Abstract
Background and Objectives
Obstructive sleep apnea is associated with increased complication rates postoperatively. Current literature does not provide adequate guidance on management of these patients. This study used the STOP-BANG questionnaire to diagnose patients with possible obstructive sleep apnea (score ≥ 3). We hypothesized that a STOP-BANG score ≥ 3 would significantly correlate with the number of oxygen desaturation episodes during the first 48 hours after total knee arthroscopy.
Methods
The STOP-BANG questionnaire was administered to 110 patients preoperatively. All patients underwent spinal-epidural anesthesia with a saphenous nerve block and sedation and were connected to the Nellcor Oximax N-600x pulse oximeter for 48 hours postoperatively.
Results
Final analysis included 98 patients. There was no significant difference in the total number of desaturation events between STOP-BANG groups (score < 3 versus ≥ 3 and score < 5 versus ≥ 5). The total number of desaturation events on postoperative day 1 was greater than on day 0 (32.8 ± 42.7 vs 4.1 ± 10.0, P < 0.0001). The total number of desaturation events correlated with length of hospital stay (r = 0.329, P = 0.0001). Patients with a preoperative serum CO2 ≥ 30 had significantly longer episodes of desaturation on postoperative day 0 compared to CO2 < 30 (233.7 ± 410.1 vs 82.0 ± 126.2 seconds, P = 0.044).
Conclusions
A high preoperative value of CO2 should be a warning for possible prolonged episodes of desaturation postoperatively. An attempt to limit postoperative desaturation events should be made to minimize length of stay.
INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by periodic, partial, or complete obstruction of the upper airway during sleep1. Compared with patients without OSA, patients with OSA tend to have a higher incidence of postoperative complications (including respiratory failure and cardiac events),2 increased intensive care unit admission, and increased hospital length of stay.3 The most recent guidelines from the American Society of Anesthesiologists suggest use of regional anesthesia in patients with OSA.4 Memtsoudis et al found a lower rate of postoperative complications in OSA patients receiving neuraxial block, indicating that regional anesthesia may provide beneficial effects, but additional evidence is currently minimal.5
The STOP-BANG questionnaire has been used to identify those at risk for OSA with high sensitivity.6–8 In the clinical setting, the STOP-BANG questionnaire may represent the only immediate way to identify potential OSA patients, based on which clinicians must make a decision regarding postoperative monitoring. However, little data is available on how this assessment correlates to actual risk of oxygen desaturation and postoperative complications in patients undergoing knee surgery under neuraxial anesthesia.
The objective of this study was to use the STOP-BANG questionnaire to identify patients at risk of OSA without previous diagnosis or treatment and correlate the STOP-BANG score with the number of episodes of desaturation and the duration of the apneic period following total knee arthroplasty (TKA) with neuraxial anesthesia. In an environment of limited health care resources, information such as this could potentially help identify patients with more severe disease and presumably higher risk for complications, who should undergo respiratory monitoring following TKA. We hypothesized that the STOP-BANG scoring model, as a predictor of OSA (score ≥ 3), would significantly correlate with the number of oxygen desaturation episodes experienced by patients during the first 48 hours following TKA under neuraxial block while on 3L nasal cannula. Therefore, our primary outcome was the number of desaturation episodes by STOP-BANG score <3 versus ≥3 and <5 versus ≥5 (low vs moderate risk and moderate vs high risk, respectively8). Our secondary outcomes were the number of complications (pulmonary and cardiac events) and length of stay in the postoperative anesthesia care unit (PACU) and hospital.
METHODS
Patient Recruitment
The study was approved by the Institutional Review Board at Hospital for Special Surgery (IRB #2012-013), where this single-center trial was conducted. Written informed consent was obtained from all patients prospectively enrolled from January 27, 2014, to December 11, 2014. Eligible patients were between 18 and 90 years of age and scheduled for primary unilateral TKA with combined spinal-epidural anesthesia, a regional nerve block, and postoperative epidural patient-controlled analgesia (PCA). Exclusion criteria included contraindication to regional anesthesia, chronic opioid use for over 1 month, prior diagnosis of OSA with planned use of continuous positive airway pressure (CPAP) postoperatively and non-English speaking patients.
Preoperative Data Collection
The STOP-BANG questionnaire was administered and scored preoperatively by a research assistant trained in administering the instrument. The results of the questionnaire were unknown to the treating anesthesiologist. Patient demographics, medical co-morbidities and preoperative serum levels of bicarbonate and hematocrit were also recorded. Following completion of the questionnaire, a disposable, adhesive sensor connected to a Nellcor OxiMax N-600x pulse oximeter (Covidien, Boulder, CO, USA) was placed on a finger of the patient’s non-dominant hand. Baseline oxygen saturation levels were recorded for a minimum of 30 minutes prior to surgery and stored on the oximeter.
Intraoperative Procedures and Postoperative Data Collection
The sedation protocol included 5–10 mg of midazolam, up to 50 mg of ketamine, up to 100 mcg of fentanyl and 15–40 ml/hr of propofol titrated to achieve sedation while maintaining spontaneous, adequate ventilation. Prior to the start of surgery, an ultrasound-guided peripheral (76 saphenous, 22 femoral) nerve block was performed with 0.25% plain bupivacaine (average 20 ml). Patients received combined spinal-epidural anesthesia with 0.5% bupivacaine (12.5 mg). In addition, 20 mg of famotidine, 4 mg of ondansetron, and 4 mg of dexamethasone were administered intraoperatively. This standardized multimodal analgesia regimen was implemented to provide optimal pain relief and enable faster recovery. Postoperatively, patient-controlled epidural analgesia [0.06% bupivacaine plus 10 mcg/ml hydromorphone] began at 4 mL/hr; 4 mL bolus dose; 10 minute lock-out; 20 mL/hr maximum. The basal rate decreased to 2 mL/hr at 07:00 on postoperative day (POD) 1, and the epidural was discontinued at 17:00 on POD 1. Oral opioids were also available as needed.
Patients were encouraged to remain continuously connected to the Nellcor OxiMax N-600x pulse oximeter (Medtronic, Minneapolis Minnesota) during surgery and postoperatively for a total of 48 hours. Sensors were disconnected during mobilization and bathroom visits. All alarms on the oximeter were disabled. Study personnel visited each patient at least twice on POD 0 and 1. Oxygen saturation levels were recorded digitally on the oximeter device and downloaded onto a secure database.
Patients were maintained on 3L nasal cannula during the study period, as per hospital protocol. Once the oximeter was removed, all data was downloaded and stored on a computer. Oxygen desaturation episodes were identified and analyzed by an investigator blinded to STOP-BANG score. For the purposes of this study, an oxygen desaturation episode was defined as oxygen saturation (SpO2) of less than 88% for a minimum of 20 seconds.
Postoperative data collection also included duration of stay in the post-anesthesia care unit (PACU), length of hospital stay, and incidence of pulmonary complications (presence of hypoxia – defined as SpO2 < 85%, pneumonia, or any type of respiratory failure requiring ventilation), cardiac complications (presence of postoperative myocardial infarction or arrthymias), and neurological complications (presence of postoperative delirium, transient ischemic attack or cerebrovascular accident). Approximately 2 years after surgery, patients with STOP-BANG score ≥ 5 and/or patients kept overnight in the PACU due to suspected OSA status (and subsequently followed for 48 hours) were contacted and asked if they had undergone any testing for OSA since their participation in the study.
Statistical Analysis
Study data were collected and managed using Research Electronic Data Capture (REDCap) hosted at Hospital for Special Surgery. REDCap is a secure, web-based application that is designed to support data capture for research studies.9
The primary outcome was the number of oxygen desaturation episodes by STOP-BANG score <3 vs ≥3 and <5 vs ≥5. Therefore, the power analysis performed was based on a kappa statistic which measures the agreement between two tests, using α = 0.05 and 80% power. A high agreement between STOP-BANG scoring and number of oxygen desaturation episodes, kappa = 0.8, was hypothesized, indicating that a high probability of correctly diagnosing patients at high risk of oxygen desaturation postoperatively using the STOP-BANG scoring model was expected. Taking into account a 10% dropout rate, the proposed sample size required was determined to be 110 patients.
Patients with a STOP-BANG score of 3 or greater were classified as having moderate risk of OSA, while patients with a score less than 3 were classified as having low risk.3 Patients with a score of 5 or greater were classified as having high risk of OSA.11 The associations between OSA status (STOP-BANG score ≥3 vs <3, or STOP-BANG score ≥5 vs <5) and patient data (ie, demographics, medical phenotype, preoperative co-morbidities, and postoperative data) were assessed using Wilcoxon rank test and Chi-square/Fisher exact test for continuous and categorical patient data, respectively. Pearson correlations were calcuated for the total number of desaturations with postanesthesia care unit (PACU) length of state (LOS) and hospital LOS. The average length of desaturation events and the longest duration of desaturation events (POD 0) were compared between two serum CO2 groups (≥30 vs <30) using Wilcoxon rank test. Patients who had both a preoperative serum CO2 value > 30 and a STOP-BANG score > 3 were compared with those who did not have both criteria in terms of the average length of desaturation events and the longest duration of desaturation events (POD 0) using Wilcoxon rank test.
All statistical analyses were performed with SAS Version 9.3 (SAS Institute, Cary, North Carolina, USA). For all statistical tests, a value of P ≤ 0.05 was considered statistically significant.
RESULTS
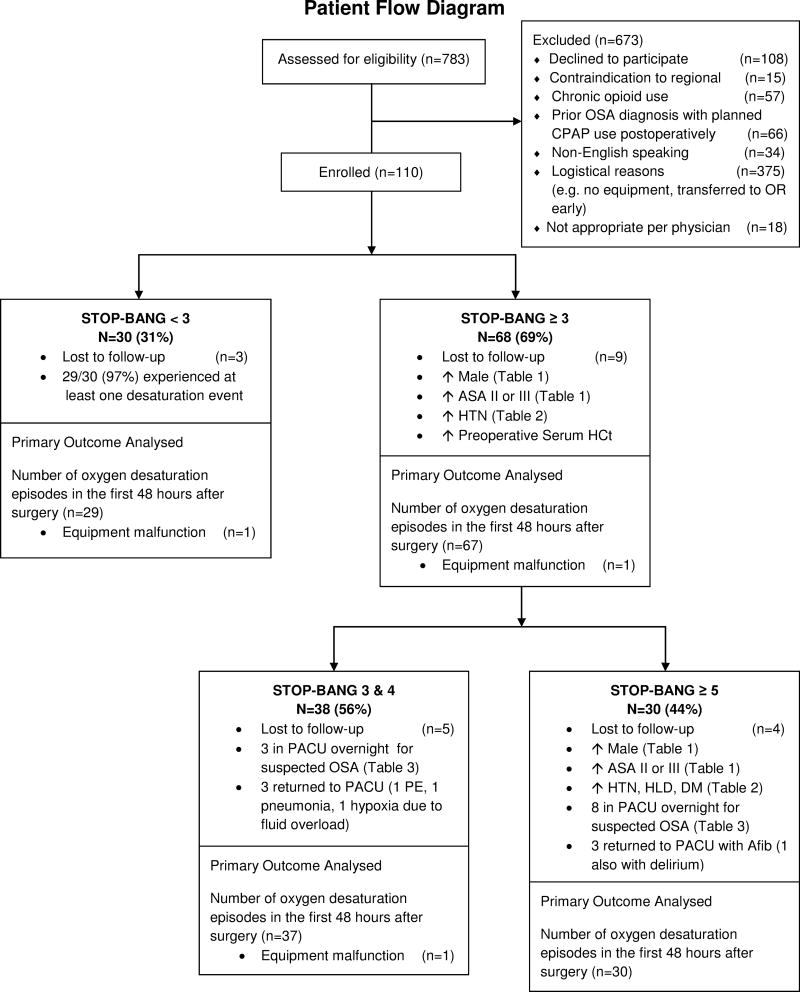
After written informed consent was obtained, a total of 110 patients were enrolled in the study. Twelve patients were excluded prior to analysis: 6 patients withdrew during the 48 hours of monitoring, 3 patients received spinal anesthesia, 2 patients received an IV PCA, and 1 patient did not receive a saphenous or femoral nerve block. A total of 98 patients were included in the final analysis (Fig. 1).
Figure 1.
Patient flow diagram. OSA = obstructive sleep apnea; CPAP = continuous positive airway pressure; ASA = American Society of Anesthesiologists; HTN = hypertension. HCt = hematocrit; PACU = post-anesthesia care unit; HLD = hyperlipidemia; DM = diabetes mellitus; Afib = atrial fibrillation.
Patient Demographics
Groups were categorized using STOP-BANG scores. Of the 98 patients analyzed, 68 patients (69% of the study population), had a score ≥ 3 whereas 30 patients (31%) had a score < 3. Of the 68 patients classified as having a score ≥ 3, 30 patients (44%) had a score ≥ 5, putting them at high risk for moderate-to-severe OSA (Table 1).
Table 1.
Patient Demographics, Medical Phenotype and Preoperative Co-morbidities
| STOP-BANG < 3 (N = 30) |
STOP-BANG ≥ 3 (N = 68) |
P-Value | STOP-BANG < 5 (N = 68) |
STOP-BANG ≥ 5 (N = 30) |
P-Value | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age (years) | 67 ± 7 | 68 ± 9 | 0.63 | 67 ± 8 | 68 ± 8 | 0.74 |
| Gender, n (%) | <0.001 | <0.001 | ||||
| Male | 3 (10%) | 46 (67.6%) | 24 (35.3%) | 25 (83.3%) | ||
| Female | 27 (90%) | 22 (32.4%) | 44 (64.7%) | 5 (16.7%) | ||
| Race, n (%) | 0.069 | 0.16 | ||||
| Asian | 1 (3%) | 2 (29.4%) | 2 (2.9%) | 1 (3.3%) | ||
| Black/African-American | 0 (0%) | 5 (7.4%) | 4 (5.9%) | 1 (3.3%) | ||
| White | 29 (96.7%) | 61 (89.7%) | 62 (91.2%) | 28 (93.4%) | ||
| Pain and Medical Phenotype | ||||||
| Body Mass Index | 26.7 ± 3.9 | 30.9 ± 5.8 | <0.0001 | 28.3 ± 5.4 | 32.6 ± 5.1 | <0.0001 |
| ASA status, n (%) | 0.031 | 0.05 | ||||
| I | 1 (3.3%) | 2 (2.9%) | 3 (4.4%) | 0 (0%) | ||
| II | 28 (93.4%) | 50 (73.5%) | 57 (83.8%) | 21 (70%) | ||
| III | 1 (3.3%) | 16 (23.5%) | 8 (11.8%) | 9 (30%) | ||
| Serum CO2 (mmol/L) | 27.0 ± 2.3 | 27.4 ± 2.6 | 0.45 | 27.4 ± 2.5 | 26.9 ± 2.4 | 0.41 |
| Serum HCt (%) | 38.5 ± 3.2 | 40.0 ± 4.2 | 0.05 | 39.6 ± 4.0 | 39.3 ± 4.1 | 0.64 |
| Preoperative Co-morbidities, n (%) | ||||||
| Hypertension | 6 (20%) | 46 (67.6%) | <0.0001 | 27 (39.7%) | 25 (83.3%) | <0.0001 |
| Hyperlipidemia | 12 (40%) | 38 (55.9%) | 0.15 | 29 (42.6%) | 21 (70%) | 0.012 |
| Myocardial infarction | 0 (0%) | 4 (5.9%) | 0.31 | 1 (1.5%) | 3 (10%) | 0.084 |
| Chronic heart failure | 0 (0%) | 1 (4.5%) | 0.99 | 1 (1.5%) | 0 (0%) | 0.99 |
| Diabetes | 2 (6.7%) | 12 (17.6%) | 0.22 | 5 (7.4%) | 9 (30%) | 0.009 |
| Asthma | 4 (13.3%) | 6 (8.8%) | 0.49 | 7 (10.3%) | 3 (10%) | 0.99 |
| Chronic obstructive pulmonary disease | 0 (0%) | 2 (2.9%) | 0.99 | 2 (2.9%) | 0 (0%) | 0.99 |
| Autoimmune disease | 1 (3.3%) | 1 (1.5%) | 0.52 | 2 (2.9%) | 0 (0%) | 0.99 |
Data presented as mean ± SD for continuous variables and frequency (percentage) for categorical variables.
ASA = American Society of Anesthesiologists; Hct = hematocrit; SD = standard deviation.
As expected, patients with a STOP-BANG score ≥ 3 were significantly more likely to be male, ASA II or III, have a higher BMI and have a history of hypertension than patients with a score < 3 (Table 1). They were also found on average to have a higher serum hematocrit (Hct) value preoperatively than females (mean ± SD; 40.0 ± 4.2 vs. 38.5 ± 3.2, P = 0.05).
Patients with a STOP-BANG score ≥ 5 were significantly more likely to be male, ASA II or III and have a higher BMI than patients with a score < 5 (Table 1). They were also significantly more likely to have a history of hypertension, hyperlipidemia, and diabetes mellitus. No significant difference was found in preoperative serum Hct values between patients with a score ≥ 5 and those with a score < 5.
STOP-BANG Score and Postoperative Desaturation Events
There was no significant difference in the total number of postoperative desaturation events in the first 48 hours after surgery between patients with a STOP-BANG score < 3 and ≥ 3 (mean ± SD; 66.6 ± 62.1 vs 58.6 ± 66.8, P = 0.40) (Table 2). Likewise, no significant difference in total number of postoperative desaturation events was found between patients with a STOP-BANG score < 5 and ≥ 5 (56.9 ± 53.6 vs 70.2 ± 85.7, P = 0.99). Each individual component of the STOP-BANG questionnaire was assessed; there was no significant difference in the total number of desaturation events between patients that were positive or negative for each component, except gender. Patients with a STOP-BANG score of < 3 and ≥ 3 and patients with score of < 5 and ≥ 5 had similar hospital length of stay.
Table 2.
Postoperative data
| STOP- BANG < 3 (N=30) |
STOP- BANG ≥ 3 (N=68) |
P Value |
STOP- BANG < 5 (N=68) |
STOP- BANG ≥ 5 (N=30) |
P Value |
|
|---|---|---|---|---|---|---|
| Total number of desaturation events | (N=29) 66.6 ± 62.1 | (N=67) 58.6 ± 66.8 | 0.40 | (N=66) 56.9 ± 53.6 | 70.2 ± 85.7 | 0.99 |
| Incidence of complications, N (%) | 2 (6.7%) | 4 (5.9%) | 0.88 | 3 (4.4%) | 3 (10.0%) | 0.29 |
| PACU LOS (min) | 591.1 ± 516.4 | 611.8 ± 517.1 | 0.82 | 543.8 ± 420.3 | 745.2 ± 669.0 | 0.51 |
| PACU overnight for suspected OSA, N (%) | 1 (3.3%) | 10 (14.7%) | 0.16 | 3 (4.4%) | 8 (26.7%) | 0.002 |
| Hospital LOS (hr) | 97.4 ± 33.1 | 91.0 ± 28.3 | 0.16 | 92.5 ± 31.2 | 94.0 ± 26.8 | 0.55 |
Data presented as mean ± SD for continuous variables and frequency (percentage) for categorical variables. PACU = postanesthesia care unit; LOS = length of stay; OSA = obstructive sleep apnea; SD = standard deviation.
When analyzing the entire patient population, not taking into account the STOP-BANG score, a significant positive correlation was found between the total number of postoperative desaturation events and hospital length of stay (r = 0.329, P = 0.001) (Table 3). The number of desaturation events on POD 1 was significantly greater than the number of events on POD 0 (mean ± SD; 32.8 ± 42.7 vs 4.7 ± 10.0, P < 0.0001) (Fig. 2). Similarly, the number of desaturation events on POD 2 was also greater than the number of events on POD 0 (22.9 ± 31.7 vs 4.7 ± 10.0, P < 0.0001).
Table 3.
Correlations between total number of desaturation events and LOS
| Variables | Sample Correlation | P Value |
|---|---|---|
| Total # desaturation events with PACU LOS | −0.16128 | 0.12 |
| Total # desaturation events with hospital LOS | 0.32927 | 0.001 |
LOS = length of stay; PACU = postanesthesia care unit.
Figure 2.
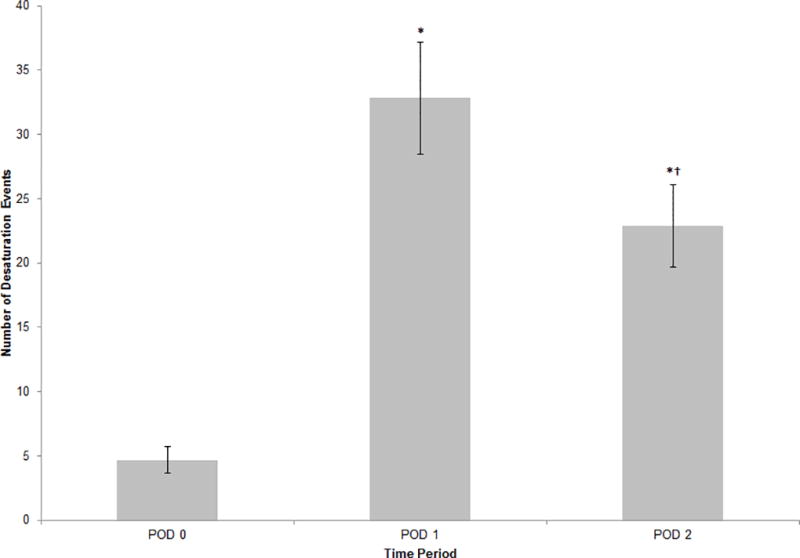
Daily number of desaturation events in postoperative study period. Data plotted as mean ± standard error; error bars extend to one standard error of the mean. *P <0.001 compared with POD 0. †P=0.04 compared with POD 1. POD = postoperative day.
Postoperative oral opioid use was greater on POD 1 when compared to POD 0 (60.0 ± 37.0 vs 13.1 ± 16.9 oral morphine equivalents, P < 0.0001) (Fig. 3). No difference was noted in postoperative opioid administration via epidural PCA between POD 0 and POD 1.
Figure 3.
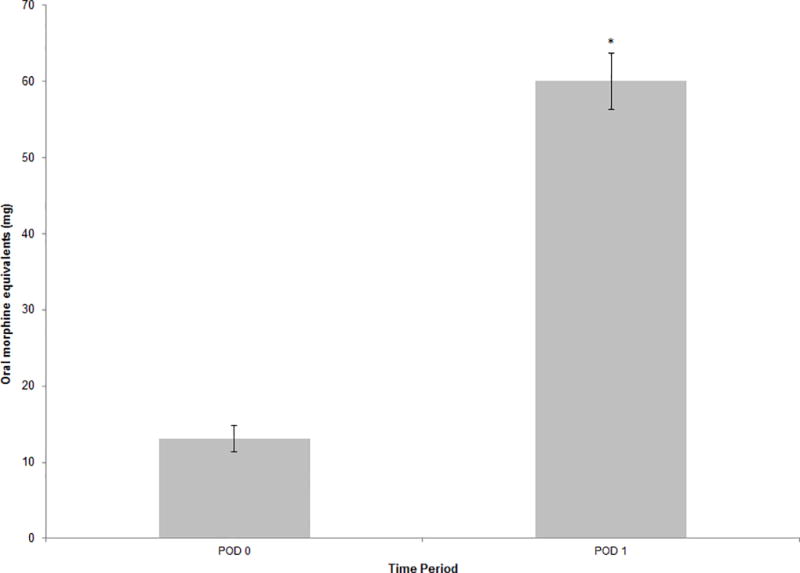
Oral opioid medication usage, as measured in oral morphine equivalents, on day of surgery and POD 1. Data plotted as mean ± standard error; error bars extend to one standard error of the mean. *P <0.001 compared with POD 0. POD = postoperative day.
Postoperative Monitoring and Complications
A total of 11 patients were observed in a monitored setting overnight due to suspected OSA, based on intraoperative respiratory behavior observed by the treating anesthesiologist (Table 2). Of these 11 patients, 10 had a STOP-BANG score ≥ 3 and 8 had a score ≥ 5. Overall, there was a higher likelihood of patients with a STOP-BANG score ≥ 5 staying overnight in the PACU from observations made in the operating room by the anesthesiologist (P = 0.002) (Table 2).
The postoperative course of three patients with a STOP-BANG score ≥ 5 was complicated by atrial fibrillation, with one concurrently developing postoperative delirium. Three patients with a score < 5 developed complications including pulmonary embolism, pneumonia, and hypoxia secondary to fluid overload. Only one patient with a score < 3 was kept in a monitored setting for suspected OSA due to significant airway obstruction episodes noted in the operating room.
Additional Subgroup Analyses
Secondary analyses were performed using preoperative serum CO2 values as grouping characteristics rather than STOP-BANG score. Patients with preoperative serum CO2 value ≥ 30 had significantly longer desaturation events on POD 0 compared to those with serum CO2 < 30, (mean ± SD; 233.7 ± 410.1 vs 82.0 ± 126.2, P < 0.044) (Table 4). No difference in duration of desaturation events was noted on POD 1 or POD 2 between groups. Patients who had both a preoperative serum CO2 value ≥ 30 and a STOP-BANG score ≥ 3 were also found to have significantly longer desaturation events on POD 0 compared to patients who did not have both criteria, 265.3 ± 439.8 seconds per event vs 81.3 ± 125.1, P < 0.027 (Table 4).
Table 4.
Postoperative desaturation data by additional subgroup
| Serum CO2 < 30 (N = 78) |
Serum CO2 ≥ 30 (N = 20) |
P Value | No combination of Serum CO2 ≥ 30 and STOP-BANG ≥ 3 (N = 81) |
Combination of Serum CO2 ≥ 30 and STOP-BANG ≥ 3 (N = 15) |
P Value |
|
|---|---|---|---|---|---|---|
| Average Length of Desaturation Event (s) | 77.7 ± 77.0 | 104.1 ± 98.6 | 0.23 | 83.5 ± 83.4 | 76.9 ± 72.1 | 0.89 |
| Longest Duration of Desaturation Event – POD 0 (s) | 82.0 ± 126.2 | 233.7 ± 410.1 | 0.044 | 81.3 ± 125.1 | 265.3 ± 439.8 | 0.027 |
Data presented as mean ± SD. POD = postoperative day.
Polysomnography Testing and OSA Diagnosis
During the follow-up to the study, we were able to contact 30 patients. Two patients (6.5% of those contacted) underwent polysomnography testing and were both diagnosed with moderate OSA (15 ≤ apnea-hypopnea index (AHI) < 30). One of these patients (STOP-BANG score 3) was kept overnight in the PACU after surgery due to suspected OSA and the other patient’s STOP-BANG score was 5. Another patient subsequently remembered that he was diagnosed with severe OSA 10 years prior to his surgery but decided not to have any therapy, including CPAP. Twenty-seven out of the 33 patients contacted (81.8%) reported that they did not undergo polysomnography testing, and out of that cohort, 2 patients (7.4%) suspected they had mild to moderate OSA because of snoring.
DISCUSSION
A STOP-BANG score ≥ 3, used as a predictor of moderate risk of OSA, did not significantly correlate with the number of oxygen desaturation events experienced during the first 48 hours after surgery. Desaturation events were common and increased in frequency on POD 1.
In this study of patients undergoing unilateral total knee arthroplasty (TKA) under regional anesthesia, the possibility of obstructive sleep apnea (OSA), as defined by a STOP-BANG score of ≥ 3 was very high (69%). In addition, 44% of these patients had a score ≥ 5, putting them at high risk of OSA. This is comparable with previous studies showing a higher prevalence of OSA in the orthopedic population compared to the general surgery patient population. Particularly, TKA patients may be more prone to OSA, since they have a higher incidence of obesity.10 Of note, one third of patients with a score ≥ 5 were suspected to have OSA from obstructive events observed in the operating room by the anesthesiologist. Only 3 patients with STOP-BANG score < 5 were similarly identified, but one of these patients (score 3) was later diagnosed with moderate OSA through polysomnography testing. Intraoperative observation can serve as an additional tool in identifying potential OSA patients while under sedation.
We used the STOP-BANG scoring model to screen for OSA, since this tool has been well validated with a sensitivity of 93% and 100% for moderate and severe OSA, respectively.6 According to Chung et al,7 a STOP-BANG score of 5 is associated with an odds ratio (OR) of 4.8 for moderate/severe OSA and an OR of 10.4 for severe OSA. Patients with a STOP-BANG score ≥ 5 had a high probability of having moderate to severe OSA.8
Patients with a STOP-BANG score < 3, or low-risk category of OSA had frequent episodes of desaturation. Ahmad et al. reported frequent episodes of desaturation in morbidly obese patients (BMI 35 – 75 kg/m2) who did or did not have sleep apnea while on supplemental oxygen.11 In a study similarly measuring the association between severity of OSA and postoperative hypoxemia, Khanna et al also found that greater STOP-BANG score was not associated with hypoxemia.12 They concluded that factors other STOP-BANG scores may positively associate with postoperative desaturation. In this study, such associations can be found between exploratory secondary outcomes and number and duration of desaturation events. The number of desaturation events increased significantly on POD 1 in all groups of patients. Oral opioid consumption also increased significantly on POD 1, raising the possibility that they may be related, although Rosenberg et al showed that rebound of REM sleep occurring beyond postoperative day one also contributes to the development of sleep-disordered breathing and nocturnal episodic hypoxemia.13
Of note, the epidural PCA was discontinued on POD 1; this may have led to a greater consumption of oral opioids. In addition, the analgesic effect of the saphenous or femoral nerve block was likely no longer in effect by POD 1. The addition of a peripheral nerve block to an anesthetic regimen has been reported to decrease the incidence of opioid-related respiratory depression while in effect.14, 15 Attempts at using non-opioid analgesics may decrease the number of desaturation events on POD 1.
Patients with a STOP-BANG score ≥ 3 had a higher serum hematocrit (Hct) value than patients with scores < 3, but there was no correlation with the number or duration of desaturation events. Serum CO2 values ≥ 30 did, however, correlate with the duration of desaturation events. Preoperative CO2 ≥ 30 should warrant further investigation as a screening tool for predicting desaturation events in the postoperative period.
According to the most recent guidelines, the use of preoperative continuous positive airway pressure (CPAP) should be considered in patients with sleep apnea.4 A recent meta-analysis by Nagappa et al found no significant difference in postoperative adverse events between the use of CPAP versus none.16 However, patients on CPAP had a lower postoperative apnea-hypopnea index (AHI) and a trend toward a shorter length of stay. Athough there was no difference in PACU or hospital length of stay between STOP-BANG groups in our study, patients with a greater number of desaturation events had a longer length of stay in the hospital. Given this correlation between the number of desaturation events and length of stay, the use of CPAP should be evaluated in these patients suspected of having OSA.
All 3 patients who developed atrial fibrillation fell in the high-risk category for OSA (score ≥ 5). In cardiac surgery, OSA has been associated with a higher incidence of atrial fibrillation.17 Inflammation, known to be significantly elevated in OSA,18, 19 plays a major role in development of atrial fibrillation.20 Further studies to evaluate the relationship between OSA and atrial fibrillation in this population may be warranted.
This study is limited by several factors. Polysomnography testing could not be obtained in all patients to confirm the diagnosis of sleep apnea. Our study contained a relatively small sample size and therefore may not be representative of a general patient population. Focusing on desaturation events may not be adequate to predict perioperative complications, as patients may desaturate at home after hospital discharge. Apnea events may have been a better measurement, especially in light of the standard use of oxygen therapy. However, the use of supplemental oxygen must be considered in the context of common practice, hospital protocol and clinical care to reduce postoperative complications. Finally, the number of complications may have been reduced by the use of regional anesthesia, which has been shown to have a positive impact on perioperative outcomes.
In conclusion, this study represents a unique population of patients undergoing TKA only under spinal-epidural anesthesia with peripheral nerve block, followed by postoperative epidural analgesia. There was no association between STOP-BANG score and number of desaturation events, but some secondary results warrant further exploration. While epidural analgesia is maintained, the number of desaturation events is low, however it significantly increases on POD 1 and POD 2 as oral opioid use also increases. A high preoperative value of serum CO2 should alert the physician about the possibility of prolonged episodes of desaturation postoperatively, and should trigger further evaluation for sleep apnea. Given the correlation between length of hospital stay and the number of desaturation events, investigations into a causal relationship should be considered.
Acknowledgments
Funding:
This study was funded by Hospital for Special Surgery Anesthesiology Department Research and Education Fund, New York, NY. The REDCap electronic data capture tools are funded by the CTSC grant (grant number UL1 TR000457-06) from the National Center for Advancing Translational Sciences, National Institutes of Health, Bethesda, MD.
Dr. Memtsoudis has received compensation for consultation from HappyMed (Vienna, Austria).
Footnotes
Prior Presentation:
This work was presented in part at the 41st Annual Regional Anesthesiology and Acute Pain Medicine Meeting (New Orleans, LA), March 31–April 2, 2016.
Conflict of Interest/Disclosure:
The other authors declare no potential conflict of interest.
References
- 1.Members of the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Practice guidelines for perioperative management of patients with obstructive sleep apnea. Anesthesiology. 2006;104:1081–1093. doi: 10.1097/00000542-200605000-00026. [DOI] [PubMed] [Google Scholar]
- 2.Kaw R, Chung F, Pasupuleti V, Mehta J, Gay PC, Hernandez AV. Meta-analysis of the association between obstructive sleep apnoea and postoperative outcome. Br J Anaesth. 2012;109:897–906. doi: 10.1093/bja/aes308. [DOI] [PubMed] [Google Scholar]
- 3.Gupta RM, Parvizi J, Hanssen AD, Gay PC. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: A case-control study. Mayo Clin Proc. 2001;76:897–905. doi: 10.4065/76.9.897. [DOI] [PubMed] [Google Scholar]
- 4.Members of the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: An updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2014;120:268–286. doi: 10.1097/ALN.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 5.Memtsoudis SG, Stundner O, Rasul R, et al. Sleep apnea and total joint arthroplasty under various types of anesthesia: A population-based study of perioperative outcomes. Reg Anesth Pain Med. 2013;38:274–281. doi: 10.1097/AAP.0b013e31828d0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: A tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812–21. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 7.Chung F, Subramanyam R, Liao P, Sasaki E, Shapiro C, Sun Y. High STOP-BANG score indicates a high probability of obstructive sleep apnoea. Br J Anaesth. 2012;108:768–775. doi: 10.1093/bja/aes022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagappa M, Liao P, Wong J, et al. Validation of the STOP-BANG Questionnaire as a screening tool for obstructive sleep apnea among different populations: A systematic review and meta-analysis. PLoS One. 2015;10:e0143697. doi: 10.1371/journal.pone.0143697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Memtsoudis S, Liu SS, Ma Y, et al. Perioperative pulmonary outcomes in patients with sleep apnea after noncardiac surgery. Anesth Analg. 2011;112:113–121. doi: 10.1213/ANE.0b013e3182009abf. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad S, Nagle A, McCarthy RJ, Fitzgerald PC, Sullivan JT, Prystowsky J. Postoperative hypoxemia in morbidly obese patients with and without obstructive sleep apnea undergoing laparoscopic bariatric surgery. Anesth Analg. 2008;107:138–143. doi: 10.1213/ane.0b013e318174df8b. [DOI] [PubMed] [Google Scholar]
- 12.Khanna AK, Sessler DI, Sun Z, Naylor AJ, You J, Hesler BD, Kurz A, Devereaux PJ, Saager L. Using the STOP-BANG questionnaire to predict hypoxaemia in patients recovring from noncardiac surgery: A prospective cohort analysis. Br J Anaesth. 2016;116:632–640. doi: 10.1093/bja/aew029. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg J, Wildschiødtz G, Pedersen MH, von Jessen F, Kehlet H. Late postoperative nocturnal episodic hypoxaemia and associated sleep pattern. Br J Anaesth. 1994;72:145–150. doi: 10.1093/bja/72.2.145. [DOI] [PubMed] [Google Scholar]
- 14.Hogan MV, Grant RE, Lee L., Jr Analgesia for total hip and knee arthroplasty: A review of lumbar plexus, femoral, and sciatic nerve blocks. Am J Orthop. 2009;38:E129–133. [PubMed] [Google Scholar]
- 15.Yadeau JT, Liu SS, Rade MC, Marcello D, Liguori GA. Performance characteristics and validation of the Opioid-Related Symptom Distress Scale for evaluation of analgesic side effects after orthopedic surgery. Anesth Analg. 2011;113:369–377. doi: 10.1213/ANE.0b013e31821ae3f7. [DOI] [PubMed] [Google Scholar]
- 16.Nagappa M, Mokhlesi B, Wong J, Wong DT, Kaw R, Chung F. The effects of continuous positive airway pressure on postoperative outcomes in obstructive sleep apnea patients undergoing surgery: A systematic review and meta-analysis. Anesth Analg. 2015;120:1013–1023. doi: 10.1213/ANE.0000000000000634. [DOI] [PubMed] [Google Scholar]
- 17.Wong JK, Maxwell BG, Kushida CA, et al. Obstructive sleep apnea is an independent predictor of postoperative atrial fibrillation in cardiac surgery. J Cardiothorac Vasc Anesth. 2015;29:1140–1147. doi: 10.1053/j.jvca.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 18.McNicholas WT. Obstructive sleep apnea and inflammation. Prog Cardiovasc Dis. 2009;51:392–399. doi: 10.1016/j.pcad.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–2667. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 20.Boos CJ, Anderson RA, Lip GY. Is atrial fibrillation an inflammatory disorder? Eur Heart J. 2006;27:136–149. doi: 10.1093/eurheartj/ehi645. [DOI] [PubMed] [Google Scholar]