Abstract
Current clinically-tested nicotine vaccines have yet shown enhanced smoking cessation efficacy due to their low immunogenicity. Achieving a sufficiently high immunogenicity is a necessity for establishing a clinically-viable nicotine vaccine. This study aims to facilitate the immunogenicity of a hybrid nanoparticle-based nicotine vaccine by rationally incorporating toll-like receptor (TLR)-based adjuvants, including monophosphoryl lipid A (MPLA), Resiquimod (R848), CpG oligodeoxynucleotide 1826 (CpG ODN 1826), and their combinations. The nanoparticle-delivered model adjuvant was found to be taken up more efficiently by dendritic cells than the free counterpart. Nanovaccine particles were transported to endosomal compartments upon cellular internalization. The incorporation of single or dual TLR adjuvants not only considerably increased total anti-nicotine IgG titers but also significantly affected IgG subtype distribution in mice. Particularly, the nanovaccines carrying MPLA+R848 or MPLA+ODN 1826 generated a much higher anti-nicotine antibody titer than those carrying none or one adjuvant. Meanwhile, the anti-nicotine antibody elicited by the nanovaccine adjuvanted with MPLA+R848 had a significantly higher affinity than that elicited by the nanovaccine carrying MPLA+ODN 1826. Moreover, the incorporation of all the selected TLR adjuvants (except MPLA) reduced the brain nicotine levels in mice after nicotine challenge. Particularly, the nanovaccine with MPLA+R848 exhibited the best ability to reduce the level of nicotine entering the brain. Collectively, rational incorporation of TLR adjuvants could enhance the immunological efficacy of the hybrid nanoparticle-based nicotine vaccine, making it a promising next-generation immunotherapeutic candidate for treating nicotine addiction.
Keywords: Nicotine addiction, nicotine vaccine, hybrid nanoparticle, toll-like receptors, molecular adjuvant, anti-nicotine antibody
1. Introduction
Tobacco smoking has constantly been one of the largest public health concerns worldwide for decades. It is the leading cause of preventable diseases and premature deaths, and results in huge socioeconomic burdens.[1, 2] In recent decades, nicotine vaccines have been studied as a promising immunotherapeutic strategy to combating nicotine addiction.[3, 4] In principle, nicotine vaccines can induce the production of nicotine-specific antibodies that can bind with nicotine in serum and thus keep nicotine from entering the brain.[5] To date, numerous conjugate nicotine vaccines (CNVs) have been reported to achieve high immunological efficacy in preclinical trials, and some of them have entered various stages of clinical trials.[6–9] However, none of these clinically tested CNVs have shown improved overall smoking cessation rate compared to the placebo, mainly due to the insufficient titers of antibodies and their low binding capacity.[5, 10]
Immunologically, the immune system prefers to recognize particulate antigens and is relatively invisible to soluble protein antigens.[11, 12] Therefore, the insufficient immunogenicity of conventional CNVs can be partially attributed to their intrinsic shortfalls, such as poor recognition and internalization by immune cells and low bioavailability. In addition, even with the help of alum to form particulate particles, CNVs cannot be easily tuned to have optimal physicochemical properties (such as size, shape, and charge) for cellular uptake.[13, 14] Moreover, molecular adjuvants cannot be easily incorporated into CNVs, and they are typically co-administered with CNVs via physical mixing. In this way, molecular adjuvants are not specifically available to immune cells and their release cannot be controlled in immune cells, thus leading to low adjuvant efficacy and systemic toxicity.[13, 15, 16]
In our previous study, by taking advantage of the superiorities of nanoparticles (NPs), such as particulate nature, tunable physicochemical properties, and controlled payload release, we developed a lipid-polymeric hybrid nanoparticle (NP)-based nicotine vaccine (NanoNicVac) as a next-generation immunotherapeutic strategy against nicotine addiction.[17, 18] We demonstrated that NanoNicVac had a significantly higher immunogenicity than the conjugate vaccine, and its immunological efficacy could be enhanced by modulating NP size,[17] hapten localization,[19] hapten density,[20] and stimulating proteins (unpublished data).
From the immunological point of view, adjuvants are an important component of a vaccine formulation, and they are necessary for the induction of a strong immune response, especially for poorly-immunogenic antigens.[15, 21] Currently, alum is the most-widely used adjuvant for vaccine development. However, alum has shown to be a relatively weak adjuvant and sometimes may cause lesions at injection sites.[22, 23] Especially for NP-based vaccines, alum may absorb vaccine NPs to form very large particles, resulting in sizes that are not optimal for cellular uptake.[14] Also, due to the high viscosity, alum may disrupt the structure of vaccine NPs. In addition, alum can limit the release of vaccine NPs from injection sites and impair their availability to antigen presenting cells (APCs).[16, 24] Our previous studies suggested that the use of alum would not significantly improve the immunogenicity of NanoNicVac.[20] As alternatives, molecular adjuvants, such as toll-like receptor (TLR) agonists, have been studied as a class of promising potent adjuvants.[25–27] TLR agonists are capable of enhancing the secretion of cytokines, promoting the activation of antigen presenting cells, and enhancing the production of antibodies.[28–30]
Based on the hypothesis that incorporation of appropriate molecular adjuvants may enhance the immunological efficacy of NanoNicVac, this study aims to further rationalize the design of NanoNicVac by developing a NanoNicVac particle capable of co-delivering nicotine antigens and TLR agonists. As shown in Figure 1A, the nicotine-protein conjugates were conjugated to the surface of lipid-polymeric hybrid NPs for presentation. The lipid-shell and PLGA-core served as hosts for cell-surface-TLR and endosomal-TLR agonists, respectively. Monophosphoryl lipid A (MPLA),[31, 32] Resiquimod (R848),[13, 33] and CpG oligodeoxynucleotide 1826 (CpG ODN 1826),[34, 35] all of which have been reported to significantly enhance immune responses, were selected as adjuvant candidates. In this study, NanoNicVac particles carrying different TLR adjuvants or combinations were fabricated, and their physicochemical properties were characterized. The cellular uptake of NanoNicVac particles was studied in dendritic cells. The immunogenicity and ability to reduce brain nicotine concentration of NanoNicVac were investigated in mice.
Figure 1.

Characterization of NPs. (A) Schematic illustration and TEM images of liposomes, PLGA NPs, lipid-PLGA hybrid NPs, and adjuvant-loaded NanoNicVac particles. Scale bars represent 100 nm. (B) Average diameters of NanoNicVac particles. (C) Zeta-potential of NanoNicVac particles.
2. Materials and methods
2.1 Materials
1,2-Dioleoyl-3-trimethylammonium-propane (DOTAP), cholesterol (CHOL), 1,2-diphytanoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2-1,3-benzoxadiazol-4-yl) (ammonium salt) (NBD-PE), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)-2000] (ammonium salt) (DSPE-PEG2000-maleimide), and MPLA were purchased from Avanti Polar Lipids (Alabaster, AL, USA). Lactel® 50:50 poly(lactic-co-glycolic acid) (PLGA) was purchased from Durect Corporation (Cupertino, CA, USA). O-Succinyl-3’-hydroxymethyl-(±)-nicotine (Nic) was purchased from Toronto Research Chemicals (North York, ON, Canada). Keyhole limpet hemocyanin (KLH), Alexa Fluor® 647 NHS ester (AF647), coumarin-6 (CM-6), 1-Ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC), and N-hydroxysulfosuccinimide (Sulfo-NHS) were purchased from Thermo Fisher Scientific (Rockford, IL, USA). CpG ODN 1826 and R848 were purchased from InvivoGen (San Diego, CA, USA). All other chemicals were of analytical grade.
2.2 Fabrication of adjuvant-loaded PLGA NPs
Adjuvant-loaded PLGA NPs were fabricated using a water/oil/water double-emulsion-solvent-evaporation method.[17] In brief, 40 mg of PLGA was dissolved in 2 mL of dichloromethane (DCM) to form an organic phase. For CpG ODN 1826- or R848-encapsulated PLGA NP preparation, 1.20 mg of CpG ODN 1826 in 200 μL of DI water or 1.50 mg of R848 in 200 μL of DI water-DMSO (9:1) was added into the organic phase. For CpG ODN 1826 and R848 co-encapsulated PLGA NP preparation, 1.20 mg of CpG ODN 1826 in 100 μL of DI water and 1.50 mg of R848 in 100 μL of DI water-DMSO (9:1) were added into the organic phase. The water-in-oil solution was mixed and emulsified by sonication for 10 min using a Branson M2800H Ultrasonic Bath Sonicator (Danbury, CT, USA). The resultant primary emulsion was added dropwise to 12 mL of 0.5% w/v poly(vinyl alcohol) solution under continuous stirring. The suspension was emulsified again by sonication using a sonic dismembrator (Model 500; Fisher Scientific, Pittsburg, PA, USA) at an amplitude of 70% for 40 s. The resultant secondary emulsion was stirred overnight to allow complete DCM evaporation. Blank PLGA NPs were prepared using a similar method, except that 200 μL of DI water was used as the first aqueous phase. Blank and adjuvant-loaded PLGA NPs were collected by centrifugation at 10,000 g, 4 °C for 30 min (Beckman Coulter Avanti J-251, Brea, CA, USA), washed three times, and stored at 4 °C for later use. To quantify the loading efficiency of R848 and ODN 1826, 20 mg of NPs were disrupted by incubating with 0.2 N NaOH for 14 h. After particles were completely dissolved, the solution was neutralized using 1 N HCl. The concentration of ODN 1826 was measured using a Quant-iT™ OliGreen™ ssDNA Assay kit (Thermo Fisher Scientific, Rockford, IL). The concentration of R848 was quantified by reverse-phase HPLC using a Luna C18 (2) reverse phase column. The loading efficiencies of adjuvants are shown in Table 1.
Table 1.
The loading efficiencies of adjuvants in NanoNicVac.
| NPs | MPLA loading (μg/mg NP) | R848 loading (μg/mg NP) | ODN 1826 loading (μg/mg NP) |
|---|---|---|---|
| NanoNicVac (No adjuvant) | / | / | / |
| NanoNicVac (MPLA) | 17.85# | / | / |
| NanoNicVac (R848) | / | 38.72 ± 4.47 | / |
| NanoNicVac (ODN 1826) | / | / | 34.01 ± 2.10 |
| NanoNicVac (MPLA+R848) | 17.85# | 37.35 ± 4.33 | / |
| NanoNicVac (MPLA+ODN 1826) | 17.85# | / | 34.52 ± 0.75 |
| NanoNicVac (R848+ODN 1826) | / | 32.82 ± 3.42 | 33.72 ± 1.21 |
It is assumed that 100% of MPLA in the lipid mixture was incorporated into the lipid-layer of NanoNicVac.
2.3 Preparation of lipid-PLGA hybrid NPs
Blank and MPLA-carrying liposomes were prepared using a lipid-film-hydration-sonication method as reported previously.[17] The lipid mixtures used for preparing blank and MPLA-carrying liposomes were composed of DOTAP, DSPE-PEG2000-maleimide, CHOL, and MPLA at molar ratios of 90:5:5:0 and 80:5:5:10, respectively. Lipid-PLGA hybrid NPs were fabricated using a sonication method as reported previously.[17] Particularly, 2.5 mg of liposomes were mixed with 25 mg of PLGA NPs for hybrid NP fabrication. Lipid-PLGA hybrid NPs were collected by centrifugation at 10,000 g, 4 °C for 30 min, washed three times, and stored at 4 °C for later use.
2.4 Assembly of NanoNicVac particles
Nic-KLH conjugates were synthesized using an EDC/NHS mediated reaction as reported previously.[20] NanoNicVac particles were assembled by conjugating an appropriate amount of Nic-KLH conjugates to the surface of lipid-PLGA hybrid NPs according to a previously reported method.[20]. NanoNicVac particles were collected by centrifugation at 10,000 g, 4 °C for 30 min. Unconjugated Nic-KLH in the supernatant was quantified by the bicinchoninic acid assay. NanoNicVac particles were stored at 4 °C for later use.
2.5 Characterization of NPs
The morphology of NPs was characterized by transmission electron microscopy (TEM) on a JEM 1400 transmission electron microscope (JEOL, Tokyo, Japan). Size distribution and zeta potential of NPs were measured on a Nano ZS Zetasizer (Malvern Instruments, Worcestershire, United Kingdom) at 25 °C.
2.6 Testing the uptake of NanoNicVac particles in dendritic cells (DCs)
The uptake of NanoNicVac particles carrying different adjuvants by DCs was studied by flow cytometry. NBD-labelled NPs were prepared by adding 2.5% of NBD-PE into a lipid mixture. JAWSII (ATCC® CRL-11904™) immature DCs (ATCC, Manassas, VA, USA) (2 × 106/well) were seeded into 35-mm petri dish and cultured overnight. Cells were treated with 20 μg of NBD-labelled NanoNicVac particles for 1, 2, or 4 h. Cells were washed 3 times using phosphate buffered saline (PBS) and detached from the petri dish using 0.25% trypsin/EDTA solution. Cells were collected by centrifugation at 200 g for 10 min and re-suspended in PBS. Samples were immediately analyzed on a flow cytometer (FACSAria I, BD Biosciences, Franklin Lakes, NJ, USA).
The intracellular distribution of NanoNicVac particles was analyzed by confocal laser scanning microscopy (CLSM). AF647- and CM-6-labeled NPs were prepared according to the method described above, except that AF647-KLH was conjugated to hybrid NPs and CM-6 was encapsulated in the PLGA core for labeling. DCs (2 × 105/chamber) were seeded into 2-well chamber slides and cultured overnight. Cells were treated with 20 μg of AF647- and CM-6-labeled nanovaccine particles for 1, 2, or 4 h. Cells were then washed using PBS and fixed with freshly prepared 4% (w/v) paraformaldehyde for 10 min. The membrane of cells was permeabilized by adding 0.5 mL of 0.1% (v/v) Triton™ X-100 for 10 min. Cell nuclei were stained by 4′,6-diamidino-2-phenylindole (DAPI). The intracellular distribution of NPs was visualized on a Zeiss LSM 510 laser scanning microscope (Carl Zeiss, Germany).
2.7 Testing the immunogenicity of NanoNicVac in mice
All animal studies were carried out following the National Institutes of Health guidelines for animal care and use. Animal protocols were approved by the Institutional Animal Care and Use Committee at Virginia Tech. Female Balb/c mice (6-7 weeks of age, 16-20 g, 5-6 per group) were immunized subcutaneously with a total volume of 200 μL of nicotine vaccines equivalent to 25 μg of KLH on days 0, 14, and 28. For the control group, mice were injected with 200 μL of sterilized PBS. Blood was collected from the retro-orbital plexus under isoflurane anesthesia on days 12, 26, and 40.
Anti-nicotine antibody titers in the serum were measured by an enzyme-linked immunosorbent assay (ELISA) as reported previously.[17] Antibody titer was defined as the dilution factor at which the absorbance at 450 nm dropped to half maximal. The relative affinity of anti-nicotine antibodies elicited by NanoNicVac carrying different adjuvants was measured using a competition ELISA method as reported previously.[19] The nicotine concentration at which 50% inhibition was achieved (IC50) was extrapolated and used as an indicator of the affinity of anti-nicotine antibodies.
2.8 Testing the distribution of nicotine in the serum and brain of mice immunized with NanoNicVac
The capability of NanoNicVac to decrease brain nicotine concentrations after nicotine challenge, was assayed using a method reported previously.[17] In brief, female Balb/c mice (6-7 weeks of age, 16-20 g, 5-6 per group) were immunized according to the same protocol as described in the above context. Mice were administered 0.06 mg/kg nicotine subcutaneously two weeks after the second booster immunization (on day 42). Brain and serum samples were collected 3 min post nicotine challenge. Nicotine levels in the brain and serum were measured by GC/MS as reported previously.[36]
2.9 Evaluating the safety of NanoNicVac by histopathological analysis
The behavioral and physical conditions of mice during the entire study were monitored. The lesions of mouse organs caused by the immunization with NanoNicVac were determined by histopathological review. In brief, on day 42, mice were euthanized, and their organs, including liver, kidney, heart, spleen, and lung, were extracted and immerged in 10% formalin. Tissue blocks were stained with hematoxylin and eosin (H&E) and imaged on a Nikon Eclipse E600 light microscope.
2.10 Statistical analyzes
Data are expressed as means ± standard error unless specified. Comparisons among multiple groups were conducted using one-way ANOVA followed by Tukey’s HSD test. Differences were considered significant when p-values were less than 0.05.
3. Results
3.1 Characterization of adjuvant-loaded NanoNicVac particles
The structure of NPs involved in the fabrication of NanoNicVac was characterized morphologically by TEM (Figure 1A). A “core-shell” structure was observed on lipid-PLGA hybrid NPs, suggesting the successful hybridization of PLGA NPs and liposomes. The various adjuvant-loaded NanoNicVac particles shared similar morphological characteristics with non-adjuvant-loaded NPs. Interestingly, a distinct black “cloud” was found on the surface of NanoNicVac particles, which was most likely caused by the efficient conjugation of Nic-KLH conjugates. Noticeably, upon Nic-KLH conjugation, NanoNicVac particles seemed to be no longer spherical compared to the lipid-PLGA NPs. This phenomenon may be attributed to the fact that KLH can form a large three-dimensional cylinder structure approximately 35 nm in diameter by 40 nm in length.[37] The conjugation of the large cylinder KLH protein to hybrid NP surface could form an overall non-spherical morphology. Meanwhile, the uneven conjugation of Nic-KLH during the preparation process may also cause a non-spherical morphology.
The size of NPs was characterized by dynamic light scattering (Figure 1B). The average diameter of PLGA NPs (without adjuvant), LP hybrid NPs (without adjuvant), and NanoNicVac particles without adjuvant was 91.3, 107.2, and 140.9 nm, respectively. The coating of lipid-layer and conjugation of Nic-KLH caused an increase in particle size. NanoNicVac particles loaded with MPLA, R848, ODN 1826, MPLA+R848, MPLA+ODN 1826, and R848+ODN 1826 have average diameters of 126.3, 194.7, 188.4, 176.3, 177.4, and 204.7 nm, respectively. Interestingly, the incorporation of MPLA into the lipid-layer did not increase the particle size. However, the inclusion of R848 and/or ODN 1826 into the PLGA-core led to considerable particle size increase. The surface charge of NPs was measured by electrophoretic light scattering (Figure 1C). PLGA NPs (without adjuvant) had an average zeta-potential of −17.5 mV. The average zeta-potential of LP hybrid NPs (without adjuvant) was 10.2 mV. The zeta-potential shifting from negative to positive suggested the successful hybridization between PLGA NPs and liposome, because the liposome is positively charged (12.0 mV). The zeta-potential of NanoNicVac particles loaded with no adjuvant, MPLA, R848, ODN 1826, MPLA+R848, MPLA+ODN 1826, and R848+ODN 1826 was −8.43, −8.77, −7.07, −13.00, −7.74, −13.20, and −10.32 mV, respectively, indicating that all NanoNicVac particles were negatively charged, likely due to the conjugation of negatively charged Nic-KLH conjugates.
3.2 Cellular uptake of adjuvant-loaded NanoNicVac particles
The impact of NanoNicVac particles on the cellular uptake efficiency of nicotine-protein antigens and adjuvants was studied using flow cytometry (Figure 2A–2C). AF647 was used as a model of hapten to be conjugated to KLH for fluorescent labelling. CM-6, a model of TLR adjuvant, was loaded into the PLGA core. DCs were treated with free AF647-KLH+CM-6 (“In free form”) or NanoNicVac particles (“In nanoparticles”) carrying the same amounts of AF647-KLH and CM-6 for 1 or 4 h. Interestingly, at both 1 h and 4 h, the mean fluorescence intensity (M.F.I.) of CM-6 and AF647 in the group of “In free form” was significantly lower than that in the group of “In nanoparticles” (Figure 2B and 2C), revealing that the use of hybrid NPs as delivery vehicles would significantly improve the internalization of both nicotine-protein antigens and molecular adjuvants.
Figure 2.
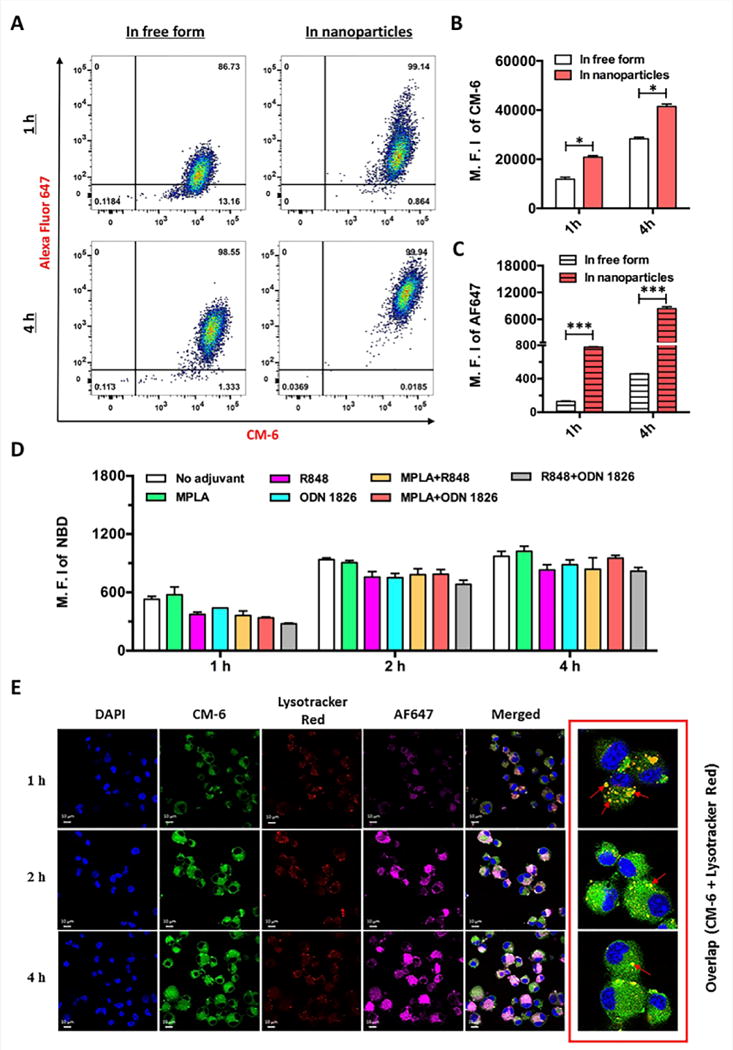
Cellular Uptake of NanoNicVac particles by dendritic cells. (A) Flow cytometry recorded events, (B) M.F.I. of CM-6, and (C) M.F.I. of AF647 of dendritic cells after being treated with free AF467-KLH+CM-6 (In free form) or NanoNicVac particles carrying AF647-KLH and CM-6 (In nanoparticles). AF647 was used to label KLH, and CM-6 was used as a model adjuvant that was loaded into the PLGA core. Significantly different: *, p < 0.05, ***, p < 0.001. (D) M.F.I. of NBD in dendritic cells after being treated with NanoNicVac particles loaded with different adjuvants. NBD was added to the lipid-layer to label NPs. (E) CLSM images of dendritic cells after being treated with NanoNicVac particles for 1, 2, or 4 h. AF647 was used as a model hapten to provide fluorescence and CM-6 was used as a model adjuvant loaded into the PLGA core. Scale bars represent 10 μm.
The cellular uptake of adjuvant-loaded NanoNicVac particles in DCs was investigated using flow cytometry (Figure 2D). DCs were treated with same amounts of NanoNicVac particles loaded with different TLR adjuvants for 1, 2, or 4 h. NanoNicVac particles were fluorescently labeled by adding NBD-PE to the lipid-layer. Consistent with the data shown in Figure 2A, the uptake of NanoNicVac particles was time-dependent. Particularly, NanoNicVac particles were rapidly taken up at 2 h, and the uptake process appeared to be saturated after that. NanoNicVac particles loaded with R848, ODN 1826, MPLA+R848, MPLA+ODN 1826, and R848+ODN 1826 exhibited similar cellular uptake efficiency at all studied time points, which may be attributed to their similar size (Figure 1B). In addition, DCs took up non-adjuvant-loaded and MPLA-loaded NPs more efficiently than the other NPs, especially at 1 h and 2 h. This higher cellular uptake efficiency may be due to the fact that non-adjuvant-loaded and MPLA-loaded NPs had a smaller size than the other adjuvant-loaded NPs (Figure 1B).
The cellular internalization of NanoNicVac particles was visualized using CLSM (Figure 2E). DCs were treated with NanoNicVac particles in which KLH was labeled by AF647 and CM-6 (a model adjuvant) was loaded in the PLGA core. The endosomes and lysosomes of cells were labeled by Lysotracker Red. Both bright AF647 and CM-6 fluorescence were found within DCs, especially at 2 h and 4 h, revealing that both protein antigens and adjuvants could be efficiently co-delivered into DCs. At 1 h, a substantial portion of CM-6 was co-localized with Lysotracker Red, suggesting NanoNicVac particles were transported to endosomes/lysosomes after being internalized by DCs. At 2 h and 4 h, most CM-6 fluorescence was distributed widely in cells and did not overlap with Lysotracker Red. This suggested that the model adjuvant CM-6 was efficiently released from NPs. As TLR 7/8 and 9 are primarily localized in the endosomal compartments of cells, the endosomal localization of NanoNicVac particles and the efficient release of adjuvant from NPs would be beneficial for promoting an effective interaction between TLRs and TLR adjuvants.
3.3 Anti-nicotine antibody response induced by adjuvant-loaded NanoNicVac
The immunogenicity of adjuvant-loaded NanoNicVac was tested in mice (Figure 3). As shown in Figure 3A, after the primary immunization, anti-nicotine antibody titers were detected in all vaccine groups on day 12. All adjuvant-loaded NanoNicVac generated comparable anti-nicotine antibody titers as NanoNicVac with no adjuvant. The first booster immunization significantly increased the antibody response in all vaccine groups (Figure 3B). On day 26, NanoNicVac loaded with MPLA or R848+ODN 1826 did not increase the antibody titers compared to NanoNicVac without adjuvant. However, the antibody titers elicited by NanoNicVac loaded with R848, ODN 1826, MPLA+R848, or MPLA+ODN 1826 increased by 69%, 127%, 305%, and 180%, respectively, compared to that induced by NanoNicVac without adjuvant. The co-delivery of MPLA and R848 or MPLA and ODN 1826 generated a higher anti-nicotine antibody response than the delivery of only one adjuvant. The second booster injection further significantly enhanced antibody titers in all vaccine groups (Figure 3C). On day 40, NanoNicVac loaded with MPLA, R848, ODN 1826, MPLA+R848, MPLA+ODN 1826, or R848+ODN 1826 induced 1.44-, 1.84-,1.89-, 3.06-, 2.64-, and 1.26-fold higher antibody titers than NanoNicVac without adjuvant. Interestingly, the co-delivery of R848 and ODN 1826 did not result in a higher antibody titer than the delivery of only either R848 or ODN 1826. However, the co-delivery of MPLA and R848 or MPLA and ODN 1826 resulted in a considerably stronger antibody response than the delivery of only one adjuvant.
Figure 3.

Immunogenicity of adjuvant-loaded NanoNicVac. The titers of anti-nicotine IgG antibodies elicited by NanoNicVac on (A) day 12, (B) day 26, and (C) day 40 were measured by ELISA. Significantly different compared to the previous studied date: & p < 0.05, && p < 0.01, and &&& p < 0.001. Significantly different compared to NanoNicVac group with no adjuvant: # p < 0.05, ## p < 0.01, and ### p < 0.001. Significantly different: * p < 0.05, ** p < 0.01, and *** p < 0.001.
3.4 Subtype distribution of anti-nicotine IgG induced by adjuvant-loaded NanoNicVac
The titers of anti-nicotine subtype IgGs on day 40 were assayed (Figure 4A–D). Evidently, the incorporation of different single TLR adjuvant to NanoNicVac significantly impacted the change of titers of specific subtype IgGs. Specifically, the incorporation of MPLA or ODN 1826 mainly increased the levels of IgG2a and IgG2b, while the inclusion of R848 considerably increased the titers of IgG1 as well as IgG2a and IgG2b. The effects of co-incorporating different TLR adjuvant combinations to NanoNicVac on enhancing the levels of subtype IgGs also appeared to be different. Specifically, the incorporation of R848+ODN 1826 considerably increased the titers of IgG2a, IgG2b, and IgG3 but decreased the levels of IgG1. The incorporation of MPLA+ODN 1826 significantly increased the titers of all subtype IgGs except IgG1. The incorporation of MPLA+R848 considerably increased the titers of all four subtype IgGs, especially IgG1 and IgG2b. The relative percentage of subtype IgGs induced by adjuvant-loaded NanoNicVac was shown in Figure 4E. Interestingly, the distribution of subtype IgGs was significantly changed by the incorporation of different TLR adjuvants. IgG1 is the only major subtype detected when no adjuvant was used. The incorporation of MPLA+R848 did not significantly alter the subtype distribution. The incorporation of MPLA or R848 increased the percentage of IgG2a but IgG1 is still the major subtype. In contrast, the inclusion of ODN 1826, MPLA+ODN 1826, or R848+ODN 1826 significantly increased the percentage of IgG2a, and IgG2a became the major IgG subtype.
Figure 4.
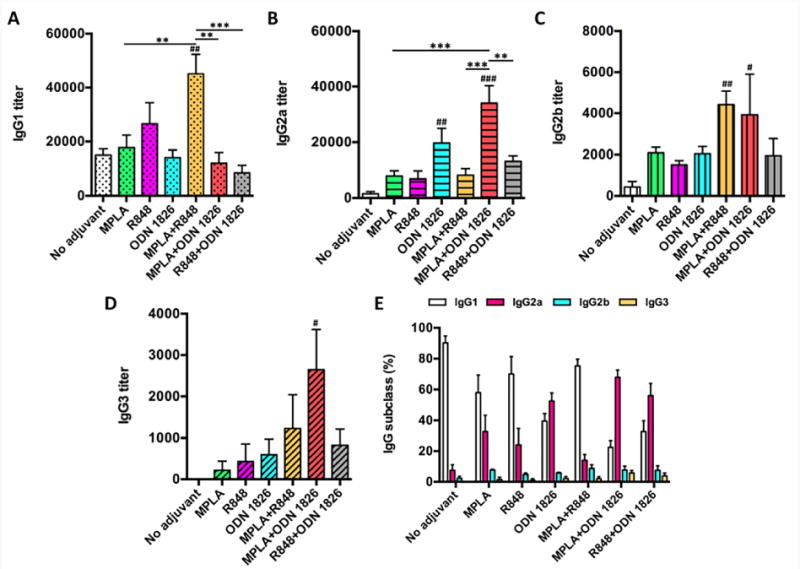
Subtype distribution of anti-nicotine IgGs. The titers of anti-nicotine IgG subtypes on day 40, including (A) IgG1, (B) IgG2a, (C) IgG2b, and (D) IgG3, were assayed. (E) shows the relative percentages of subtype anti-nicotine IgGs. Significantly different compared to NanoNicVac with no adjuvant: # p < 0.05, ## p < 0.01, and ### p < 0.001. Significantly different: ** p < 0.01, *** p < 0.001.
3.5 Relative affinity of anti-nicotine antibodies induced by adjuvant-loaded NanoNicVac
The affinity of anti-nicotine antibodies was estimated by competition ELISA (Figure 5). As shown in Figure 5A, 12 days after the primary immunization (on day 12), the IC50 values of all vaccine groups were high (>540 μM), suggesting the antibodies had not matured sufficiently to have a high affinity to nicotine. However, the IC50 values of antibodies of NanoNicVac loaded with no adjuvant, MPLA, R848, MPLA+R848, or R848+ODN 1826 were significantly lower on day 26 than that on day 12 (Figure 5B). In addition, although no statistically significant differences were detected, the IC50 values of NanoNicVac loaded with ODN 1826 or MPLA+ODN 1826 were considerably lower on day 26 compared to the values on day 12. These data suggest that the first booster immunization promoted the affinity maturation of anti-nicotine antibodies. Interestingly, as shown in Figure 5C, the second booster immunization exhibited different effects on the affinity maturation of anti-nicotine antibodies among NanoNicVac loaded with different TLR adjuvants. Specifically, the average IC50 values of antibodies induced by NanoNicVac loaded with no adjuvant, MPLA, or MPLA+ODN 1826 were higher on day 40 than those on day 26. However, for NanoNicVac loaded with R848, ODN 1826, or MPLA+R848, the IC50 values were slightly lower on day 40 than those on day 26. In terms of the end-point affinity, compared to NanoNicVac loaded with no adjuvant, NanoNicVac loaded with R848 or MPLA+R848 resulted in a slightly lower average IC50 while NanoNicVac loaded with the other adjuvants caused a higher average IC50.
Figure 5.
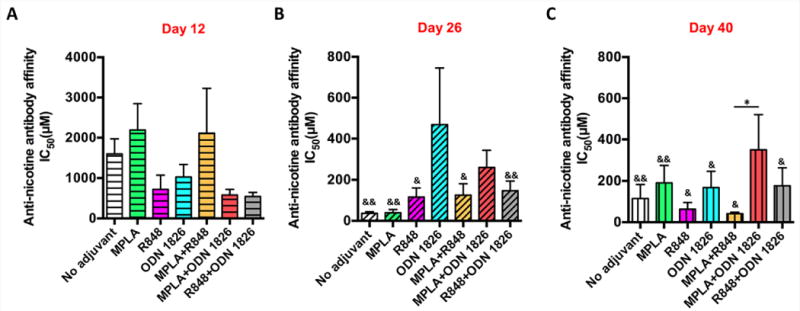
Affinity of anti-nicotine antibodies elicited by NanoNicVac on (A) day 12, (B) day 26, and (C) day 40. The antibody affinity was estimated by competition ELISA. Significantly different compared to the IC50 on day 12: & p < 0.05, && p < 0.01. Significantly different: * p < 0.05.
3.6 Ability of adjuvant-loaded NanoNicVac to change nicotine distribution in the serum and brain after nicotine challenge
The ability of adjuvant-loaded NanoNicVac to reduce nicotine levels in the brain was studied in mice (Figure 6). Mice received subcutaneous administration of 0.06 mg/kg nicotine on day 42. The serum and brain nicotine levels after nicotine challenge were analyzed. As shown in Figure 6A, the serum nicotine levels in all NanoNicVac groups were much higher than that in the PBS (control) group, suggesting that the immunization with NanoNicVac led to enhanced serum nicotine sequestration. In addition, compared to that of NanoNicVac loaded with no adjuvant, the serum nicotine levels increased by 15.3%, 38.4%, 62.9%, 295.5%, and 44.3% in the groups of NanoNicVac loaded with MPLA, R848, ODN 1826, MPLA+R848, and MPLA+ODN 1826, respectively. Particularly, NanoNicVac loaded with MPLA+R848 resulted in a considerably higher serum nicotine sequestration than NanoNicVac loaded with MPLA or R848 alone. As shown in Figure 6B, the incorporation of MPLA to NanoNicVac did not reduce brain nicotine levels. In contrast, the brain nicotine levels of NanoNicVac loaded with R848, ODN 1826, MPLA+R848, MPLA+ODN 1826, and R848+ODN 1826 were 19.6%, 21.0%, 54.0%, 32.0%, 16.7% lower than that of NanoNicVac with no adjuvant, respectively, suggesting the incorporation of these adjuvants facilitated the ability of NanoNicVac to keep nicotine from entering the brain. Particularly, NanoNicVac loaded with MPLA+R848 exhibited the best ability to reduce nicotine levels in the brain of mice, which was much better than that of NanoNicVac loaded with just MPLA or R848.
Figure 6.
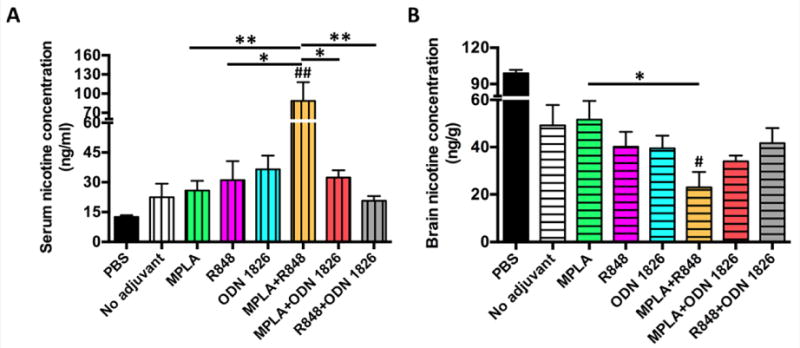
Pharmacokinetic efficacy of adjuvant-loaded NanoNicVac in mice. (A) Serum nicotine concentration. (B) Brain nicotine concentration. Mice were administered 0.06 mg/kg nicotine on day 42, and the brain and serum samples were collected 3 min after nicotine administration. Significantly different compared to NanoNicVac with no adjuvant: # p < 0.05, ## p < 0.01. Significantly different: * p < 0.05, ** p < 0.01.
3.7 Preliminary safety of adjuvant-loaded NanoNicVac
The behavioral and physical conditions of immunized mice during the entire study period were monitored. In all mouse groups immunized with NanoNicVac loaded with various adjuvants, no abnormal behavioral changes were observed compared to the mice injected with PBS. In addition, no short-term reactions, such as local reaction near the injection sites, apparent body temperature increase, and abnormal food and water consumption, were detected in any group. The body weight of mice was also monitored (Figure 7A). No weight loss was observed in any group of mice. In addition, there were no significant differences of body weight change between the PBS and all NanoNicVac groups. NanoNicVac had no effect on major organs of mice, including spleen, liver, lung, kidney, and heart, as determined by histopathological examination (Figure 7B). In all mouse groups injected with various NanoNicVac, no detectable lesions were found in any of the studied organs. All the above data suggest that NanoNicVac, regardless of the adjuvants, were safe for mice.
Figure 7.
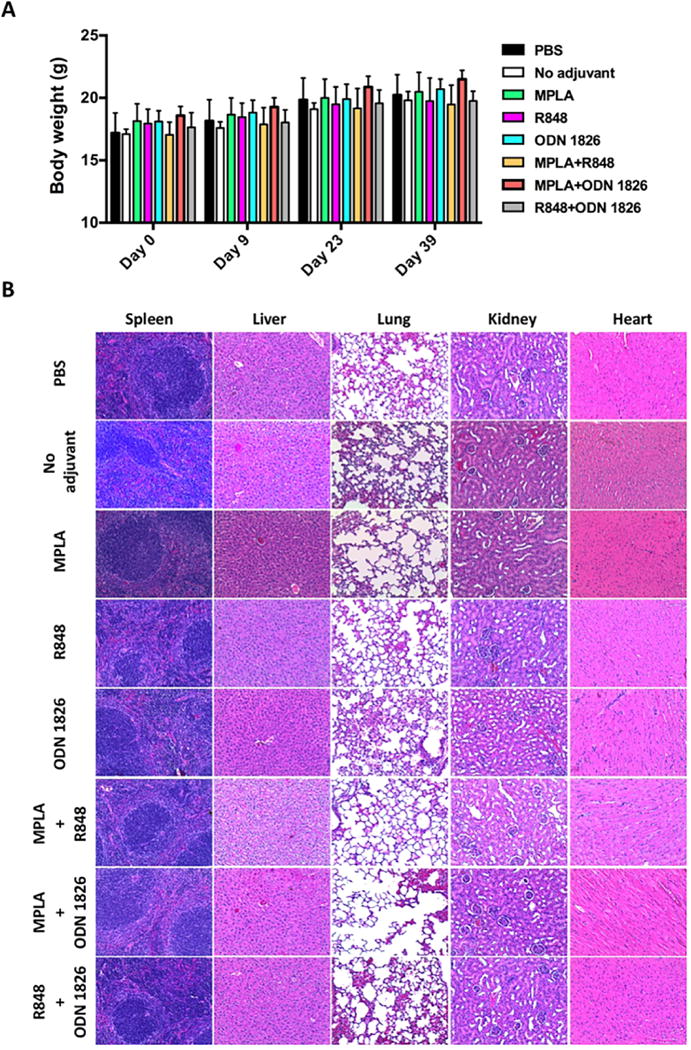
Preliminary safety of adjuvant-loaded NanoNicVac particles. (A) Body weight of immunized mice. (B) Representative H&E staining images of major organs of mice immunized with NanoNicVac carrying different adjuvants.
4. Discussion
The clinical trials of NicVAX and NicQb revealed that the top 30% subjects with the highest antibody levels showed enhanced smoking cessation rate than placebo.[6, 7] On one hand, this information suggests that the basic concept of using nicotine vaccines for treating nicotine addiction is sound. However, on the other hand, this information also indicates that more powerful nicotine vaccines that have sufficiently high immunogenicity are required so as to achieve an enhanced overall smoking cessation rate. The failure of the first-generation nicotine-protein conjugate nicotine vaccines (CNVs) inspired researchers to develop completely new nicotine vaccine platforms that can circumvent the innate shortfalls of conjugate vaccines. Because of their excellent properties, such as particulate nature, tunable physicochemical property, and high payload loading capacity,[38–41] NPs can be a basis for the development of the next-generation nicotine vaccines that can induce a stronger immune response. In our previous study, we developed a lipid-polymeric hybrid NP-based nicotine nanovaccine (NanoNicVac) and demonstrated that NanoNicVac could induce a significantly higher anti-nicotine antibody titer than CNVs.[17] In other studies, we demonstrated that the immunogenicity of NanoNicVac could be facilitated by modulating multiple factors, such as particle size,[17] hapten density,[20] hapten localization,[19] and stimulating protein (unpublished data). Adjuvants are a critical component of a nicotine vaccine to induce a strong and long-lasting immune response. In this current study, we rationally incorporated potent TLR-based molecular adjuvants to NanoNicVac particles and studied the impact of different TLR adjuvants on the vaccine’s immunological efficacy.
Efficient and specific delivery of nicotine vaccine components (hapten, T help protein, and adjuvant) to APCs is a prerequisite to initiate an effective immune response.[5] In our experiments, flow cytometry assay suggested that the design of NanoNicVac particles significantly enhanced the delivery efficiency of both nicotine antigens and adjuvants. These data are in agreement with a previous report showing that the use of NPs as delivery vehicles could improve the cellular internalization of soluble proteins and small molecules.[42] APCs have a relatively poor ability in recognizing and taking up soluble protein antigens,[11, 12] and thus CNVs cannot be internalized by APCs efficiently. Meanwhile, molecular adjuvants cannot be easily integrated with CNVs and are typically added to vaccine formulations by physical mixing. As a result, adjuvants cannot be targeted to APCs specifically and thus are poorly available to APCs.[13] The design of NanoNicVac particles can overcome the abovementioned drawbacks of CNVs. On one hand, NanoNicVac particles provide a host for the loading of protein antigens and molecular adjuvants. On the other hand, the particulate nature of NanoNicVac can achieve an enhanced recognition and internalization by APCs, increasing the availability of nicotine antigens and adjuvants to APCs.
It has been reported that co-localization of antigens and adjuvants within the same APCs can augment antigen presentation and T helper cell activation, which are required for B cell activation and maturation. Our CLSM results suggest that nicotine antigen and model adjuvant were efficiently co-delivered to the same DCs by NanoNicVac particles. Therefore, NanoNicVac may induce enhanced antigen presentation and T cell activation, thus helping elicit a strong immune response. Moreover, our CLSM results also revealed that NanoNicVac particles were transported to endosomes/lysosomes upon cellular internalization, and model adjuvant was efficiently released from NPs. In the design of NanoNicVac, MPLA, an agonist to TLR4, which is primarily localized on cell surface was loaded to the outer lipid-layer. R848 and ODN 1826 that are agonists to TLR7/8 and TLR9, respectively, which are localized in endosomal compartments, were loaded in the inner PLGA-core. The specified localization of adjuvants based on their relevant TLRs, the endosomal transportation of NPs, and the efficient release of adjuvants would promote effective interactions between TLRs and TLR adjuvants.
Our antibody titer results demonstrated that the incorporation of single TLR adjuvant (MPLA, R848, or OND 1826) increased the antibody titers but the enhancement was not significant. This suggests that the use of single TLR adjuvant may not be sufficient to significantly improve the immunogenicity of NanoNicVac. Also, the incorporation of different TLR adjuvant combinations exhibited dramatically different impacts on the antibody titers. Specifically, the combination of R848 and ODN 1826 exhibited an antagonistic effect on the antibody titers compared to R848 or ODN 1826 alone. However, the combination of MPLA and R848 or MPLA and ODN 1826 synergistically induced a much higher antibody titer than the corresponding single adjuvant. The difference in the adjuvant effects of different TLR adjuvant combinations may be attributed to the fact that MPLA, R848, and ODN 1826 act through different signaling pathways. MPLA, a TLR4 agonist, was reported to act in a TRIF pathway biased manner.[43] R848 and ODN 1826, which are TLR7/8 and TLR 9 agonists, respectively, are predominantly acting through MyD88-dependent signaling pathways.[44] It has been reported that the co-activation of these different pathways has the potential to induce complementary or synergistic effects, while antagonism more commonly occurs with agonists that act through the same pathway.[44, 45] The combination of MPLA and R848 or MPLA and ODN 1826 may co-activate both TRIF and MyD88 pathways. The cross-talk between MyD88 and TRIF may lead to enhanced cytokine production, reciprocal upregulation of each receptor,[46] and enhanced activation of T-helper cell responses,[47, 48], thus promoting antibody production.[49, 50] In contrast, as TLR7/8 and TLR9 signal via the same pathway, the combination of R848 and ODN 1826 may only stimulate the MyD88 pathway. As a result, the induction of immune responses cannot be enhanced due to the lack of MyD88-TRIF cross-talk. Moreover, in this current study, a NanoNicVac mixed with free TLR adjuvants was not tested in mice, as it has been reported that compared to nanoparticle-delivered TLR adjuvants, the free TLR counterparts exhibited lower immunostimulatory effect and higher risk of systemic toxicity.[33]
Our antibody affinity results reveal that compared to non-adjuvant-loaded NanoNicVac, NanoNicVac adjuvanted with MPLA, ODN 1826, MPLA+ODN 1826, or R848+ODN 1826 resulted in a comparable or lower antibody affinity while NanoNicVac adjuvanted with R848 or MPLA+R848 led to a higher antibody affinity. Surprisingly, the antibodies elicited by NanoNicVac adjuvanted with MPLA+R848 or MPLA+ODN 1826 had comparable quantity but significantly different affinity. This phenomenon indicates that the incorporation of different TLR adjuvants to NanoNicVac not only significantly influences the production of anti-nicotine antibodies but also significantly affects the quality of the produced anti-nicotine antibodies. The reduction of brain nicotine levels seen in our experiments were in agreement with the antibody titer data and antibody affinity data. Noticeably, NanoNicVac with MPLA+R848 adjuvant had a significantly better ability to reduce brain nicotine levels compared to non-adjuvant-loaded NanoNicVac. However, NanoNicVac with MPLA+ODN 1826 or R848+ODN 1826 did not result in significantly different brain nicotine concentrations than non-adjuvant-loaded NanoNicVac. These results were not unexpected. NanoNicVac adjuvanted with MPLA+R848 induced both significantly higher titer and much higher affinity of anti-nicotine antibodies, so more nicotine could be blocked from entering the brain. However, the anti-nicotine antibodies elicited by NanoNicVac with MPLA+ODN 1826 or R848+ODN 1826 had either lower affinity or comparable titer compared to that induced by non-adjuvant-loaded NanoNicVac. Therefore, the ability of NanoNicVac to reduce brain nicotine levels could not be significantly improved by the incorporation of MPLA+ODN 1826 or R848+ODN 1826. It should be pointed out that a NanoNicVac loaded with MPLA+R848+ODN 1826 was not tested in this study, considering that the incorporation of too many adjuvants might cause safety issues that could impair future clinical translation. Moreover, the co-incorporation of R848 and ODN 1826 did not resulted in an enhanced immunogenicity, further supporting the unnecessity of incorporating the combination of triple adjuvants. In conclusion, a series of lipid-polymeric hybrid NP-based nicotine nanovaccines, in which various TLR adjuvants or their combinations were incorporated, were successfully fabricated and tested. The impacts of TLR adjuvants on the immunogenicity and ability to reduce brain nicotine concentration of the nicotine nanovaccines were examined. Mouse trial results suggested that the use of single TLR adjuvant (MPLA, R848, or ODN 1826) was not sufficient to significantly enhance the immunogenicity of NanoNicVac. The co-incorporation of appropriate TLR adjuvant combinations (MPLA+R848 or MPLA+ODN 1826) exhibited a complementary effect and thus significantly improved the immunogenicity of NanoNicVac. Particularly, the incorporation of MPLA+R848 induced the highest titers of anti-nicotine antibody that had a high affinity to nicotine, and thus exhibited the best capability to block nicotine from entering the brain of mice. The findings of this work demonstrated that incorporating appropriate TLR adjuvants could be a novel strategy to improve the immunological efficacy of the next-generation NP-based nicotine vaccines. Based on all the reported results, hybrid NP-based nicotine nanovaccines can be a promising next-generation immunotherapeutic candidate for treating nicotine addiction. As previous clinical trials indicated that the induction of a sufficiently strong immune response against nicotine is a prerequisite for a clinically-viable nicotine vaccine, future studies will be focused on further improving the immunogenicity of the hybrid nanoparticle-based nicotine vaccines by integrating nanoparticle engineering strategies and hapten design. Once a leading nicotine nanovaccine that can result in a desirable antibody binding capacity and pharmacokinetic efficacy is established, efforts will be devoted to the clinical translation of the hybrid nanoparticle-based nicotine vaccine. If successful, the hybrid nanoparticle-based nicotine vaccine may provide smokers with a safer and more effective immunotherapeutic method for combating nicotine addiction, significantly contributing to solving the unmet demands for smoking cessation. Meanwhile, the hybrid nanoparticle-based nicotine vaccine also has potential to be co-administered with current pharmacological medications as a combinatorial intervention, considerably changing the current landscape of smoking cessation.
Acknowledgments
This work was financially supported by National Institute on Drug Abuse (U01DA036850).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no competing financial interests.
References
- 1.Prochaska JJ, Benowitz NL. The Past, Present, and Future of Nicotine Addiction Therapy. Annu Rev Med. 2016;67:467–86. doi: 10.1146/annurev-med-111314-033712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362(24):2295–303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lisy K. Nicotine vaccines for smoking cessation. Clin Nurse Spec. 2013;27(2):71–2. doi: 10.1097/NUR.0b013e31828191a3. [DOI] [PubMed] [Google Scholar]
- 4.Kitchens CM, Foster SL. Nicotine conjugate vaccines: A novel approach in smoking cessation. J Am Pharm Assoc (2003) 2012;52(1):116–8. doi: 10.1331/JAPhA.2012.12505. [DOI] [PubMed] [Google Scholar]
- 5.Pentel PR, LeSage MG. New Directions in Nicotine Vaccine Design and Use. Adv Pharmacol. 2014;69:553–580. doi: 10.1016/B978-0-12-420118-7.00014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatsukami DK, Jorenby DE, Gonzales D, Rigotti NA, Glover ED, Oncken CA, Tashkin DP, Reus VI, Akhavain RC, Fahim RE, Kessler PD, Niknian M, Kalnik MW, Rennard SI. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther. 2011;89(3):392–9. doi: 10.1038/clpt.2010.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornuz J, Zwahlen S, Jungi WF, Osterwalder J, Klingler K, van Melle G, Bangala Y, Guessous I, Muller P, Willers J, Maurer P, Bachmann MF, Cerny T. A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS One. 2008;3(6):e2547. doi: 10.1371/journal.pone.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tonstad S, Heggen E, Giljam H, Lagerback PA, Tonnesen P, Wikingsson LD, Lindblom N, de Villiers S, Svensson TH, Fagerstrom KO. Niccine(R), a nicotine vaccine, for relapse prevention: a phase II, randomized, placebo-controlled, multicenter clinical trial. Nicotine Tob Res. 2013;15(9):1492–501. doi: 10.1093/ntr/ntt003. [DOI] [PubMed] [Google Scholar]
- 9.McCluskie MJ, Thorn J, Gervais DP, Stead DR, Zhang N, Benoit M, Cartier J, Kim IJ, Bhattacharya K, Finneman JI, Merson JR, Davis HL. Anti-nicotine vaccines: Comparison of adjuvanted CRM197 and Qb-VLP conjugate formulations for immunogenicity and function in non-human primates. Int Immunopharmacol. 2015;29(2):663–671. doi: 10.1016/j.intimp.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Raupach T, Hoogsteder PH, Onno van Schayck CP. Nicotine vaccines to assist with smoking cessation: current status of research. Drugs. 2012;72(4):e1–16. doi: 10.2165/11599900-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Storni T, Kundig TM, Senti G, Johansen P. Immunity in response to particulate antigen-delivery systems. Adv Drug Deliv Rev. 2005;57(3):333–55. doi: 10.1016/j.addr.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Benne N, van Duijn J, Kuiper J, Jiskoot W, Slutter B. Orchestrating immune responses: How size, shape and rigidity affect the immunogenicity of particulate vaccines. J Control Release. 2016;234:124–134. doi: 10.1016/j.jconrel.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 13.Ilyinskii PO, Johnston LPM. Nanoparticle-Based Nicotine Vaccine. 1st. Springer; 2016. [Google Scholar]
- 14.Li XR, Aldayel AM, Cui ZR. Aluminum hydroxide nanoparticles show a stronger vaccine adjuvant activity than traditional aluminum hydroxide microparticles. J Control Release. 2014;173:148–157. doi: 10.1016/j.jconrel.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coffman RL, Sher A, Seder RA. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity. 2010;33(4):492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregory AE, Titball R, Williamson D. Vaccine delivery using nanoparticles. Front Cell Infect Mi. 2013:3. doi: 10.3389/fcimb.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Z, Hu Y, Hoerle R, Devine M, Raleigh M, Pentel P, Zhang C. A nanoparticle-based nicotine vaccine and the influence of particle size on its immunogenicity and efficacy. Nanomedicine. 2017;13(2):443–454. doi: 10.1016/j.nano.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu Y, Smith D, Frazier E, Hoerle R, Ehrich M, Zhang C. The next-generation nicotine vaccine: a novel and potent hybrid nanoparticle-based nicotine vaccine. Biomaterials. 2016;106:228–39. doi: 10.1016/j.biomaterials.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao ZM, Hu Y, Harmon T, Pentel P, Ehrich M, Zhang CM. Rationalization of a nanoparticle-based nicotine nanovaccine as an effective next-generation nicotine vaccine: A focus on hapten localization. Biomaterials. 2017;138:46–56. doi: 10.1016/j.biomaterials.2017.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Z, Powers K, Hu Y, Raleigh M, Pentel P, Zhang CM. Engineering of a hybrid nanoparticle-based nicotine nanovaccine as a next-generation immunotherapeutic strategy against nicotine addiction: A focus on hapten density. Biomaterials. 2017;123:107–117. doi: 10.1016/j.biomaterials.2017.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonam SR, Partidos CD, Halmuthur SKM, Muller S. An Overview of Novel Adjuvants Designed for Improving Vaccine Efficacy. Trends Pharmacol Sci. 2017 doi: 10.1016/j.tips.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Petrovsky N. Comparative Safety of Vaccine Adjuvants: A Summary of Current Evidence and Future Needs. Drug Saf. 2015;38(11):1059–74. doi: 10.1007/s40264-015-0350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomljenovic L, Shaw CA. Aluminum Vaccine Adjuvants: Are they Safe? Curr Med Chem. 2011;18(17):2630–2637. doi: 10.2174/092986711795933740. [DOI] [PubMed] [Google Scholar]
- 24.Ghimire TR. The mechanisms of action of vaccines containing aluminum adjuvants: an in vitro vs in vivo paradigm. Springerplus. 2015;4:181. doi: 10.1186/s40064-015-0972-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinhagen F, Kinjo T, Bode C, Klinman DM. TLR-based immune adjuvants. Vaccine. 2011;29(17):3341–55. doi: 10.1016/j.vaccine.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaisho T, Akira S. Toll-like receptors as adjuvant receptors. Bba-Mol Cell Res. 2002;1589(1):1–13. doi: 10.1016/s0167-4889(01)00182-3. [DOI] [PubMed] [Google Scholar]
- 27.Mbow ML, De Gregorio E, Valiante NM, Rappuoli R. New adjuvants for human vaccines. Curr Opin Immunol. 2010;22(3):411–6. doi: 10.1016/j.coi.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Toussi DN, Massari P. Immune Adjuvant Effect of Molecularly-defined Toll-Like Receptor Ligands. Vaccines (Basel) 2014;2(2):323–53. doi: 10.3390/vaccines2020323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maisonneuve C, Bertholet S, Philpott DJ, De Gregorio E. Unleashing the potential of NOD- and Toll-like agonists as vaccine adjuvants. P Natl Acad Sci USA. 2014;111(34):12294–12299. doi: 10.1073/pnas.1400478111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seya T, Akazawa T, Tsujita T, Matsumoto M. Role of Toll-like receptors in adjuvant-augmented immune therapies. Evid Based Complement Alternat Med. 2006;3(1):31–8. doi: 10.1093/ecam/nek010. discussion 133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lockner JW, Ho SO, McCague KC, Chiang SM, Do TQ, Fujii G, Janda KD. Enhancing nicotine vaccine immunogenicity with liposomes. Bioorg Med Chem Lett. 2013;23(4):975–8. doi: 10.1016/j.bmcl.2012.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alving CR, Matyas GR, Torres O, Jalah R, Beck Z. Adjuvants for vaccines to drugs of abuse and addiction. Vaccine. 2014;32(42):5382–9. doi: 10.1016/j.vaccine.2014.07.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ilyinskii PO, Roy CJ, O’Neil CP, Browning EA, Pittet LA, Altreuter DH, Alexis F, Tonti E, Shi J, Basto PA, Iannacone M, Radovic-Moreno AF, Langer RS, Farokhzad OC, von Andrian UH, Johnston LPM, Kishimoto TK. Adjuvant-carrying synthetic vaccine particles augment the immune response to encapsulated antigen and exhibit strong local immune activation without inducing systemic cytokine release. Vaccine. 2014;32(24):2882–2895. doi: 10.1016/j.vaccine.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bremer PT, Schlosburg JE, Lively JM, Janda KD. Injection Route and TLR9 Agonist Addition Significantly Impact Heroin Vaccine Efficacy. Mol Pharmaceut. 2014;11(3):1075–1080. doi: 10.1021/mp400631w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimishima A, Wenthur CJ, Eubanks LM, Sato S, Janda KD. Cocaine Vaccine Development: Evaluation of Carrier and Adjuvant Combinations That Activate Multiple Toll-Like Receptors. Mol Pharmaceut. 2016;13(11):3884–3890. doi: 10.1021/acs.molpharmaceut.6b00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Villiers SHL, Cornish KE, Troska AJ, Pravetoni M, Pentel PR. Increased efficacy of a trivalent nicotine vaccine compared to a dose-matched monovalent vaccine when formulated with alum. Vaccine. 2013;31(52):6185–6193. doi: 10.1016/j.vaccine.2013.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaenicke E, Buchler K, Decker H, Markl J, Schroder GF. The refined structure of functional unit h of keyhole limpet hemocyanin (KLH1-h) reveals disulfide bridges. IUBMB Life. 2011;63(3):183–7. doi: 10.1002/iub.435. [DOI] [PubMed] [Google Scholar]
- 38.Courant T, Bayon E, Reynaud-Dougier HL, Villiers C, Menneteau M, Marche PN, Navarro FP. Tailoring nanostructured lipid carriers for the delivery of protein antigens: Physicochemical properties versus immunogenicity studies. Biomaterials. 2017;136:29–42. doi: 10.1016/j.biomaterials.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Rincon-Restrepo M, Mayer A, Hauert S, Bonner DK, Phelps EA, Hubbell JA, Swartz MA, Hirosue S. Vaccine nanocarriers: Coupling intracellular pathways and cellular biodistribution to control CD4 vs CD8 T cell responses. Biomaterials. 2017;132:48–58. doi: 10.1016/j.biomaterials.2017.03.047. [DOI] [PubMed] [Google Scholar]
- 40.Pavot V, Climent N, Rochereau N, Garcia F, Genin C, Tiraby G, Vernejoul F, Perouzel E, Lioux T, Verrier B, Paul S. Directing vaccine immune responses to mucosa by nanosized particulate carriers encapsulating NOD ligands. Biomaterials. 2016;75:327–39. doi: 10.1016/j.biomaterials.2015.10.034. [DOI] [PubMed] [Google Scholar]
- 41.Chavez-Santoscoy AV, Roychoudhury R, Pohl NLB, Wannemuehler MJ, Narasimhan B, Ramer-Tait AE. Tailoring the immune response by targeting C-type lectin receptors on alveolar macrophages using “pathogen-like” amphiphilic polyanhydride nanoparticles. Biomaterials. 2012;33(18):4762–4772. doi: 10.1016/j.biomaterials.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Xu Y, Yu T, Clifford C, Liu Y, Yan H, Chang Y. A DNA nanostructure platform for directed assembly of synthetic vaccines. Nano Lett. 2012;12(8):4254–9. doi: 10.1021/nl301877k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316(5831):1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 44.Tan RS, Ho B, Leung BP, Ding JL. TLR cross-talk confers specificity to innate immunity. Int Rev Immunol. 2014;33(6):443–53. doi: 10.3109/08830185.2014.921164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKay PF, King DF, Mann JF, Barinaga G, Carter D, Shattock RJ. TLR4 and TLR7/8 Adjuvant Combinations Generate Different Vaccine Antigen-Specific Immune Outcomes in Minipigs when Administered via the ID or IN Routes. PLoS One. 2016;11(2):e0148984. doi: 10.1371/journal.pone.0148984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Makela SM, Strengell M, Pietila TE, Osterlund P, Julkunen I. Multiple signaling pathways contribute to synergistic TLR ligand-dependent cytokine gene expression in human monocyte-derived macrophages and dendritic cells. J Leukocyte Biol. 2009;85(4):664–672. doi: 10.1189/jlb.0808503. [DOI] [PubMed] [Google Scholar]
- 47.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6(8):769–76. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bohnenkamp HR, Papazisis KT, Burchell JM, Taylor-Papadimitriou J. Synergism of Toll-like receptor-induced interleukin-12p70 secretion by monocyte-derived dendritic cells is mediated through p38 MAPK and lowers the threshold of T-helper cell type 1 responses. Cell Immunol. 2007;247(2):72–84. doi: 10.1016/j.cellimm.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 49.Mitsdoerffer M, Lee Y, Jager A, Kim HJ, Korn T, Kolls JK, Cantor H, Bettelli E, Kuchroo VK. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A. 2010;107(32):14292–7. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Milpied PJ, McHeyzer-Williams MG. High-affinity IgA needs TH17 cell functional plasticity. Nat Immunol. 2013;14(4):313–5. doi: 10.1038/ni.2567. [DOI] [PubMed] [Google Scholar]


