Abstract
BACKGROUND
Multiple subpial transections (MST) are a treatment for seizure foci in nonresectable eloquent areas.
OBJECTIVE
To systematically review patient-level data regarding MST.
METHODS
Studies describing patient-level data for MST procedures were extracted from the Medline and PubMed databases, yielding a synthetic cohort of 212 patients from 34 studies. Data regarding seizure outcome, patient demographics, seizure type, surgery type, and complications were extracted and analyzed.
RESULTS
Seizure freedom was achieved in 55.2% of patients undergoing MST combined with resection, and 23.9% of patients undergoing MST alone. Significant predictors for seizure freedom were a temporal lobe focus (odds ratio 4.9; 95% confidence interval 1.71, 14.3) and resection of portions of the focus, when feasible (odds ratio 3.88; 95% confidence interval 2.02, 7.45). Complications were frequent, with transient mono- or hemiparesis affecting 19.8% of patients, transient dysphasia 12.3%, and permanent paresis or dysphasia in 6.6% and 1.9% of patients, respectively.
CONCLUSION
MST is an effective treatment for refractory epilepsy in eloquent cortex, with greater chances of seizure freedom when portions of the focus are resected in tandem with MST. The reported rates of seizure freedom with MST are higher than those of existing neuromodulatory therapies, such as vagus nerve stimulation, deep brain stimulation, and responsive neurostimulation, though these latter therapies are supported by randomized-controlled trials, while MST is not. The reported complication rate of MST is higher than that of resection and neuromodulatory therapies. MST remains a viable option for the treatment of eloquent foci, provided a careful risk-benefit analysis is conducted.
Keywords: Refractory epilepsy, Seizures, Eloquent, Palliative, Extratemporal lobe epilepsy
ABBREVIATIONS
- CI
confidence interval
- DBS
deep brain stimulation
- MST
multiple subpial transections
- OR
odds ratio
- RNS
responsive neurostimulation
- VNS
vagus nerve stimulation
Epilepsy becomes refractory in roughly one-third of newly diagnosed patients.1 Surgical resection is an option for some of these patients, but is problematic when the seizure focus resides in eloquent cortex (such as language, motor, or visual areas).2 For these patients, nondestructive neuromodulatory operations, such as vagus nerve stimulation (VNS), deep brain stimulation (DBS), and responsive neurostimulation (RNS), are alternative therapies that have generated increasing interest in the past decade.3 Yet, one of the original techniques for treating eloquent seizure foci is sometimes overlooked: multiple subpial transections (MST).
MST were first described by Frank Morrell and colleagues in 19894 as a means of treating refractory epilepsy when the focus lay in what they termed unresectable cortex.4 The technique uses a small metal wire with a right-angle hook at its end, extending ∼4 mm. This hook is inserted into 1 side of a cortical gyrus, as close to the sulcus as possible, and then driven to the far side subcortically, toward the next sulcus. The bent end of the wire is subsequently raised to the pial surface, and the hook dragged under the pia back to the wire's entry point. This maneuver severs intracortical fibers along the wire's course, but spares subcortical white matter and U-fibers. Cuts are made perpendicular to the gyrus, from one sulcus to the other, and repeated roughly every 5 mm. The extent of the cuts spans the unresectable epileptic focus. The concept behind this technique is to prevent ictal activity from spreading throughout the focus via intracortial connections, but preserve the major subcortical inputs and outputs of the eloquent region. In their initial study of 20 patients, Morrell et al4 reported a seizure freedom rate (Engel class I) of 55% with no significant induced deficits.
Motivated by these good outcomes, more than 100 studies of MST followed, all showing various rates of success. Yet, despite this large literature base, no systematic review of this technique was ever conducted to summarize the published data, or provide more general estimates of the technique's efficacy and rate of complications. While Spencer et al5 produced an informative meta-analysis in 2002, their study compiled mostly unpublished data from 6 high-volume surgical centers.5 It is unknown whether the select results from those few high-volume centers generalize to the wider neurosurgical community, as represented in the myriad articles published on MST before and after the Spencer meta-analysis. The upshot is that there remains no comprehensive literature review of all the work published on MST, which is the motivation of our work herein.
METHODS
The Medline and PubMed databases were queried on 6/13/2016 with the following Boolean search terms: (“multiple subpial transection” OR “multiple subpial transections”) AND epilepsy. Nonhuman animal studies were excluded, and results were limited to English language publications (Figure 1). No time limits were placed on the searches. All abstracts were reviewed independently by 2 authors (JDR and HD) and those not directly pertaining to MST were excluded. Further, articles were excluded when the results of MST could not be separated from the results of other interventions. Only publications with patient-level reporting were included. References were analyzed of all relevant articles to find additional articles on MST. Individual data for each patient were extracted and combined, including patient age, gender, seizure semiology, surgical procedure, complications, outcome, follow-up, and epileptic focus location (Excel, Microsoft Inc, Redmond, Washington). Outcomes were divided into Engel class I vs Engel class IIto IV, since seizure freedom (class I) is the most common predictor of epilepsy surgery satisfaction.6,7 Duplicate reports of the same patient were avoided by screening for patients with identical ages at surgery, genders, semiologies, procedure types, and epileptic focus location.
FIGURE 1.
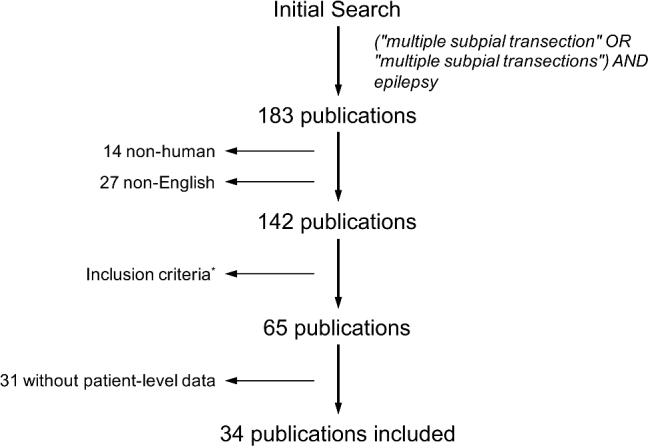
Search results. All abstracts were reviewed and those not directly pertaining to MST were excluded. Further, articles were excluded when the results of MST could not be separated from the results of other interventions.
Statistical analysis was performed with SPSS Statistics version 23 (IBM Corp, Armonk, New York) and Matlab r2015a (Mathworks, Natick, Massachusetts). Summary statistics are presented with mean ± standard deviation. Numeric data were compared with Student's t-test, and categorical data with Pearson's χ2 statistic. For multivariate analysis, logistic regression with backward conditional removal using a 0.10 cutoff was used for a maximum of 200 iterations.
RESULTS
One hundred thirty-nine articles concerning MST were identified through the Medline and Pubmed databases. After excluding irrelevant articles and articles with insufficient classification of results, 34 articles remained that described individual patient outcomes and characteristics (Figure 1).8-41
After disaggregating patient-level data from these studies, a synthetic cohort of 212 patients undergoing MST with or without adjunctive surgery was generated (Table 1). The mean age was 20.9 ± 13.7 yr and 36.9% were female; 54.4% had left-sided surgery, 42.4% had right-sided surgery, and 2.4% had bilateral surgery. Of these patients, only 47 (18.7%) had isolated MST without a concomitant surgery (eg, amygdalohippocampectomy, lesionectomy, corpus callosotomy, or VNS). Mean follow-up was 33.0 ± 20.0 mo.
TABLE 1.
Studies Evaluating MST with Patient-Level Data
| Study | Year | # Patients | Mean age | Mean follow-up | # Seizure free (%) |
|---|---|---|---|---|---|
| Devinsky et al8 | 1994 | 3 | 39.7 ± 10.2 | 12.7 ± 0.6 | 2 (67) |
| Wyler et al9 | 1995 | 6 | 23.2 ± 11.5 | 14.3 ± 3.9 | 1 (16.7) |
| Rougier et al10 | 1996 | 7 | 26.0 ± 10.7 | 21.4 ± 15.4 | 1 (14.3) |
| Patil et al11 | 1997 | 27 | 22.2 ± 15.4 | 26.9 ± 11.7 | 11 (40.7) |
| Hufnagel et al12 | 1997 | 22 | 23.0 ± 11.8 | 18.1 ± 6.9 | 9 (40.1) |
| Oguni et al13 | 1998 | 1 | 15 | 25 | 1 (100) |
| Molyneux et al14 | 1998 | 1 | 19 | 9 | 1 (100) |
| Asano et al15 | 1999 | 1 | 14 | 36 | 1 (100) |
| Akimura et al16 | 2000 | 1 | 25 | 22 | 1 (100) |
| Arita et al17 | 2000 | 1 | 17 | 30 | 1 (100) |
| Schramm et al18 | 2001 | 1 | 39 | 7 | 0 |
| Mittal et al19 | 2001 | 1 | 8 | 21 | 1 (100) |
| Irwin et al20 | 2001 | 5 | 7.9 ± 2.3 | 53.8 ± 20.6 | 5 (100) |
| Ma et al21 | 2001 | 1 | 22 | 2 | 1 (100) |
| Cheng et al22 | 2001 | 1 | 14 | 16 | 1 (100) |
| Shimizu et al23 | 2001 | 1 | 12 | 24 | 0 |
| D’Giano et al24 | 2001 | 1 | 6 | 12 | 1 (100) |
| Otsubo et al25 | 2001 | 7 | 12.1 ± 3.9 | 30a | 3 (42.9) |
| Bernasconi et al27 | 2001 | 2 | 22.5 ± 16.3 | 48.0 ± 50.9 | 1 (50.0) |
| Otsubo et al26 | 2001 | 3 | 10.0 ± 3.6 | 33.3 ± 1.2 | 3 (100) |
| Schramm et al28 | 2002 | 21 | 25.6 ± 10.1 | 48.2 ± 18.5 | 1 (4.8) |
| Romanelli et al29 | 2002 | 1 | 10 | 26 | 1 (100) |
| Onal et al30 | 2003 | 30 | 11.1 ± 4.5 | 32.4 ± 12.2 | 11 (36.7) |
| Devinsky et al31 | 2003 | 13 | 24.4 ± 11.3 | 59.2 ± 17.1 | 4 (30.8) |
| Bauman et al32 | 2005 | 11 | 11.5 ± 5.5 | 67.3 ± 21.6 | 5 (45.5) |
| Iida et al33 | 2005 | 6 | 10.7 ± 4.3 | 25.0 ± 7.3 | 3 (50.0) |
| Chuang et al34 | 2006 | 2 | 1.5 ± 0.8 | 6.0 ± 0 | 0 |
| Costello et al35 | 2005 | 1 | 36 | 16 | 1 (100) |
| Behdad et al36 | 2009 | 6 | 15.2 ± 1.5 | 26.0 ± 14.5 | 3 (50.0) |
| Nakayama et al37 | 2010 | 1 | 7 | 10 | 0 |
| Patil et al38 | 2010 | 10 | 39.2 ± 9.6 | 20.7 ± 8.1 | 6 (66.7) |
| Wang et al39 | 2013 | 1 | 33 | 17 | 1 (100) |
| Patil et al40 | 2013 | 15 | 44.4 ± 9.1 | 39.3 ± 12.2 | 14 (87.5) |
| Chen et al41 | 2015 | 1 | 11 | 17 | 1 (100) |
| Total | 212 | 20.9 ± 13.7 | 33.0 ± 20.0 | 96 (45.3) |
aOnly mean given, so standard deviation not calculable.
Overall, 96 patients (45.3%) who underwent MST achieved seizure freedom (Engel class I). However, when MST was performed alone, without an adjunctive surgery (such as resection), only 16 of 68 patients (23.9%) were seizure-free (Figure 2). This is compared to 80 of 146 patients (55.2%) that achieved seizure freedom when MST was combined with resection or another procedure, such as callosotomy (Figure 1). When examining patient-level data, 2 significant predictors of seizure freedom (Engel class I) were found: (1) including a resection along with the MST (odds ratio [OR] 3.88; 95% confidence interval [CI] 2.02, 7.45), and (2) a seizure focus within the temporal lobe (OR 4.9; 95% CI 1.71, 14.3; Table 2).
FIGURE 2.
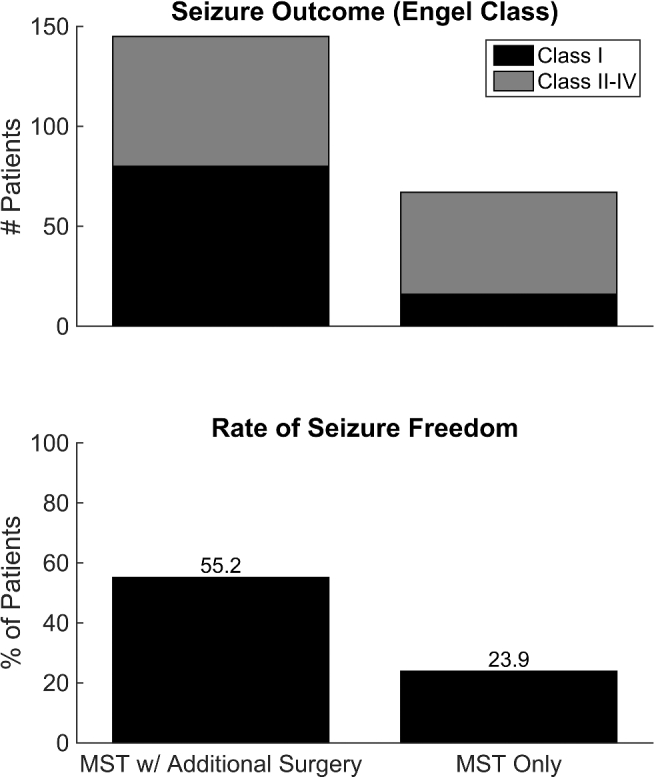
Seizure freedom rates for MST alone and MST combined with surgical resection. The number of patients with different Engel class outcomes for both surgery types (MST + resection and MST alone) are shown in the top panel, with the percentages of each depicted in the bottom panel.
TABLE 2.
Patient Characteristics and seizure Outcomes for MST
| Characteristic | # Class I (%) | # Class II-IV (%) | Total (%) | Odds ratio (95% CI) |
|---|---|---|---|---|
| Age | 23.6 ± 15.8 | 19.9 ± 11.8 | 21.6 ± 13.9 | P = .06 |
| Gender | ||||
| Female | 36 (37.5) | 57 (43.9) | 93 (43.9) | 1 [reference] |
| Male | 60 (72.5) | 59 (50.9) | 119 (56.1) | 1.61 (0.93, 2.79) |
| Seizure focus laterality | ||||
| Right | 38 (39.6) | 51 (44.0) | 89 (42.2)a | 1 [reference] |
| Left | 56 (58.3) | 60 (51.7) | 116 (55.0)a | 1.25 (0.72, 2.18) |
| Bilateral | 2 (0.1) | 4 (0.3) | 6 (2.8)a | 0.67 (0.12, 3.86) |
| Operation | ||||
| MST only | 16 (16.7) | 51 (44.0) | 67 (31.6) | 1 [reference] |
| Without biopsy | 14 (14.6) | 44 (37.9) | 58 (27.4) | |
| With biopsy | 2 (2.1) | 7 (6.0) | 9 (4.2) | 0.90 (0.17, 4.83) |
| MST + resection | 78 (81.3) | 64 (55.2) | 142 (70.0) | 3.88 (2.02, 7.45) |
| MST + disconnection | 2 (2.1) | 0 | 2 (0.9) | n/a |
| MST + VNS | 2 (2.1) | 0 | 2 (0.9) | n/a |
| Focus location | ||||
| Frontal | 21 (21.9) | 24 (20.7) | 45 (21.2) | 1 [reference] |
| Temporal | 26 (27.1) | 6 (5.2) | 32 (15.1) | 4.95 (1.71, 14.3) |
| Parietal | 3 (3.1) | 6 (5.2) | 9 (4.2) | 0.57 (0.13, 2.57) |
| Occipital | 0 | 1 (0.9) | 1 (0.5) | n/a |
| Frontal-parietal | 20 (20.8) | 33 (28.4) | 53 (25.0) | 0.69 (0.31, 1.55) |
| Frontal-temporal | 9 (9.4) | 19 (16.4) | 28 (13.2) | 0.54 (0.20, 1.45) |
| Temporal-parietal | 4 (4.2) | 4 (3.4) | 8 (3.8) | 1.14 (0.25, 5.15) |
| Temporal-occipital | 2 (2.1) | 2 (1.7) | 4 (1.9) | 1.14 (0.15, 8.84) |
| Parietal-occipital | 0 | 1 (0.9) | 1 (0.5) | n/a |
| Frontal-temporal-parietal | 6 (6.3) | 14 (12.1) | 20 (9.4) | 0.49 (0.16, 1.50) |
| Frontal-temporal-occipital | 1 (1.0) | 3 (2.6) | 4 (1.9) | 0.38 (0.04, 3.95) |
| Temporal-parietal-occipital | 2 (2.1) | 0 | 2 (0.9) | n/a |
| Frontal-parietal-occipital | 0 | 2 (1.7) | 2 (0.9) | n/a |
| All lobes | 2 (2.1) | 1 (0.9) | 3 (1.4) | 2.29 (0.19, 27.05) |
aOne patient did not have a laterality reported, so the total number of patients is lower for this category than others. Statistically significant differences are indicated in bold.
Data describing seizure semiology were available for 167 patients (78.8%; Table 3). The most prevalent seizures were complex partial (59.7%), followed by simple partial (14.7%). The seizure types associated with the best outcomes were epilepsia partialis continua (66.7% Engel class I), syndromic epilepsies (such as Lennox-Gastaut and Landau-Kleffner; 62.5% Engel Class I), and simple partial seizures (57.9%), though these categories had very small sample sizes compared to more common complex and simple partial seizures. Complex partial seizures had some of the worst outcomes in comparison (39.0% Engel class I; Table 3).
TABLE 3.
Seizure Types and Outcome. Only Patients With Clear Documented Seizure Types were Included (n = 167; 78.8% of All Patients)
| Seizure type | # Patients (%) | # Class I outcome (%) |
|---|---|---|
| Simple partial | 19 (14.7) | 11 (57.9) |
| Complex partial | 77 (59.7) | 30 (39.0) |
| Generalized | 10 (7.8) | 5 (50.0) |
| Status | 6 (4.7) | 3 (50.0) |
| Epilepsia partialis continua | 6 (4.7) | 4 (66.7) |
| Syndromica | 8 (6.2) | 5 (62.5) |
| Otherb | 3 (2.3) | 1 (33.3) |
aIncludes Lennox-Gastaut and Landau-Kleffner.
bIncludes drop attacks, atonic seizures, and myoclonic seizures.
The most frequent recorded complications were hemi- and monoparesis, which occurred transiently in 42 patients (26.4%) and remained permanent in 14 patients (6.6%). Language difficulties were the second most common, and were transient in 26 patients (12.3%) and permanent in 4 (1.9%). Other complications are quantified in Table 4.
TABLE 4.
Complications of MST
| Complication | # Patients (%) |
|---|---|
| Paresis | 56 (26.4) |
| Transient | 42 (19.8) |
| Permanent | 14 (6.6) |
| Dysphasia | 30 (14.2) |
| Transient | 26 (12.3) |
| Permanent | 4 (1.9) |
| Visual Field Deficit | 14 (6.6) |
| CSF leak | 8 (3.8) |
| Hematoma | 6 (2.8) |
| Infection | 5 (2.4) |
CSF, cerebrospinal fluid.
DISCUSSION
Per guidelines from the American Academy of Neurology, the American Epilepsy Society, and the American Association of Neurological Surgeons, patients with newly diagnosed refractory epilepsy should all be evaluated for potentially curative epilepsy surgery.42 Though before surgical resection can proceed, the epileptic focus must be adequately localized, typically through imaging, seizure semiology, and electrophysiology.43,44 Foci localized in noneloquent areas generally can be safely resected, and surgery provides a good chance of seizure freedom for many of these patients.45-47 The issue becomes complicated, however, when seizure foci arise in eloquent areas, making resection untenable given the possibility of causing pronounced permanent neurological deficits.2
For these patients with foci in eloquent areas, the technique of MST was introduced by Morrell et al in 1989.4 As described above, the procedure uses multiple passes of a bent wire probe to interrupt intracortical connections within an epileptic focus, but preserves the subcortical efferent and afferent fibers. This disruption of the focus putatively prevents lateral spread of seizures, but maintains the area's eloquent function. While MST was one of the original treatments for eloquent foci, many novel neuromodulatory treatments now exist, such as VNS, RNS, and DBS, all of which have class I evidence supporting their use.3 Yet, despite predating each of these neuromodulatory therapies, no comprehensive review of the MST literature exists.
Herein, we conducted a systematic review of literature to create a synthetic cohort of 212 patients undergoing MST. Most of these patients had MST as an adjunct to standard resection, and most of these patients were seizure-free (55.2%). A sizeable portion also had MST alone, without resection, and had a seizure freedom rate of 23.9%. This is notably different than the prior meta-analysis of Spencer et al,5 wherein 62% of patients with complex partial seizures and 71% of patients with generalized seizures had excellent outcomes following isolated MST (without resection), where “excellent outcome” is defined as a ≥95% reduction in seizures.5 Even if the Engel class I and II outcomes are combined in our systematic review, the improvement rate we found is significantly lower (41.8% class I or II for isolated MST) than the 62% to 71% reported by Spencer et al.5 Part of this difference could be related to a volume–outcome relationship, whereby the 6 high-volume centers used in the Spencer et al5 meta-analysis outperformed the more varied centers represented in the present systematic review, though we cannot analyze this in detail given the lack of volume data for the reviewed studies.48
When data were available describing seizure semiology, complex partial seizures had worse outcomes (39.0% Engel class I; Table 3) than simple partial seizures (57.9%), syndromic epilepsy (62.5%), and epilepsia partialis continua (66.7%). Anatomically, however, temporal lobe foci had the best outcomes (OR 4.95, 95% CI 1.71-14.3; Table 2). It should be emphasized that complex partial seizures in general do not arise exclusively from temporal lobe foci (eg, in this cohort 52% of complex partial seizures have nontemporal foci), which helps explain this difference.
Comparison to Other Modalities
Importantly, even if we accept the lower seizure freedom rate of 23.9% reported herein (as opposed to the 62%-71% reported by Spencer et al5), this rate is still higher than that of any current neuromodulatory therapy, such as VNS (8.2% seizure freedom at 24 mo49), RNS (0% overall at a mean follow-up of 5.4 yr, though 23% of patients have transient 6-mo periods of seizure freedom50), or DBS of the anterior nucleus (0% after 5 yr, but 16% with transient 6-mo periods of seizure freedom51). Yet, as noted before, the MST patients do worse than standard resection patients, whether for mesial temporal sclerosis (60%-90% seizure freedom) or neocortical epilepsy (40%-70% seizure freedom).47 However, patients undergoing standard surgical resection generally do not have foci in eloquent locations. There is no patient cohort, to our knowledge, where resection alone was used in eloquent areas, as compared to MST or MST and resection.
While none of the neuromodulation studies above specifically delineate patients with eloquent foci (allowing for a more direct comparison with MST), some inferences can be drawn. For instance, it has been shown that VNS is less likely to be successful with focal epilepsy (eloquent or not), as compared to generalized epilepsy (OR 1.38 favoring generalized, 95% CI 1.06-1.81)49. RNS, unlike VNS, requires identification of seizure foci before placement and has not been applied to generalized seizures. Roughly half of the RNS patients treated in the pivotal trial had lateral temporal or extratemporal foci, and many of these foci were likely in eloquent locations (given the choice of RNS over resection), though the publications describing RNS do not specify whether foci were eloquent or not. Nevertheless, there was no difference in outcome for patients with temporal vs extratemporal foci, suggesting the overall rate of seizure freedom applies equally to eloquent and noneloquent areas.50,52 Like RNS, papers describing DBS of the anterior thalamic nucleus do not specify whether the treated seizure foci were eloquent, though ∼20% of patients had “diffuse” or “other” specified as the seizure onset location in the SANTE trial, leaving ∼80% as potential eloquent cases.53 Again, as for RNS, many of the DBS patients likely had foci in eloquent areas, prompting the choice of DBS over resection, though the exact breakdown is unknown.
Though MST provides a good chance for seizure freedom, neurological complications with MST were more frequent than complications from resective epilepsy surgery. This is likely due to the direct manipulation of eloquent tissue during MST, tissue that is typically spared during traditional resections. For example, transient hemi- and monoparesis afflicted 19.8% of patients, and transient dysphasias 12.3% of patients—these deficits were permanent in 6.6% and 1.9% of patients, respectively (Table 4). In contrast, Hader et al54 conducted a systematic review of epilepsy surgery complications and found a 3.7% rate of transient dysphasia and 0.8% for permanent dysphasia.54
The use of MST should therefore be viewed with the typical risk vs benefit lens of all surgery—while there are greater risks of injury with MST than standard resection, there are potentially greater rewards in terms of seizure freedom if the eloquent area can also be treated, instead of avoided. If a medically refractory seizure focus lies within eloquent cortex, the options are to do nothing, use some form of neuromodulation, attempt MST, or resect the eloquent area and accept a neurological deficit. Each option adds progressively more risk, but offers progressively higher odds of seizure freedom. If the goal is weighted toward seizure freedom, the studies reviewed herein would suggest the focus be resected to the limits of eloquent cortex, followed by MST in the remaining eloquent areas. However, if the potential for neurological deficits is too worrisome, then a form of nonresective neuromodulation will be more appropriate.
Limitations
There are several limitations to these conclusions. First, there is no class I evidence for the use of MST. This is not the case for open surgical resection,45,46 VNS,55 DBS,53 or RNS,52 which all have randomized-controlled trials supporting their efficacy.3 While many studies argue for the utility of MST, the lack of randomized trials suggests that the efficacy of MST might be inflated by the selection and reporting biases inherent to small case series. However, a funnel plot of the data (Figure 3) shows a symmetric distribution with all but 3 studies within the 95% confidence limits of the expected average, suggesting no obvious reporting bias. As another means of testing for reporting bias, we excluded the 16 smallest case series, which negligibly changed the seizure freedom rate from 45.3% to 42.3%, largely because these small studies only account for a small portion of the reported cases (16 of 212, or 7.5%).
FIGURE 3.

Funnel plot of study data. Studies are plotted based on study size (# patients) and reported seizure freedom rate. The average across the synthetic cohort (45.3%) is plotted as a vertical dashed line. The 95% confidence intervals are shown as dotted lines and 99% confidence intervals as dashed lines.
Second, the reporting of complications in the reviewed studies was not standardized or verified, and, in particular, many studies did not document neuropsychological testing. The actual presence of neurological deficits postoperatively might therefore be higher than presented here, particularly for subtler and harder to detect cognitive issues. Prospective trials with strict criteria for monitoring complications and for evaluating neuropsychological outcomes is the best means for addressing this.
Third, while the above data compare resection plus MST to MST alone, there is no comparable group of patients undergoing resection alone in eloquent areas (without any MST). Without that comparison, it is difficult to know what value MST adds to resection, and therefore whether it is worth the additional risks. On the other hand, because the above data show that MST in isolation is quite powerful—23.9% seizure freedom—MST likely does function as a useful adjunct, though again without direct comparisons there is no way to know the magnitude of this effect.
Lastly, all literature reviews have inherent limitations when combining heterogeneous patient populations, surgical techniques, reporting standards, and follow-up times, which can bias the resulting outcomes.56 Again, the best alternative is to conduct controlled studies in the future, and to require strict reporting standards for future case series.
CONCLUSION
Patients with seizure foci in eloquent areas are often not candidates for traditional surgical resection. MST is one method for treating these patients, with a seizure freedom rate of 55.2% when MST is combined with resection and 23.9% when used alone. Importantly, these seizure freedom rates are far higher than those of neuromodulatory therapies such as VNS, DBS, or RNS, though the surgery clearly carries higher risk: neurological complications from MST appear to be more frequent than with traditional resection or neuromodulatory therapies, with transient mono- or hemiparesis occurring in 19.8% of patients, transient dysphasias in 12.3%, and permanent paresis and dysphasis in 6.6% and 1.9% of patients, respectively. The greatest limitation of MST is the lack of class I evidence validating its efficacy and complication rates, which would help establish its place in the treatment of eloquent seizure foci.
Disclosure
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
COMMENT
The authors present a well written systematic review on multiple subpial transections for medically intractable epilepsy using the PRISMA reporting guideline. The systematic review fulfills the PRISMA guideline well, though the available data in the literature is sparse. The review covers an important topic that has not received the research attention it deserves as of late. It is a reminder that while neuromodulation has its merits, there remains another option.
Omar Zalatimo
Hummelstown, Pennsylvania
REFERENCES
- 1. French JA. Refractory epilepsy: clinical overview. Epilepsia. 2007;48(s1):3-7. [DOI] [PubMed] [Google Scholar]
- 2. Rolston JD. Surgical strategies for epilepsy in eloquent areas. J Epilepsy. 2016;2:103. [Google Scholar]
- 3. Rolston JD, Englot DJ, Wang DD, Shih T, Chang EF. Comparison of seizure control outcomes and the safety of vagus nerve, thalamic deep brain, and responsive neurostimulation: evidence from randomized controlled trials. Neurosurg Focus. 2012;32(3):E14. [DOI] [PubMed] [Google Scholar]
- 4. Morrell F, Whisler WW, Bleck TP. Multiple subpial transection: a new approach to the surgical treatment of focal epilepsy. J Neurosurg. 1989;70(2):231-239. [DOI] [PubMed] [Google Scholar]
- 5. Spencer SS, Schramm J, Wyler A et al. . Multiple subpial transection for intractable partial epilepsy: an international meta-analysis. Epilepsia. 2002;43(2):141-145. [DOI] [PubMed] [Google Scholar]
- 6. Macrodimitris S, Sherman EMS, Williams TS, Bigras C, Wiebe S. Measuring patient satisfaction following epilepsy surgery. Epilepsia. 2011;52(8):1409-1417. [DOI] [PubMed] [Google Scholar]
- 7. Elliott I, Kadis DS, Lach L et al. . Quality of life in young adults who underwent resective surgery for epilepsy in childhood. Epilepsia. 2012;53(9):1577-1586. [DOI] [PubMed] [Google Scholar]
- 8. Devinsky O, Perrine K, Vazquez B, Luciano DJ, Dogali M. Multiple subpial transections in the language cortex. Brain. 1994;117(2):255-265. [DOI] [PubMed] [Google Scholar]
- 9. Wyler AR, Wilkus RJ, Rostad SW, Vossler DG. Multiple subpial transections for partial seizures in sensorimotor cortex. Neurosurgery. 1995;37(6):1122-1128. [DOI] [PubMed] [Google Scholar]
- 10. Rougier A, Sundstrom L, Claverie B et al. . Multiple subpial transection: report of 7 cases. Epilepsy Res. 1996;24(1):57-63. [DOI] [PubMed] [Google Scholar]
- 11. Patil AA, Andrews R, Torkelson R. Isolation of dominant seizure foci by multiple subpial transections. Stereotact Funct Neurosurg. 1997;69(1-4):210-215. [DOI] [PubMed] [Google Scholar]
- 12. Hufnagel A, Zentner J, Fernandez G, Wolf HK, Schramm J, Elger CE. Multiple subpial transection for control of epileptic seizures: effectiveness and safety. Epilepsia. 1997;38(6):678-688. [DOI] [PubMed] [Google Scholar]
- 13. Oguni H, Hayashi K, Usui N, Osawa M, Shimizu H. Startle epilepsy with infantile hemiplegia: report of two cases improved by surgery. Epilepsia. 1998;39(1):93-98. [DOI] [PubMed] [Google Scholar]
- 14. Molyneaux PD, Barker RA, Thom M, Van Paesschen W, Harkness WF, Duncan JS. Successful treatment of intractable epilepsia partialis continua with multiple subpial transections. J Neurol Neurosurg Psychiatr. 1998;65(1):137-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Asano E, Ishikawa S, Otsuki T, Nakasato N, Yoshimoto T. Surgical treatment of intractable epilepsy originating from the primary sensory area of the hand - Case report. Neurol Med Chir (Tokyo). 1999;39(3):246-250. [DOI] [PubMed] [Google Scholar]
- 16. Akimura T, Fujii M, Adachi H, Ito H. Intractable epilepsy associated with multiple cavernous malformations: case report. Neurosurgery. 2000;46(3):740. [DOI] [PubMed] [Google Scholar]
- 17. Arita K, Kurisu K, Iida K et al. . Surgical treatment for intractable epilepsy caused by cavernous angioma in the temporal lobe of the dominant hemisphere–three case reports. Neurol Med Chir (Tokyo). 2000;40(8):439-445. [DOI] [PubMed] [Google Scholar]
- 18. Schramm J, Kral T, Grunwald T, Blumcke I. Surgical treatment for neocortical temporal lobe epilepsy: clinical and surgical aspects and seizure outcome. J Neurosurg. 2001;94(1):33-42. [DOI] [PubMed] [Google Scholar]
- 19. Mittal S, Farmer JP, Rosenblatt B, Andermann F, Montes JL, Villemure JG. Intractable epilepsy after a functional hemispherectomy: important lessons from an unusual case. Case report. J Neurosurg. 2001;94(3):510-514. [DOI] [PubMed] [Google Scholar]
- 20. Irwin K, Birch V, Lees J et al. . Multiple subpial transection in Landau-Kleffner syndrome. Dev Med Child Neurol. 2001;43(4):248-252. [DOI] [PubMed] [Google Scholar]
- 21. Ma XP, Liporace J, O’Connor MJ, Sperling MR. Neurosurgical treatment of medically intractable status epilepticus. Epilepsy Res. 2001;46(1):33-38. [DOI] [PubMed] [Google Scholar]
- 22. Cheng WW, Otsubo H, Snead OC. Surgery for intractable epilepsy in a 14-year-old girl. Hong Kong Med J. 2001;7(1):97-100. [PubMed] [Google Scholar]
- 23. Shimizu T, Maehara T, Hino T, Komori T, Shimizu H. Effect of multiple subpial transection on motor cortical excitability in cortical dysgenesis. Brain. 2001;124(7):1336-1349. [DOI] [PubMed] [Google Scholar]
- 24. D’Giano CH, Del C García M, Pomata H, Rabinowicz AL. Treatment of refractory partial status epilepticus with multiple subpial transection: case report. Seizure. 2001;10(5):382-385. [DOI] [PubMed] [Google Scholar]
- 25. Otsubo H, Chitoku S, Ochi A et al. . Malignant rolandic-sylvian epilepsy in children: diagnosis, treatment, and outcomes. Neurology. 2001;57(4):590-596. [DOI] [PubMed] [Google Scholar]
- 26. Otsubo H, Ochi A, Elliott I et al. . MEG predicts epileptic zone in lesional extrahippocampal epilepsy: 12 pediatric surgery cases. Epilepsia. 2001;42(12):1523-1530. [DOI] [PubMed] [Google Scholar]
- 27. Bernasconi A, Martinez V, Rosa Neto P et al. . Surgical resection for intractable epilepsy in “double cortex” syndrome yields inadequate results. Epilepsia. 2001;42(9):1124-1129. [DOI] [PubMed] [Google Scholar]
- 28. Schramm J, Aliashkevich AF, Grunwald T. Multiple subpial transections: outcome and complications in 20 patients who did not undergo resection. J Neurosurg. 2002;97(1):39-47. [DOI] [PubMed] [Google Scholar]
- 29. Romanelli P, Najjar S, Weiner HL, Devinsky O. Epilepsy surgery in tuberous sclerosis: multistage procedures with bilateral or multilobar foci. J Child Neurol. 2002;17(9):689-692. [DOI] [PubMed] [Google Scholar]
- 30. Onal Ç, Otsubo H, Araki T et al. . Complications of invasive subdural grid monitoring in children with epilepsy. J Neurosurg. 2003;98(5):1017-1026. [DOI] [PubMed] [Google Scholar]
- 31. Devinsky O, Romanelli P, Orbach D, Pacia S, Doyle W. Surgical treatment of multifocal epilepsy involving eloquent cortex. Epilepsia. 2003;44(5):718-723. [DOI] [PubMed] [Google Scholar]
- 32. Bauman JA, Feoli E, Romanelli P, Doyle WK, Devinsky O, Weiner HL. Multistage epilepsy surgery: safety, efficacy, and utility of a novel approach in pediatric extratemporal epilepsy. Neurosurgery. 2005;56(2):318-332. [DOI] [PubMed] [Google Scholar]
- 33. Iida K, Otsubo H, Matsumoto Y et al. . Characterizing magnetic spike sources by using magnetoencephalography-guided neuronavigation in epilepsy surgery in pediatric patients. J Neurosurg. 2005;102(2 suppl):187-196. [DOI] [PubMed] [Google Scholar]
- 34. Chuang MF, Harnod T, Wang PJ, Chen YH, Hsin YL. Effect of multiple subpial transection on patients with uncontrolled atypical infantile spasms. Epilepsia. 2006;47(3):659-660. [DOI] [PubMed] [Google Scholar]
- 35. Costello DJ, Simon MV, Eskandar EN et al. . Efficacy of surgical treatment of de novo, adult-onset, cryptogenic, refractory focal status epilepticus. Arch Neurol. 2006;63(6):895-901. [DOI] [PubMed] [Google Scholar]
- 36. Behdad A, Limbrick DD Jr, Bertrand ME, Smyth MD. Epilepsy surgery in children with seizures arising from the rolandic cortex. Epilepsia. 2009;50(6):1450-1461. [DOI] [PubMed] [Google Scholar]
- 37. Nakayama T, Otsuki T, Kaneko Y et al. . Repeat magnetoencephalography and surgeries to eliminate atonic seizures of non-lesional frontal lobe epilepsy. Epilepsy Res. 2009;84(2-3):263-267. [DOI] [PubMed] [Google Scholar]
- 38. Patil AA, Andrews RV. Nonresective hippocampal surgery for epilepsy. World Neurosurg. 2010;74(6):645-649. [DOI] [PubMed] [Google Scholar]
- 39. Wang DD, Deans AE, Barkovich AJ et al. . Transmantle sign in focal cortical dysplasia: a unique radiological entity with excellent prognosis for seizure control. J Neurosurg. 2013;118(2):337-344. [DOI] [PubMed] [Google Scholar]
- 40. Patil AA, Andrews R. Long term follow-up after multiple hippocampal transection (MHT). Seizure. 2013;22(9):731-734. [DOI] [PubMed] [Google Scholar]
- 41. Chen PC, Baumgartner J, Seo JH, Korostenskaja M, Lee KH. Bilateral intracranial EEG with corpus callosotomy may uncover seizure focus in nonlocalizing focal epilepsy. Seizure. 2015;24:63-69. [DOI] [PubMed] [Google Scholar]
- 42. Engel J, Wiebe S, French J et al. . Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the quality standards subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 2003;60(4):538-547. [DOI] [PubMed] [Google Scholar]
- 43. Rolston JD, Englot DJ, Cornes S, Chang EF. Major and minor complications in extraoperative electrocorticography: a review of a national database. Epilepsy Res. 2016;122:26-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rolston JD, Ouyang D, Englot DJ, Wang DD, Chang EF. National trends and complication rates for invasive extraoperative electrocorticography in the USA. J Clin Neurosci. 2015;22(5):823-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311-318. [DOI] [PubMed] [Google Scholar]
- 46. Engel J, McDermott MP, Wiebe S et al. . Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA. 2012;307(9):922-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Englot DJ, Chang EF. Rates and predictors of seizure freedom in resective epilepsy surgery: an update. Neurosurg Rev. 2014;37(3):389-404 discussion 404–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Davies JM, Ozpinar A, Lawton MT. Volume-outcome relationships in neurosurgery. Neurosurg Clin N Am. 2015;26(2):207-218. [DOI] [PubMed] [Google Scholar]
- 49. Englot DJ, Rolston JD, Wright CW, Hassnain KH, Chang EF. Rates and predictors of seizure freedom with vagus nerve stimulation for intractable epilepsy. Neurosurgery. 2016;79(3):345-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bergey GK, Morrell MJ, Mizrahi EM et al. . Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology. 2015;84(8):810-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Salanova V, Witt T, Worth R et al. . Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84(10):1017-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Morrell MJ, RNS System in Epilepsy Study Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77(13):1295-1304. [DOI] [PubMed] [Google Scholar]
- 53. Fisher R, Salanova V, Witt T et al. . Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899-908. [DOI] [PubMed] [Google Scholar]
- 54. Hader WJ, Tellez-Zenteno J, Metcalfe A et al. . Complications of epilepsy surgery: a systematic review of focal surgical resections and invasive EEG monitoring. Epilepsia. 2013;54(5):840-847. [DOI] [PubMed] [Google Scholar]
- 55. Handforth A, DeGiorgio CM, Schachter SC et al. . Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology. 1998;51(1):48-55. [DOI] [PubMed] [Google Scholar]
- 56. Sampson JH, Barker FGI. Methodology and reporting of meta-analyses in the neurosurgical literature. J Neurosurg. 2014;120(4):791-794. [DOI] [PubMed] [Google Scholar]


