Abstract
Regular use of colorectal cancer screening can reduce incidence and mortality, but participation rates remain low among low-income, Spanish-speaking Latino adults. We conducted two distinct pilot studies testing the implementation of evidence-based interventions to promote fecal immunochemical test (FIT) screening among Latinos aged 50–75 years who were not up-to-date with CRC screening (n=200) at a large Federally-Qualified Health Center (FQHC) in San Diego, CA. One pilot focused on an opportunistic clinic visit “in-reach” intervention including a 30 minute session with a patient navigator, review of an educational “flip-chart”, and a take-home FIT kit with instructions. The second pilot was a system-level “outreach” intervention consisting of mailed materials (i.e., FIT kit, culturally and linguistically tailored instructions, and a pre-paid return envelope). Both received follow-up calls to promote screening completion and referrals for additional screening and treatment if needed. The primary outcome was FIT kit completion and return within three months assessed through electronic medical records. The in-reach pilot consisted of mostly insured (85%), women (82%) and Spanish-speaking (88%) patients. The outreach pilot consisted of mostly of Spanish-speaking (73%) women (64%), half of which were insured (50%). At three months follow-up, screening completion was 76% for in-reach, and 19% for outreach. These data demonstrate that evidence-based strategies to promote CRC screening can be implemented successfully within FQHCs, but implementation (particularly of mailed outreach) may require setting and population-specific optimization. Patient, provider and healthcare system related implementation approaches and lessons learned from this study may be implemented in other primary care settings.
Keywords: Latino health, colorectal cancer screening, community health center, primary care
Colorectal cancer (CRC) is one of the most common cancer diagnoses and causes of cancer related mortality among Latinos1–6. Latinos experience a greater burden of cancer disparities, in that they are more likely to have late stage cancers at time of diagnosis which is related to havinge lower survival rates compared to non-Hispanic whites7. Research shows that regular use of CRC screening is associated with a decreased risk of developing invasive cancer8 and an increased rate of survival9–13. Latinos have lower CRC screening rates compared to non-Hispanic whites, which may explain these disparities6,14,15.
The 2016 United States Preventive Services Task Force (USPSTF) recommends regular CRC screening for all individuals age 50 to 75, and noted that annual high-sensitivity guaiac fecal occult blood testing (FOBT), annual fecal immunochemical testing (FIT), FIT-DNA every 3 years, sigmoidoscopy every 5 years alone, or every 10 years with annual FIT, or colonoscopy every 10 years16.
Evidence-based clinical strategies have been shown to improve CRC screening rates from 10–40%, and include: individual (e.g., education), provider (e.g., physician recommendation, patient navigation, or case management) and system interventions, (e.g., mailed reminders, decision aides, access to services)9,12,17–20. However, many primary care settings have little to no organized CRC screening programs, which require “explicit policy in a defined target population with a defined implementation and quality assurance structure, and tracking of cancer in the population.”21,22 Organized screening programs can be broadly categorized as in-reach (i.e., promotion of screening at the point of routine medical care) vs. outreach (i.e., targeting all eligible individuals within a defined population regardless of scheduled visits).
In-reach strategies take advantage of in-person encounters with age-eligible individuals. However, in-reach strategies may be limited in their ability to reach all patients, and especially those who do not utilize care on a regular basis. As such, outreach strategies may be a way for health systems to conceptualize care delivery beyond the clinical visit encounter23. There are few randomized trials that examined the effectiveness of either in-reach or outreach strategies for promoting screening that have primarily focused on Latino populations. Few studies that have included some Latino representation have been shown to increase FOBT screening completion from 7–35%24–27. The aim of the study was to test the implementation of two CRC screening strategies (in-reach and outreach) in a Federally Qualified Health Center (FQHC) primary care setting without a current organized CRC screening program among a predominantly Latino population.
Methods
Participants
From 2014–2015, we conducted two separate pilot studies of 1) an in-reach and 2) an outreach intervention for increasing CRC screening. Both pilot studies focused on Mexican-heritage Latino adults receiving continuity of care at San Ysidro Health Center, Inc. (SYHC), a FQHC in the Southern border region of San Diego, California. Within this region, Latinos experience cancer disparities in terms of poor access to care, screening 28, time to treatment, and survival2. SYHC has 12 clinic sites and serves over 90,000 registered patients, most of whom are low income and Spanish-speaking Latinos. In 2015, the CRC screening rate at SYHC was 42.9%29.
Participants in these studies included 200 SYHC patients of ages 50–75 years, who were not up-to-date with CRC screening (i.e., verified by electronic medical record (EMR) review, and based on either an absence of FOBT or FIT in the past 12 months, flexible sigmoidoscopy in the past 5 years, or colonoscopy in the past 10 years), who self-identified as Latino, and had at least one primary care visit in the preceding year. Those with a family history of CRC and inflammatory bowel diseases (e.g., ulcerative colitis or Crohn’s disease) were excluded as these individuals are at higher than average risk for CRC, and require specialized screening regimens.
Recruitment
In-reach recruitment was carried out in-person by trained promotoras (culturally- and linguistically- aligned lay health educators who are part of the patient care team)30 in the SYHC clinic waiting rooms at one of SYHC’s clinic sites. First, the EMR was reviewed for potential participants who had a scheduled visit and who met eligibility criteria. Potential participants who were aged 50–75 and not up-to-date with screening were approached before their encounter with the primary care provider (PCP). Additional eligibility was verified in discussions with the patient.
The outreach pilot randomly selected and enrolled 100 patients aged 50–75 who were not up to date with screening, (50% insured and 50% uninsured). Given the significant clinical cost associated with screening and treatment for uninsured patients, the rationale for stratified sampling based on insurance status was to understand the needs of those who were insured versus uninsured in inform the development of scalable infrastructure for delivering screening in similar settings. Given that this study involved no more than minimal risk, a waiver of documentation of written informed consent and Health Insurance Portability and Accountability Act (HIPAA) authorization was obtained for use of the EMR to screen and enroll all study participants. As described below, potential participants were given a research factsheet that included a description of the study, and a plan for medical and research staff to view participants’ electronic record data for colorectal cancer screening results.
In-reach and Outreach Intervention Descriptions
The in-reach study was adapted from previous work in the SYHC setting31,32, and took place before or after a regularly scheduled PCP clinical visit and consisted of a 10 minute one-on-one educational session with a promotora, who: 1) reviewed CRC and the importance of early detection, 2) reviewed risks and benefits of CRC screening tests, 3) modeled steps to complete an at-home FIT kit, and 4) gave the patient an at-home FIT kit with instructions. Navigation was provided in English or Spanish, based on participant preference.
The outreach study was adapted from prior work26,33,34, and consisted of a: 1) mailed one page English and Spanish invitation, 2) an at-home FIT kit, 3) FIT completion instructions in English and Spanish, and 4) a self-addressed return envelope.
Both in-reach and outreach interventions were delivered by trained promotoras who utilized standardized patient education materials, handouts, in-person scripts, and phone scripts to ensure fidelity of the interventions. Both in-reach and outreach intervention groups were given a research fact sheet that detailed the nature of the study, voluntary participation, and steps involved in the research. Both interventions were supplemented by a follow-up phone call from a promotora to support FIT screening completion within 1–3 weeks of the initial session or package mailing. All participants had a working phone number on file and had secondary contact numbers listed in their electronic health records in case the primary numbers were not working. Once the participant returned the FIT test and the results were in the EMR, the promotora sent a letter home and alerted the PCP following clinic protocol. If the test was abnormal, the promotora alerted the participant to schedule a visit with their PCP to discuss the results and obtain a colonoscopy referral. The promotora facilitated the colonoscopy scheduling by providing the referral information and emphasizing the importance of diagnostic follow up. The promotora also monitored results of diagnostic colonoscopy in the EMR, and alerted the PCP to any findings.
Data Analysis
Descriptive analyses describing participant demographics were stratified by intervention group. The primary outcome was the proportion of individuals who completed their FIT test within a three month follow-up time period assessed by reviewing the EMR for lab results. Data are presented for within-group analyses only, since between-group analyses were not possible due to the distinct sampling and recruitment methods for each group. Secondary outcomes included whether participants receive guideline appropriate follow up, (e.g., results reporting, referral for and completion of diagnostic colonoscopy after abnormal FIT, and specialist referral if cancer was found). All analyses were conducted with SAS statistical software (version 9.4; SAS Institute Inc, Cary, NC). Institutional Review Board approval was obtained for the study from San Diego State University and UC San Diego.
Results
Baseline characteristics of participants in both in-reach and outreach intervention groups are summarized in Table 1.
Table 1.
Sociodemographic characteristics of participants by study intervention, N=200
| Characteristics | In-reach (N=100) n (%) |
Outreach (N=100) n (%) |
|---|---|---|
| Age (years), mean (SD) | 58.7 (6.3) | 59.6 (6.0) |
| Sex | ||
| Female | 82 (82) | 64 (64) |
| Had Health Insurance | ||
| Yes | 85 (85) | 50 (50) |
| Health Insurance Type | ||
| Medical (Medical managed Care, Medical MC CAP)* | 48 (48) | 25 (25) |
| Medicare | 35 (35) | 21 (21) |
| Self-Pay (Self Pay & Sliding scale) | 15 (15) | 44 (44) |
| Other(San Diego Physicians Medical Group & Low Income Health Program) | 1(1) | 8 (8) |
| Primary language | ||
| Spanish | 88 (88) | 73 (73) |
| English | 9 (9) | 26 (26) |
| Not reported | 2 (2) | 1 (1) |
MEDI-CAL Medi-Cal= is free or low-cost health coverage for children and adults with limited income and resources; MEDI-CAL MANAGED CARE=Medi-Cal Managed Care provides high quality, accessible, and cost-effective health care through managed care delivery systems; MEDICARE MANAGED CARE=A type of Medicare health plan offered by a private company that contracts with Medicare to provide you with all your Part A and Part B benefits.
In-reach Group
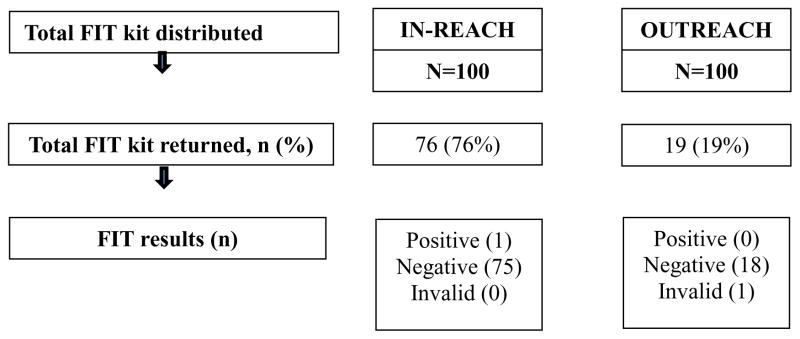
In-reach participants had average age of 58.7 years (SD=6.3), and most (85%) had health insurance. Most participants were female (82%) and reported their primary language as Spanish (88%) (Table 1). The screening rate for participants in the in-reach intervention was 76%. The median time from the in-reach visit to having the results in the EMR was 16 days (range 9–32 days) (Table 2). One participant had an abnormal FIT test result. This patient received a referral to a diagnostic colonoscopy which was completed within two months from referral, and results indicated no abnormality (Figure 1).
Table 2.
FIT return information for outreach intervention, N=100
| Frequency (n) | |
|---|---|
| Patient recall of mailing receipt based on telephone follow up (n=96)* | |
| Yes | 54 |
| No | 12 |
| Not Sure | 2 |
| Never reached by phone | 26 |
| Time from mailing date to result date (days) | |
| Range | 13–136 |
| Median [IQR] | 46 [21–83] |
| Time from initial mailing to FIT collection date*(days) | |
| Range | 7–135 |
| Median [IQR] | 36 [10–82] |
Excludes 4 individuals with returned mail; IQR-Interquartile range
Figure 1. Primary outcome results by study intervention, N=200.
Note: Among the 100 individuals in the in-clinic intervention who completed the FIT test, one (1%) was positive. A diagnostic colonoscopy was completed after 2 months, and it was declared normal.
Outreach Group
Outreach participants had average age of 59.6 years (SD= 6.0) and 50% had health insurance. Most were female (64%) and reported their primary language as Spanish (73%) (Table 1). The screening rate in the outreach group was 19%. Of those who did not return a FIT kit within three months, 14% reported that they did not recall receiving the FIT kit mailing and 26% could not be reached by phone. Four of the mailings were returned to the sender. A median of 46 days was recorded from the time the mailing was sent and the result was reported in the EMR (Table 3). No participants in the outreach group had an abnormal FIT (Figure 1).
Table 3.
FIT return information for in-reach intervention, N=100
| Frequency (n) | |
|---|---|
| Time from in-clinic visit date to FIT result date (days) | |
| Range | 4–11 |
| Median [IQR] | 16 [9–31.5] |
| Time from in-clinic visit date to FIT collection date*(days) | |
| Range | 1–108 |
| Median [IQR] | 8.5 [5–28] |
Includes actual collection date or date designated by lab; IQR-Interquartile range
Discussion
This study took place in a large FQHC that did not have an organized CRC screening program at the time of the study. Results showed that the in-reach intervention (e.g., point of service inclinic educational encounter delivered by promotoras in addition to handing out the FIT kit) resulted in a higher proportion completing FIT screening (76%) as compared to mailed outreach (e.g., print media plus FIT kit mailing; 19%) (Table 1). Phone reminders were utilized in both interventions that at the time of the study were not part of usual care. Proactive phone reminders are an effective way to help minimize clinic no-show rates and lack of patient follow-up for abnormal test results. These data demonstrate that evidence-based strategies to promote CRC screening can be implemented successfully within FQHCs, but implementation (particularly of mailed outreach) may require setting and population-specific optimization. The distinct recruitment approaches used for each intervention preclude direct formal statistical comparison of these strategies. While patient-level responses in our pilot study appear to be much higher for the in-reach vs. outreach strategy, we postulate a randomized trial is required to determine the comparative population benefits of these strategies on screening completion, and, based on our pilot work have initiated such a trial (clinicaltrials.gov ID NCT02870049).
There are few randomized trials that have focused on in-reach or outreach strategies for promoting screening using samples that included substantial (≥25%) Latino representation. For example, Jandorf and colleagues24 studied the effects of in-reach strategy after physician recommendation for FOBT and/or endoscopic screening (sigmoidoscopy or colonoscopy) in a New York City FQHC setting and found that the in-reach navigation resulted in a non-statistically significant 17% increase in FOBT completion, and a statistically significant 19% increase in endoscopic screening completion. Other researchers have examined mailed outreach strategies in samples of Latino patients. For example, Walsh et al., Coronado et al., and Gupta et al., have both reported increased screening completion (from 7% to 35% compared to usual care) associated with mailed invitation to complete FOBT with and without phone follow up25–27.
Our study was based at an FQHC serving a population of predominantly low-income Latinos in the border region of San Diego, California, most of which were women participants, which may limit the generalizability of findings to other geographically distinct Latino groups, non-Latino populations, or those with higher SES. This particular FQHC currently has 90,000 patients. Nationwide, FQHCs serve over 21 million patients annually, of which approximately 5 million are uninsured. Methods that work in this FQHC may be disseminated to other FQHCs serving similar low income Latino populations35. This study only utilized recruitment and study-related data from the EHR, without self-report data, and thus was limited in terms of being able to analyze patient-level mediators to CRC screening (e.g., cancer knowledge, beliefs, and health behaviors31,36–38). Time constraints limited our ability to assess CRC screening beyond three month follow-up. Selection bias may have influenced recruitment for the in-reach intervention because patients who were in obvious distress or pain were not approached. Implementing the interventions in tandem limited our ability to truly compare the two interventions directly. In addition, the clinic only offered stool tests or referrals to specialty screening for CRC, which presents barriers for uninsured patients. These barriers were minimized by the study covering the FIT costs and the development of a partnership with a local charity care network that provides screening colonoscopies at no cost for uninsured patients and linkages to treatment.
Conclusion
Regular use of CRC screening increases early detection and reduces the morbidity of late-stage diagnoses. This study demonstrates that evidence-based CRC screening strategies can be successfully implemented in FQHC environtments and thereby have great potential to reduce disparities through dissemination in the national FQHC and FQHC-look alikes nationwide. Future studies are needed to assess long-term outcomes of clinic-based CRC screening interventions within the context of the patient-centered medical home.
Acknowledgments
This research was made possible by the SDSU/UCSD Cancer Center Comprehensive Partnership NCI U54 CA13238406A1 (Arredondo, PI) and U54 CA13237906A1 (Martinez, PI); NCI R21 CA112368 (Talavera, PI); Redes en Acción NCI U54 CA153511 (Ramirez, PI/Talavera, PI); California Colorectal Cancer Coalition (C4); “Primary Care Systems” Walgreen’s for Change/American Cancer Society (ACS) award. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, C4, or the ACS. Special thanks go to patients at San Ysidro Health Center, Inc. who made this research possible.
Footnotes
Conflicts of interest: Authors Castañeda, Bharti, Espinoza-Giacinto, Sanchez, O’Connell, Muñoz, Mercado, Meza, Rojas, Talavera and Gupta declare that they have no conflicts of interest.
Ethical Standards Statement: This research study involved human subjects and was approved by the San Diego State University and San Ysidro Health Center, Inc. institutional review boards. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all participants for being included in the study.
References
- 1.American Cancer Society (ACS) [Accessed February 14, 2008];California Cancer Facts & Figures, 2008. 2008 http://www.ccrcal.org/PDF/ACS2008.pdf.
- 2.California Cancer Registry. [Accessed August 1, 2008];Public Use Data Set: California Cancer Incidence and Mortality Rates Plus Interactive Maps. 2008 http://www.ccrcal.org/dataquery.html.
- 3.American Cancer Society. Cancer Facts & Figures, 2014. 2014 http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf.
- 4.American Cancer Society (ACS) Cancer Facts & Figures for Hispanics/Latinos 2012–2014. Atlanta, GA: American Cancer Society; 2012. [Google Scholar]
- 5.American Cancer Society (ACS) [Accessed February 1, 2008];Colorectal Cancer Facts & Figures, 2014–2016. 2014 http://www.cancer.org/acs/groups/content/documents/document/acspc-042280.pdf.
- 6.American Cancer Society. Cancer Facts and Figures for Hispanics/Latinos 2015–2017. 2017. [Google Scholar]
- 7.Zambrana RE, Breen N, Fox SA, Gutierrez-Mohamed ML. Use of cancer screening practices by Hispanic women: analyses by subgroup. Preventive medicine. 1999;29(6 Pt 1):466–477. doi: 10.1006/pmed.1999.0566. [DOI] [PubMed] [Google Scholar]
- 8.National Cancer Institute. [Accessed December 7, 2007];Breast Cancer Prevention (PDQ®) 2007 http://www.cancer.gov/cancertopics/pdq/prevention/breast/Patient/
- 9.Elmore JG, Armstrong K, Lehman CD, Fletcher SW. Screening for breast cancer. JAMA. 2005;293(10):1245–1256. doi: 10.1001/jama.293.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elmore JG, Reisch LM, Barton MB, et al. Efficacy of breast cancer screening in the community according to risk level. J Natl Cancer Inst. 2005;97(14):1035–1043. doi: 10.1093/jnci/dji183. [DOI] [PubMed] [Google Scholar]
- 11.Wells KJ, Roetzheim RG. Health disparities in receipt of screening mammography in latinas: a critical review of recent literature. Cancer Control. 2007;14(4):369–379. doi: 10.1177/107327480701400407. [DOI] [PubMed] [Google Scholar]
- 12.Vernon SW, McQueen A, Tiro JA, del Junco DJ. Interventions to promote repeat breast cancer screening with mammography: a systematic review and meta-analysis. J Natl Cancer Inst. 2010;102(14):1023–1039. doi: 10.1093/jnci/djq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151(10):738–747. doi: 10.1059/0003-4819-151-10-200911170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frazier EL, Jiles RB, Mayberry R. Use of screening mammography and clinical breast examinations among black, Hispanic, and white women. Preventive medicine. 1996;25(2):118–125. doi: 10.1006/pmed.1996.0037. [DOI] [PubMed] [Google Scholar]
- 15.Hubbell FA, Chavez LR, Mishra SI, Valdez RB. Differing beliefs about breast cancer among Latinas and Anglo women. West J Med. 1996;164(5):405–409. [PMC free article] [PubMed] [Google Scholar]
- 16.U.S. Preventive Services Task Force. [Accessed December 15, 2016];Final Update Summary: Colorectal Cancer: Screening. 2016 https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/colorectal-cancer-screening2?ds=1&s=colorectal.
- 17.Phillips CE, Rothstein JD, Beaver K, Sherman BJ, Freund KM, Battaglia TA. Patient navigation to increase mammography screening among inner city women. Journal of general internal medicine. 2011;26(2):123–129. doi: 10.1007/s11606-010-1527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramirez AG, Perez-Stable EJ, Talavera GA, et al. Time to definitive diagnosis of breast cancer in Latina and non-Hispanic white women: the six cities study. Springerplus. 2013;2(1):84. doi: 10.1186/2193-1801-2-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabatino SA, Lawrence B, Elder R, et al. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: nine updated systematic reviews for the guide to community preventive services. American journal of preventive medicine. 2012;43(1):97–118. doi: 10.1016/j.amepre.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Corcoran J, Dattalo P, Crowley M. Interventions to increase mammography rates among U.S. Latinas: a systematic review. Journal of women’s health. 2010;19(7):1281–1288. doi: 10.1089/jwh.2009.1621. [DOI] [PubMed] [Google Scholar]
- 21.Levin TR, Jamieson L, Burley DA, Reyes J, Oehrli M, Caldwell C. Organized colorectal cancer screening in integrated health care systems. Epidemiologic reviews. 2011;33(1):101–110. doi: 10.1093/epirev/mxr007. [DOI] [PubMed] [Google Scholar]
- 22.Gupta S, Sussman DAC, et al. Challenges and Possible Solutions to Colorectal Cancer Screening for the Underserved. J Natl Cancer Inst. 2014;106(4) doi: 10.1093/jnci/dju032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quinones AR, Talavera GA, Castaneda SF, Saha S. Interventions that Reach into Communities--Promising Directions for Reducing Racial and Ethnic Disparities in Healthcare. J Racial Ethn Health Disparities. 2015;2(3):336–340. doi: 10.1007/s40615-014-0078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jandorf L, Gutierrez Y, Lopez J, Christie J, Itzkowitz SH. Use of a patient navigator to increase colorectal cancer screening in an urban neighborhood health clinic. Journal of urban health: bulletin of the New York Academy of Medicine. 2005;82(2):216–224. doi: 10.1093/jurban/jti046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coronado GD, Golovaty I, Longton G, Levy L, Jimenez R. Effectiveness of a clinic-based colorectal cancer screening promotion program for underserved Hispanics. Cancer. 2011;117(8):1745–1754. doi: 10.1002/cncr.25730. [DOI] [PubMed] [Google Scholar]
- 26.Gupta S, Halm EA, Rockey DC, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Intern Med. 2013;173(18):1725–1732. doi: 10.1001/jamainternmed.2013.9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh JM, Salazar R, Terdiman JP, Gildengorin G, Perez-Stable EJ. Promoting use of colorectal cancer screening tests. Can we change physician behavior? J Gen Intern Med. 2005;20(12):1097–1101. doi: 10.1111/j.1525-1497.2005.0245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Susan G. Community Profile Report. 4699 Murphy Canyon Road, Suite #207, San Diego, CA 921232011: Komen for the Cure San Diego Affiliate. [Google Scholar]
- 29.Health Resources & Service Administration. 2015 Health Center Profile. CENTRO DE SALUD DE LA COMUNIDAD SAN YSIDRO, INC; SAN YSIDRO, CALIFORNIA: 2016. https://bphc.hrsa.gov/uds/datacenter.aspx?q=d&bid=091080&state=CA. [Google Scholar]
- 30.Findley S, Matos S, Hicks A, Chang J, Reich D. Community health worker integration into the health care team accomplishes the triple aim in a patient-centered medical home: a Bronx tale. The Journal of ambulatory care management. 2014;37(1):82–91. doi: 10.1097/JAC.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 31.Castaneda SF, Giacinto RE, Medeiros EA, et al. Academic-Community Partnership to Develop a Patient-Centered Breast Cancer Risk Reduction Program for Latina Primary Care Patients. J Racial Ethn Health Disparities. 2016;3(2):189–199. doi: 10.1007/s40615-015-0125-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castaneda SF, Xiong Y, Gallo LC, et al. Colorectal cancer educational intervention targeting latino patients attending a community health center. J Prim Care Community Health. 2012;3(3):164–169. doi: 10.1177/2150131911427731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta S, Miller S, Koch M, et al. Financial Incentives for Promoting Colorectal Cancer Screening: A Randomized, Comparative Effectiveness Trial. Am J Gastroenterol. 2016;111(11):1630–1636. doi: 10.1038/ajg.2016.286. [DOI] [PubMed] [Google Scholar]
- 34.Singal AG, Gupta S, Tiro JA, et al. Outreach invitations for FIT and colonoscopy improve colorectal cancer screening rates: A randomized controlled trial in a safety-net health system. Cancer. 2016;122(3):456–463. doi: 10.1002/cncr.29770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaiser Family Foundation. Community Health Centers: A 2013 Profile and Prospects as ACA Implementation Proceeds. 2015 http://kff.org/report-section/community-health-centers-a-2013-profile-and-prospects-as-aca-implementation-proceeds-issue-brief/
- 36.Castaneda SF, Holscher J, Mumman MK, et al. Dimensions of community and organizational readiness for change. Prog Community Health Partnersh. 2012;6(2):219–226. doi: 10.1353/cpr.2012.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goebel M, Singal AG, Nodora J, et al. How can we boost colorectal and hepatocellular cancer screening among underserved populations? Curr Gastroenterol Rep. 2015;17(6):22. doi: 10.1007/s11894-015-0445-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez P, Castaneda SF, Mills PJ, Talavera GA, Elder JP, Gallo LC. Determinants of breast, cervical and colorectal cancer screening adherence in Mexican-American women. J Community Health. 2012;37(2):421–433. doi: 10.1007/s10900-011-9459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]