Abstract
Background & Aims
Upon intestinal epithelial damage a complex wound healing response is initiated to restore epithelial integrity and defend against pathogenic invasion. Epithelium-derived Indian Hedgehog (Ihh) functions as a critical sensor in this process. Signaling occurs in a paracrine manner because the receptor for Ihh is expressed only in the mesenchyme, but the exact Hedgehog target cell has remained elusive. The aim of this study was to elucidate further the nature of this target cell in the context of intestinal inflammation.
Methods
Hedgehog activity was modulated genetically in both cell type–specific and body-wide models and the resulting animals were analyzed for gene expression profiles and sensitivity for dextran sodium sulfate (DSS) colitis. To characterize the Hedgehog target cell, Gli1-CreERT2-Rosa26-ZsGreen animals were generated, which express ZsGreen in all Hedgehog-responsive cells. These cells were characterized using flow cytometry and immunofluorescence.
Results
Loss of Indian Hedgehog from the intestinal epithelium resulted in a rapid increase in expression of inflammation-related genes, accompanied by increased influx of immune cells. Animals with epithelium-specific deletion of Ihh or lacking the Hedgehog receptor Smoothened from Hedgehog target cells were more sensitive to DSS colitis. In contrast, specific deletion of Smoothened in the myeloid compartment did not alter the response to DSS. This suggests that Hedgehog signaling does not repress intestinal immunity through an effect on myeloid cells. Indeed, we found that Hedgehog-responsive cells expressed gp38, smooth muscle actin, and desmin, indicating a fibroblastic nature. Ihh signaling inhibited expression of C-X-C motif chemokine ligand 12 (CXCL12) in fibroblasts in vitro and in vivo, thereby impairing the recruitment of immune cells.
Conclusions
We show that epithelium-derived Indian Hedgehog signals exclusively to fibroblasts in the intestine. Loss of Ihh leads to a rapid immune response with up-regulation of fibroblast-derived CXCL12, and migration of immune cells into the lamina propria.
Keywords: Intestine, Hedgehog, Stroma, Inflammation, CXCL12
Abbreviations used in this paper: α-SMA, α smooth muscle actin; CXCL, C-X-C motif chemokine ligand; CXCR, C-X-C motif chemokine receptor; DMEM, Dulbecco's modified Eagle medium; DSS, dextran sodium sulfate; FCS, fetal calf serum; Gli, glioma-associated oncogene proteins; Hhip, Hedgehog interacting protein; IBD, inflammatory bowel disease; Ihh, Indian Hedgehog; Ihh+/+, Villin-CreERT2-ZsGreen-Ihh+/+; IhhΔ, Villin-CreERT2-ZsGreen-Ihhfl/fl; IL, interleukin; MPO, myeloperoxidase; PBT, PBS/BSA/Triton; Ptch1, Patched1; RT-PCR, reverse-transcription polymerase chain reaction; Smo, Smoothened
Graphical abstract
See editorial on page 63.
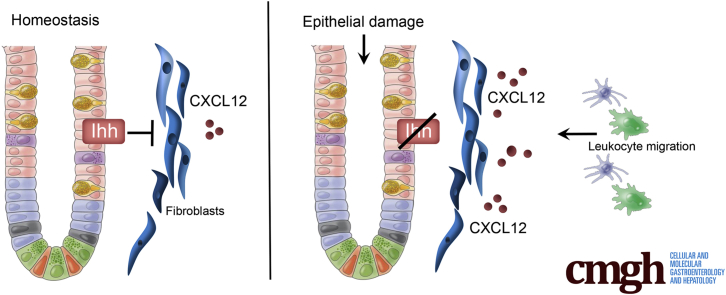
Summary.
We show that epithelium-derived Indian Hedgehog signals exclusively to fibroblasts in the intestine. Short-term loss of Indian Hedgehog leads to a rapid immune response with up-regulation of fibroblast-derived C-X-C motif chemokine ligand 12, and migration of immune cells into the lamina propria.
Immune cells in the intestinal lamina propria are in a state of basal tolerance. In response to epithelial damage, a switch to an activated status is required to limit the consequences of exposure to potentially dangerous luminal content. This control is maintained in part by the immune system itself and in part by the epithelial tissue. The intact epithelium provides factors that mediate immune suppression under steady-state conditions. Upon tissue damage, the loss of epithelial cells results in loss of these factors and relief of the active immunosuppression. Several epithelium-derived factors have been identified that can suppress the mucosal immune response, including thymic stromal lymphopoietin, transforming growth factor-β, semaphorin 7a, and interleukin 25 (IL25).1, 2, 3, 4 These factors mainly exert their effects through their influence on the development of dendritic cells and macrophages with tolerogenic properties such as production of IL10 or inhibition of IL17 and tumor necrosis factor-α.
One of the key epithelium-derived factors required to maintain immune tolerance in the intestine is Indian Hedgehog (Ihh).5 Ihh is secreted exclusively by intestinal epithelial cells6, 7 and signals in a paracrine manner to the inhibitory receptor Patched1 (Ptch1) on cells in the mesenchyme.8 Binding of Ihh to Ptch1 alleviates the inhibitory effect on the second Hedgehog receptor Smoothened (Smo), resulting in translocation of the glioma-associated oncogene proteins (Gli) into the nucleus and subsequent transcription of the Hedgehog target genes such as Gli1, Ptch1, and the Hedgehog interacting protein (Hhip).9
Conditional loss of Ihh from the intestinal epithelium activates many aspects of an epithelial repair response such as crypt expansion and increased proliferation of progenitor cells.10, 11 Ultimately, unresolved loss of Ihh results in the development of severe enteritis with extensive fibrosis, mucosal damage, and the infiltration of macrophages and leukocytes. The Hedgehog pathway already was linked to mucosal inflammation when a single-nucleotide polymorphism in the gene that encodes transcription factor Gli1 was found to predispose to inflammatory bowel disease (IBD).12 This polymorphism results in a hypomorphic protein with diminished capacity for transcriptional activation. Furthermore, the expression of the Hedgehog targets Gli1, Ptch1, and Hhip is down-regulated in patients with IBD with active disease.12, 13 In addition, the association of reduced Hedgehog signaling and the risk of developing IBD was functionally tested in mice that were heterozygous mutant for Gli1. These mice developed more severe disease compared with controls in a murine model of colitis.12 In vitro, the role of Ihh as an immune suppressor has been studied using cultured embryonic tissue.11 Culturing intestinal lamina propria in the absence of epithelial cells, and therefore in the absence of Hedgehog, resulted in loss of Hedgehog signaling and significant activation of inflammatory genes such as IL1β, IL6, and Toll-like receptor 2, which was corrected by the addition of recombinant Hedgehog protein.
Despite the fact that Ihh seems to be a crucial sensor of epithelial integrity in the intestine, the identity of the Hedgehog-responsive cells and a precise anti-inflammatory pathway via which Ihh signals remain to be shown. Although it was suggested that lamina propria macrophages and dendritic cells can respond directly to Hedgehog signaling, functional evidence for such direct effects is lacking.11, 12 Other stromal cells such as fibroblasts, smooth muscle cells, blood vessels, and lymphatic vessels also may play a role. In fact, it has been shown that Hedgehog is a critical regulator of smooth muscle homeostasis8, 14 and is important for the maintenance of myofibroblasts in the intestine.15
Here, we identify fibroblast-like cells as the exclusive Hedgehog-responsive cells in the intestine. Short-term loss of Hedgehog signaling resulted in up-regulation of chemokine production by these cells, in particular C-X-C motif chemokine ligand 12 (CXCL12). Subsequently, various subsets of immune cells were recruited to the intestine that predisposed to the development of colitis.
Materials and Methods
Animals
Villin-CreERT2 mice16 were crossed with Ihhfl/fl mice17 and Rosa26-ZsGreen mice (007906; The Jackson Laboratory, Bar Harbor, ME) to generate Villin-CreERT2-ZsGreen-Ihhfl/fl animals. Rosa26-CreERT2 mice18 were crossed with Ptch1fl/fl animals19 to generate the previously described Rosa26-CreERT2-Ptch1fl/fl animals.7 LysM-Cre mice20 (004781; The Jackson Laboratory) were crossed with Rosa26-YFP (006148; The Jackson Laboratory) and Smofl/fl mice (004288; The Jackson Laboratory) to generate LysM-Cre-YFP-Smofl/fl mice. CD11cCre-GFP mice (008068; The Jackson Laboratory) were crossed with Smofl/fl mice to generate CD11c-Cre-GFP-Smofl/fl mice. Gli1-CreERT2 (007913; The Jackson Laboratory) and Rosa26-ZsGreen animals were crossed to generate Gli1-CreERT2-Rosa26-ZsGreen mice. Gli1-CreERT2 and Smofl/fl mice were crossed to generate Gli1-CreERT2-Smofl/fl animals. Activation of CreERT2 and thus induction of the respective gene manipulations was performed by intraperitoneal administration of 1 mg tamoxifen (Sigma-Aldrich, St. Louis, MO) for 5 consecutive days.
For dextran sodium sulfate (DSS) colitis, drinking water was supplemented with 2% DSS and animals were killed on day 6 or 7. The experiments with Rosa26-CreERT2-Ptch1fl/fl, Villin-CreERT2-ZsGreen-Ihhfl/fl, LysM-Cre-YFP-Smofl/fl, CD11c-Cre-GFP-Smofl/fl, and Gli1-CreERT2-Rosa26-ZsGreen animals were performed in the same mouse facility, whereas experiments with Gli1-CreERT2-Smofl/fl animals were performed in a different mouse facility. For all experiments, littermate control animals negative for the floxed allele but carrying the cre allele were used. All experimental groups were treated with the same concentration of tamoxifen. A standardized scoring system was used to assess the severity of colitis both clinically and histopathologically.21 Table 1 shows a detailed description of the scoring system. All animals were housed at the Experimental Animal Center of the Leiden University Medical Center, at the Animal Research Institute of the Academic Medical Center Amsterdam, or at the Institutional Animal Care facility of the University of Göttingen. All experiments were approved by the relevant local ethical committees.
Table 1.
Scoring System for DSS-Induced Colitis
| Score | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Area involved | 0% | 1%–10% | 10%–25% | 25%–50% | >50% |
| Follicles | Normal (0–1) | Minimal (2–3) | Mild (4–5) | Moderate (6–7) | Severe (>7) |
| Edema | Absent | Minimal | Mild | Moderate | Severe |
| Fibrosis | Absent | Minimal | Mild | Moderate | Severe |
| Erosion/ulceration | 0% | 1%–10% | 10%–25% | 25%–50% | >50% |
| Crypt loss | 0% | 1%–10% | 10%–25% | 25%–50% | >50% |
| Granulocytes | Normal | Minimal increase | Mild increase | Moderate increase | Severe increase |
| Mononuclear cells | Normal | Minimal increase | Mild increase | Moderate increase | Severe increase |
RNA Isolation, Complementary DNA Synthesis, and Quantitative Reverse-Transcription Polymerase Chain Reaction
Cells or tissue were lysed in 1 mL TRI Reagent (Sigma-Aldrich). Intestinal tissue was homogenized and RNA extraction was performed according to the manufacturer's instructions. RNA from isolated or cultured primary cells was isolated using the RNAeasy mini kit (Qiagen, Hilden, Germany). For complementary DNA synthesis, 1 μg of RNA was transcribed using Revertaid (Thermo Fisher Scientific, Waltham, MA). Quantitative reverse-transcription polymerase chain reaction (RT-PCR) was performed using SybrGreen (Roche, Basel, Switzerland) according to the manufacturer's protocol. Cyclophilin or glyceraldehyde-3-phosphate dehydrogenase was used for normalization according to the delta delta Ct (ΔΔCt) method. Table 2 shows all primer sets used. The RT2 profiler PCR array for mouse chemokines and receptors (PAMM-022ZA-2; Qiagen) was performed according to the manufacturer’s protocol. Results were analyzed using the PCR Array Data Analysis Web Portal provided by Qiagen.
Table 2.
Primers used for Quantitative RT-PCR Experiments
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Gli1 | CTTGTGGTGGAGTCATTGGA | GAGGTTGGGATGAAGAAGCA |
| Ptch1 | TTAGCGCCTTCTTCTTTTGG | ATCTCGAGACCAACGTGGAG |
| Hhip | CGTTCCTGGTTGGTGGTATAA | TCAAGGAGCCTTACTTGGACA |
| CXCL12 | TTTCAGATGCTTGACGTTGG | GCGCTCTGCATCAGTGAC |
| CXCL1 | TCTCCGTTACTTGGGGACAC | CCACACTCAAGAATGGTCGC |
Transcriptional Analysis
Two platforms were used to perform transcriptional analysis. For microarray analysis in the Ptch mutant animals, tissue was lysed in TRI Reagent (Sigma-Aldrich) and RNA was extracted from the colons according to the manufacturer’s protocol. RNA clean up was performed with the RNeasy kit (Qiagen). RNA was labeled using a complementary RNA labeling kit according to the protocol of the Affymetrix kit (Thermo Fisher Scientific) and hybridized with the U 430 2.0 array from Affymetrix. For Ihh mutant animals, tissue was lysed in TRI Reagent (Sigma-Aldrich) and RNA was extracted from the colons according to the manufacturer’s protocol. RNA clean up was performed using the RNeasy kit (Qiagen). RNA was labeled using the complementary RNA labeling kit for Illumina Arrays (Illumina, San Diego, CA) and hybridized with Illumina Ref8 v2.0 mouse slides. After initial normalization by using Genomestudio software (Illumina) and Robust Multichip Average (Affymetrix), all probe sets expressed were used in the Gene Set Enrichment Analysis (GSEA) for the gene set Hallmark_Inflammatory_Response using GSEA software (Broad Institute of Massachusetts Institute of Technology and Harvard).22, 23 Enrichment was considered significant for a false discovery rate (FDR) q-value less than 0.05. The data discussed in this publication have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus24 and are accessible through GEO series accession number GSE103172 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE103172).
Immunohistochemistry
Tissue was fixed in 4% ice-cold formalin and embedded in paraffin. Sections of 4.5 μm thickness were deparaffinized in xylene and rehydrated. Endogenous peroxidase was blocked using 0.3% H2O2 in methanol for 30 minutes. The following methods of antigen retrieval were used: sodium citrate (slides were cooked at 100°C for 20 minutes in 0.01 mol/L sodium citrate, pH 6); Tris/EDTA (slides were cooked at 100°C for 20 minutes in a Tris/EDTA buffer, 10 mmol/L Tris, 1 mmol/L EDTA, pH 9.0); proteinase K (slides were incubated with proteinase K [S302030; Dako, Santa Clara, CA] for 5 minutes at room temperature). After antigen retrieval, slides were blocked in phosphate buffered saline with 1% bovine serum albumin and 0.1% Triton X-100 (Sigma-Aldrich) (PBS/BSA/Triton [PBT]) for 30 minutes, followed by incubation overnight at 4°C with a primary antibody in PBT. Table 3 shows the antibodies used. Antibody binding was visualized using Powervision horseradish-peroxidase–labeled secondary antibodies from Immunologic or biotinylated anti-rat (E0468; Dako) and streptavidin/horseradish-peroxidase (P039701; Dako) and diaminobenzidine (Sigma-Aldrich) for substrate development. All sections were counterstained with Mayer’s hematoxylin (Sigma-Aldrich).
Table 3.
Antibodies Used for Immunohistochemistry and Immunofluorescence
| Antigen | Company | Catalogue number |
Clone | Concentration | Antigen retrieval |
|---|---|---|---|---|---|
| CD3 | Dako | A0425 | Polyclonal | 1:1000 | Tris/EDTA |
| F4-80 | Bio-Rad, Hercules, CA | MCA497GA | Cl:A3-1 | 1:1000 | Proteinase K |
| CD45 | R&D, Minneapolis, MN | AF114 | Polyclonal | 1:50 | None |
| Lyve-1 | R&D | AF2125 | Polyclonal | 1:100 | NaCi |
| Collagen III | Southern Biotech, Birmingham, AL | 1330-01 | Polyclonal | 1:200 | NaCi |
| PDGFRα | Novus Biologicals, Littleton, CO | NBP1-10473 | Polyclonal | 1:100 | NaCi |
| gp38 | R&D | AF3244 | Polyclonal | 1:50 | NaCi |
| α-SMA | Lifespan Biosciences, Seattle, WA | B3933 | Polyclonal | 1:500 | NaCi |
| Desmin | Abcam, Cambridge, UK | Ab15200 | Polyclonal | 1:750 | NaCi |
PDGFRα, platelet-derived growth factor receptor-α.
Immunofluorescent Staining
Tissue was fixed in 4% ice-cold formalin and embedded in paraffin. Sections of 4.5 μm were deparaffinized in xylene and rehydrated. Antibodies were diluted in PBT and slides were incubated overnight. Table 3 shows the antibodies used. The next day, slides were incubated for 1 hour using fluorescently labeled secondary antibodies (all Alexa Fluor secondary antibodies from Invitrogen, Waltham, MA), diluted 1:500 in PBT at room temperature. Slides were washed and mounted with Slowfade Gold Antifade reagent with 4′,6-diamidino-2-phenylindole (s36938; Invitrogen). Images were obtained on a Leica DM6000 digital microscope equipped with LAS AF software (Leica, Wetzler, Germany). For analysis, the software program ImageJ (rsbweb.nih.gov/ij/; National Institutes of Health, Bethesda, MD) was used.
Isolation of Intestinal Cells
Murine small intestine and colon were harvested, opened longitudinally, thoroughly washed, and cut into 5-mm pieces. Pieces were incubated in 5 mmol/L Tris/EDTA in Hank's balanced salt solution for 20 minutes at 37°C. After washing, tissue was cut into very thin pieces and incubated in Hank's balanced salt solution containing 125 μg/mL Liberase TL (05401020001; Roche) and 40 μg/mL DNAse (11284932001; Roche) for 40 minutes at 37°C. Cells were passed through a 100-μm cell strainer and used for further analysis and culture.
Flow Cytometry Analysis
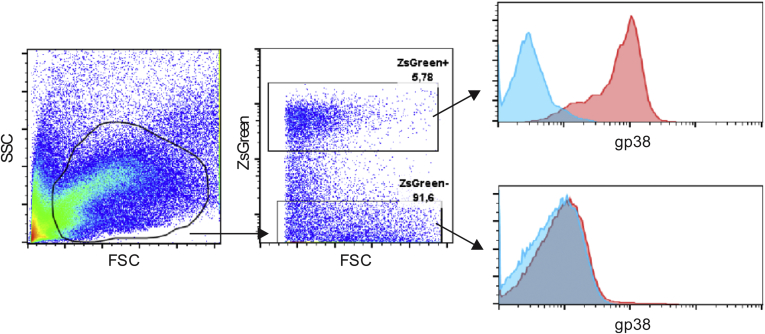
Single-cell suspension from small intestine or colon was stained for 30 minutes on ice using an antibody cocktail enabling us to distinguish between immune cells, epithelial cells, lymphatics/endothelial cells, and fibroblasts. Table 4 shows the antibodies used. Supplementary Figure 1 shows the flow cytometry gating strategy. Viable cells were gated by forward and side scatter and fibroblasts were gated as CD45-Epcam-CD31-gp38+ cells. Cells were acquired using a FACS Fortessa (BD Biosciences, San Jose, CA) and FlowJo software (Tree Stars, Inc, Ashland, OR), or 50–300*103 cells were sorted using a FACS ARIA III (BD Biosciences) sorter for further RNA isolations.
Table 4.
Antibodies Used for Flow Cytometry
| Antigen/label | Company | Catalogue number | Clone | Concentration |
|---|---|---|---|---|
| Epcam (FITC) | Santa Cruz Biotechnology, Dallas, TX | sc-53532 | G8.8 | 1:400 |
| CD45 (FITC) | eBioscience, San Diego, CA | 11-0451-81 | 30-F11 | 1:800 |
| CD31 (eFluor-450) | eBioscience | 48-0311-82 | 390 | 1:400 |
| gp38 (PE) | Biolegend | 127407 | 8.1.1. | 1:400 |
| α-SMA | Lifespan Biosciences | B3933 | Polyclonal | 1:200 |
| Desmin | Abcam | Ab32362 | Polyclonal | 1:50 |
FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Supplementary Figure 1.
Flow cytometry gating strategy example for expression of gp38 in ZsGreen+ and ZsGreen- cells in the colon of an induced Gli1-CreERT2-Rosa26-ZsGreen animal.Blue lines indicate isotype control, red lines indicate specific staining. FSC, forward scatter; SSC, side scatter.
For intracellular staining, cells were fixed using 2% paraformaldehyde (PFA) and permeabilized in 2% saponine. All antibodies were diluted in 2% saponine and incubated for 30 minutes at room temperature. For unlabeled primary antibodies, cells were incubated with fluorescently labeled secondary antibodies (donkey anti-goat Alexa Fluor 647; Invitrogen; and donkey anti-rabbit brilliant violet 421; Biolegend, San Diego, CA).
Cell Culture Experiments
The HEK293 cell line stably transfected with N-terminal fragment of Shh without cholesterol modification or control vector was described previously.25 The cells were grown to 70%–80% confluence in Dulbecco’s modified Eagle medium (DMEM) with 10% fetal calf serum (FCS), 1% penicillin/streptomycin, and 400 μg/mL G418. The medium replaced was for DMEM with 10% FCS and 1% penicillin/streptomycin, and 24 hours after supernatant from Shh-expressing cells (Hh+) and control cells (Hh-) was harvested and filtered through a 0.22 μm filter.
For generation of mouse dendritic cells or macrophages, bone marrow was isolated from femur and cultured in RMPI 1640 culture medium (Invitrogen) supplemented with 10% FCS and 20 ng/mL recombinant mouse granulocyte-macrophage colony–stimulating factor or macrophage colony–stimulating factor, respectively (R&D Systems, Minneapolis, MN) for 7–9 days. Afterward, cells were cultured in control media (Hh-) or 10% Hedgehog conditioned media (Hh+) for 3–7 days.
The C3H10T1/2 cell line was obtained from American Type Culture Collection (CCL-226) and grown in DMEM with 10% FCS and 1% penicillin/streptomycin. Cells were cultured in control media (Hh-) or 10% Hedgehog conditioned media (Hh+) for 3–7 days. Primary mouse fibroblasts were isolated as described earlier and cultured in RMPI 1640 culture medium (Invitrogen) supplemented with 10% FCS, 1% penicillin/streptomycin, 40 μg/mL G418, and 0.025 μg/mL amphotericin B. Primary cells were treated with Smoothened Agonist (2.5 μmol/L; Selleckchem, Houston, TX) for 7 days. Supernatant of both cell lines was stored at -80°C to perform migration assays.
Migration Assay
The 3-dimensional chemotaxis μ-slide (80326; Ibidi, Martinsried, Germany) was used to measure the migration of Jurkat T cells toward supernatant of fibroblasts. The procedure was performed according to the manufacturer’s protocol. Cold bovine collagen I gel (3 mg/mL, A10644-01; Thermo Fisher Scientific) was supplemented with fibronectin (Sanquin, Amsterdam, Netherlands). To establish a gradient, supernatant of fibroblasts exposed to Hedgehog conditioned or control medium was placed at contralateral sides of the migration chamber. To inhibit cell migration, anti-CXCL12 (MAB310, 100 μg/mL; R&D Systems) was added to supernatant before use. Migration chambers were placed inside a temperature-controlled chamber (Tokaihit, Shizuoka-ken, Japan) and maintained at 37°C. Images were obtained every 3 minutes for 15 hours using a Leica DMi8 microscope with a DFC365FX camera (Leica). Cell tracking was performed using the ImageJ software plugin “Manual Tracking” (Fabrice Cordelières, Institut Curie, Orsay, France). Each experiment was repeated 3 times, and 25–30 cells were tracked per condition in each experiment.
Cytokine Bead Array, Myeloperoxidase, Serum Amyloid A, and Enzyme-Linked Immunosorbent Assay
Intestinal tissue was weighed and homogenized (100 mg tissue/mL) in cell lysis buffer (Cell Signaling Technology, Beverly, MA) with protease inhibitors (Roche) using Precellys tissue homogenizer tubes (Bertin Technologies, Montigny, France). For cytokine bead array, the Cytometric Bead Array Mouse Cytokine Kit (560485; BD Biosciences) was used. Serum amyloid A and myeloperoxidase (MPO) concentration were measured by enzyme-linked immunosorbent assay kits for MPO (HK210-02; Hycult Biotech, Uden, Netherlands) and serum amyloid A (TP 802M; Tridelta Development, Ltd, Maynooth, Ireland). MPO concentrations in tissue lysates were corrected for total protein content as measured by bicinchoninic acid (BCA) assay (Pierce, Thermo Fisher Scientific). The concentration of CXCL12 in the supernatant of fibroblasts was determined using a sandwich enzyme-linked immunosorbent assay kit (DY460; R&D Systems).
Statistics
Statistical analysis was performed using Prism 5.0 (GraphPad Software, La Jolla, CA). All values are represented as means ± SEM. Samples were analyzed using the Student t test or 2-way analysis of variance followed by the Bonferroni post-test. Differences were considered statistically significant at a P value less than .05.
All authors had access to the study data, and have reviewed and approved the final manuscript.
Results
Loss of Indian Hedgehog Results in a Rapid Immune Response
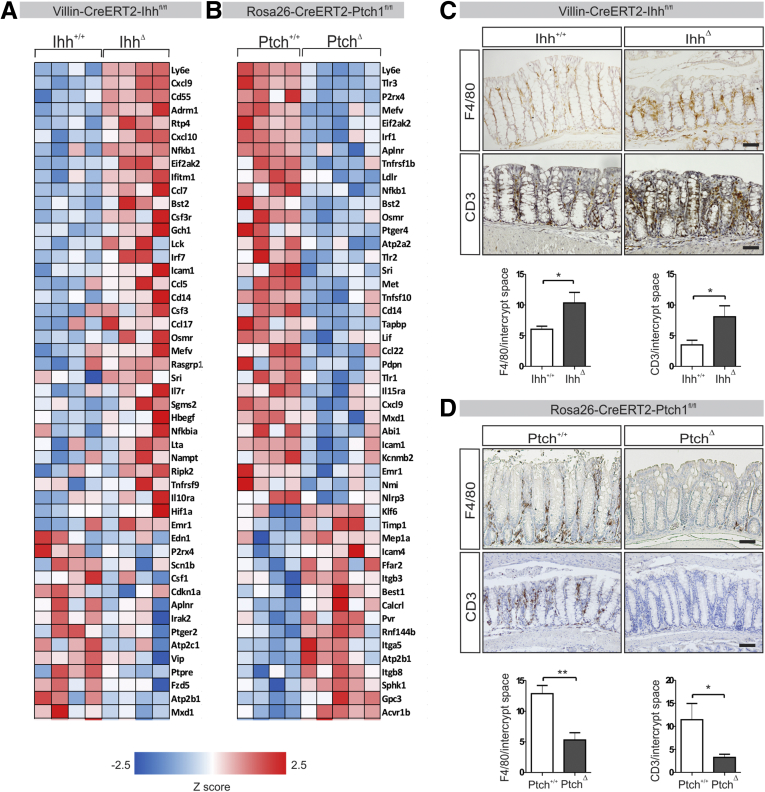
We generated Villin-CreERT2-ZsGreen-Ihh+/+ control animals and Villin-CreERT2-ZsGreen-Ihhfl/fl animals (henceforth referred to as Ihh+/+ and IhhΔ), which lack Ihh expression in the intestinal epithelium after induction with tamoxifen. Ablation of Ihh abrogates an important component of Hedgehog activity in the intestinal mucosa. Prolonged loss of Ihh from the intestinal epithelium leads to the gradual development of a severe enteritis that aggravates over the course of several months.10 To investigate the short-term effects of Ihh deletion, we performed gene expression profiling followed by gene set enrichment analysis on whole colons of Ihh+/+ and IhhΔ animals 7 days after induction. Even at this early time point, significant enrichment of inflammation-related genes was apparent in IhhΔ animals, including genes associated with an interferon response, chemokines, and components of the nuclear factor-κB pathway (Figure 1A). This was confirmed further using the inverse model, Rosa26-CreERT2-Ptch1+/+ (Ptch+/+) control animals and Rosa26-CreERT2-Ptch1fl/fl (PtchΔ) animals in which the Hedgehog signaling pathway constitutively is activated in a body-wide manner on deletion of Ptch1. These animals showed a significant down-regulation of transcripts involved in immune responses compared with their littermate controls, many of which were up-regulated in the signature seen in the IhhΔ animal pathway (Figure 1B). Although no macroscopic inflammation was apparent at this time point in the IhhΔ mouse model, the observed gene signature suggests the onset of an inflammatory process. This confirms previous in vitro data showing that intestinal mesenchyme cultured in the absence of epithelial cells shows activation of an inflammatory response that can be suppressed by recombinant Hedgehog protein.11
Figure 1.
Deletion of intestinal Hedgehog results in up-regulation of inflammation-mediated genes, whereas activation of Hedgehog signaling results in down-regulation.Villin-CreERT2-Ihh+/+ (Ihh+/+, n = 4) and Villin-CreERT2-Ihhfl/fl animals (IhhΔ, n = 4), or Rosa26-CreERT2-Ptch1+/+ (Ptch+/+, n = 4) and Rosa26-CreERT2-Ptch1fl/fl animals (PtchΔ, n = 4) were injected intraperitoneally with 1 mg tamoxifen for 5 consecutive days. Seven and 12 days after the start of induction for each genotype, respectively, animals were killed and transcriptional profiling and histologic analyses were performed. (A and B) Transcriptional analysis of (A) IhhΔ animals using Affymetrix and (B) PtchΔ animals using Illumina platforms. Gene set enrichment analyses of the Hallmark gene set Inflammatory_Response. Gene expression heatmaps of the 50 most differentially regulated transcripts within the Inflammatory_Response gene set in each mouse model are shown. (C and D) Immunohistochemistry for CD3 and F4/80 expression (positive cells/crypt) in the colons of (C) IhhΔ and (D) Ptch1Δ animals. Scale bars: 50 μm. Bars represent means, error bars represent SEM. *P < .05, **P < .01.
Given the increase in various chemotactic factors in IhhΔ animals, we hypothesized that the increased expression of proinflammatory genes may be the result of an increased recruitment of immune cells. Indeed, immunohistochemistry showed increased numbers of both T cells and macrophages in the colons of these animals (Figure 1C). Conversely, both cell types were almost completely absent from the colons of the Ptch1Δ animals (Figure 1D). In summary, loss of Ihh from the intestinal epithelium results in a rapidly arising proinflammatory environment with the induction of chemoattractants resulting in increased numbers of immune cells.
Loss of Indian Hedgehog Results in Increased Sensitivity to DSS-Induced Colitis
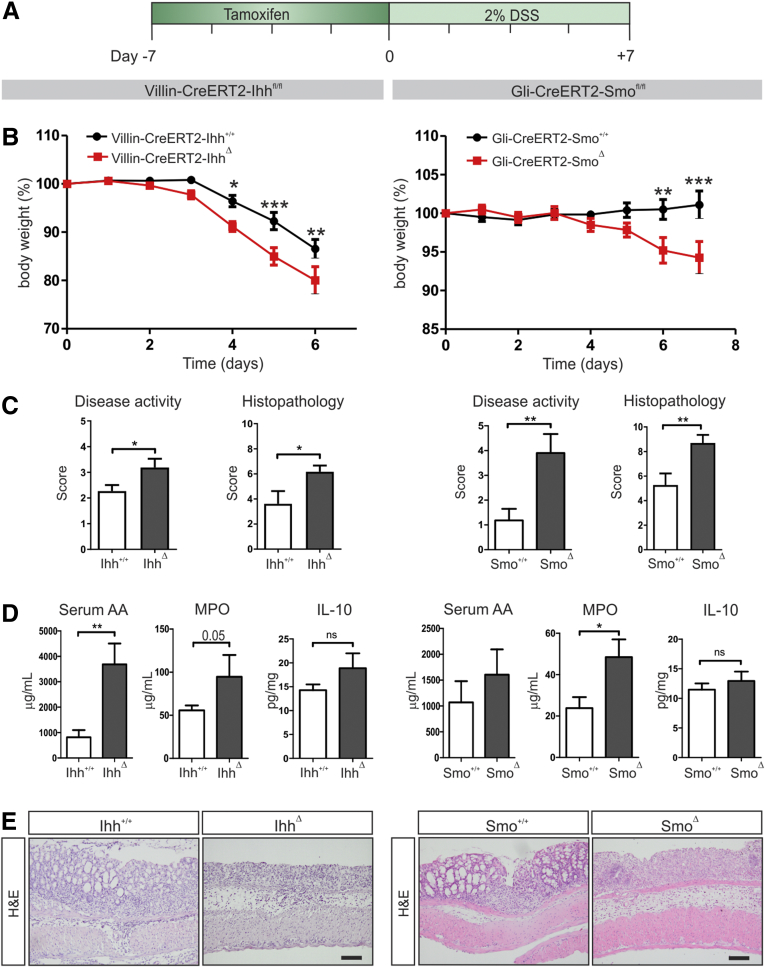
To show direct functional consequences of the induced proinflammatory state upon Ihh deletion from the intestinal epithelium, we exposed the Ihh+/+ and IhhΔ animals to DSS. Seven days after induction of the Ihh mutation, drinking water was supplemented with 2% DSS for 7 consecutive days (Figure 2A). Mice lacking Ihh in the intestine lost significantly more weight and developed more severe colitis both in terms of disease activity and histologic pathology (Figure 2B and C, left panels). Furthermore, systemic levels of serum amyloid A were enhanced significantly and intestinal MPO protein levels were increased, suggesting an increase in the presence of neutrophils (Figure 2D, left panels). These data were confirmed in an independent second model using Gli-CreERT2-Smo+/+ (Smo+/+) control animals and Gli-CreERT2-Smo fl/fl (SmoΔ) mice. In this model, the critical Hedgehog signaling receptor Smoothened is deleted from all Hedgehog-responsive cells that express the Hedgehog target Gli1, preventing pathway activation. Administration of 2% DSS led to significant weight loss and an increase in disease activity and histopathology score in SmoΔ animals compared with their littermate controls (Figure 2B and C, right panels). Again, systemic levels of amyloid A and intestinal MPO levels were higher in SmoΔ animals (Figure 2D, right panels). Recently, a role was shown for the anti-inflammatory cytokine IL10 as a mediator of Hedgehog-dependent immunosuppression.26 However, in the colons of the IhhΔ and SmoΔ animals treated with DSS, we did not observe differences in IL10 expression (Figure 2D). Histologic examination of colons of both mouse models confirmed increased disease activity upon Hedgehog signaling abrogation (Figure 2E). This shows that either deletion of Ihh from the intestinal epithelium or removal of its receptor Smoothened from Hedgehog-responsive cells lead to an increased sensitivity to DSS-induced colitis as early as 1 week after induction.
Figure 2.
Decreased Hedgehog signaling results in increased sensitivity to DSS-induced colitis. (A) Experimental set up: Villin-CreERT2-Ihh+/+ (Ihh+/+, n = 10) and Villin-CreERT2-Ihhfl/fl animals (IhhΔ, n = 10), or Gli1-CreERT2-ZsGreen-Smo+/+ (Smo+/+, n = 11) and Gli1-CreERT2-ZsGreen-Smofl/fl animals (SmoΔ, n = 10) were injected intraperitoneally with 1 mg tamoxifen for 5 consecutive days. Animals were provided with drinking water containing 2% DSS for 6 or 7 days, starting 7 days after induction of recombination. Experiments were performed in 2 separate mouse facilities. For both mouse models, (B) the relative weight to the weight at the start of DSS administration is shown, (C) disease and histologic scores were determined as described in the Materials and Methods section, (D) MPO levels and IL10 protein levels were determined in colon lysates and are shown as corrected for total protein content, and serum amyloid A levels were measured in the serum at death. (E) Representative images of H&E-stained sections of the colons of each mouse model. Scale bars: 50 μm. Bars represent means, error bars represent SEM. *P < .05, **P < .01, ***P < .001.
Intestinal Immune Cells Are Not Hedgehog Responsive
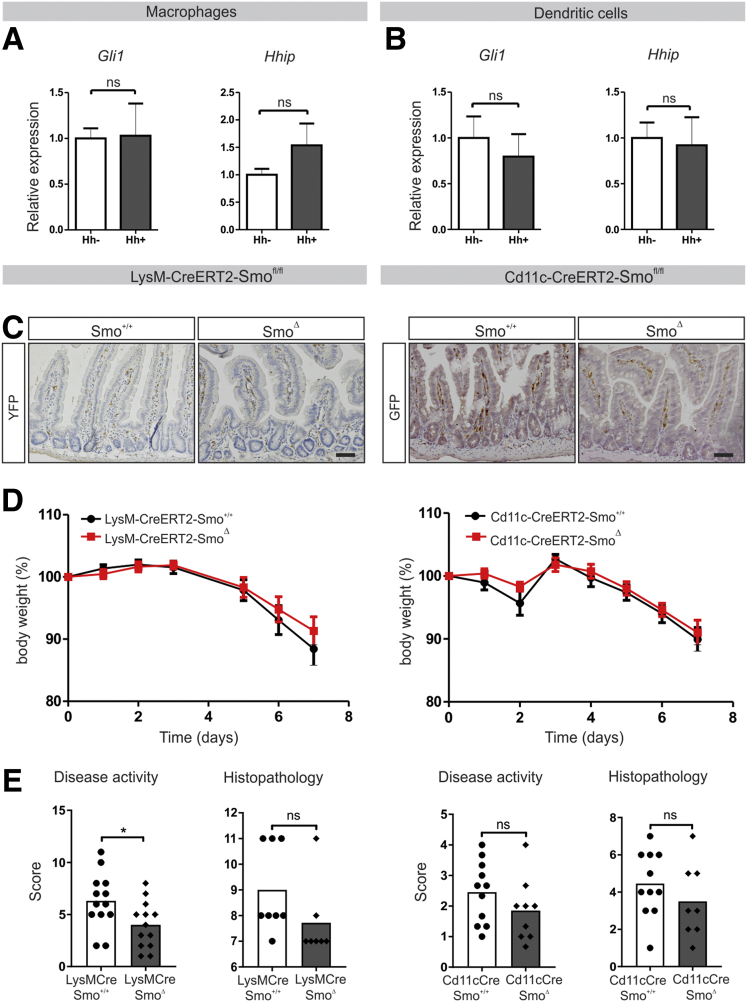
Next, we sought to identify the cell type responsible for the immune-modulatory effect of Hedgehog. Because the literature has suggested immune cells are Hedgehog target cells,11, 12 and given their abundant presence in the intestinal mesenchyme after 1 week of loss of Ihh, myeloid cells would be potential candidates. However, treatment of primary mouse macrophages or dendritic cells with Hedgehog-conditioned medium did not result in up-regulation of the Hedgehog targets Gli1 and Hhip, suggesting a lack of Hedgehog responsiveness (Figure 3A and B). Intestinal myeloid cells are known to differ from those in other tissues, for example, by being more refractory to proinflammatory stimuli. It therefore is possible that in vitro–generated bone marrow–derived macrophages or dendritic cells do not fully reflect their intestinal counterparts. To address this issue, we generated mice specifically lacking the Hedgehog signaling receptor Smoothened in myeloid cells. To target macrophages, we used LysM-Cre-YFP-Smo+/+ (Smo+/+) control animals and LysM-Cre-YFP-Smofl/fl (SmoΔ) animals. To target dendritic cells, we used CD11c-Cre-GFP-Smo+/+ (Smo+/+) control animals and CD11c-Cre-GFP-Smofl/fl (SmoΔ) animals. Expression of Cre in both mouse models was confirmed by immunohistochemistry for the reporter constructs YFP and GFP (Figure 3C). Neither animal model showed an increased sensitivity to DSS colitis on either weight loss or histologic parameters upon loss of Smo from these myeloid lineages (Figure 3D and E). Together, these data suggest that intestinal myeloid cells are not the Hedgehog target cells responsible for enhanced sensitivity to intestinal inflammation.
Figure 3.
Intestinal myeloid cells are not Hedgehog responsive. (A and B) Quantitative RT-PCR for Hedgehog targets Gli1 and Hhip in bone marrow–derived macrophages and dendritic cells stimulated using 10% Hedgehog conditioned or control medium for 24 hours. Expression relative to the household gene cyclophilin is shown. Data are representative of 3 independent experiments. (C) Immunohistochemistry for GFP and YFP to confirm expression of Cre recombinase. Scale bars: 50 μm. (D and E) LysM-Cre-Smo+/+ (Smo+/+, n = 13) and LysM-Cre-SmoΔ animals (SmoΔ, n = 13) or CD11c-Cre-Smo+/+ (Smo+/+, n = 11) and CD11c-Cre-Smofl/fl animals (SmoΔ, n = 9) were provided with drinking water containing 2% DSS after induction with tamoxifen and killed on day 7. (D) Relative weight to the weight of start was measured, and (E) disease and histologic scores were determined as described in the Materials and Methods section. Bars represent means, error bars represent SEM. ∗P < .05.
Indian Hedgehog–Responsive Cells Show Fibroblast-Like Characteristics
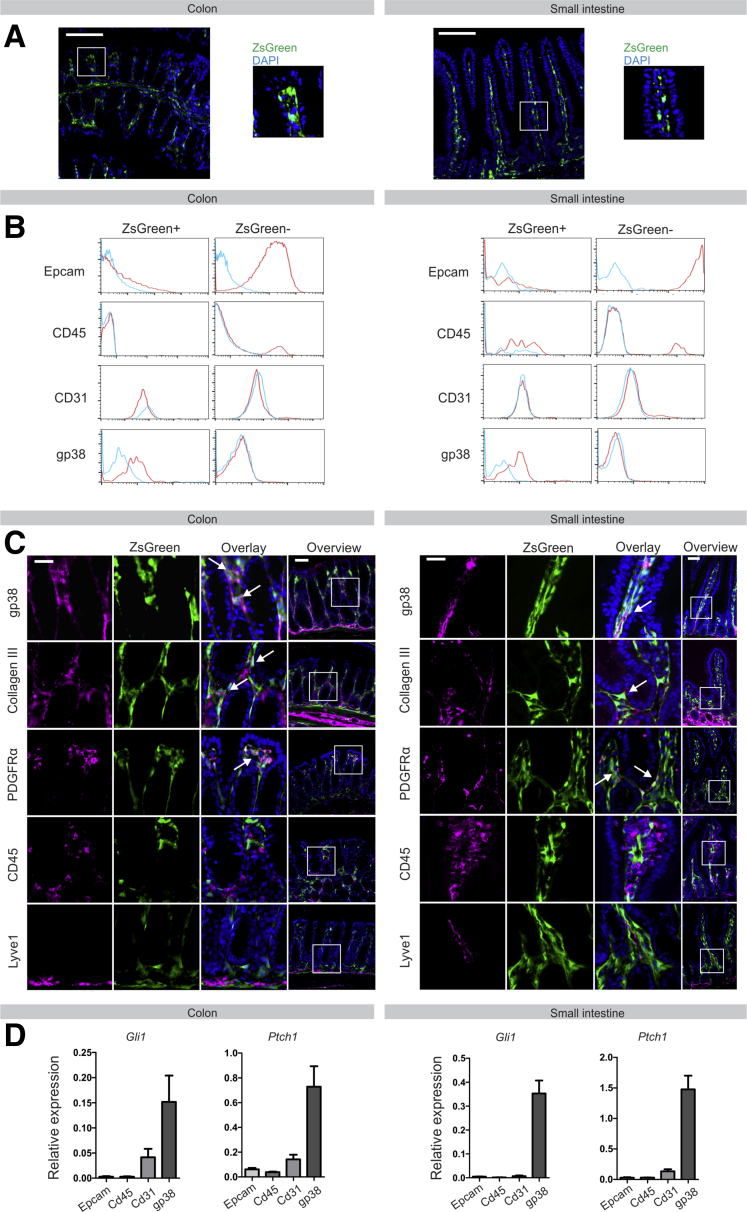
To trace Hedgehog responsiveness in vivo, we crossed Gli1-CreERT2 mice into Rosa26-Stopfl/fl-ZsGreen animals, generating Gli1-CreERT2-Rosa26-ZsGreen mice, which express ZsGreen under the control of the endogenous Gli1 promotor in a tamoxifen-inducible manner. Induction of these animals with tamoxifen resulted in clear ZsGreen expression in the mesenchymal compartment, both in colon and small intestine (Figure 4A). As previously described, no Hedgehog-responsive cells were observed in the epithelial layer.7 This was corroborated further by flow cytometric analysis, which showed that expression of the epithelial marker Epcam, the myeloid marker CD45, and the lymphatics/endothelial cell marker CD31 were restricted to the GliZsGreen negative (GliZsGreen-), and therefore the Hedgehog-nonresponsive population (Figure 4B). In contrast, the majority of GliZsGreen+ cells expressed the fibroblast marker gp38 (Figure 4B). This was confirmed by immunofluorescence, which showed a large overlap between GliZsGreen and the pan-fibroblast markers collagen III, platelet-derived growth factor receptor-α (PDGFRα), and gp38, and no co-localization with CD45 or lymphatics marker Lyve1 (Figure 4C). Finally, we isolated CD45+ immune, Epcam+ epithelial, CD31+ lymphatic/endothelial cells, and gp38+ fibroblasts from both colon and small intestine by flow cytometry sorting and analyzed the expression of Hedgehog targets Gli1 and Ptch1 in these populations. Again, this confirmed the fibroblast population as the hedgehog-responsive cells (Figure 4D).
Figure 4.
Hedgehog-responsive cells in the intestine are fibroblast-like cells. (A) ZsGreen expression in small intestine and colon of Gli1-CreERT2-Rosa26-ZsGreen animals upon induction with tamoxifen. Nuclei were visualized by 4′,6-diamidino-2-phenylindole (DAPI) (blue). Scale bars: 50 μm. (B) Flow cytometry for expression of Epcam, CD45, CD31, and gp38 in ZsGreen+ and ZsGreen- cells of induced Gli1-CreERT2-Rosa26-ZsGreen animals. Blue lines indicate isotype control, red line indicates specific staining. (C) Immunofluorescent staining for gp38, collagen III, platelet-derived growth factor receptor-α (PDGFRα), CD45, and Lyve1 (magenta) on sections generated from induced Gli1-CreERT2-Rosa26-ZsGreen animals. Nuclei were visualized by DAPI (blue). Arrows indicate co-localization. Scale bars: 25 μm; scale bars in overview, 50 μm. (D) Quantitative RT-PCR for Hedgehog targets Gli1 and Ptch1 in intestinal cell populations positive for Epcam, CD45, CD31, or gp38, respectively, isolated from wild-type mice (N = 3).
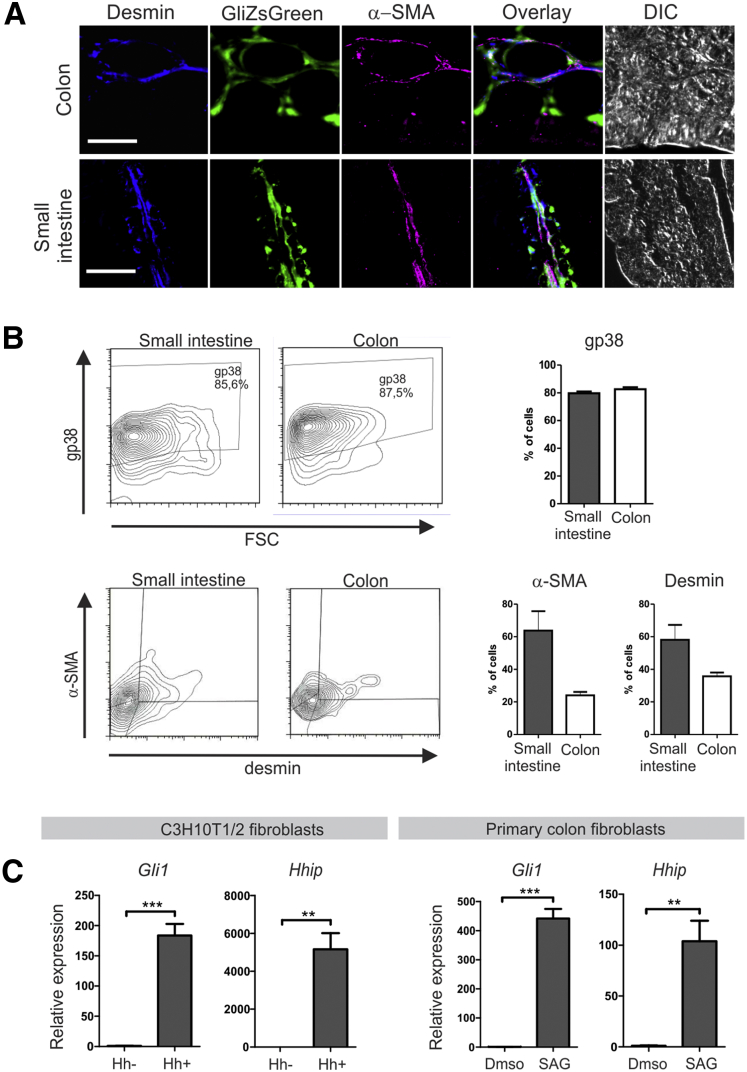
Further analysis showed a partial overlap of Gli1-driven ZsGreen expression with desmin, α smooth muscle actin (α-SMA), and desmin/α-SMA double expression, markers for smooth muscle precursors, myofibroblasts, and smooth muscle cells, respectively, showing the heterogeneity of the stromal Hedgehog-responsive cells (Figure 5A). Although the vast majority of GliZsGreen+ cells expressed gp38 (∼85%), expression of α-SMA and desmin was restricted to approximately 60% in the small intestine and approximately 30% in the colon (Figure 5B).
Figure 5.
Hedgehog-responsive cells are a heterogeneous population of fibroblasts. (A) Immunofluorescent staining for desmin (blue) and α-SMA (magenta) on sections generated from induced Gli1-CreERT2-Rosa26-ZSGreen animals. For topographic reference, a differential interference contrast (DIC) image is shown. Scale bars: 50 μm. (B) Flow cytometry analysis and quantification of expression of gp38, desmin, and α-SMA in ZsGreen+ cells isolated from induced Gli1-CreERT2-Rosa26-ZsGreen animals. (C) Quantitative RT-PCR for Hedgehog targets Gli1 and Hhip in C3H10T1/2 fibroblasts or primary mouse colon fibroblasts that were incubated in 10% Hedgehog conditioned medium or stimulated with a Smoothened agonist (SAG), respectively, for 7 days. Expression relative to the household gene cyclophilin is shown. Data are representative of 3 independent experiments. Bars represent means, error bars represent SEM. **P < .01, ***P < .0001.
To further confirm Hedgehog responsiveness of mesenchymal cells in vitro, the murine mesenchymal cell line C3H10T1/2 was incubated with either Hedgehog conditioned or control medium and induction of the Hedgehog target genes Gli1 and Hhip were measured. Incubation for 7 days resulted in a strong induction of Hedgehog signaling (Figure 5C). This was corroborated using primary mouse colon fibroblasts, where the Hedgehog targets were up-regulated significantly after activation of the Smoothened receptor upon administration of the Smoothened agonist. Collectively, these data indicate that Ihh signals to the stroma specifically to a heterogeneous population of fibroblasts.
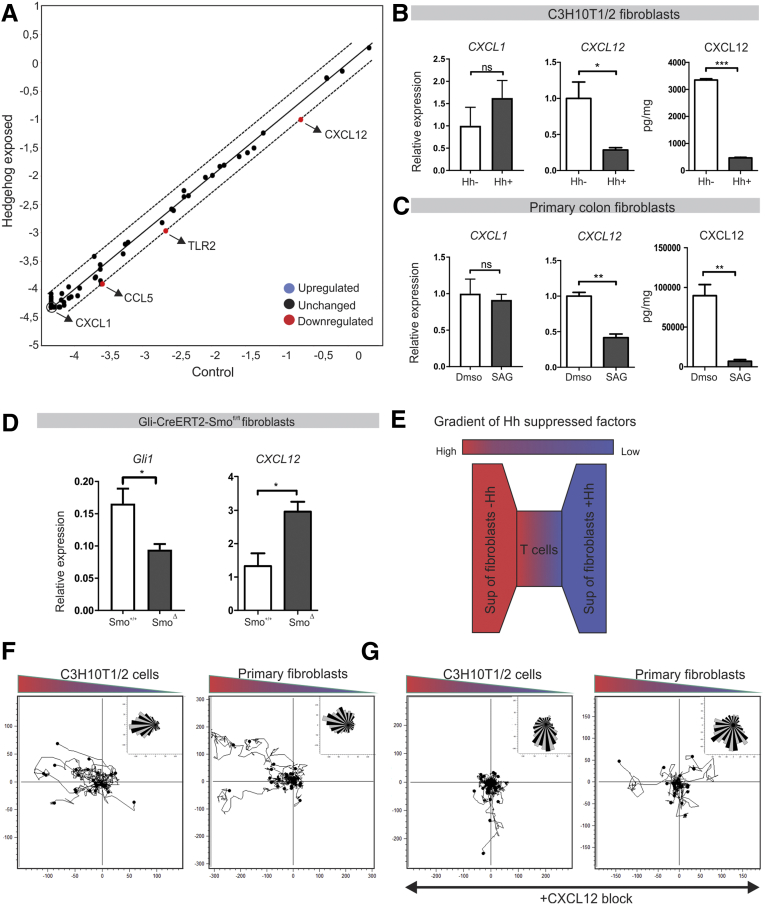
Indian Hedgehog Inhibits Immune Cell Migration Through Suppression of Stromal CXCL12
Given the fact that Hedgehog-responsive cells are fibroblasts and chemokine expression is increased in the colon in the absence of Hedgehog signaling, we hypothesized that Hedgehog suppresses chemokine expression in fibroblasts. To investigate this, an array of 84 chemokine-related transcripts was analyzed by a quantitative RT-PCR array in C3H10T1/2 fibroblasts exposed to Hedgehog-containing medium. Among these 84 chemokines, the chemokine CXCL12 was down-regulated prominently in C3H10T1/2 cells upon stimulation of the Hedgehog pathway (Figure 6A and B). We further confirmed these data in primary mouse colon fibroblasts (Figure 6C). As a control, CXCL1 expression was not altered in the PCR array and this could be verified in both C3H10T1/2 and primary cells (Figure 6A–C).
Figure 6.
Hedgehog suppresses immune cell migration through inhibition of fibroblast-derived CXCL12. (A) Quantitative RT-PCR array for 84 chemokines and receptors in C3H10T1/2 cells incubated in 10% Hedgehog conditioned medium for 48 hours. (B and C) Quantitative RT-PCR for CXCL1 and CXCL12 and enzyme-linked immunosorbent assay for CXCL12 in C3H10T1/2 cells or primary mouse colon fibroblasts that were incubated in 10% Hedgehog conditioned medium or stimulated with a Smoothened agonist (SAG), respectively, for 7 days. Data are representative of 3 independent experiments. (D) Quantitative RT-PCR for Gli1 and CXCL12 in Epcam-CD45-gp38+ fibroblasts isolated from Gli1-CreERT2-ZsGreen-Smo+/+ (Smo+/+, n = 3) and Gli1-CreERT2-ZsGreen-Smofl/fl animals (SmoΔ, n = 3). (E) Experimental set up: Jurkat T cells were cultured in a collagen/fibronectin matrix and exposed to a gradient of supernatant of fibroblasts with active Hedgehog signaling and control fibroblasts. Migration was tracked overnight. (F) Migration assay for Jurkat T cells in response to supernatant of C3H10T1/2 cells or primary mouse colon fibroblasts that were incubated in 10% Hedgehog conditioned medium or stimulated with SAG, respectively, for 7 days. Data are representative of 3 independent experiments. (G) Migration assay for Jurkat T cells in response to supernatant of fibroblasts supplemented with CXCL12 neutralizing antibody. Data are representative of 3 independent experiments. Bars represent means, error bars represent SEM. *P < .05, **P < .01, ***P < .001. DMSO, dimethyl sulfoxide; Sup, supernatant.
In addition, CXCL12 protein released by fibroblasts was analyzed. Again, a high level of CXCL12 was present in the supernatant of these cells, which could be suppressed by activation of the Hedgehog pathway (Figure 6B and C). Furthermore, we observed an increase in CXCL12 expression in gp38+ fibroblasts isolated from induced Gli1-CreERT2-Smofl/fl animals vs control animals (Figure 6D). The key role of CXCL12 secretion was shown in a migration assay (Figure 6E). Exposing Jurkat T cells to a gradient of supernatant obtained from either Hedgehog stimulated (and thus CXCL12 low) or control (and thus CXCL12 high) fibroblasts showed a clear preferential migration toward the control supernatant (Figure 6F). Critically, migration toward supernatant of unstimulated C3H10T1/2 cells was inhibited when using a CXCL12 neutralizing antibody (Figure 6G). In all, we show that activation of the Hedgehog pathway leads to inhibition of CXCL12 expression by fibroblasts, and thereby suppression of immune cell migration.
Discussion
Loss of Indian Hedgehog from the intestinal epithelium results in an epithelial phenotype that closely resembles a wound healing response with increased stem cell proliferation.10, 11 Ultimately, unresolved loss of intestinal Hedgehog signaling results in the development of a chronic enteritis with villous atrophy. Here, we focus on the short-term effects of deletion of Ihh from the intestinal epithelium and find that down-regulation of intestinal Hedgehog signaling results in a rapidly developing inflammatory response. We observe a rapid increase in chemokine expression accompanied by infiltration of immune cells and increased susceptibility to DSS-induced colitis. Conversely, activation of Hedgehog signaling resulted in decreased inflammatory markers and rescued recruitment of immune cells. Although it has to be noted that this is a body-wide activation, the results complement the results obtained by intestinal-specific deletion of Hedgehog signaling. Tamoxifen was administered to both knock-out and wild-type animals to control for tamoxifen-induced effects. Although we cannot formally exclude the possibility that tamoxifen itself affects Hedgehog-deficient animals differently than wild types, this appears unlikely. Despite the increasing interest in the role of Hedgehog signaling in both intestinal immune responses and carcinogenesis, the nature of the Hedgehog-responsive cells has not been defined precisely. We show that Hedgehog-responsive cells are fibroblast-like cells. By signaling to these cells, Ihh inhibits the expression of chemokine CXCL12. In in vitro experiments we show that fibroblast-directed immune cell migration is suppressed by Hedgehog signaling in a CXCL12-dependent manner.
Both within the small intestine and colon, the vast majority of Gli1+ cells express the pan-fibroblast markers gp38, collagen III, and platelet-derived growth factor receptor-β. Expression of desmin and α-SMA was detected, albeit not on all cells, confirming the heterogeneity of the responsive fibroblast-like cells. Earlier data also indicated the importance of Hedgehog signaling for intestinal stromal cells. For example, several studies have shown that decreased Hedgehog signaling leads to depletion of smooth muscle cells from the villus core,10, 14 whereas overexpression of Hedgehog results in expansion of the smooth muscle compartment.7, 8, 15 Interestingly, VillinCre-Ihhfl/fl mice not only show disruption of the mesenchymal compartment after birth, but also reduced proliferation of pericryptal myofibroblasts.15 In addition, a more recent study showed the presence of Gli1+ mesenchymal cells that lie in close proximity to Lgr5+ stem cells and secrete high levels of the Wnt/β-catenin signaling activator Wnt2b.27 This is in line with work published by another group, showing that Foxl1-expressing stromal cells are Hedgehog responsive and a crucial part of the intestinal stem cell niche.28, 29 Altogether these interesting data suggest the existence of a Hedgehog-responsive subpopulation of fibroblasts via which Ihh is able to directly maintain stem cell homeostasis.
Earlier, mesenchymal myeloid cells were suggested as Hedgehog targets because double-immunofluorescence staining on lacZ reporter mice for the Hedgehog target Gli1 indicated that CD11b+ and CD11c+ myeloid cells might be Hedgehog responsive.11 However, we found both macrophages and dendritic cells to be unresponsive to Hedgehog in vitro, and deleting Hedgehog signaling in these cell types in vivo did not alter the animals’ sensitivity to DSS. This is in line with earlier data that showed that deletion of either Ptch1 or Smoothened (and therefore activation or inhibition of Hedgehog signaling, respectively) in the hematopoietic compartment of adult mice did not result in an aberrant immunophenotype.30, 31
Interestingly, our data show a link between intestinal Hedgehog signaling and expression of the well-studied chemokine CXCL12. CXCL12 not only plays a crucial role in homing and mobilization of bone marrow progenitor cells,32, 33 but also is up-regulated in inflammatory diseases.34 It was shown that CXCL12 expression is increased in inflamed colonic tissue of mice with DSS-induced colitis35, 36 and up-regulated in patients with IBD.34 This correlates with the fact that both in human beings and mice the Hedgehog targets are down-regulated in the inflamed intestine.12, 13, 26 It was shown that CXCL12 attracts both peripheral blood T cells as lamina propria T cells through its receptor CXCR4, resulting in accumulation of CXCR4+ cells in the intestinal lamina propria.34, 37 In line with these observations, administration of a CXCR4 antagonist was able to alleviate disease activity in 2 different murine models of colitis.36
Our previous work suggested that the absence of Hedgehog may serve as a danger signal for epithelial damage, and results in a wound repair response to quickly replace damaged epithelial cells that are the source of Ihh.10 However, epithelial damage also results in decreased barrier function and increased likelihood of pathogenic invasion. These new data now indicate that lack of Hedgehog not only stimulates epithelial repair, but also primes the local immune system for rapid response to invasion, even in the absence of a clear proinflammatory signal. In our model, we propose an important role for fibroblast-derived CXCL12 in rapid recruitment of leukocytes to the colonic mucosa upon epithelial damage and thus loss of Hedgehog signaling. Although this local priming may be beneficial in the case of localized epithelial damage, intestinal-wide priming as a result of genetic polymorphisms that diminish Hedgehog signaling may contribute to an excessive immune response and enhanced epithelial damage upon normally minor tissue insults.
During preparation of this manuscript, a new study by Lee et al26 was published online that independently confirmed part of our results. These investigators also showed that a decrease in Hedgehog pathway activity, by inhibition of the Hedgehog receptor Smoothened, increases susceptibility to DSS-induced colitis. Conversely, activation of the Hedgehog pathway protected animals against colitis. This study focused on the pathway as a whole, and did not address the relative contribution of Sonic hedgehog and Ihh in suppressing intestinal inflammation. By specifically deleting Ihh from the intestinal epithelium, we show that Ihh is responsible for the anti-inflammatory role of the Hedgehog pathway in the intestine. When looking for an underlying mechanism, Lee et al26 showed that the protective effect of Hedgehog is owing partly to induction of the anti-inflammatory cytokine IL10 in fibroblasts. However, mice lacking IL10 still showed a partial protection against DSS colitis after stimulation of Smoothened, suggesting additional mechanisms for Hedgehog signaling in immunosuppression. The data described here may provide such an alternative mechanism.
In summary, these data show that in the intestine the direct targets of Hedgehog signaling are a heterogeneous population of fibroblasts. Active signaling through these cells serves as a no-danger signal for both the epithelium and the immune compartments. Abrogation of signaling results not only in wound healing responses, but also in a heightened activation state of the intestinal immune system.
Footnotes
Author contributions B. Florien Westendorp, Nikè V. J. A. Büller, Olga N. Karpus, Manon E. Wildenberg, Vanesa Muncan, and Gijs R. van den Brink were responsible for conceptualization and methodology; B. Florien Westendorp, Nikè V. J. A. Büller, Manon E. Wildenberg, and Vanesa Muncan were responsible for validation; B. Florien Westendorp, Nikè V. J. A. Büller, Willemijn A. van Dop, Manon E. Wildenberg, Vanesa Muncan, Joris J. T. H. Roelofs, Emiel Ver Loren van Themaat, Rogier Versteeg, and Jan Koster performed the formal analysis; B. Florien Westendorp, Nikè V. J. A. Büller, Olga N. Karpus, Willemijn A. van Dop, Pim J. Koelink, Clinton Y. Snel, Sander Meisner, and Jarom Heijmans performed the investigation; Anja Uhmann and Heidi Hahn were responsible for resources; B. Florien Westendorp and Nikè V. J. A. Büller wrote the original draft; Olga N. Karpus, Manon E. Wildenberg, Vanesa Muncan, and Gijs R. van den Brink performed the review and editing; B. Florien Westendorp, Nikè V. J. A. Büller, and Manon E. Wildenberg were responsible for visualization; Manon E. Wildenberg, Vanesa Muncan, and Gijs R. van den Brink were responsible for supervision; B. Florien Westendorp and Nikè V. J. A. Büller were responsible for project administration; and Gijs R. van den Brink was responsible for funding acquisition.
Conflicts of interest The authors disclose no conflicts.
Funding This work was financially supported by a Vici grant 016.140.605 (G.R.B.) from The Netherlands Organisation for Scientific Research (NWO).
Supplementary Material
References
- 1.Liu Y.J., Soumelis V., Watanabe N., Ito T., Wang Y.H., Malefyt Rde W., Omori M., Zhou B., Ziegler S.F. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 2.Zeuthen L.H., Fink L.N., Frokiaer H. Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-beta. Immunology. 2008;123:197–208. doi: 10.1111/j.1365-2567.2007.02687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang S., Okuno T., Takegahara N., Takamatsu H., Nojima S., Kimura T., Yoshida Y., Ito D., Ohmae S., You D.J., Toyofuku T., Jang M.H., Kumanogoh A. Intestinal epithelial cell-derived semaphorin 7A negatively regulates development of colitis via alphavbeta1 integrin. J Immunol. 2012;188:1108–1116. doi: 10.4049/jimmunol.1102084. [DOI] [PubMed] [Google Scholar]
- 4.Saenz S.A., Siracusa M.C., Perrigoue J.G., Spencer S.P., Urban J.F., Jr., Tocker J.E., Budelsky A.L., Kleinschek M.A., Kastelein R.A., Kambayashi T., Bhandoola A., Artis D. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martini E., Krug S.M., Siegmund B., Neurath M.F., Becker C. Mend your fences. Cell Mol Gastroenterol Hepatol. 2017;4:33–46. doi: 10.1016/j.jcmgh.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Brink G.R., Bleuming S.A., Hardwick J.C., Schepman B.L., Offerhaus G.J., Keller J.J., Nielsen C., Gaffield W., van Deventer S.J., Roberts D.J., Peppelenbosch M.P. Indian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nat Genet. 2004;36:277–282. doi: 10.1038/ng1304. [DOI] [PubMed] [Google Scholar]
- 7.van Dop W.A., Uhmann A., Wijgerde M., Sleddens-Linkels E., Heijmans J., Offerhaus G.J., van den Bergh Weerman M.A., Boeckxstaens G.E., Hommes D.W., Hardwick J.C., Hahn H., van den Brink G.R. Depletion of the colonic epithelial precursor cell compartment upon conditional activation of the hedgehog pathway. Gastroenterology. 2009;136:2195–2203. doi: 10.1053/j.gastro.2009.02.068. e1–e7. [DOI] [PubMed] [Google Scholar]
- 8.Kolterud A., Grosse A.S., Zacharias W.J., Walton K.D., Kretovich K.E., Madison B.B., Waghray M., Ferris J.E., Hu C., Merchant J.L., Dlugosz A.A., Kottmann A.H., Gumucio D.L. Paracrine Hedgehog signaling in stomach and intestine: new roles for hedgehog in gastrointestinal patterning. Gastroenterology. 2009;137:618–628. doi: 10.1053/j.gastro.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buller N.V., Rosekrans S.L., Westerlund J., van den Brink G.R. Hedgehog signaling and maintenance of homeostasis in the intestinal epithelium. Physiology (Bethesda) 2012;27:148–155. doi: 10.1152/physiol.00003.2012. [DOI] [PubMed] [Google Scholar]
- 10.van Dop W.A., Heijmans J., Buller N.V., Snoek S.A., Rosekrans S.L., Wassenberg E.A., van den Bergh Weerman M.A., Lanske B., Clarke A.R., Winton D.J., Wijgerde M., Offerhaus G.J., Hommes D.W., Hardwick J.C., de Jonge W.J., Biemond I., van den Brink G.R. Loss of Indian Hedgehog activates multiple aspects of a wound healing response in the mouse intestine. Gastroenterology. 2010;139:1665–1676. doi: 10.1053/j.gastro.2010.07.045. 1676 e1–e10. [DOI] [PubMed] [Google Scholar]
- 11.Zacharias W.J., Li X., Madison B.B., Kretovich K., Kao J.Y., Merchant J.L., Gumucio D.L. Hedgehog is an anti-inflammatory epithelial signal for the intestinal lamina propria. Gastroenterology. 2010;138:2368–2377. doi: 10.1053/j.gastro.2010.02.057. 2377 e1–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lees C.W., Zacharias W.J., Tremelling M., Noble C.L., Nimmo E.R., Tenesa A., Cornelius J., Torkvist L., Kao J., Farrington S., Drummond H.E., Ho G.T., Arnott I.D., Appelman H.D., Diehl L., Campbell H., Dunlop M.G., Parkes M., Howie S.E., Gumucio D.L., Satsangi J. Analysis of germline GLI1 variation implicates hedgehog signalling in the regulation of intestinal inflammatory pathways. PLoS Med. 2008;5:e239. doi: 10.1371/journal.pmed.0050239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noble C.L., Abbas A.R., Cornelius J., Lees C.W., Ho G.T., Toy K., Modrusan Z., Pal N., Zhong F., Chalasani S., Clark H., Arnott I.D., Penman I.D., Satsangi J., Diehl L. Regional variation in gene expression in the healthy colon is dysregulated in ulcerative colitis. Gut. 2008;57:1398–1405. doi: 10.1136/gut.2008.148395. [DOI] [PubMed] [Google Scholar]
- 14.Zacharias W.J., Madison B.B., Kretovich K.E., Walton K.D., Richards N., Udager A.M., Li X., Gumucio D.L. Hedgehog signaling controls homeostasis of adult intestinal smooth muscle. Dev Biol. 2011;355:152–162. doi: 10.1016/j.ydbio.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kosinski C., Stange D.E., Xu C., Chan A.S., Ho C., Yuen S.T., Mifflin R.C., Powell D.W., Clevers H., Leung S.Y., Chen X. Indian hedgehog regulates intestinal stem cell fate through epithelial-mesenchymal interactions during development. Gastroenterology. 2010;139:893–903. doi: 10.1053/j.gastro.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.el Marjou F., Janssen K.P., Chang B.H., Li M., Hindie V., Chan L., Louvard D., Chambon P., Metzger D., Robine S. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39:186–193. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- 17.Razzaque M.S., Soegiarto D.W., Chang D., Long F., Lanske B. Conditional deletion of Indian hedgehog from collagen type 2alpha1-expressing cells results in abnormal endochondral bone formation. J Pathol. 2005;207:453–461. doi: 10.1002/path.1870. [DOI] [PubMed] [Google Scholar]
- 18.Hameyer D., Loonstra A., Eshkind L., Schmitt S., Antunes C., Groen A., Bindels E., Jonkers J., Krimpenfort P., Meuwissen R., Rijswijk L., Bex A., Berns A., Bockamp E. Toxicity of ligand-dependent Cre recombinases and generation of a conditional Cre deleter mouse allowing mosaic recombination in peripheral tissues. Physiol Genomics. 2007;31:32–41. doi: 10.1152/physiolgenomics.00019.2007. [DOI] [PubMed] [Google Scholar]
- 19.Uhmann A., Dittmann K., Nitzki F., Dressel R., Koleva M., Frommhold A., Zibat A., Binder C., Adham I., Nitsche M., Heller T., Armstrong V., Schulz-Schaeffer W., Wienands J., Hahn H. The Hedgehog receptor Patched controls lymphoid lineage commitment. Blood. 2007;110:1814–1823. doi: 10.1182/blood-2007-02-075648. [DOI] [PubMed] [Google Scholar]
- 20.Clausen B.E., Burkhardt C., Reith W., Renkawitz R., Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 21.Cooper H.S., Murthy S.N., Shah R.S., Sedergran D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 22.Mootha V.K., Lindgren C.M., Eriksson K.F., Subramanian A., Sihag S., Lehar J., Puigserver P., Carlsson E., Ridderstrale M., Laurila E., Houstis N., Daly M.J., Patterson N., Mesirov J.P., Golub T.R., Tamayo P., Spiegelman B., Lander E.S., Hirschhorn J.N., Altshuler D., Groop L.C. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 23.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., Mesirov J.P. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edgar R., Domrachev M., Lash A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J.K., Taipale J., Young K.E., Maiti T., Beachy P.A. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci U S A. 2002;99:14071–14076. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J.J., Rothenberg M.E., Seeley E.S., Zimdahl B., Kawano S., Lu W.J., Shin K., Sakata-Kato T., Chen J.K., Diehn M., Clarke M.F., Beachy P.A. Control of inflammation by stromal Hedgehog pathway activation restrains colitis. Proc Natl Acad Sci U S A. 2016;113:E7545–E7553. doi: 10.1073/pnas.1616447113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valenta T., Degirmenci B., Moor A.E., Herr P., Zimmerli D., Moor M.B., Hausmann G., Cantu C., Aguet M., Basler K. Wnt ligands secreted by subepithelial mesenchymal cells are essential for the survival of intestinal stem cells and gut homeostasis. Cell Rep. 2016;15:911–918. doi: 10.1016/j.celrep.2016.03.088. [DOI] [PubMed] [Google Scholar]
- 28.Madison B.B., McKenna L.B., Dolson D., Epstein D.J., Kaestner K.H. FoxF1 and FoxL1 link hedgehog signaling and the control of epithelial proliferation in the developing stomach and intestine. J Biol Chem. 2009;284:5936–5944. doi: 10.1074/jbc.M808103200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aoki R., Shoshkes-Carmel M., Gao N., Shin S., May C.L., Golson M.L., Zahm A.M., Ray M., Wiser C.L., Wright C.V., Kaestner K.H. Foxl1-expressing mesenchymal cells constitute the intestinal stem cell niche. Cell Mol Gastroenterol Hepatol. 2016;2:175–188. doi: 10.1016/j.jcmgh.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hofmann I., Stover E.H., Cullen D.E., Mao J., Morgan K.J., Lee B.H., Kharas M.G., Miller P.G., Cornejo M.G., Okabe R., Armstrong S.A., Ghilardi N., Gould S., de Sauvage F.J., McMahon A.P., Gilliland D.G. Hedgehog signaling is dispensable for adult murine hematopoietic stem cell function and hematopoiesis. Cell Stem Cell. 2009;4:559–567. doi: 10.1016/j.stem.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siggins S.L., Nguyen N.Y., McCormack M.P., Vasudevan S., Villani R., Jane S.M., Wainwright B.J., Curtis D.J. The Hedgehog receptor Patched1 regulates myeloid and lymphoid progenitors by distinct cell-extrinsic mechanisms. Blood. 2009;114:995–1004. doi: 10.1182/blood-2009-03-208330. [DOI] [PubMed] [Google Scholar]
- 32.Morrison S.J., Scadden D.T. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugiyama T., Kohara H., Noda M., Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Dotan I., Werner L., Vigodman S., Weiss S., Brazowski E., Maharshak N., Chen O., Tulchinsky H., Halpern Z., Guzner-Gur H. CXCL12 is a constitutive and inflammatory chemokine in the intestinal immune system. Inflamm Bowel Dis. 2010;16:583–592. doi: 10.1002/ibd.21106. [DOI] [PubMed] [Google Scholar]
- 35.Mikami S., Nakase H., Yamamoto S., Takeda Y., Yoshino T., Kasahara K., Ueno S., Uza N., Oishi S., Fujii N., Nagasawa T., Chiba T. Blockade of CXCL12/CXCR4 axis ameliorates murine experimental colitis. J Pharmacol Exp Ther. 2008;327:383–392. doi: 10.1124/jpet.108.141085. [DOI] [PubMed] [Google Scholar]
- 36.Xia X.M., Wang F.Y., Xu W.A., Wang Z.K., Liu J., Lu Y.K., Jin X.X., Lu H., Shen Y.Z. CXCR4 antagonist AMD3100 attenuates colonic damage in mice with experimental colitis. World J Gastroenterol. 2010;16:2873–2880. doi: 10.3748/wjg.v16.i23.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Werner L., Elad H., Brazowski E., Tulchinsky H., Vigodman S., Kopylov U., Halpern Z., Guzner-Gur H., Dotan I. Reciprocal regulation of CXCR4 and CXCR7 in intestinal mucosal homeostasis and inflammatory bowel disease. J Leukoc Biol. 2011;90:583–590. doi: 10.1189/jlb.0111101. [DOI] [PubMed] [Google Scholar]