Abstract
The calmodulin-binding transcription activators (CAMTAs) transcription factor family plays an important role in normal plant growth and development, as well as in biotic and abiotic stress resistance. In this study, we identified seven CAMTA genes across the whole genome of Populus trichocarpa and analyzed the expression patterns of PtCAMTAs in the root and leaf tissues. Promoter cis-element analysis indicated that most CAMTA genes contained stress- or phytohormone-related cis-elements. Quantitative real-time reverse transcription-PCR (qRT-PCR) indicated indicated that PtCAMTAs were induced by mannitol, NaCl, cold stress, pathogenic infection with A. alternata, and phytohormone treatments with abscisic acid, salicylic acid, and methyl jasmonate. We analyzed the expression of homologous genes between P. trichocarpa and P. ussuriensis and alternative splicing forms of PtCAMTA genes under cold stress. We also performed a network interaction analysis for PtCAMTA proteins to predict their interactions and associations. The results of the present study serve as a basis for future functional studies on the Populus CAMTA family.
Introduction
The divalent cation Ca2+, a universal secondary messenger in eukaryotic organisms, is employed by plants. Ca2+ signals are sensed through the actions of Ca2+-binding proteins, which contain the highly conserved Ca2+-binding ‘EF-hand’ motif that exhibits different configurations depending on the Ca2+ loading status1. In recent years, three classes of Ca2+ messenger, involving calmodulins and calmodulin-like proteins (CaMs and CMLs), calcium-dependent protein kinases (CDPKs), and calcineurin B-like proteins (CBLs) have been characterized in plants2. To date, it has been determined that CaMs can regulate at least 90 transcription factors, including calmodulin-binding transcription activators (CAMTAs)3.
CAMTAs, as a family of conserved transcription factors, have been found to exist in all multicellular eukaryotes and constitute the main transcription factors regulated by calmodulin. CAMTAs contain a specific CG-1 DNA-binding domain in the N terminus, followed by a TIG domain involved in non-specific DNA binding, an ankyrin (ANK) repeats domain, a Ca2+ dependent CaM binding domain (CaMBD) that is located between the N terminus and C terminus, and a different number of IQ motifs that interact with CaM in a Ca2+-independent pattern4,5. To date, several CAMTA genes from different plant species have been characterized, including Arabidopsis, Medicago truncatula, soybean, and maize6–8. The Arabidopsis CAMTA proteins group into four classes, namely, Ia, IIa, IIIc and IIIe, and the IIIe CAMTAs mostly constitute the non-TIG type with duplication occurring only in some species.
CAMTA transcription factors play an important role in plant growth and development, as well as in biotic and abiotic stress responses, particularly cold stress. Studies indicate that AtCAMTA3 is a positive regulator of CBF2 expression, which is known to be involved in the cold stress response. AtCAMTA1 and the AtCAMTA3 double mutant were discovered to have low tolerance to freezing9. In addition, AtCAMTA3 was found to possess 10 interactors, including CBF2. AtCAMTA1 is also involved in the expression regulation of a broad spectrum of membrane integrity response genes via the generating of an ABA response to drought stress10. The CAMTA homologs were able to recognize the (A/C)CGTGT DNA motif, which encompasses a classic abscisic acid (ABA)-responsive element (ABRE, ACGTGT)11,12. Furthermore, the maize ZmCAMTA1 was significantly reduced by cold treatment in the roots. The majority of MtCAMTA genes have been proven to respond to hormones such as SA, MeJA, and ABA, suggesting that CAMTA-mediated abiotic and biotic stress tolerance may exist in different plant species.
Populus trichocarpa is widely used in functional forest tree genomics studies and has significant commercial and ecological value13. Populus trichocarpa is threatened by a multitude of environmental stresses and biotic stresses such as drought and fungal disease during growth and development. Although CAMTA genes have been studied in some plants, research into the CAMTA gene family in Populus is limited. We identified seven CAMTA genes in P. trichocarpa and analyzed their phylogenetic relationships, chromosomal locations, gene duplication events, and gene structures. The expression mode of PtCAMTAs under abiotic stress (mannitol, NaCl, 4 °C), biotic stress (Alternaria alternate infection), and phytohormone treatment, including abscisic acid (ABA), salicylic acid (SA), and methyl jasmonate (MeJA), were analyzed using quantitative real-time RT-PCR (qRT-PCR). We also analyzed the expression of homologous PtCAMTA genes between P. trichocarpa and P. ussuriensis and alternative splicing forms of PtCAMTA genes under cold stress. The results may support further functional gene research through the study of these candidate CAMTA genes in response to abiotic and biotic stress.
Results
Identification, phylogenetic relationships, gene structure, conserved domain and alternative splicing analyses of PtCAMTA genes
We identified a total of seven CAMTA genes in P. trichocarpa, which encoded proteins that varied in length from 907 to 1,116 amino acids (aa), with an average length of 1,000 aa. The CAMTA protein sequences showed large variations in isoelectric point (pI) values (ranging from 5.45 to 8.27) and molecular weight (ranging from 102.195 kDa to 126.102 kDa). The location of the PtCAMTA proteins was predicted to be the cell nucleus using Wolf PSORT (Table 1).
Table 1.
The CAMTA gene family in Populus trichocarpa.
| Gene name | Transcript Name | Accession number | NCBI locus ID | Length (aa) | MW (Da) | pI | Localization |
|---|---|---|---|---|---|---|---|
| PtCAMTA1 | Potri.001G057800.1 | POPTR_0001s13700 | XM_006368809.1 | 998 | 111418.7 | 5.45 | nucl |
| PtCAMTA2 | Potri.005G075100.1 | POPTR_0005s07660 | XM_002307047.2 | 1116 | 126102.3 | 5.52 | nucl |
| PtCAMTA3 | Potri.007G093400.1 | POPTR_0007s05410 | XM_002310526.2 | 1091 | 122444.9 | 5.49 | nucl |
| PtCAMTA4 | Potri.010G141700.1 | POPTR_0010s15160 | XM_002314890.2 | 915 | 102868.2 | 8.27 | nucl |
| PtCAMTA5 | Potri.010G153100.1 | POPTR_0010s16290 | XM_002316035.2 | 999 | 111276 | 7.14 | nucl |
| PtCAMTA6 | Potri.008G107900.1 | POPTR_0008s10730 | XM_002311358.2 | 907 | 102194.5 | 6.70 | nucl |
| PtCAMTA7 | Potri.003G170600.1 | POPTR_0003s16910 | XM_002303751.2 | 980 | 109542.2 | 5.53 | nucl |
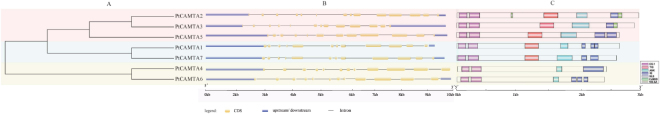
To examine the phylogenetic relationships among the CAMTA domain proteins in P. trichocarpa, an unrooted phylogenetic tree was constructed from the full-length CAMTA sequence alignments. We classified seven CAMTA genes into three subgroups according to their homology (Fig. 1A). A comparison of the exon/intron organization of the coding sequences of individual PtCAMTA genes showed a similar exon-intron structural pattern, indicating a necessary conservation of the genomic structure of PtCAMTA genes (Fig. 1B). The conserved domains of the CAMTAs, involving a CG-1 DNA binding domain, a TIG domain, ankyrin repeats, and one or two copies of IQ motifs were predicted in the PtCAMTA proteins (Fig. 1C).
Figure 1.
Phylogenetic relationships and gene structure of Populus CAMTAs. (A) Multiple alignment of full-length amino acid sequences of Populus CAMTA genes, executed in ClustalX 1.83. The phylogenetic tree was constructed using the neighbor-joining method in MEGA 5.0. Support values from a bootstrap analysis with 1,000 replicates are specified at each node. The three major phylogenetic subgroups are marked with different colored backgrounds. (B) Exon/intron structures. Exons and introns are represented by particular colored boxes and black lines, respectively. (C) Bioinformatics analysis of the conserved domains was conducted in the Pfam database (http://pfam.janelia.org/). Cam-binding domains (CaMBD) were specifically searched in the Calmodulin Target Database (http://calcium.uhnres.utoronto.ca/ctdb/ctdb/).
We analyzed the PtCAMTAs primary cDNAs and the genomic DNA sequences. The results showed that PtCAMTA1-7 produced splice variants. The number of unigenes corresponding to splice variants was two in PtCAMTA1, 4, 6, and 7; three in PtCAMTA3; four in PtCAMTA2 and five in PtCAMTA5. There are four major alternative splicing types of PtCAMTAs: intron retention (PtCAMTA1.2, 2.3, 3.2, 3.3, 4.2, 5.2 and 5.5); alternative 5′ splice site (PtCAMTA2.2,5.3, 6.3); alternative 3′ splice site (PtCAMTA6.2, 2.4); and cassette exons (PtCAMTA7.2) (Supplementary Figure S1).
Chromosomal location and duplication of PtCAMTA genes
To verify the relationship between genetic divergence and gene duplication, we identified the chromosomal locations of PtCAMTA genes. As shown in Supplementary Figure S2, PtCAMTA genes were characterized by an obvious feature whereby all the genes were distributed on chromosomes I, III, V, VII, VIII, and X, respectively. There were two PtCAMTA genes (PtCAMTA3 and PtCAMTA6) on chromosome X. The rest of the PtCAMTA genes were detected on each of chromosomes I, III, V, VII, and VIII. In order to confirm the relationship between the CAMTA genes and potential segmental duplications, we used the duplicated blocks set up in a previous study. The distribution of the duplicate blocks related to CAMTA genes is illustrated in Supplementary Figure S2. We discovered that two of the seven PtCAMTA (PtCAMTA1 and PtCAMTA7) genes were present in both duplicated regions and were thus prioritized, as the others were only present in one of the blocks. The history of selection acting on coding sequences can be measured on the basis of the ratio of nonsynonymous to synonymous substitutions (Ka/Ks). A pair of homologous sequences will have Ka/Ks < 1 if one sequence has been under the select of purification but the other has been drifting neutrally, while Ka/Ks = 1 when both sequences are drifting neutrally and peculiarly, and Ka/Ks > 1 at specific sites that are under positive selection14. A summary of Ka/Ks for three CAMTA duplicated pairs shown in Supplementary Table S1 was less than 0.7. This result suggests that all gene pairs have evolved mainly under the influence of purifying selection. Based on the divergence rate of 6.1 × 10−9 synonymous mutations per synonymous site per year as previously presented for populus15, duplications of these three paralogous pair genes was estimated to have taken place between14.7 to 147.4 Mya (Supplementary Table S1).
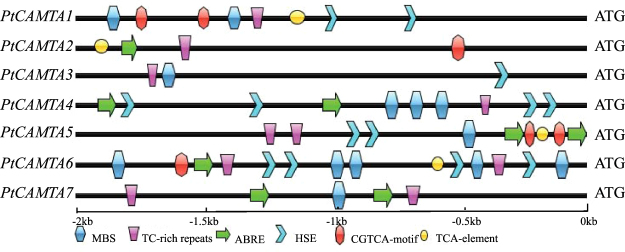
Promoter cis-element analysis
We identified putative cis-acting regulatory DNA elements via the promoter sequences of PtCAMTA genes (2,000 bp upstream of the translation start site) based on the Phytozome version 12.1 database. The CAMTA gene family promoter sequences demonstrated that several cis-elements were related to biotic and abiotic stress responsiveness (Fig. 2). In total, 10 types of abiotic stress elements were identified. Nearly all the PtCAMTA genes had MBS elements and five had W-box in their promoters, which showed that the MYB binding site is involved in drought inducibility. Three of the PtCAMTA genes (PtCAMTA4, PtCAMTA5, and PtCAMTA6) possessed AREB-responsive elements (ABREs). Nearly all the PtCAMTA genes possessed MeJA-responsive elements (CGTCA-motif, G-Box, TGACG-motif), except PtCAMTA6. Four of the PtCAMTA genes (PtCAMTA1, PtCAMTA3, PtCAMTA5, and PtCAMTA6) possessed SA-responsive elements (TCA-element; Supplementary Table S2).
Figure 2.
Abiotic stress and phytohormone response elements in PtCAMTA genes promoter.
Tissue-specific expression profile
We observed specific expression patterns in the different tissues of PtCAMTA genes from the Affymetrix (GSE6422) microarray data in PopGeneIE version 3.0 (Supplementary Figure S3). The microarray data showed high expression levels of PtCAMTA genes in the roots, with PtCAMTA3 exhibiting particularly high expression levels among all the PtCAMTA genes. In the leaves, only PtCAMTA1 was highly expressed in the mature leaves. We found that PtCAMTA2 and PtCAMTA3 displayed low expression levels in the mature leaves and high expression levels in the roots, simultaneously. Notably, the expression level of all PtCAMTAs in the young leaves was very low.
Expression analysis of PtCAMTA genes under mannitol and NaCl stress
In order to understand how PtCAMTA genes react under osmotic stress, we analyzed the expression of the PtCAMTAs in the roots and leaves under treatment with 200 mM mannitol and 150 mM NaCl for 3 h, 6 h, 12 h, 24 h, and 7d, respectively. Genes that were up or downregulated by more than 2.0-fold were considered significantly differentially expressed16. Under mannitol stress, all the genes were upregulated in both the roots and leaves in the short-term treatments (3 h, 6 h, 12 h, and 24 h). Changes in the expression of PtCAMTA3, 6, and 7 were not obvious in the roots and leaves in the long term (7 d). Notably, PtCAMTA1, 4, and 5 were significantly upregulated (>5.0-fold relative to the control) in the roots. PtCAMTA1-6 was significantly upregulated in the leaves. Under NaCl stress, all the PtCAMTA genes were suppressed in the roots at all time points. PtCAMTA genes were upregulated in the leaves under short-term stress and showed no change under long-term stress (Fig. 3).
Figure 3.
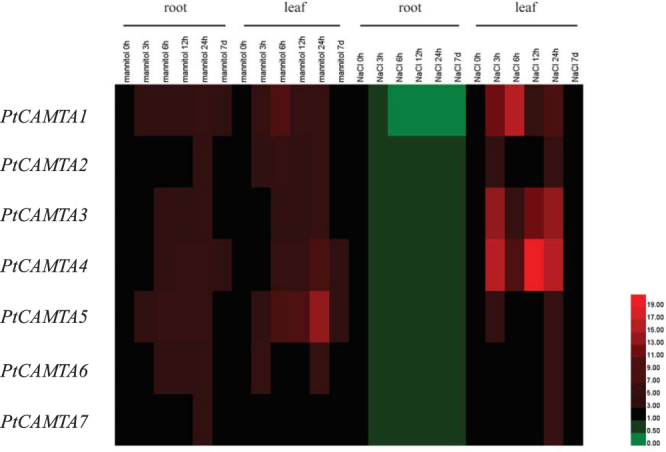
Expression analysis of PtCAMTA genes in the roots and leaves under mannitol and NaCl stress by qRT-PCR. Red and green indicate high and low levels of transcript abundances, respectively.
The expression of PtCAMTA genes induced by pathogenic infection with A. alternata
Poplar leaf blight, caused by the pathogen A. alternata, is a common disease in northeast China. In order to study the expression of PtCAMTA genes under biotic stress, we infected the leaves of P. trichocarpa with A. alternata in vitro. Following infection, two CAMTA genes (PtCAMTA1 and 2) were induced, four CAMTA genes (PtCAMTA3, 4, 6, and 7) were suppressed, and PtCAMTA5 showed no change. PtCAMTA1, 2, and 4 were induced at 24 h after infection (Fig. 4).
Figure 4.
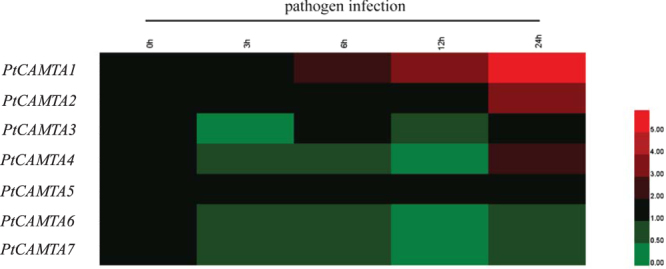
Expression analysis of PtCAMTA genes in leaves under pathogen infection by qRT-PCR. Red and green indicate high and low levels of transcript abundances, respectively.
Expression levels of PtCAMTA genes in response to phytohormone stimuli
To understand how PtCAMTA genes participate in stress-related hormone responses, we analyzed the expression of PtCAMTA genes under 200 μM ABA, 100 μM SA, and 100 μM MeJA in the leaves and roots for 3 h, 6 h, 12 h, 24 h, and 7d using qRT-PCR. Under ABA stress, three genes were upregulated before 6 h stress, and four genes were downregulated in the roots, while in the leaves all the genes were upregulated at all time points. Five CAMTA genes (PtCAMTA1, 2, 3, 6, and 7) were significantly upregulated in the short term. Under SA stress, all genes were significantly downregulated in the roots. Four genes (PtCAMTA1, 5, 6, and 7) were upregulated in the leaves, whereas PtCAMTA2, 3, and 4 were downregulated. Under MeJA stress, four genes (PtCAMTA1, 5, 6, and 7) were upregulated, two (PtCAMTA3 and 4) were downregulated, and PtCAMTA2 exhibited no change in the roots. With respect to the leaves, three genes (PtCAMTA1, 4, and 7) were upregulated and three (PtCAMTA2, 3, and 5) were downregulated. PtCAMTA1 and 7 were induced in both the roots and leaves. Most CAMTA genes were downregulated in the roots under ABA, SA, and MeJA treatments (Fig. 5).
Figure 5.
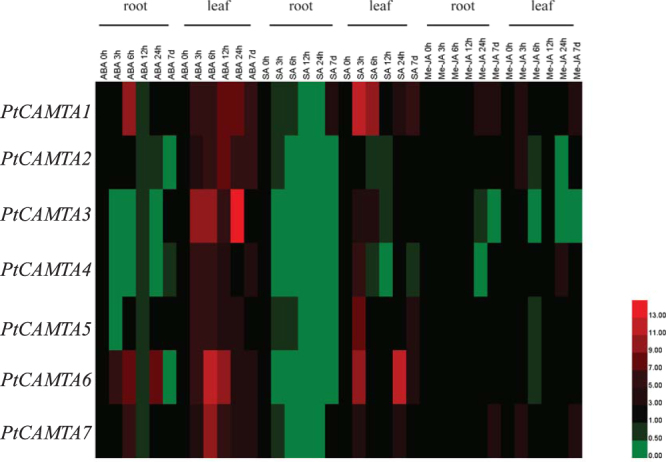
Expression analysis of PtCAMTA genes in the roots and leaves under ABA, SA, and MeJA treatments by qRT-PCR. Red and green indicate high and low levels of transcript abundances, respectively.
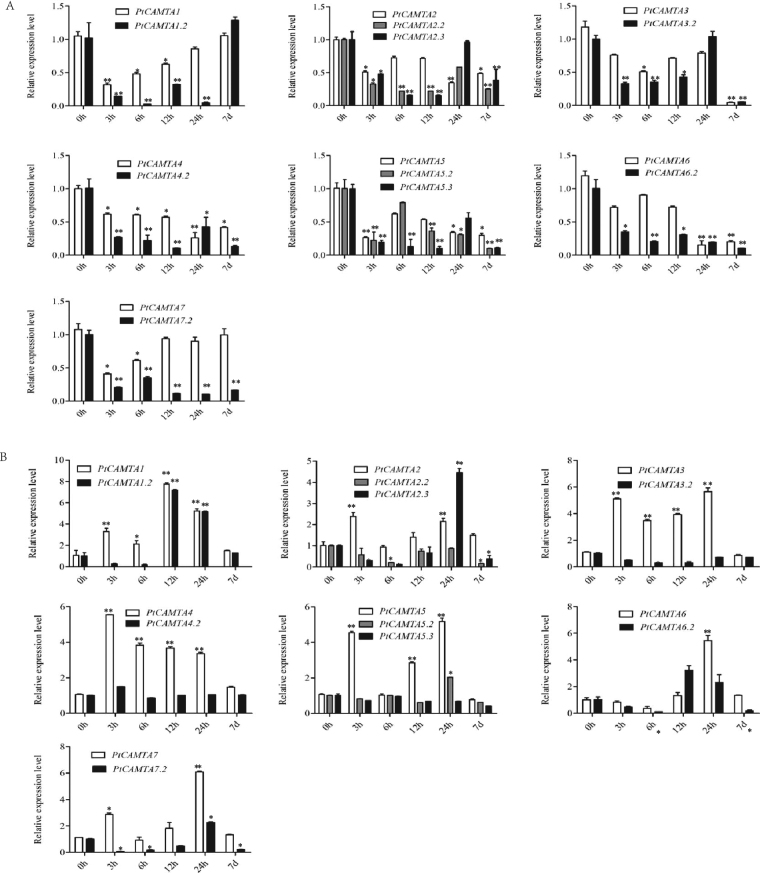
Expression analysis of PtCAMTA alternative splicing forms under cold stress
Alternative splicing, as a post-transcriptional mechanism of precursor-mRNA, plays a significant role in transcriptome and proteome diversity as well as transcript and protein abundance17,18. As the CAMTA gene family is mainly involved in the cold stress response, we studied the expression of several alternative splicing forms of PtCAMTA genes under cold stress in detail.
The analysis of qRT-PCR using splice variant-specific primers showed that specific amplicons could be obtained for PtCAMTA1.2, PtCAMTA2.2, 2.3, PtCAMTA3.2, PtCAMTA4.2, PtCAMTA5.2, 5.3, PtCAMTA6.2, and PtCAMTA7.2. Under cold treatment, most of the genes were downregulated in the roots in the short term, with the exception of PtCAMTA1 and PtCAMTA7. At 7 d, the expression of PtCAMTA2-6 in the roots was distinctly downregulated. The expression patterns of the splice variants in the roots were similar to the normal transcripts under cold treatment. Most of the splice variants of PtCAMTAs were downregulated in the roots under cold treatment. In the leaves, most of the genes were induced under cold treatment in the short time. PtCAMTA1.2, 2.3, and 6.2 were induced in the leaves in the short time. The expression of PtCAMTAs in the leaves showed no significant change in the long term, but all the splice variants of PtCAMTAs in the leaves exhibited a negative trend at the same condition. In conjunction, these results suggest that PtCAMTAs are important in cold-regulated gene expression (Fig. 6).
Figure 6.
Expression analysis of alternative splicing forms of PtCAMTA genes under 4 °C stress. (A) expression analysis of alternative splicing forms of PtCAMTA genes in the roots under 4 °C stress. (B) expression analysis of alternative splicing forms of PtCAMTA genes in the leaves under 4 °C stress. The x-axis represents time after the onset of stress treatments. Error bars represent the standard deviations of three biological replicates. Asterisks indicate stress treatment groups that showed a significant difference in transcript abundance compared with the control group (*P < 0.05, **P < 0.01).
Homologous PtCAMTA genes in two Populus varieties under cold stress
In order to gain further insight into the relationship between PtCAMTA gene expression and cold stress resistance, we compared the expression patterns of P. trichocarpa and P. ussuriensis (Sect. Tacamahaca: the same as P. trichocarpa. P. ussuriensis Kom), which are mainly distributed in the cold temperature zone of Northeast China and the far east region of Russia. Populus trichocarpa is a cold-tolerant species that can survive an annual average temperature of −3.8 °C as well as an extremely low temperature environment (−46.9 °C). Exhibiting strong resistance to cold, it constitutes the ideal candidate for studying the molecular mechanisms of woody plants. The results of the qRT-PCR revealed that the CAMTAs were differentially expressed in P. trichocarpa and P. ussuriensis under cold stress. For P. ussuriensis, the expression of most PuCAMTA genes was downregulated in the roots (except PuCAMTA4) and the leaves (except PuCAMTA1). The same trends were observed in P. trichocarpa, in that most of the PtCAMTAs were downregulated in the roots under cold treatment. However, most of the PtCAMTA genes were upregulated in the leaves in P. trichocarpa in the short term (Fig. 7).
Figure 7.

Expression analysis of CAMTA genes in the roots and leaves in P. trichocarpa and P. ussuriensis under 4 °C stress. Red and green indicate high and low levels of transcript abundances, respectively.
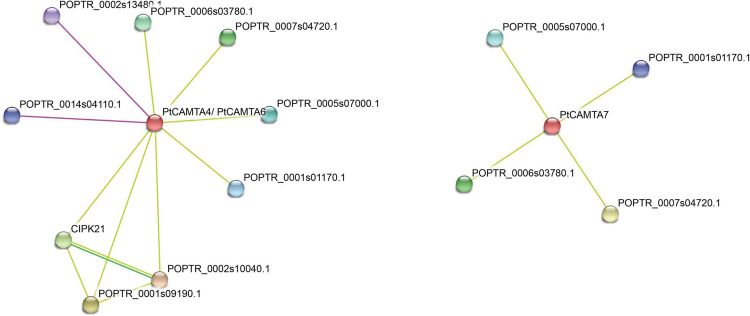
Protein interaction network analysis
In order to predict the interactions and associations of all the PtCAMTA proteins, we performed a network interaction analysis based on Arabidopsis proteins using STRING software, with the confidence value set at 0.5. An Arabidopsis CAMTA proteins network was created and 38 interactive proteins (confidence value = 0.5) were identified with the STRING database19. Then, the homologs of these 38 proteins in Populus were identified using Phytozome version 12.1. A total of eight unique proteins were predicted as potential interactors of PtCAMTA4, 6, and 7. The partners of PtCAMTA4 and 6 were predicted to be the Ca2+/CaM-regulated protein kinases CIPK5 and CIPK21. Moreover, PtCAMTA4 and PtCAMTA6 were identified as homologous proteins, which were predicted by STRING to directly interact or function in the same pathway (Fig. 8).
Figure 8.
Protein interaction network of PtCAMTA proteins. The potential interactors of PtCAMTA4/6 and PtCAMTA7 were predicted using STRING software. The various interactions are illustrated with different colored connective lines.
Studies have shown that CAMTA regulates the expression of the target gene by directly binding to the CGCG cis-elements of their promoter20. Rahman et al. have reported AtCAMTA3 protein can interacte with 10 proteins which contain at least one CGCG cis-element in their promoters in Arabidopsis 21. We found 16 homologous genes of the 10 AtCAMTA3 target genes in P. trichocarpa using the Phytozome version 12.1 database, six of which were homologous genes. However, we discovered that only four homologous genes possessed CGCG cis-elements in their promoters (2,000 bp upstream of the translation start site) of the AtCAMTA3 target genes in P. trichocarpa. (Supplementary Table S3)
Discussion
In this report, seven P. trichocarpa CAMTA family gene members were identified, each of which contained conserved domains related to CAMTA proteins. Previous studies have suggested that the Populus genome experienced at least three rounds of genome-wide duplication, including multiple segmental duplication, tandem duplication, and transposition events such as retroposition and replicative transposition. Tandem and segmental duplication plays an important role in genomic expansions and realignments22,23. In our study, all the PtCAMTA genes were located in the duplicated regions. We discovered that PtCAMTA1 and PtCAMTA7 belonged to the same branch of the phylogenetic tree and were present in the homologous regions of chromosomes I and III, respectively. This finding might be explained by the homology of the two genes. As found in the CAMTAs that have been characterized in other species, all seven of the PtCAMTAs contained conserved domains. In one of the subgroups that contained CaMBD, we identified variation in PtCAMTA2 and PtCAMTA3 that was not present in the other genes. Based on the phylogenetic tree, we discovered that a close relationship exists between PtCAMTA2 and PtCAMTA3. Interestingly, PtCAMTA2 and 3 were found to be closely associated with AtCAMTA3. These have been well studied and are known to participate together in SA-mediated defense responses and cold tolerance24. These results suggest that CAMTA genes have divergent functions in P. trichocarpa.
Promoter cis-elements play crucial roles in the response to biotic and abiotic stresses25. In this study, we identified many abiotic stress responsiveness cis-elements in the promoters of PtCAMTA family genes, including MBS, ABRE, TCA-element, G-Box, and W-Box. In particular, PtCAMTA5 was found to possess seven abiotic stresses responsiveness cis-elements, suggesting important functions under abiotic stress. Interestingly, PtCAMTA1, 2, and 3 did not contain ABRE elements in their −2kb promoters, but their responsiveness to ABA treatment was clear. We can thus speculate that it is inaccurate to assume a correlation between the existence and responsiveness to the related stress treatments. The same conditions were found in soybean, in that GmCAMTA7 and GmCAMTA9 contained ABRE elements in their promoters, but their responsiveness to ABA treatment was not obvious7. In particular, most PtCAMTA genes have SA and MeJA related cis-elements (TCA-element, and G-Box, CGTCA-motif, and TGACG-motif), but no fungus related cis-element. The qRT-PCR results showed that PtCAMTA2 and PtCAMTA3 were induced not only by SA and MeJA, but also by infection with A. alternata. This result suggests that a close relationship exists between SA and MeJA with regards to biotic stress.
Plants are often affected by abiotic and biotic stress in the process of growth and development. Under mannitol and NaCl stress, all the PtCAMTAs were induced in the leaves. Conversely, all the PtCAMTA genes were downregulated in the roots under NaCl stress. We suggested that all the PtCAMTA genes have significant functions under mannitol and NaCl stress. Previous studies have shown that all the GmCAMTA genes and the majority of ZmCAMTA genes are induced in the roots under drought and NaCl conditions, indicating that different expression patterns exist between woody plants and crops8. Alternaria alternata is a fungus that has been recorded to cause leaf spot and other diseases on over 380 host species of plant26. In the present study, all PtCAMTA genes were differentially expressed in the leaves under A. alternata infection. Three PtCAMTA genes were upregulated under the 24 h infection period, while four were downregulated. A previous study showed that the expression of all but one of the MtCAMTA genes was significantly downregulated under biotic stress (Sinorhizobium meliloti infection)6. These results indicate different expression patterns of CAMTA genes under biotic stress across a variety of species. In particular, four PtCAMTA genes that were downregulated under A. alternata infection exhibited similar expression patterns under SA and MeJA stress. This result indicates that PtCAMTA genes are involved in the pathogen expression network related to SA and MeJA pathways.
Research has indicated that a variety of hormone responses to biotic and abiotic stress could mediate the expression of CAMTA in Arabidopsis, including ABA (AtCAMTA2, 4, 5, and 6), SA (AtCAMTA2, 4, 5, and 6), and MeJA (AtCAMTA1, 3, and 4)27. It has been reported that ABA is involved in the mediation of drought stress, and that it is an important regulatory factor during drought stress28. Research has also demonstrated that SA and MeJA constitute the two major plant hormones that regulate plant biotic stress signal transduction29. In our study, most of the PtCAMTA genes were differentially expressed in the roots and leaves under ABA, MeJA, and SA treatments. This result indicates that phytohormones regulate the expression of PtCAMTA genes. PtCAMTA2 and PtCAMTA3, as homologous genes, were downregulated in the roots under ABA, MeJA, and SA treatment. Conversely, two soybean homolog genes (Glyma05g31190 and Glyma08g14370) in PtCAMTA2 and PtCAMTA3 were induced in the roots under ABA, SA, and MeJA treatment7. These variations indicate that different expression patterns of CAMTA genes under abiotic stress and phytohormone treatment exist between woody plants and crops.
Cold stress is a major environmental factor that can affect plant growth and development. Previous studies have shown that CAMTA transcription factors play an important role in cold regulation. AtCAMTA genes have been reported to be involved in cold stress, and the AtCAMTA1 and AtCAMTA3 double mutant in particular was found to have a negative effect on freezing tolerance30. Previous studies have shown that AtCAMTA genes might regulate freezing tolerance via the SA signaling pathway. AtCAMTA3 was found to be a negative regulator of the SA signaling pathway, and elevated levels of endogenous SA can enhance plant defense responses31. A study has shown that AtCAMTA3 is induced under cold stress, while AtCAMTA1 and AtCAMTA2 are downregulated under cold stress. Conversely, PtCAMTA2 and PtCAMTA3, the homologs of AtCAMTA1, 2 and 3, were reduced in P. trichocarpa. According to the expression of several alternative splicing forms of PtCAMTA genes, we found that most of the splice variants of the PtCAMTAs were also downregulated in the roots, but induced in the leaves under cold stress. Alternative splicing plays a key regulatory role in modulating gene expression during development, and in response to environmental stimuli32. In this study, PtCAMTA4.2 was more clearly expressed in leaves under cold stress. However, the expression of PtCAMTA4 was not significant. These might be related to the absence motifs of PtCAMTA4.2 alternative splicing variants which shown in Supplementary Figure S4. In conjunction, we believe these results indicate that PtCAMTA genes play an important role in the cold stress response, and that there are differences between P. trichocarpa and Arabidopsis in the response of CAMTA to cold stress. Furthermore, the expression patterns of CAMTA in P. trichocarpa differ from that of P. ussuriensis, in that most of the genes were clearly downregulated in the leaves under cold stress. We suggest that the differences in expression of the CAMTA genes under cold stress may be closely related to the differential cold tolerance of P. trichocarpa and P. ussuriensis.
In this study, we predicted eight potential PtCAMTA interactors using STRING software. The most consistent and interesting finding of this analysis was the different protein interactions between PtCAMTAs and AtCAMTAs. PtCAMTA2 and PtCAMTA3 share a high degree of homology with AtCAMTA3, which is known to be involved in both plant disease resistance and abiotic stress responses33,34. Rahman, H. et al. reported that AtCAMTA3 possessed 10 interactors that are DNA-binding transcription factors, including SRS, CBP60G, CM2, ICE1, XLG2, RHL41/ZAT12, CBF1, CBF2, EDS1, and EDS16/ICS1. However, no protein that interacts with PtCAMTA2 and PtCAMTA3 was predicted using STRING software in the present study. This might be related to the absence of the CGCG CAMTA-binding element in the promoter of the homologous genes of the AtCAMTA3 interactors in P. trichocarpa. This result indicates that the regulatory pathway of PtCAMTA proteins is not the same as AtCAMTA proteins. An earlier study showed that the 38 potential interactors of AtCAMTAs are related to Ca2+ signaling components, such as Ca2+/CaM-regulated protein kinases, Ca2+-dependent phospholipids, and CaM-binding proteins35. According to the protein interactions analyzed in this study, none were related to Ca2+ signaling components among the eight potential interactors of PtCAMTA proteins. We boldly speculate that other binding sites exist besides the CGCG CAMTA-binding element in the promoter of the homologous genes of the AtCAMTA3 interactors in P. trichocarpa that act with PtCAMTA proteins. Another speculation is that PtCAMTA proteins act with the other DNA binding transcription factors to regulate Ca2+-related biological processes. The initial results of the present study have provided essential information on the Populus CAMTA family that may serve as basis for future functional studies, and will facilitate future mechanistic research aiming to investigate the divergent roles of these genes.
Materials and Methods
Identification of PtCAMTA genes
Populus trichocarpa genome sequence data were downloaded from the Phytozome version 12.1 (http://phytozome.jgi.doe.gov/pz/portal.html) and NCBI (http://www.ncbi.nlm.nih.gov/) databases. WoLFPSORT (http://wolfpsort.org/)36 was used to predict the subcellular localization of the CAMTA proteins. The ExPasy site (http://web.expasy.org/protparam/)37 was used to calculate the molecular weight and isoelectric point (pI) of the deduced polypeptides. Multiple alignment of the PtCAMTA full-length protein sequences was performed using ClustalX (version 1.83) and aligned manually using BioEdit 7.1 software38.
Gene structure, chromosome localization, and gene duplications
The coding domain sequences (CDS) and DNA sequences of the P. trichocarpa CAMTA genes were used to reveal the gene structure using the Gene Structure Display Server (http://gsds.cbi.pku.edu.cn/index.php)39. The Multiple Expectation Maximization for Motif Elucidation (MEME) system (Version 4.9.1, http://meme.nbcr.net/meme/) was used to identify conserved motifs for each CAMTA gene40. The Softberry (http://linux1.softberry.com/berry.phtml?topic=fgenesh&group=programs&subgroup=gfind) was used to generate the exon/intron organization. Bioinformatics analysis of the conserved domains was conducted using the Pfam database (http://pfam.janelia.org/). The domain structures of the PtCAMTAs were drawn using Domain Illustrator software (http://dog.biocuckoo.org/)41. In order to confirm the chromosomal locations of the CAMTA genes, all PtCAMTA genes were obtained from the PopGenIE version 3 database (http://www.popgenie.org/). The PtCAMTA genes defined as separate by five or fewer gene loci within a genetic distance of 100 kb were considered to be tandem duplicates42. A schematic view of the reorganization of homologous chromosome segments was based on the most recent account of whole-genome duplication in P. trichocarpa 43.
Calculation of Ka/Ks values
Pairs from the homologous genes were aligned by MEGA5.0. Subsequently, the aligned sequences were analyzed by the DnaSP program to calculated Ks and Ka rates. For each gene pair, the Ks value was translated into divergence time in millions of years based on a rate of 6.1 × 10−9 substitutions per site per year. The divergence time (T) was calculated as T = Ks/2 × 6.1 × 10−9 Mya.
Promoter cis-element analysis
Promoter sequences (2 kb upstream of the translation start site) of all CAMTA genes were obtained from the Phytozome version 12.1 database. PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/)44 was used to analyze the sequences of the CAMTA gene promoters and to predict and locate their cis-elements.
Plant materials, abiotic stress, and phytohormone treatments
Clonally propagated P. trichocarpa (genotype Nisqually-1) and Populus ussuriensis (originated from seeds collected from 20-year-old trees growing at Liangshui Forest Farm of Northeast Forestry University, Yichun city, Heilongjiang province, China) were grow in half-strength Murashige and Skoog medium (1/2 MS) under long day conditions (16 h light, 8 h dark) at 25°C. P. trichocarpa plants were exposed to the following: 200 mM mannitol, 150 mM NaCl, 4 °C, 200 μM abscisic acid (ABA), 100 μM salicylic acid (SA), and 100 μM methyl jasmonate (MeJA). Each treatment lasted for 0 h, 3 h, 6 h, 12 h, 24 h, and 7 d, and samples were collected at each time point. Each experiment was repeated at least three times. Non-treated plants were used as controls. Additionally, P. ussuriensis plants were treated under a 4 °C stress treatment, as was P. trichocarpa. Following sampling, all samples were immediately frozen in liquid nitrogen and stored at −80 °C until analysis.
In vitro pathogen inoculation assay
To induce fungal infection, mycelial plugs of the Populus leaf blight pathogen Alternaria alternata were placed on excised leaves and cultured in 1/2 MS medium45. The leaves were collected at 0 h, 3 h, 6 h, 12 h, and 24 h following fungal treatment. Three independent biological replicates were performed for each treatment. All collected samples were instantly frozen and stored at −80 °C for RNA isolation.
RNA extraction and qRT-PCR analysis
Total RNA was extracted using the CTAB method46. cDNA was obtained using the PrimeScript™ RT reagent kit (Perfect Real Time; Takara, Dalian, China). SYBR Premix Ex Taq II (TaKaRa, Dalian, China) was used to perform qRT-PCR in 96-well optical reaction plates (Applied Biosystems, Foster City, CA, USA). Reactions were prepared in 20 μL volumes containing the following: 10 μL of 2× SYBR Premix, 6 μL of ddH2O, 2 μL of template, and 1 μL of each specific primer, prepared to a final concentration of 10 µM. In order to ensure the accuracy of the results, we performed three technical replicates for each sample. All primers mentioned above are listed in Supplementary Tables S4 and S5.
Protein interaction network analysis
The online database resource search tool STRING 10 (http://string-db.org/) was used to predict the PtCAMTA protein interaction network. The STRING database integrated information from different datasets, including test and manually curated gene neighborhoods, gene fusion, gene coexistence, protein-protein interactions, and co-expressed genes to calculate the statistics and semantic links between proteins47.
Accession numbers
P. trichocarpa genome sequence data (PtCAMTA1-7) were downloaded from the Phytozome version 12.1 (http://phytozome.jgi.doe.gov/pz/portal.html) and NCBI (http://www.ncbi.nlm.nih.gov/) databases with the following accession number: POPTR_0001s137001, POPTR_0007s054102, POPTR_0005s076601, POPTR_0010s151601, POPTR_0010s162901, POPTR_0008s107302, POPTR_0003s169101. P. ussuriensis sequence data (PuCAMTA1-7) can be found at NCBI with the following number: MF372148-54.
Electronic supplementary material
Acknowledgements
This work was supported by the fundamental research funds for the central universities (Grant number 2572015EA05) and the 111 project (B16010). We thank LetPub for its linguistic assistance during the preparation of this manuscript.
Author Contributions
M.W. and C.L. conceived and designed the experiments. M.W. performed the experiments. M.W. and X.X. analyzed the data. M.W., X.X and C.L. wrote the paper. All authors read and approved the final version of manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-18219-8.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kim MC, Chung WS, Yun DJ, Cho MJ. Calcium and calmodulin-mediated regulation of gene expression in plants. Mol Plant. 2009;2:13–21. doi: 10.1093/mp/ssn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ludwig AA, Romeis T, Jones J. CDPK-mediated signalling pathways: Specificity and cross-talk. J Exp Bot. 2004;55:181–188. doi: 10.1093/jxb/erh008. [DOI] [PubMed] [Google Scholar]
- 3.Du L, Poovaiah BW. A novel family of Ca2+/calmodulin-binding proteins involved in transcriptional regulation: Interaction with fsh/Ring3 class transcription activators. Plant Mol Biol. 2004;54:549–569. doi: 10.1023/B:PLAN.0000038269.98972.bb. [DOI] [PubMed] [Google Scholar]
- 4.Du L, et al. Ca(2+)/calmodulin regulates salicylic-acid-mediated plant immunity. Nature. 2009;457:1154–1158. doi: 10.1038/nature07612. [DOI] [PubMed] [Google Scholar]
- 5.Yang T, Peng H, Whitaker BD, Conway WS. Characterization of a calcium/calmodulin-regulated SR/CAMTA gene family during tomato fruit development and ripening. Bmc Plant Biol. 2012;12:19. doi: 10.1186/1471-2229-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y, et al. Genome-wide identification of CAMTA gene family members in Medicago truncatula and their expression during root nodule symbiosis and hormone treatments. Front Plant Sci. 2015;6:459. doi: 10.3389/fpls.2015.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang GP, et al. Identification and expression analyses of calmodulin-binding transcription activator genes in soybean. Plant and Soil. 2015;386:205–221. doi: 10.1007/s11104-014-2267-6. [DOI] [Google Scholar]
- 8.Yue RQ, et al. Identification and expression profiling analysis of calmodulin-binding transcription activator genes in maize (Zea mays L.) under abiotic and biotic stresses. Front Plant Sci. 2015;6:576. doi: 10.3389/fpls.2015.00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim Y, Park S, Gilmour SJ, Thomashow MF. Roles of CAMTA transcription factors and salicylic acid in configuring the low-temperature transcriptome and freezing tolerance of Arabidopsis. Plant J. 2013;75:364–376. doi: 10.1111/tpj.12205. [DOI] [PubMed] [Google Scholar]
- 10.Pandey N, et al. CAMTA1 regulates drought responses in Arabidopsis thaliana. Bmc Genomics. 2013;14:216. doi: 10.1186/1471-2164-14-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finkler A, Kaplan B, Fromm H. Ca2+-responsive cis-elements in plants. Plant Signaling & Behavior. 2007;2:17–19. doi: 10.4161/psb.2.1.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi MS, et al. Isolation of a calmodulin-binding transcription factor from rice (Oryza sativa L.) J Biol Chem. 2005;280:40820–40831. doi: 10.1074/jbc.M504616200. [DOI] [PubMed] [Google Scholar]
- 13.Taylor G. Populus: Arabidopsis for forestry. Do we need a model tree? Ann Bot. 2002;90:681–689. doi: 10.1093/aob/mcf255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, et al. Genome-wide analysis of soybean HD-Zip gene family and expression profiling under salinity and drought treatments. Plos One. 2014;9:e87156. doi: 10.1371/journal.pone.0087156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lynch M, Conery JS. The evolutionary fate and consequences of duplicate genes. Science. 2000;290:1151–1155. doi: 10.1126/science.290.5494.1151. [DOI] [PubMed] [Google Scholar]
- 16.Xiang Y, Tang N, Du H, Ye H, Xiong L. Characterization of OsbZIP23 as a key player of the basic leucine zipper transcription factor family for conferring abscisic acid sensitivity and salinity and drought tolerance in rice. Plant Physiol. 2008;148:1938–1952. doi: 10.1104/pp.108.128199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbazuk WB, Fu Y, Mcginnis KM. Genome-wide analyses of alternative splicing in plants: Opportunities and challenges. Genome Res. 2008;18:1381–1392. doi: 10.1101/gr.053678.106. [DOI] [PubMed] [Google Scholar]
- 18.Filichkin S, Priest HD, Megraw M, Mockler TC. Alternative splicing in plants: Directing traffic at the crossroads of adaptation and environmental stress. Curr Opin Plant Biol. 2015;24:125–135. doi: 10.1016/j.pbi.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Rahman H, Yang J, Xu Y, Munyampundu J, Cai X. Phylogeny of Plant CAMTAs and Role of AtCAMTAs in nonhost resistance to Xanthomonas oryzae pv. Oryzae. Front Plant Sci. 2016;7:177. doi: 10.3389/fpls.2016.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang T, Poovaiah BW. A calmodulin-binding/CGCG box DNA-binding protein family involved in multiple signaling pathways in plants. J Biol Chem. 2002;277:45049–45058. doi: 10.1074/jbc.M207941200. [DOI] [PubMed] [Google Scholar]
- 21.Rahman H, Xu Y, Zhang X, Cai X. Brassica napus genome possesses extraordinary high number of CAMTA genes and CAMTA3 contributes to PAMP triggered immunity and resistance to sclerotinia sclerotiorum. Front Plant Sci. 2016;7:581. doi: 10.3389/fpls.2016.00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vision TJ, Brown DG, Tanksley SD. The origins of genomic duplications in Arabidopsis. Science. 2000;290:2114–2117. doi: 10.1126/science.290.5499.2114. [DOI] [PubMed] [Google Scholar]
- 23.Kumar R, Tyagi AK, Sharma AK. Genome-wide analysis of auxin response factor (ARF) gene family from tomato and analysis of their role in flower and fruit development. Mol Genet Genomics. 2011;285:245–260. doi: 10.1007/s00438-011-0602-7. [DOI] [PubMed] [Google Scholar]
- 24.Doherty CJ, Van Buskirk HA, Myers SJ, Thomashow MF. Roles for Arabidopsis CAMTA transcription factors in Cold-regulated gene expression and freezing tolerance. Plant Cell. 2009;21:972–984. doi: 10.1105/tpc.108.063958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujita M, et al. Crosstalk between abiotic and biotic stress responses: A current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol. 2006;9:436–442. doi: 10.1016/j.pbi.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Wiest PM, et al. Alternaria infection in a patient with acquired immunodeficiency syndrome: Case report and review of invasive alternaria infections. Reviews of infectious diseases. 1987;9:799–803. doi: 10.1093/clinids/9.4.799. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi-Shinozaki K, Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol. 2006;57:781–803. doi: 10.1146/annurev.arplant.57.032905.105444. [DOI] [PubMed] [Google Scholar]
- 28.Luan S. Signalling drought in guard cells. Plant Cell Environ. 2002;25:229–237. doi: 10.1046/j.1365-3040.2002.00758.x. [DOI] [PubMed] [Google Scholar]
- 29.Huang D, Wu W, Abrams SR, Cutler AJ. The relationship of drought-related gene expression in Arabidopsis thaliana to hormonal and environmental factors. J Exp Bot. 2008;59:2991–3007. doi: 10.1093/jxb/ern155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doherty CJ, van Buskirk HA, Myers SJ, Thomashow MF. Roles for Arabidopsis CAMTA transcription factors in Cold-Regulated gene expression and freezing tolerance. Plant Cell. 2009;21:972–984. doi: 10.1105/tpc.108.063958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nie HZ, et al. SR1, a calmodulin-binding transcription factor, modulates plant defense and ethylene-induced senescence by directly regulating NDR1 and EIN3. Plant Physiol. 2012;158:1847–1859. doi: 10.1104/pp.111.192310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shizuka K, Yuki H, Yuki K, Taro M, Reiko U. Expression and characterization of protein disulfide isomerase family proteins in bread wheat. BMC Plant Biology. 2015;15:73. doi: 10.1186/s12870-015-0460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang YX, et al. Control of salicylic acid synthesis and systemic acquired resistance by two members of a plant-specific family of transcription factors. Proc Natl Acad Sci USA. 2010;107:18220–18225. doi: 10.1073/pnas.1005225107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, et al. CBP60g and SARD1 play partially redundant critical roles in salicylic acid signaling. Plant J. 2011;67:1029–1041. doi: 10.1111/j.1365-313X.2011.04655.x. [DOI] [PubMed] [Google Scholar]
- 35.Rahman H, Yang J, Xu Y, Munyampundu J, Cai X. Phylogeny of plant CAMTAs and role of AtCAMTAs in nonhost resistance to Xanthomonas oryzae pv. Oryzae. Front Plant Sci. 2016;7:177. doi: 10.3389/fpls.2016.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horton P, et al. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007;35:W585–W587. doi: 10.1093/nar/gkm259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilkins MR, et al. Protein identification and analysis tools in the ExPASy server. Methods Mol Biol. 1999;112:531–552. doi: 10.1385/1-59259-584-7:531. [DOI] [PubMed] [Google Scholar]
- 38.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu B, et al. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics. 2015;31:1296–1297. doi: 10.1093/bioinformatics/btu817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.T L. Bailey & C Elkan, Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proceedings of the Second International Conference on Intelligent Systems for Molecular Biology. AAAI Press. pp, 28–36 (1994) [PubMed]
- 41.Ren J, et al. DOG 1.0: Illustrator of protein domain structures. Cell Res. 2009;19:271–273. doi: 10.1038/cr.2009.6. [DOI] [PubMed] [Google Scholar]
- 42.Hu RB, et al. Comprehensive analysis of NAC domain transcription factor gene family in Populus trichocarpa. Bmc Plant Biol. 2010;10:145. doi: 10.1186/1471-2229-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou L, Bawa R, Holliday JA. Exome resequencing reveals signatures of demographic and adaptive processes across the genome and range of black cottonwood (Populus trichocarpa) Mol Ecol. 2014;23:2486–2499. doi: 10.1111/mec.12752. [DOI] [PubMed] [Google Scholar]
- 44.Lescot M, et al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30:325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boedo C, et al. Impact of carrot resistance on development of the Alternaria leaf blight pathogen (Alternaria dauci) Eur J Plant Pathol. 2008;121:55–66. doi: 10.1007/s10658-007-9241-6. [DOI] [Google Scholar]
- 46.Jaakola L, Pirttila AM, Halonen M, Hohtola A. Isolation of high quality RNA from bilberry (Vaccinium myrtillus L.) fruit. Mol Biotechnol. 2001;19:201–203. doi: 10.1385/MB:19:2:201. [DOI] [PubMed] [Google Scholar]
- 47.Szklarczyk D, et al. STRINGv10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.