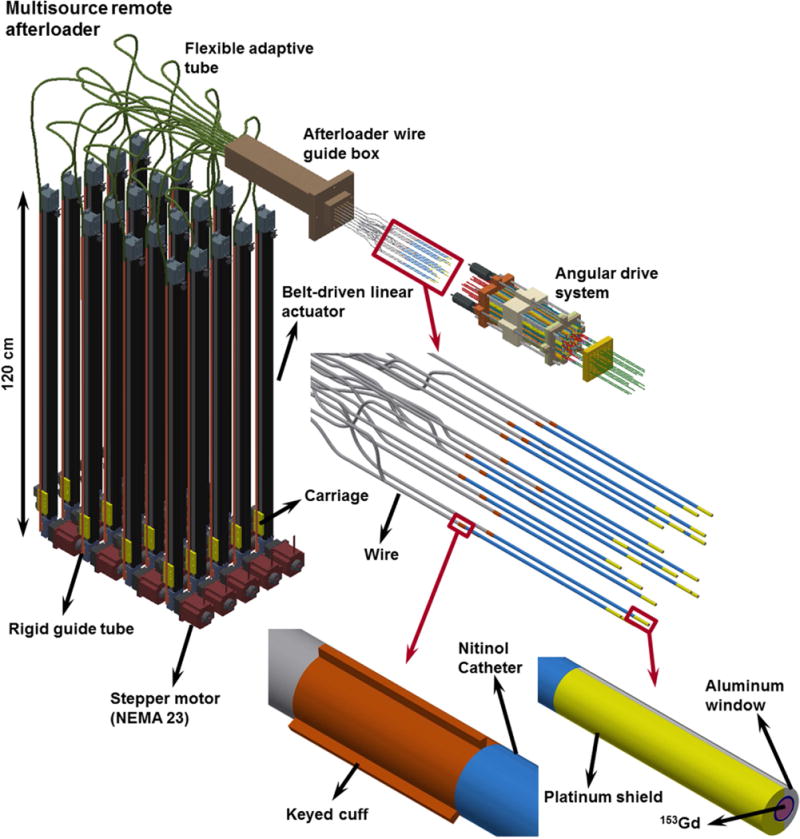
The depth positions of the radiation sources are determined via a remote afterloader (Figure 4) consisting of twenty Parker-Hannifin® (PH) (Parker, Rohnert Park, CA, USA) 1,000-mm travel belt-driven linear actuators that have 0.2 mm positional reproducibility and are assembled in the vertical orientation. A 1.8-mm-diameter 7×7 flexible stainless steel braided wire is attached to the carriage (yellow) of each actuator and moved back and forth into a guide box through a rigid guide tube and a flexible adaptive tube, connected to each other. The wire is rigidly attached to a flexible nitinol catheter by means of keyed cuff. The angular orientation of the shielded source, which is attached to the other side of the nitinol catheter, is fixed and known for every dwell position inside each needle, through the engagement of proximal keyed cuffs and the keyways cut into the connector. The overall multisource RSBT system enables controlling the depth positioning as well as the rotations of the shielded sources in a decoupled manner.
Figure 4.
Multisource remote afterloader, consisting of four stacks of five linear actuators in the vertical orientation. A flexible stainless steel braided wire from each actuator is attached to the nitinol catheter (blue) via a keyed cuff and is fed into a guide box. Each shielded source is attached to a nitinol catheter which travels and rotates inside its corresponding needle. The braided wires attached to the linear actuators’ carriage require guide tubes as well.
2.D. Dose delivery methodology
The delivery process occurs by having the moving template control the angular directions of all the source/shield combinations and using the remote afterloader to independently control the longitudinal position of all the sources in all the needles simultaneously. Once the source angles are changed by translating the moving template, the multisource afterloader moves the sources to all of the necessary depths in each needle. The process is repeated for all of the sixteen evenly-spaced emission directions per dwell position. This has been found to be sufficient to ensure CTV coverage and urethral sparing, with 5 mm spacing between dwell positions. Thus, in this technique the catheters are rotated by 22.5°, and only a single 360° catheter rotation is needed for a full treatment. The designed angular drive apparatus forces the sources to be at the same angle at the same time while the longitudinal depth of the sources inside needles can be adjusted in different positions. Therefore the total amount of time spent on a specific emission direction is dictated by the catheter that requires the greatest total dwell time to deliver.
The shielded source for each needle can be retracted back into the afterloader within less than 10 seconds in the event of an emergency. Each implanted needle has its own partially-shielded RSBT source, and the radiation from all sources is delivered simultaneously. RSBT delivery with this novel multisource apparatus involves inserting the needles, without catheters, through the perineum under ultrasound guidance. The needles will be CT/MRI compatible, and sterilizable.
2.E. Treatment planning
In order to assess the dosimetric effectiveness, delivery times, and robustness to uncertainties of the proposed RSBT approach, comparative treatment plans for RSBT and HDR-BT were generated on computed tomography (CT) images of a previously treated anonymous patient for whom 19 needles were used. Based on the methods of the California Endocurietherapy Institute for treatment planning,38 a 5-mm margin is added to the prostate boundary, excluding the regions adjacent to the bladder, rectum, and the proximal seminal vesicles, in order to contour the CTV. The urethral margin of 3 mm was included in the model as a relaxed prescription dose constraint in order to provide a spatial location for the dose gradient about the urethra. The CTV D90 (minimum dose to the hottest 90% of the CTV) is set to 110% of the prescribed dose (20 Gy). CTV V100 and V150 are required to be in the range of 90% to 100% and <35%, respectively. D0.1cc values for the rectal wall, the bladder wall, and the urethra were limited to less than 85%, 100%, and 110%, respectively. D1cc values for the same set of organs at risk were also limited to less than 80%, 90%, and 105%, respectively. The optimization method is the same as that described in a previous study.31
2.F. Uncertainty tolerance
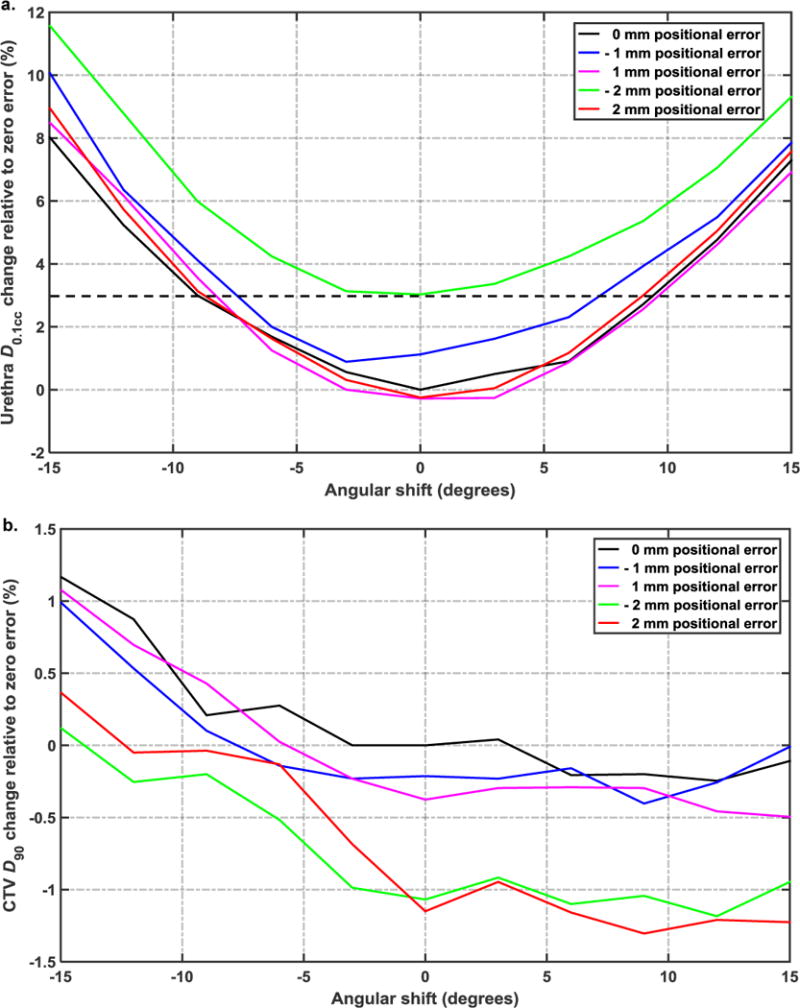
A sensitivity analysis was performed in order to estimate the dosimetric impact of uncertainty in both longitudinal positions and emission angles of the shielded catheters. A combination of multiple different systematic longitudinal positioning errors (≤ 2 mm) as well as rotational orientation errors (≤ 15°) of the shielded catheters is considered in order to determine the uncertainty tolerance of the RSBT multisource delivery system. CTV D90 and urethra D0.1cc were evaluated following error application to quantify plan degradation due to uncertainty, with ±3% accuracy considered tolerable. The assumption is based on the plausible scenario of rotating all of the catheters the same incorrect angle as well as translating all of the catheters the same incorrect distance.
3. RESULTS
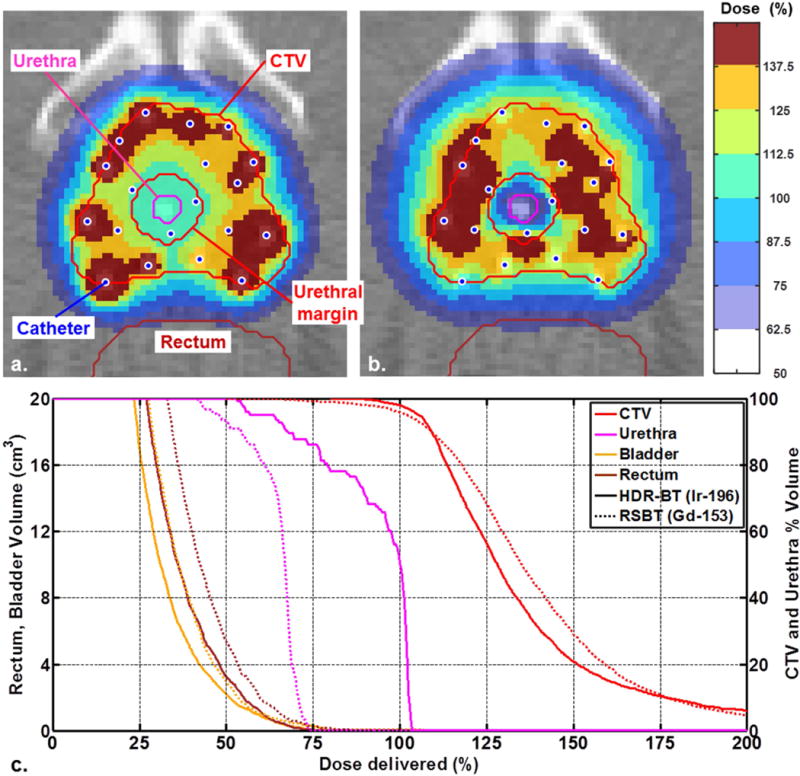
Planned conventional HDR-BT and RSBT dose distributions as well as dose volume histograms (DVH) are shown in Figure 5a–b and 5c, respectively, for the urethral gradient margin of 3 mm. For the same CTV D90 of the patient considered and 3 mm urethral margin, the planned treatment with the multisource RSBT apparatus reduced urethral D0.1cc below that of 192Ir-based HDR-BT by 31% relative to the prescribed dose of 100%. Plots of the urethral D0.1cc and CTV D90 percentage variations as a function of emission direction rotational error and catheter positional error are shown in Figure 6a and 6b, respectively. The urethral dose change relative to zero error is within the ±3% tolerance for ±1 mm positioning error and ±7° angular errors.
Figure 5.
Dose distributions for 3-mm urethral margin of (a) 192Ir based HDR-BT and (b) 153Gd based multisource RSBT apparatus sampled onto a CT scan of a prostate cancer patient. (c) Dose-volume histograms for conventional 192Ir HDR-BT and 153Gd based multisource RSBT apparatus for urethral margin of 3 mm.
Figure 6.
Dosimetric impact of positional and rotational uncertainty of catheters on (a) urethral D0.1cc and (b) CTV D90. The dashed line in (a) represents 3% error which is considered as the tolerable accuracy.
With the increasing values of angular deviation of the source’s emission direction from the ideal state of zero degree error, urethral D0.1cc value increases as shown in Figure 6 (a). The systematic angular error of 15° from the baseline increased the urethral D0.1cc by 8%. For the case considered, urethral D0.1cc increased by 3% with 2 mm positional error. Figure 6 (a) shows that when all of the catheters have 2 mm longitudinal errors and are rotated 15° incorrectly, there is a 11.6% increase in urethral D0.1cc. Figure 6 (b) shows that the catheters’ emission direction error could either increase or decrease the value of CTV D90. For the same urethral dose gradient volume peri-apical D0.1cc was reduced by 25%. The delivery time for 20 Gy to the CTV for the case considered was 15.8 min with HDR-BT using a 370 GBq 192Ir source and 121.7 min with RSBT.
4. DISCUSSION
A urethral margin of 3 mm was added to the boundary of the urethra for the case considered, irrespective of the prostate size, and no constraints were applied to the dose inside the margin in the treatment planning process. The resulting dose distributions indicate that RSBT with the proposed multisource apparatus induced cold spots only inside and adjacent to the urethra, which is desirable in terms of minimizing normal tissue toxicity. Besides that, the DVH plots exhibit a shift to the left in the urethral DVH relative to that of conventional HDR-BT technique. However the physician would need to select the appropriate margin for a given patient.
The proposed mechanism in Figure 4 offers a number of unique attributes. First, the dimensions of the unit are small enough that the system can be used in common procedure rooms. Second, as the connectors have the freedom to move longitudinally prior to the connection between the angular drive mechanism and the needles, the depth of needles entry into the patient’s perineum does not matter. Third, although the angular drive mechanism dictates all of the 20 emission windows in the patient to be at the same direction during the irradiation process, the independent depth control for each shielded source enabled by the multisource remote afterloader provides efficient treatments. Fourth, the control over longitudinal translation and the control over rotation of the shielded sources into the needles are independent.
All of the shielded sources’ aluminium emission windows are oriented in the same emission direction at the same time during the irradiation process. Therefore the delivery scheme would be based on completing all of the dwell positions of all the catheters in a single rotational angle and then switching to the next rotational angle by means of translating the moving template one sixteenth of the distance needed to create a single full rotation of the catheters. Accordingly the total amount of time spent on a single emission direction is dictated by the catheter with the longest cumulative dwell time for that direction. The treatment time for delivering RSBT dose with multisource RSBT apparatus shows an increase by a factor of about 7 relative to the conventional HDR-BT treatment time due to the lower dose rate of 153Gd relative to 192Ir. As all of the shielded sources have the same orientation in a single translational position of the moving template, inter-source attenuation is a potential concern with the proposed approach. Our strategy for addressing this issue is to develop a delivery optimization approach in which the longitudinal positions for all of the sources for a given delivery angle are intelligently ordered in time to minimize inter-source attenuation.
The precision of radiation dose delivery of multisource RSBT apparatus as well as safe delivery of high radiation doses to the prostate should be guaranteed during the treatment. For the presented mechanism it is dependent on the precise longitudinal and rotational positioning of the catheters during the irradiation process in which the catheter angles are simultaneously incremented. Therefore, a catheter position monitoring and control system is needed in order to empirically verify that the catheter angles and depths are within the required tolerances for safe radiation delivery. This is accomplished with a mechanism using feedback from multiple cameras to measure and correct for catheter longitudinal and angular positioning errors in real time.
It is expected that RSBT delivery would take place under trans-rectal ultrasound (TRUS) guidance. The current version of the RSBT angular drive mechanism is designed to demonstrate mechanical feasibility of the approach and is not yet compatible with a commercially available TRUS system. It is expected that modifications can be successfully made to the angular drive mechanism to enable TRUS usage, and the associated workflows can later be defined.
To the authors’ knowledge, the concept of using a rotating shield brachytherapy approach similar to that proposed in the current work to treat prostate cancer dates back to the work of Ebert.37 Ebert identified the potential of the method but no isotope, shielded catheter design, or practical method for achieving delivery was presented. The system described by the authors in a previously published paper and the current manuscript fills those major gaps. Stranded prostate seeds do not contain partial shields, and, once implanted, they cannot be rotated to the knowledge of the authors. The same holds for iridium ribbons, which have an isotope with an energy that is too high for partial shielding in an interstitial setting.
As the focus of this study is on presenting a novel apparatus for controlling multiple shielded radiation sources simultaneously in terms of both depth and angle, only a single previously treated prostate cancer patient was considered as the case study for comparing RSBT and 192Ir-based HDR-BT, as shown in Figure 5 and described in the Results section. An extensive (> 20 patients) treatment planning study comparing RSBT and 192Ir-based HDR-BT needs to be conducted in order to thoroughly evaluate the proposed technique, which is planned as future work. As designing a feasible RSBT delivery apparatus was a major undertaking that needed to be completed prior to conducting the RSBT versus HDR-BT treatment planning study, the scope of the current work was limited to the delivery apparatus itself and a simple HDR-BT to RSBT comparison to demonstrate feasibility.
There is inter-patient variation in the urethral dose delivered with HDR-BT, and White et al (2012)38 reported a mean urethra D0.1cc value of 107.3% with a standard deviation of only 3%, over 208 separate implants (104 patients). Based on the presented result that urethral D0.1cc can be reduced by 31% when using RSBT versus HDR-BT, it is expected that the reduction in urethral D0.1cc will greatly exceed the inter-patient variability for HDR-BT.
Treatment times for the proposed RSBT system are longer than for conventional HDR-BT, thus the optimizing the associated clinical workflow will require further investigation. However, its extended treatment times may be not so long as to make the approach clinically infeasible. For instance, with conventional HDR-BT using intra-operative TRUS based treatment planning,6,39–42 a single procedure, starting with needle implantation, can be completed within 2–3 hours.43 With the proposed system, the process would be extended to 4–5 hours.
5. CONCLUSIONS
The proposed multisource RSBT delivery apparatus enables a mechanically feasible urethra-sparing treatment technique for prostate cancer in a clinically reasonable timeframe two hours.
A novel multisource rotating shield brachytherapy system is proposed as a mechanically feasible alternative to high dose rate brachytherapy for the treatment of locally-advanced prostate cancer. The design consists of a multisource remote afterloader and an angular drive mechanism which can simultaneously control depths and angles of multiple partially shielded radiation sources, respectively. The resulting apparatus can be used to spare the urethra with minimal compromise of the prostate dose.
Acknowledgments
This study was supported by the National Institute of Health through a grant from the National Institute of Biomedical Imaging and Bioengineering (R01 EB020665) and a National Cancer Institute Phase I Small Business Technology Transfer grant (1 R41 CA210737-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
RTF has ownership interest in pxAlpha, LLC, which is developing a rotating shield brachytherapy delivery method.
References
- 1.Grills IS, Martinez AA, Hollander M, Huang R, Goldman K, Chen PY, Gustafson GS. High dose rate brachytherapy as prostate cancer monotherapy reduces toxicity compared to low dose rate palladium seeds. J Urol. 2004;171:1098, 1104. doi: 10.1097/01.ju.0000113299.34404.22. [DOI] [PubMed] [Google Scholar]
- 2.Yoshioka Y, Konishi K, Sumida I, Takahashi Y, Isohashi F, Ogata T, Koizumi M, Yamazaki H, Nonomura N, Okuyama A, Inoue T. Monotherapeutic high-dose-rate brachytherapy for prostate cancer: five-year results of an extreme hypofractionation regimen with 54 Gy in nine fractions. Int J Radiat Oncol Biol Phys. 2011;80:469–475. doi: 10.1016/j.ijrobp.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Barkati M, Williams SG, Foroudi F, Tai KH, Chander S, van Dyk A, Duchesne GM. High-dose-rate brachytherapy as a monotherapy for favorable-risk prostate cancer: a Phase II trial. International journal of radiation oncology, biology, physics. 2012;82:1889–1896. doi: 10.1016/j.ijrobp.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Ghilezan M, Martinez A, Gustason G, Krauss D, Antonucci JV, Chen P, Fontanesi J, Wallace M, Ye H, Casey A, Sebastian E, Kim L, Limbacher A. High-dose-rate brachytherapy as monotherapy delivered in two fractions within one day for favorable/intermediate-risk prostate cancer: preliminary toxicity data. International journal of radiation oncology, biology, physics. 2012;83:927–932. doi: 10.1016/j.ijrobp.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Hoskin P, Rojas A, Lowe G, Bryant L, Ostler P, Hughes R, Milner J, Cladd H. High-dose-rate brachytherapy alone for localized prostate cancer in patients at moderate or high risk of biochemical recurrence. International journal of radiation oncology, biology, physics. 2012;82:1376–1384. doi: 10.1016/j.ijrobp.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 6.Demanes DJ, Ghilezan MI. High-dose-rate brachytherapy as monotherapy for prostate cancer. Brachytherapy. 2014;13:529–541. doi: 10.1016/j.brachy.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Dattoli M, Wallner K, True L, Cash J, Sorace R. Long-term outcomes after treatment with brachytherapy and supplemental conformal radiation for prostate cancer patients having intermediate and high-risk features. Cancer. 2007;110:551–555. doi: 10.1002/cncr.22810. [DOI] [PubMed] [Google Scholar]
- 8.Pieters BR, Rezaie E, Geijsen ED, Koedooder K, van der Grient JN, Blank LE, de Reijke TM, Koning CC. Development of late toxicity and International Prostate Symptom Score resolution after external-beam radiotherapy combined with pulsed dose rate brachytherapy for prostate cancer. International journal of radiation oncology, biology, physics. 2011;81:758–764. doi: 10.1016/j.ijrobp.2010.05.044. [DOI] [PubMed] [Google Scholar]
- 9.Lettmaier S, Lotter M, Kreppner S, Strnad A, Fietkau R, Strnad V. Long term results of a prospective dose escalation phase-II trial: interstitial pulsed-dose-rate brachytherapy as boost for intermediate- and high-risk prostate cancer. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2012;104:181–186. doi: 10.1016/j.radonc.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Martinez AA, Gustafson G, Gonzalez J, Armour E, Mitchell C, Edmundson G, Spencer W, Stromberg J, Huang R, Vicini F. Dose escalation using conformal high-dose-rate brachytherapy improves outcome in unfavorable prostate cancer. International journal of radiation oncology, biology, physics. 2002;53:316–327. doi: 10.1016/s0360-3016(02)02733-5. [DOI] [PubMed] [Google Scholar]
- 11.Demanes DJ, Rodriguez RR, Schour L, Brandt D, Altieri G. High-dose-rate intensity-modulated brachytherapy with external beam radiotherapy for prostate cancer: California endocurietherapy’s 10-year results. Int J Radiat Oncol Biol Phys. 2005;61:1306–1316. doi: 10.1016/j.ijrobp.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Deger S, Boehmer D, Roigas J, Schink T, Wernecke KD, Wiegel T, Hinkelbein W, Budach V, Loening SA. High dose rate (HDR) brachytherapy with conformal radiation therapy for localized prostate cancer. European urology. 2005;47:441–448. doi: 10.1016/j.eururo.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Akimoto T, Ito K, Saitoh J, Noda SE, Harashima K, Sakurai H, Nakayama Y, Yamamoto T, Suzuki K, Nakano T, Niibe H. Acute genitourinary toxicity after high-dose-rate (HDR) brachytherapy combined with hypofractionated external-beam radiation therapy for localized prostate cancer: correlation between the urethral dose in HDR brachytherapy and the severity of acute genitourinary toxicity. Int J Radiat Oncol Biol Phys. 2005;63:463–471. doi: 10.1016/j.ijrobp.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 14.Hoskin PJ, Motohashi K, Bownes P, Bryant L, Ostler P. High dose rate brachytherapy in combination with external beam radiotherapy in the radical treatment of prostate cancer: initial results of a randomised phase three trial. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2007;84:114–120. doi: 10.1016/j.radonc.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Hoskin PJ, Rojas AM, Bownes PJ, Lowe GJ, Ostler PJ, Bryant L. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2012;103:217–222. doi: 10.1016/j.radonc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Hoskin PJ, Colombo A, Henry A, Niehoff P, Paulsen Hellebust T, Siebert FA, Kovacs G. GEC/ESTRO recommendations on high dose rate afterloading brachytherapy for localised prostate cancer: an update. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2013;107:325–332. doi: 10.1016/j.radonc.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Chen RC, Clark JA, Talcott JA. Individualizing quality-of-life outcomes reporting: how localized prostate cancer treatments affect patients with different levels of baseline urinary, bowel, and sexual function. J Clin Oncol. 2009;27:3916–3922. doi: 10.1200/JCO.2008.18.6486. [DOI] [PubMed] [Google Scholar]
- 18.Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, Lin X, Greenfield TK, Litwin MS, Saigal CS, Mahadevan A, Klein E, Kibel A, Pisters LL, Kuban D, Kaplan I, Wood D, Ciezki J, Shah N, Wei JT. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 19.Merrick GS, Butler WM, Wallner KE, Galbreath RW, Anderson RL, Allen ZA, Adamovich E. Risk factors for the development of prostate brachytherapy related urethral strictures. The Journal of urology. 2006;175:1376–1380. doi: 10.1016/S0022-5347(05)00681-6. discussion 1381. [DOI] [PubMed] [Google Scholar]
- 20.Mohammed N, Kestin L, Ghilezan M, Krauss D, Vicini F, Brabbins D, Gustafson G, Ye H, Martinez A. Comparison of acute and late toxicities for three modern high-dose radiation treatment techniques for localized prostate cancer. International journal of radiation oncology, biology, physics. 2012;82:204–212. doi: 10.1016/j.ijrobp.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Hindson BR, Millar JL, Matheson B. Urethral strictures following high-dose-rate brachytherapy for prostate cancer: analysis of risk factors. Brachytherapy. 2013;12:50–55. doi: 10.1016/j.brachy.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan L, Williams SG, Tai KH, Foroudi F, Cleeve L, Duchesne GM. Urethral stricture following high dose rate brachytherapy for prostate cancer. Radiother Oncol. 2009;91:232–236. doi: 10.1016/j.radonc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Elliott SP, Meng MV, Elkin EP, McAninch JW, Duchane J, Carroll PR, Ca PI. Incidence of urethral stricture after primary treatment for prostate cancer: data From CaPSURE. The Journal of urology. 2007;178:529–534. doi: 10.1016/j.juro.2007.03.126. discussion 534. [DOI] [PubMed] [Google Scholar]
- 24.Zwahlen DR, Andrianopoulos N, Matheson B, Duchesne GM, Millar JL. High-dose-rate brachytherapy in combination with conformal external beam radiotherapy in the treatment of prostate cancer. Brachytherapy. 2010;9:27–35. doi: 10.1016/j.brachy.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Zelefsky MJ, Chan H, Hunt M, Yamada Y, Shippy AM, Amols H. Long-term outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. The Journal of urology. 2006;176:1415–1419. doi: 10.1016/j.juro.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Fonteyne V, Villeirs G, Lumen N, De Meerleer G. Urinary toxicity after high dose intensity modulated radiotherapy as primary therapy for prostate cancer. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2009;92:42–47. doi: 10.1016/j.radonc.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Ishiyama H, Kitano M, Satoh T, Kotani S, Uemae M, Matsumoto K, Okusa H, Tabata K, Baba S, Hayakawa K. Genitourinary toxicity after high-dose-rate (HDR) brachytherapy combined with Hypofractionated External beam radiotherapy for localized prostate cancer: an analysis to determine the correlation between dose-volume histogram parameters in HDR brachytherapy and severity of toxicity. International journal of radiation oncology, biology, physics. 2009;75:23–28. doi: 10.1016/j.ijrobp.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Hsu IC, Bae K, Shinohara K, Pouliot J, Purdy J, Ibbott G, Speight J, Vigneault E, Ivker R, Sandler H. Phase II trial of combined high-dose-rate brachytherapy and external beam radiotherapy for adenocarcinoma of the prostate: preliminary results of RTOG 0321. Int J Radiat Oncol Biol Phys. 2010;78:751–758. doi: 10.1016/j.ijrobp.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morton GC, Loblaw DA, Chung H, Tsang G, Sankreacha R, Deabreu A, Zhang L, Mamedov A, Cheung P, Batchelar D, Danjoux C, Szumacher E. Health-related quality of life after single-fraction high-dose-rate brachytherapy and hypofractionated external beam radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2011;80:1299–1305. doi: 10.1016/j.ijrobp.2010.04.046. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Flynn RT, Kim Y, Yang W, Wu X. Dynamic rotating-shield brachytherapy. Med Phys. 2013;40:121703. doi: 10.1118/1.4828778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adams QE, Xu J, Breitbach EK, Li X, Enger SA, Rockey WR, Kim Y, Wu X, Flynn RT. Interstitial rotating shield brachytherapy for prostate cancer. Med Phys. 2014;41:051703. doi: 10.1118/1.4870441. [DOI] [PubMed] [Google Scholar]
- 32.Dadkhah H, Kim Y, Wu X, Flynn RT. Multihelix rotating shield brachytherapy for cervical cancer. Med Phys. 2015;42:6579–6588. doi: 10.1118/1.4933244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Flynn RT, Kim Y, Dadkhah H, Bhatia SK, Buatti JM, Xu W, Wu X. Paddle-based rotating-shield brachytherapy. Med Phys. 2015;42:5992–6003. doi: 10.1118/1.4930807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y, Flynn RT, Yang W, Kim Y, Bhatia SK, Sun W, Wu X. Rapid emission angle selection for rotating-shield brachytherapy. Med Phys. 2013;40:051720. doi: 10.1118/1.4802750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Flynn RT, Kim Y, Wu X. Asymmetric dose-volume optimization with smoothness control for rotating-shield brachytherapy. Med Phys. 2014;41:111709. doi: 10.1118/1.4897617. [DOI] [PubMed] [Google Scholar]
- 36.Enger SA, Fisher DR, Flynn RT. Gadolinium-153 as a brachytherapy isotope. Phys Med Biol. 2013;58:957–964. doi: 10.1088/0031-9155/58/4/957. [DOI] [PubMed] [Google Scholar]
- 37.Ebert MA. Potential dose-conformity advantages with multi-source intensity-modulated brachytherapy (IMBT) Australas Phys Eng Sci Med. 2006;29:165–171. doi: 10.1007/BF03178889. [DOI] [PubMed] [Google Scholar]
- 38.White EC, Kamrava MR, Demarco J, Park SJ, Wang PC, Kayode O, Steinberg ML, Demanes DJ. High-Dose-Rate Prostate Brachytherapy Consistently Results in High Quality Dosimetry. Int J Radiat Oncol Biol Phys. 2012;85:543–548. doi: 10.1016/j.ijrobp.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 39.Crook J, Ots A, Gaztanaga M, Schmid M, Araujo C, Hilts M, Batchelar D, Parker B, Bachand F, Milette MP. Ultrasound-planned high-dose-rate prostate brachytherapy: dose painting to the dominant intraprostatic lesion. Brachytherapy. 2014;13:433–441. doi: 10.1016/j.brachy.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Prada PJ, Cardenal J, Blanco AG, Anchuelo J, Ferri M, Fernandez G, Arrojo E, Vazquez A, Pacheco M, Fernandez J. High-dose-rate interstitial brachytherapy as monotherapy in one fraction for the treatment of favorable stage prostate cancer: Toxicity and long-term biochemical results. Radiother Oncol. 2016;119:411–416. doi: 10.1016/j.radonc.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Krauss DJ, Ye H, Martinez AA, Mitchell B, Sebastian E, Limbacher A, Gustafson GS. Favorable Preliminary Outcomes for Men With Low- and Intermediate-risk Prostate Cancer Treated With 19-Gy Single-fraction High-dose-rate Brachytherapy. Int J Radiat Oncol Biol Phys. 2017;97:98–106. doi: 10.1016/j.ijrobp.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 42.Morton G, Chung HT, McGuffin M, Helou J, D’Alimonte L, Ravi A, Cheung P, Szumacher E, Liu S, Al-Hanaqta M, Zhang L, Mamedov A, Loblaw A. Prostate high dose-rate brachytherapy as monotherapy for low and intermediate risk prostate cancer: Early toxicity and quality-of life results from a randomized phase II clinical trial of one fraction of 19Gy or two fractions of 13.5Gy. Radiother Oncol. 2017;122:87–92. doi: 10.1016/j.radonc.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 43.Batchelar D, Gaztanaga M, Schmid M, Araujo C, Bachand F, Crook J. Validation study of ultrasound-based high-dose-rate prostate brachytherapy planning compared with CT-based planning. Brachytherapy. 2014;13:75–79. doi: 10.1016/j.brachy.2013.08.004. [DOI] [PubMed] [Google Scholar]


