Abstract
Background
Lung cancer is one of the leading cancer sites diagnosed among Asian Americans, Pacific Islanders, and Native Hawaiians (AANHPI). To better understand the patterns of lung cancer incidence among AANHPIs, we examined the incidence trends of five histologic cell types of lung cancer across ten AANHPI populations in comparison with non-Hispanic Whites.
Methods
Lung cancer incidence data from 1990 through 2010 were obtained from 13 U.S. population-based cancer registries. Age-adjusted histologic cell-type–specific incidence rates and 95% confidence intervals were calculated. Joinpoint regression models and annual percentage change (APC) statistics were used to characterize the magnitude and direction of trends.
Results
From 1990 through 2010, incidence rates of adenocarcinoma increased significantly for Filipino and Korean women with a 2.6% and 3.0% annual percentage increase, respectively. More recently, a significant rise in the incidence of adenocarcinoma was observed for Chinese men (1996–2010; APC = 1.3%). Squamous cell carcinoma (SCC) increased 2.4% per year among Japanese women. For SCC, small cell lung carcinoma, large cell and other specified carcinoma, and unspecified types, stable or decreasing trends were observed in most AANHPI groups and non-Hispanic Whites.
Conclusions
AANHPIs demonstrate a range in the burden of lung cancer across histologies and specific populations.
Impact
These findings illustrate the importance of disaggregating AANHPIs into their specific populations. The rise in incidence of adenocarcinoma and SCC among certain AANHPIs demonstrates the need for research into non-tobacco associated risk factors for these populations and targeted efforts for tobacco prevention.
Introduction
Asian Americans are the fastest growing racial/ethnic group in the United States (1–3), reaching more than 17 million in the 2010 Census (2, 3). In the first nationwide report of cancer incidence trends among specific Asian American populations, our group identified lung cancer as one of the top four cancers diagnosed among these populations from 1990 through 2008 (4). Lung cancer was consistently the leading cancer diagnosis among Kampuchean men and the third ranking cancer diagnosed among Chinese, Filipina, and Japanese women (4). Moreover, although non-Hispanic Whites (NHW) demonstrated a decline in the incidence of lung cancer, such a decline was not observed in any Asian American population. Notably, increasing incidence trends of lung cancer were observed among South Asian men and Filipina and Korean women (4).
Native Hawaiians and pacific islanders are a small but growing population in the United States (5). Lung cancer was among the top three cancer sites in these populations from 1990 through 2008 (6). Although both Native Hawaiian men and women demonstrated steady declines in the incidence rates of lung cancer (6), Native Hawaiian and Samoan men consistently displayed higher incidence rates in comparison with NHW (6).
It is well known that tumor histology plays an important role in lung cancer etiology and prognosis. The incidence rates of the histologic subtypes vary (7–10) due to differences in exposure of smoking and other lung carcinogens. Because of decreased smoking behavior, from 1992 through 2005, the overall incidence trends of squamous cell carcinoma (SCC), lung adenocarcinoma, and small cell carcinoma declined in the United States, with more rapid declines observed for SCC than adenocarcinoma (11). Yet, there is little information on these detailed histologic trends across specific racial/ethnic populations. This is particularly relevant for Asian American, Native Hawaiian, and Pacific Islander (AANHPI) populations such as Koreans, Vietnamese, and Samoans, who have persistently high prevalence of smoking (12, 13), and for particular AANHPI women, such as Chinese women, who have an exceedingly low prevalence of smoking yet a high burden of lung adenocarcinoma (14).
To further understand the patterns of lung cancer incidence across AANHPIs, we examined the incidence trends of lung cancer by five histologic cell types across ten AANHPI populations by gender. The assembly of nationwide population-based histologic-specific incidence data by the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program enables a high quality and comprehensive investigation across these important populations with varying incidence rates of disease.
Materials and Methods
Cancer incidence data
Cancer incidence data for all invasive lung cancers (excluding mesothelioma, Kaposi Sarcoma, and lymphomas and leukemias) during the 21-year period from 1990 through 2010 were obtained from 13 U.S. population-based SEER cancer registries (Table 1). Histologic cell types were classified as defined by Lewis and colleagues (15) for small cell lung cancer (“SCLC”), adenocarcinoma, “SCC”, large cell and other specified carcinoma (“LC+OSC”), and unspecified malignant neoplasms (“unspecified”). A total of 100 AANHPI cases and 1,475 NHW cases specified as a noncarcinoma or seemed to be a metastasis were excluded. The SEER registries cover 50.4% of the Asian American population and 66.5% of the NHPI population (16).
Table 1.
Annual population estimates and percentages, nativity, and language for each Asian American, Native Hawaiian, Pacific Islander population, and non-Hispanic Whites by SEER Registry Geographic Region and Census Year (1990, 2000, and 2010)
| White non-Hispanic | Chinese | Japanese | Filipino | Vietnamese | Korean | Native Hawaiian | Kampuchean | Laotian | Samoan | Asian Indian, Pakistani | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1990
| |||||||||||
| All SEER regions | 38,062,478 | 943,014 | 660,378 | 1,040,610 | 345,004 | 391,190 | 248,722 | 89,112 | 81,419 | 49,684 | 319,350 |
| Californiaa | 44.9% | 78.4% | 49.5% | 73.9% | 85.8% | 69.8% | 14.6% | 81.2% | 75.7% | 68.2% | 56.8% |
| Connecticut | 7.2% | 1.2% | <1% | <1% | 1.2% | 1.4% | <1% | 2.0% | 3.8% | <1% | 4.1% |
| Atlanta (metropolitan) | 3.7% | 1.0% | <1% | <1% | 1.7% | 2.5% | <1% | 2.3% | 3.6% | <1% | 2.5% |
| Hawaii | <1% | 7.3% | 39.7% | 14.7% | 1.6% | 5.2% | 82.4% | <1% | 2.1% | 19.6% | <1% |
| Iowa | 7.0% | <1% | <1% | <1% | <1% | 1.2% | <1% | <1% | 4.1% | <1% | 1.1% |
| Detroit (metropolitan) | 7.4% | 1.0% | <1% | <1% | <1% | 1.7% | <1% | <1% | 1.1% | <1% | 5.7% |
| New Jersey | 15.1% | 6.4% | 2.7% | 5.2% | 2.2% | 10.1% | <1% | <1% | <1% | <1% | 27.6% |
| New Mexico | 2.0% | <1% | <1% | <1% | <1% | <1% | <1% | <1% | <1% | <1% | <1% |
| Utah | 4.1% | <1% | 1.0% | <1% | <1% | <1% | <1% | 1.2% | 2.2% | 3.3% | <1% |
| Seattle (Puget Sound) | 7.7% | 3.3% | 4.6% | 4.0% | 4.9% | 7.0% | 1.7% | 11.8% | 6.1% | 7.9% | 1.7% |
|
2000 | |||||||||||
| % Foreign-bornb | — | 63.9% | 29.2% | 55.8% | 73.5% | 69.9% | 1.9% | 64.4% | 65.8% | 16.6% | 73.0% |
| % Limited Englishc,d | — | 45.0% | 20.7% | 20.5% | 60.5% | 46.0% | 3.3% | 52.6% | 51.6% | 16.2% | 23.9% |
| All SEER regions | 37,943,386 | 1,396,938 | 660,856 | 1,420,523 | 591,877 | 557,799 | 293,023 | 100,909 | 85,658 | 75,620 | 685,041 |
| Californiaa | 43.1% | 75.6% | 52.2% | 71.6% | 79.3% | 65.2% | 13.9% | 77.2% | 70.9% | 58.2% | 53.2% |
| Connecticut | 7.0% | 1.5% | <1% | <1% | 1.4% | 1.4% | <1% | 2.6% | 3.6% | <1% | 4.1% |
| Atlanta (metropolitan) | 4.0% | 1.6% | <1% | <1% | 4.1% | 3.9% | <1% | 2.8% | 4.0% | <1% | 4.2% |
| Hawaii | <1% | 6.4% | 34.6% | 14.1% | 1.5% | 5.1% | 81.8% | <1% | 2.5% | 21.4% | <1% |
| Iowa | 7.2% | <1% | <1% | <1% | 1.3% | 1.0% | <1% | <1% | 5.2% | <1% | <1% |
| Detroit (metropolitan) | 7.3% | 1.3% | 1.1% | 1.0% | <1% | 1.6% | <1% | <1% | 1.4% | <1% | 6.6% |
| New Jersey | 14.9% | 7.6% | 2.6% | 6.4% | 2.7% | 12.2% | <1% | <1% | <1% | <1% | 27.8% |
| New Mexico | 2.2% | <1% | <1% | <1% | 0.6% | <1% | <1% | <1% | <1% | <1% | <1% |
| Utah | 5.1% | <1% | 1.2% | <1% | 1.1% | <1% | <1% | 1.5% | 2.9% | 7.4% | <1% |
| Seattle (Puget Sound) | 8.6% | 4.4% | 6.0% | 5.1% | 7.3% | 8.4% | 2.4% | 14.0% | 8.4% | 10.9% | 2.6% |
| % Foreign-bornb | — | 61.2% | 27.4% | 52.8% | 63.6% | 64.6% | 1.3% | 55.3% | 54.4% | 10.4% | 69.5% |
| % Limited Englishc,d | — | 41.0% | 17.3% | 18.2% | 50.3% | 40.9% | 1.7% | 41.7% | 40.0% | 11.7% | 22.2% |
| All SEER regions | 36,786,858 | 1,764,410 | 715,300 | 1,793,997 | 788,310 | 729,707 | 392,188 | 125,941 | 92,331 | 95,658 | 1,179,826 |
| Californiaa | 42.1% | 77.0% | 49.3% | 74.8% | 78.3% | 65.9% | 12.3% | 75.1% | 69.4% | 53.3% | 51.9% |
| Connecticut | 7.0% | 1.9% | <1% | <1% | 1.3% | 1.5% | <1% | 2.4% | 4.0% | <1% | 4.7% |
| Atlanta (metropolitan) | 3.8% | 2.0% | <1% | <1% | 4.4% | 5.6% | <1% | 3.0% | 4.0% | <1% | 4.3% |
| Hawaii | <1% | 2.4% | 37.5% | 9.5% | 1.5% | 1.0% | 83.1% | <1% | 2.4% | 19.1% | <1% |
| Iowa | 7.4% | <1% | <1% | <1% | 1.1% | <1% | <1% | <1% | 5.7% | <1% | 1.1% |
| Detroit (metropolitan) | 6.9% | 1.3% | 1.1% | 1.0% | <1% | 1.5% | <1% | <1% | <1% | <1% | 5.5% |
| New Jersey | 14.4% | 8.1% | 2.3% | 6.7% | 2.8% | 13.4% | <1% | 1.2% | <1% | <1% | 28.0% |
| New Mexico | 2.3% | <1% | <1% | <1% | 0.6% | <1% | <1% | <1% | <1% | <1% | <1% |
| Utah | 6.1% | <1% | 1.3% | <1% | 1.1% | <1% | 1.1% | 1.7% | 3.1% | 11.3% | <1% |
| Seattle (Puget Sound) | 9.2% | 5.5% | 6.2% | 5.7% | 8.0% | 8.9% | 2.3% | 15.2% | 9.2% | 14.8% | 4.1% |
| New Mexico | 2.2% | <1% | <1% | <1% | 0.6% | <1% | <1% | <1% | <1% | <1% | <1% |
| Utah | 5.1% | <1% | 1.2% | <1% | 1.1% | <1% | <1% | 1.5% | 2.9% | 7.4% | <1% |
| Seattle (Puget Sound) | 8.6% | 4.4% | 6.0% | 5.1% | 7.3% | 8.4% | 2.4% | 14.0% | 8.4% | 10.9% | 2.6% |
Including Los Angeles, San Francisco/Oakland and San Jose/Monterey, and the Greater California registries.
Source: U.S. Census Bureau, Census 2000 Summary File 4, Matrices PCT38 (language), and PCT43 (nativity) for Asian detailed group or Native Hawaiian and Other Pacific Islander alone or in any combination; Chinese definition includes Taiwanese.
Source: U.S. Census Bureau, 2006–2010 American Community Survey for Asian detailed group or Native Hawaiian and Other Pacific Islander alone or in any combination; Chinese definition includes Taiwanese.
Speak English less than “very well”; among population 5 years and over.
SEER data on race and Hispanic ethnicity were generally based on patients’ medical records (17, 18). AANHPIs were included in this analysis regardless of Hispanic ethnicity. Information on birthplace and surname was used when a specific race designation was lacking (19). Nonetheless, approximately 4.7% (increase of 0.16% in 1990 to 7.4% in 2010) of the AANHPI lung cancer cases were classified as “other Asian; Asian, not otherwise specified” and could not be included in a specific AANHPI category. Asian Indians and Pakistanis were combined on the basis of SEER coding.
Population data
Detailed population data for AANHPI populations are available from decennial U.S. censuses. Individuals could report a single race in the 1990 Census and multiple races in the 2000 and 2010 Censuses; due to this incompatibility, we developed the following methodology for producing a consistent set of denominators. April 1, 1990, April 1, 2000, and April 1, 2010 Census population distributions by age, sex, and detailed AANHPI race/ethnicity of a given geography were applied as percentages to the mid-year (July) estimates to yield estimates for the AANHPI populations. Because the 1990 Census did not publish county-level population counts for Pakistanis, we used the 1990 public-use microdata samples (20). The April 2000 and April 2010 estimates were derived by calculating an average of the single race alone count (i.e., those who self-identified with one AANHPI population) and the count for single race alone or in combination with other race(s). Intercensal estimates were developed from linear interpolation between the three censuses. Because of the high percentage of AANHPIs of mixed ethnicity in Hawaii, as well as concerns that the NH population has been undercounted in previous censuses, the Hawaii Tumor Registry has developed improved population estimates derived from sample survey data collected by the Hawaii Department of Health (21). These estimates were used for the Hawaii populations for 1990 through 2005 and estimated for 2006–2010 based on linear projections from the 2000 through 2005 data.
County-level population estimates were used for sub-state SEER regions. The Census Bureau does not disclose race/ethnic-specific population counts below 100 for any geographic area (22). When Census population data were suppressed for an AANHPI population for an entire registry, the registry was excluded from rate calculations for that particular group. When population data were suppressed for some counties within a multicounty metropolitan region, rates were calculated for the remaining counties with available population data (Supplementary Table S1). Population counts for each AANHPI population by SEER region for the three censal years are shown in Table 1.
Statistical analysis
Lung cancer histologic cell-type–specific incidence rates and 95% confidence intervals (CI) were calculated as cases per 100,000 persons and age-adjusted to the 2000 U.S. standard population using SEER Stat software (http://seer.cancer.gov/seerstat/). Rates were suppressed for case counts <10 (23). Annual rates are shown graphically as trends (24, 25), except for smaller groups, where 2 or 5 year averaged rates are shown (Figs. 1 and 2). Joinpoint regression models and annual percentage change (APC) statistics (two-side P values) were used to characterize the magnitude and direction of trends (26). A maximum of three joinpoints were allowed on the basis of single year data. Plots of rate estimates and trend lines produced by the joinpoint analysis are shown in semi-logarithmic scale and 2:1 y:x axis aspect ratio for comparison of trends by histology among populations (24, 25).
Figure 1.
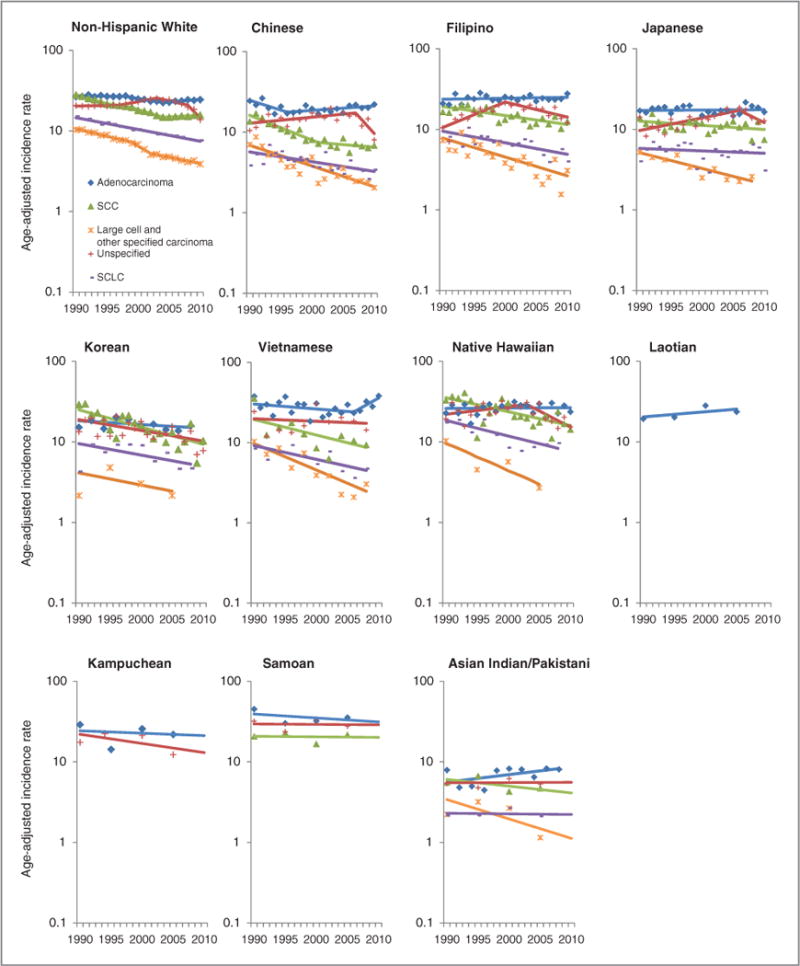
Male trends of lung cancer incidence rates and APC by race/ethnicity and histological cell type, 1990–2010, United States.
Figure 2.
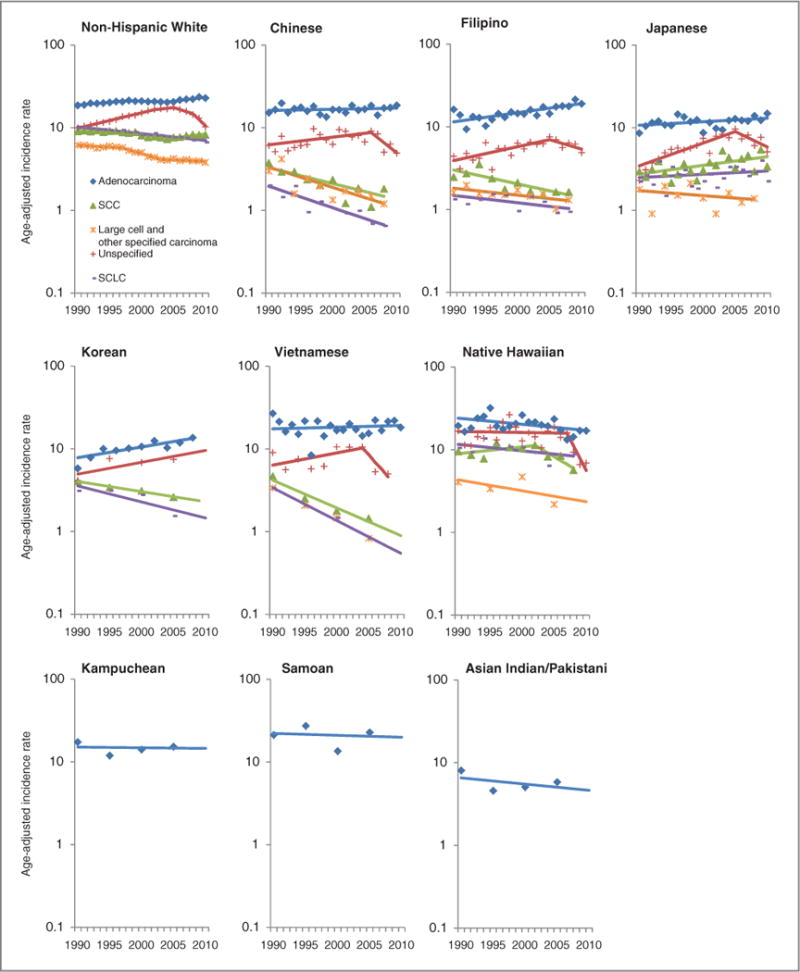
Female trends of lung cancer incidence rates and APC by race/ethnicity and histologic cell type, 1990–2010, United States.
Results
Characteristics of lung cancer cases
Table 2 describes the characteristics of incident lung cancer cases diagnosed from 1990 through 2010. With the exception of Japanese and native Hawaiians, the majority of cases were diagnosed in California. Adenocarcinoma was the most common histologic cell type, followed by unspecified types and SCC across all racial/ethnic groups.
Table 2.
Characteristics of incident lung cancer cases by Asian American, Native Hawaiian, Pacific Islander population, and non-Hispanic Whites and sex, 1990–2010a
| Characteristics | NHW | Chinese | Japanese | Filipino | Vietnamese | Korean | Native Hawaiian | Kampuchean | Laotian | Samoan | Asian Indian, Pakistani |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Total | 622,974 | 9,847 | 7,092 | 10,457 | 3,513 | 2,884 | 2,800 | 374 | 376 | 453 | 1,162 |
| Men | 333,540 No. (%) |
5,817 No. (%) |
4,018 No. (%) |
6,801 No. (%) |
2,334 No. (%) |
1,695 No. (%) |
1,598 No. (%) |
234 No. (%) |
263 No. (%) |
300 No. (%) |
800 No. (%) |
| Year of diagnosis | |||||||||||
| 1990–1992 | 51,732 (15.5) | 633 (10.9) | 523 (13) | 680 (10) | 155 (7.4) | 164 (9.7) | 215 (13.5) | 20 (8.6) | 33 (12.6) | 36 (12) | 47 (5.9) |
| 1993–1995 | 49,842 (14.9) | 666 (11.5) | 531 (13.2) | 841 (12.4) | 217 (11.8) | 175 (10.3) | 200 (12.5) | 30 (12.8) | 33 (12.6) | 33 (11) | 57(7.1) |
| 1996–1998 | 48,811 (14.6) | 690 (11.9) | 603 (15) | 950 (14) | 272 (14.7) | 241 (14.2) | 218 (13.6) | 34 (14.5) | 26 (9.9) | 35 (11.7) | 78 (9.8) |
| 1999–2001 | 47,841 (14.3) | 806 (13.9) | 529 (13.2) | 1,011 (14.9) | 325 (8.8) | 258 (15.2) | 243 (15.2) | 34 (14.5) | 38 (14.5) | 45 (15) | 112 (14) |
| 2002–2004 | 45,914 (13.8) | 919 (15.8) | 613 (15.3) | 1,104 (16.2) | 356 (17.7) | 254 (15) | 226 (14.1) | 42 (18) | 43 (16.4) | 43 (14.3) | 150 (18.8) |
| 2005–2007 | 45,430 (13.6) | 1,058 (18.2) | 643 (16) | 1,068 (15.7) | 456 (17.7) | 287 (16.9) | 237 (14.8) | 28 (12) | 38 (14.5) | 48 (16) | 164 (20.5) |
| 2008–2010 | 43,970 (13.2) | 1,045 (18) | 576 (14.3) | 1,147 (16.9) | 553 (22.1) | 316 (18.6) | 259 (16.2) | 46 (19.7) | 52 (19.8) | 60 (20) | 192 (24) |
|
| |||||||||||
| SEER registry region | |||||||||||
| California | 145,799 (43.7) | 4,606 (79.2) | 1,587 (39.5) | 4,722 (69.4) | 1,953 (75) | 1,166 (68.8) | 123 (7.7) | 194 (82.9) | 197 (74.9) | 169 (56.3) | 371 (46.4) |
| Connecticut | 25,448 (7.6) | 49 (0.8) | <5 (0.1) | 32 (0.5) | 15 (2.9) | 7 (0.4) | <5 (0.1) | 6 (2.6) | <5 (1.5) | 0(0) | 40 (5) |
| Atlanta (metro) | 10,272 (3.1) | 37 (0.6) | 9 (0.2) | 10 (0.2) | 66 (0) | 33 (2) | 0(0) | <5 (1.3) | 8(3) | 0(0) | 33 (4.1) |
| Hawaii | 2,328 (0.7) | 537 (9.2) | 2,216 (55.2) | 1,409 (20.7) | 31 (8.8) | 173 (10.2) | 1,424 (89.1) | 0(0) | 11 (4.2) | 101 (33.7) | 0(0) |
| Iowa | 27,327 (8.2) | 7 (0.1) | 0(0) | <5 (0.1) | 28 (0) | 6 (0.4) | 0(0) | 0(0) | 14 (5.3) | <5 (0.3) | 8(1) |
| Detroit (metro) | 27,835 (8.4) | 78 (1.3) | 14 (0.4) | 41 (0.6) | <5(0) | 19(1.1) | 0(0) | <5 (0.4) | <5 (1.5) | 0(0) | 56(7) |
| New Jersey | 55,470 (16.6) | 253 (4.4) | 33 (0.8) | 295 (4.3) | 36 (0) | 161 (9.5) | 16(1) | 0(0) | <5 (0.8) | 0(0) | 256 (32) |
| New Mexico | 7,215 (2.2) | 12 (0.2) | <5 (0.1) | <5(0) | 7(0) | <5 (0.1) | <5 (0.2) | 0(0) | 0(0) | 0(0) | 12(1.5) |
| Utah | 5,732 (1.7) | 19 (0.3) | 16 (0.4) | <5(0) | 22 (1.5) | <5 (0.2) | <5 (0.2) | 0(0) | <5 (1.1) | <5 (0.7) | 0(0) |
| Seattle (Puget Sound) | 26,114 (7.8) | 219 (3.8) | 135 (3.4) | 284 (4.2) | 172 (11.8) | 125 (7.4) | 28 (1.8) | 30 (12.8) | 20 (7.6) | 27(9) | 24 (3) |
|
| |||||||||||
| Age at diagnosis, y | |||||||||||
| <54 | 30,548 (9.2) | 554 (9.5) | 242 (6) | 731 (10.8) | 478 (17.7) | 188 (11.1) | 254 (15.9) | 41 (17.5) | 56 (21.3) | 59 (19.7) | 169 (21.1) |
| 55–64 | 69,293 (20.8) | 988 (17) | 557 (13.9) | 1,551 (22.8) | 561 (35.3) | 374 (22.1) | 468 (29.3) | 49 (20.9) | 62 (23.6) | 106 (35.3) | 195 (24.4) |
| 65–74 | 116,546 (34.9) | 1,914 (32.9) | 1,332 (33.2) | 2,410 (35.4) | 736 (36.8) | 571 (33.7) | 544 (34) | 75 (32.1) | 67 (25.5) | 96 (32) | 245 (30.6) |
| 75–84 | 94,570 (28.4) | 1,791 (30.8) | 1,445 (36) | 1,668 (24.5) | 465 (10.3) | 452 (26.7) | 292 (18.3) | 60 (25.6) | 67 (25.5) | 36 (12) | 158 (19.8) |
| 85+ years | 22,583 (6.8) | 570 (9.8) | 442 (11) | 441 (6.5) | 94 (0) | 110 (6.5) | 40 (2.5) | 9 (3.9) | 11 (4.2) | <5 (1) | 33 (4.1) |
|
| |||||||||||
| Men |
333,540 No. (%) |
5,817 No. (%) |
4,018 No. (%) |
6,801 No. (%) |
2,334 No. (%) |
1,695 No. (%) |
1,598 No. (%) |
263 No. (%) |
300 No. (%) |
800 No. (%) |
No. (%) |
|
| |||||||||||
| Tumor histology | |||||||||||
| Adenocarcinoma | 102,584 (30.8) | 2,368 (40.7) | 1381 (34.4) | 2,559 (37.6) | 1,036 (0) | 549 (32.4) | 470 (29.4) | 96 (41) | 96 (36.5) | 100 (33.3) | 306 (38.3) |
| Squamous cell | 77,138 (23.1) | 976 (16.8) | 901 (22.4) | 1,454 (21.4) | 374 (0) | 462 (27.3) | 404 (25.3) | 44 (18.8) | 41 (15.6) | 65 (21.7) | 158 (19.8) |
| LC + OSC | 26,958 (8.1) | 428 (7.4) | 271 (6.7) | 458 (6.7) | 162 (0) | 100 (5.9) | 98 (6.1) | 17(7.3) | 17 (6.5) | 25 (8.3) | 77 (9.6) |
| NOS | 83,670 (25.1) | 1,571 (27) | 1037 (25.8) | 1,650 (24.3) | 569 (0) | 367 (21.7) | 409 (25.6) | 59 (25.2) | 72 (27.4) | 79 (26.3) | 172 (21.5) |
| Small cell | 43,190 (12.9) | 474 (8.1) | 428 (10.7) | 680 (10) | 193 (0) | 217 (12.8) | 217 (13.6) | 18 (7.7) | 37 (14.1) | 31 (10.3) | 87 (10.9) |
|
| |||||||||||
| Stage at presentationa | |||||||||||
| Localized | 47,515 (14.3) | 810 (13.9) | 594 (14.8) | 885 (13) | 332 (13.2) | 214 (12.6) | 229 (14.3) | 20 (8.6) | 14 (5.3) | 27(9) | 116 (14.5) |
| Regional | 69,256 (20.8) | 1,241 (21.3) | 989 (24.6) | 1,474 (21.7) | 501 (19.1) | 372 (22) | 401 (25.1) | 45 (19.2) | 42 (16) | 74 (24.7) | 179 (22.4) |
| Distant | 139,635 (41.9) | 2,988 (51.4) | 1,980 (49.3) | 3,553 (52.2) | 1,253 (54.4) | 830 (49) | 832 (52.1) | 136 (58.1) | 157 (59.7) | 166 (55.3) | 363 (45.4) |
| Unstaged | 45,447 (13.6) | 598 (10.3) | 386 (9.6) | 693 (10.2) | 206 (13.2) | 176 (10.4) | 128 (8) | 29 (12.4) | 49 (18.6) | 28 (9.3) | 66 (8.3) |
| Missing | 31,687 (9.5) | 180 (3.1) | 69 (1.7) | 196 (2.9) | 42 (0) | 103 (6.1) | 8 (0.5) | <5 (1.7) | <5 (0.4) | 5(1.7) | 76 (9.5) |
|
| |||||||||||
| Women |
289,434 No. (%) |
4,030 No. (%) |
3,074 No. (%) |
3,656 No. (%) |
1,179 No. (%) |
1,189 No. (%) |
1,202 No. (%) |
140 No. (%) |
113 No. (%) |
153 No. (%) |
362 No. (%) |
|
| |||||||||||
| Year of diagnosis | |||||||||||
| 1990–1992 | 37,200 (12.9) | 383 (9.5) | 271 (8.8) | 284 (7.8) | 85 (7.2) | 77 (6.5) | 116 (9.7) | 14 (10) | 10 (8.9) | 16 (10.5) | 19 (5.3) |
| 1993–1995 | 39,174 (13.5) | 402 (10) | 356 (11.6) | 321 (8.8) | 97 (8.2) | 93 (7.8) | 150 (12.5) | 12 (8.6) | 7 (6.2) | 13 (8.5) | 26 (7.2) |
| 1996–1998 | 41,491 (14.3) | 494 (12.3) | 422 (13.7) | 380 (10.4) | 115 (9.8) | 144 (12.1) | 154 (12.8) | 17 (12.1) | 15 (13.3) | 24 (15.7) | 35 (9.7) |
| 1999–2001 | 42,063 (14.5) | 547 (13.6) | 422 (13.7) | 491 (13.4) | 163 (13.8) | 175 (14.7) | 179 (14.9) | 17 (12.1) | 29 (25.7) | 21 (13.7) | 47 (13) |
| 2002–2004 | 42,864 (14.8) | 659 (16.4) | 483 (15.7) | 607 (16.6) | 215 (18.2) | 199 (16.7) | 200 (16.6) | 19 (13.6) | 18 (15.9) | 20 (13.1) | 62 (17.1) |
| 2005–2007 | 43,823 (15.1) | 747 (18.5) | 555 (18.1) | 707 (19.3) | 231 (19.6) | 224 (18.8) | 212 (17.6) | 28 (20) | 18 (15.9) | 36 (23.5) | 84 (23.2) |
| 2008–2010 | 42,819 (14.8) | 798 (19.8) | 565 (18.4) | 866 (23.7) | 273 (23.2) | 277 (23.3) | 191 (15.9) | 33 (23.6) | 16 (14.2) | 23 (15) | 89 (24.6) |
|
| |||||||||||
| SEER registry region | |||||||||||
| California | 131,786 (45.5) | 3,211 (79.7) | 1,459 (47.5) | 2,512 (68.7) | 1,005 (85.2) | 711 (59.8) | 101 (8.4) | 117 (83.6) | 92 (81.4) | 65 (42.5) | 189 (52.2) |
| Connecticut | 23,011 (8) | 25 (0.6) | 16 (0.5) | 23 (0.6) | 11 (0.9) | 13(1.1) | <5 (0.1) | 6 (4.3) | <5 (2.7) | 0(0) | 8 (2.2) |
| Atlanta (metro) | 8,611 (3) | 25 (0.6) | 14 (0.5) | 7 (0.2) | 21 (1.8) | 25 (2.1) | 0(0) | <5 (1.4) | <5 (1.8) | 0(0) | 10 (2.8) |
| Hawaii | 1,677 (0.6) | 377 (9.4) | 1,242 (40.4) | 696 (19) | 21 (1.8) | 177 (14.9) | 1,072 (89.2) | 0(0) | <5 (1.8) | 60 (39.2) | 0(0) |
| Iowa | 18,775 (6.5) | 5 (0.1) | 17 (0.6) | 9 (0.3) | 5 (0.4) | 5 (0.4) | <5 (0.2) | 0(0) | <5 (3.5) | 0(0) | <5 (0.6) |
| Detroit (metro) | 23,728 (8.2) | 37 (0.9) | 18 (0.6) | 39 (1.1) | 8 (0.7) | 16 (1.4) | 0(0) | 0(0) | <5 (0.9) | 0(0) | 15 (4.1) |
| New Jersey | 49,011 (16.9) | 179 (4.4) | 60 (2) | 200 (5.5) | 19 (1.6) | 95 (8) | 9 (0.8) | 0(0) | 0(0) | 0(0) | 126 (34.8) |
| New Mexico | 5,638 (2) | 6 (0.2) | 19 (0.6) | 7 (0.2) | <5 (0.3) | 5 (0.4) | 0(0) | 0(0) | <5 (0.9) | 0(0) | <5 (0.3) |
| Utah | 3,748 (1.3) | 10 (0.3) | 20 (0.7) | <5 (0.1) | <5 (0.3) | <5 (0.3) | <5 (0.2) | <5 (0.7) | <5 (0.9) | <5 (2.6) | 0(0) |
| Seattle (Puget Sound) | 23,449 (8.1) | 155 (3.9) | 209 (6.8) | 159 (4.4) | 82(7) | 139 (11.7) | 15 (1.3) | 14 (10) | 7 (6.2) | 24 (15.7) | 11 (3) |
|
| |||||||||||
| Age at diagnosis, y | |||||||||||
| <54 | 28,034 (9.7) | 564 (14) | 177 (5.8) | 613 (16.8) | 242 (20.5) | 195 (16.4) | 174 (14.5) | 37 (26.4) | 25 (22.1) | 43 (28.1) | 78 (21.6) |
| 55–64 | 56,041 (19.4) | 632 (15.7) | 512 (16.7) | 777 (21.3) | 273 (23.2) | 260 (21.9) | 335 (27.9) | 35 (25) | 32 (28.3) | 40 (26.1) | 85 (23.5) |
| 65–74 | 95,962 (33.2) | 1,154 (28.6) | 1,090 (35.5) | 1,140 (31.2) | 309 (26.2) | 355 (29.9) | 418 (34.8) | 32 (22.9) | 24 (21.2) | 38 (24.8) | 102 (28.2) |
| 75–84 | 85,157 (29.4) | 1,173 (29.1) | 982 (32) | 875 (23.9) | 270 (22.9) | 268 (22.5) | 215 (17.9) | 28 (20) | 23 (20.4) | 27 (17.7) | 83 (22.9) |
| 85+ years | 24,240 (8.4) | 507 (12.6) | 313 (10.2) | 251 (6.9) | 85 (7.2) | 111 (9.3) | 60 (5) | 8 (5.7) | 9(8) | 5 (3.3) | 14 (3.9) |
|
| |||||||||||
| Tumor histology | |||||||||||
| Adenocarcinoma | 104,947 (36.3) | 2,378 (59) | 1,383 (45) | 2,249 (61.5) | 734 (62.3) | 567 (47.7) | 424 (35.3) | 86 (61.4) | 61 (54) | 71 (46.4) | 204 (56.4) |
| Squamous cell | 43,056 (14.9) | 283 (7) | 434 (14.1) | 278 (7.6) | 81 (6.9) | 150 (12.6) | 191 (15.9) | 13 (9.3) | 11 (9.7) | 13 (8.5) | 33 (9.1) |
| LC + CSC | 24,401 (8.4) | 259 (6.4) | 172 (5.6) | 216 (5.9) | 65 (5.5) | 58 (4.9) | 74 (6.2) | 8 (5.7) | 7 (6.2) | 9 (5.9) | 36 (9.9) |
| NOS | 74,526 (25.7) | 967 (24) | 755 (24.6) | 735 (20.1) | 249 (21.1) | 296 (24.9) | 301 (25) | 27 (19.3) | 29 (25.7) | 47 (30.7) | 79 (21.8) |
| Small cell | 42,504 (14.7) | 143 (3.5) | 330 (10.7) | 178 (4.9) | 50 (4.2) | 118 (9.9) | 212 (17.6) | 6 (4.3) | 5 (4.4) | 13 (8.5) | 10 (2.8) |
|
| |||||||||||
| Stage at presentationa | |||||||||||
| Localized | 50,684 (17.5) | 597 (14.8) | 539 (17.5) | 607 (16.6) | 160 (13.6) | 185 (15.6) | 191 (15.9) | 18 (12.9) | 11 (9.7) | 13 (8.5) | 58 (16) |
| Ragionai | 58,433 (20.2) | 733 (18.2) | 690 (22.5) | 685 (18.7) | 174 (14.8) | 231 (19.4) | 308 (25.6) | 24 (17.1) | 17(15) | 23 (15) | 64 (17.7) |
| Distant | 115,689 (40) | 2,194 (54.4) | 1,533 (49.9) | 1,957 (53.5) | 695 (59) | 613 (51.6) | 619 (51.5) | 76 (54.3) | 66 (58.4) | 101 (66) | 173 (47.8) |
| Unstaged | 39,944 (13.8) | 407 (10.1) | 258 (8.4) | 312 (8.5) | 130 (11) | 120 (10.1) | 79 (6.6) | 16 (11.4) | 18 (15.9) | 14 (9.2) | 35 (9.7) |
| Missing | 24,684 (8.5) | 99 (2.5) | 54 (1.8) | 95 (2.6) | 20 (1.7) | 40 (3.4) | 5 (0.4) | 6 (4.3) | <5 (0.9) | <5 (1.3) | 32 (8.8) |
Counts less than five cases are suppressed for privacy consideration.
Lung cancer incidence trends in males
Among NHW men, incidence rates for most histologic cell types declined overall, with the exceptions of SCC and adenocarcinoma, which showed a slight upward trend after 2004 (Fig. 1; Table 3). SCLC showed a significantly steady −3.3% (95% CI, −3.5 to −3.1) decline per year, while rates for LC+OSC showed a −4.1% (95% CI, −4.8 to −3.5) decrease per year from 1990 through 1999, followed by a steep decline of −10.3% (95% CI, −18 to −2.4) per year from 1999 through 2002, and then slowing down to −3.5% (95% CI, −4.5 to −2.5) decrease per year thereafter. Unlike trends for other histologic cell types, rates for unspecified types were stable from 1990 through 1997, then increased 3.5% (95% CI, 2.2 to 4.8) per year from 1997 through 2003, followed by a decrease of −4.0% (95% CI, −5.7 to −2.3) per year from 2003 to 2008, and then a much steeper decrease of −17% (95% CI, −22 to −11) per year from 2008 through 2010.
Table 3.
Male trends of lung cancer incidence rates and APC by race/ethnicity and histologic cell type, 1990–2010, United States
| SCLC | Adenocarcinoma | SCC | LC and OSC | Unspecified |
|---|---|---|---|---|
| NHW | ||||
| 1990–2010 −3.3a (−3.5 to −3.1) | 1990–1998 −0.2 (−0.7 to 0.4) | 1990–1995 −5.1a (−5.8 to −4.3) | 1990–1999 −4.1a (−4.8 to −3.5) | 1990–1997 0.3 (−0.5 to 1.2) |
| 1998–2004 −2.9a (−4 to −1.7) | 1995–1999 −2.6a (−4.5 to −0.7) | 1999–2002 −10.3a (−18 to −2.4) | 1997–2003 3.5a (2.2 to 4.8) | |
| 2004–2010 1.5a (0.7 to 2.4) | 1999–2004 −5.4a (−6.7 to −4.2) | 2002–2010 −3.5a (−4.5 to −2.5) | 2003–2008 −4.0a (−5.7 to −2.3) | |
| 2004–2010 1.0a (0.3 to 1.7) | 2008–2010 −17a (−22 to −11) | |||
|
| ||||
| Chinese | ||||
| 1990–2010 −2.7a (−4.1 to −1.3) | 1990–1996 −5.5a (−11 to −0.1) | 1990–2001 −6.8a (−9 to −4.6) | 1990–2010 −5.7a (−7.1 to −4.4) | 1990–2007 1.8(0 to 3.6) |
| 1996–2010 1.3a (0 to 2.5) | 2001–2010 −1.4 (−4.3 to 1.5) | 2007–2010 −18 (−34 to 3.0) | ||
|
| ||||
| Filipino | ||||
| 1990–2010 −3.3a (−4.4 to−2.1) | 1990–2010 0.3 (−0.4 to 1.0) | 1990–2010 −2.5a (−3.5 to−1.6) | 1990–2010 −5.3a (−7.2 to −3.3) | 1990–2000 7.5a (3.9 to 11.4) |
| 2000–2010 −4.2a (−6.7 to −1.6) | ||||
|
| ||||
| Japanese | ||||
| 1990–2010 −0.7 (−2.4 to 1.1) | 1990–2010 0.1(−0.9 to 1.1) | 1990–2010 −1.2(−2.4 to 0.1) | 1990–2010b −4.4a (−6.1 to −2.7) | 1990–2006 3.7a (1.8 to 5.5) |
| 2006–2010 −8.3 (−19 to 3.7) | ||||
|
| ||||
| Korean | ||||
| 1990–2010b −3.2 (−6.5 to 0.3) | 1990–2010b −1.1 (−2.6 to 0.5) | 1990–2010 −4.8a (−6.7 to −2.8) | 1990–2010c −3.4 (−19 to 15) | 1990–2010 −3.0a (−4.7 to−1.2) |
|
| ||||
| Vietnamese | ||||
| 1990–2010b −3.9a (−6.5 to −1.2) | 1990–2006 −1.4 (−3.7 to 1.0) | 1990–2010b −4.3a (−7.9 to −0.7) | 1990–2010b −7.3a (−10 to −4.4) | 1990–2010b −0.7 (−4.7 to 3.4) |
| 2006–2010 11 (−1.5 to 24.2) | ||||
|
| ||||
| Native Hawaiian | ||||
| 1990–2010b −4.3a (−6.7 to−1.9) | 1990–2010 0.1 (−1.0 to 1.3) | 1990–2010 −3.9a (−5.8 to −2.0) | 1990–2010c −7.5 (−18 to 3.8) | 1990–2003 2.2 (−1.1 to 5.6) |
| 2003–2010 −8.8a (−15 to −2.1) | ||||
|
| ||||
| Laotian | ||||
| 1990–2010c 1.5 (−4.7 to 8.1) | ||||
|
| ||||
| Kampuchean | ||||
| 1990–2010c −0.7 (−11 to 11) | 1990–2010c −2.7 (−13 to 9.2) | |||
|
| ||||
| Samoan | ||||
| 1990–2010c −1.1 (−7.4 to 5.5) | 1990–2010c −0.1 (−6.5 to 6.6) | 1990–2010c −0.1 (−7.3 to 7.7) | ||
|
| ||||
| Asian Indian/Pakistani | ||||
| 1990–2010c −0.2 (−5.7 to 5.6) | 1990–2010b 2.2 (−0.2 to 4.7) | 1990–2010c −1.9 (−8.7 to 5.3) | 1990–2010c −5.4 (−21 to 13) | 1990–2010c 0.1 (−5.0 to 5.4) |
The 95% CI for the APC does not include zero (P < 0.05).
Joinpoint and observed rates are based on 2-year groups.
Joinpoint and observed rates are based on 5-year groups.
Overall, lung cancer incidence trends in Chinese men declined during the period 1990–2010, with the exception of a slight 1.3% increase (95% CI, 0 to 2.5) in adenocarcinoma from 1996 through 2010. Rates of unspecified types declined dramatically by −18% per year (95% CI, −34.5 to 3.0) from 2007 through 2010. For SCC, incidence rates also declined overall, with the steepest decline observed from 1990 through 2001 (APC = −6.8; 95% CI, −9 to −4.6). Chinese men also experienced a significant annual decrease of SCLC (APC 1990-2010 = −2.7; 95% CI, −4.1 to −1.3) and a more striking decrease in LC+OSC (APC 1990-2010 = −5.7; 95% CI, −7.1 to −4.4).
For Filipino men, trends in adenocarcinoma were stable; however, incidence of unspecified types increased significantly from 1990 through 2000 (APC = 7.5; 95% CI, 3.9 to 11.4) and declined sharply from 2000 through 2010 (APC = −4.2; 95% CI, −6.7 to −1.6). From 1990 through 2010, Filipino men also experienced a statistically significant annual decrease in SCC (APC = −2.5; 95% CI, −3.5 to −1.6), SCLC (APC = −3.3; 95% CI, −4.4 to −2.1), and LC+OSC (APC = −5.3; 95% CI, −7.2 to −3.3).
Although Japanese men experienced stable rates of adenocarcinoma from 1990 through 2010, they experienced a statistically significant −4.4% (95% CI, −6.1 to −2.7) annual decrease in LC+OSC. Incidence rates of unspecified types showed a divergent trend unlike those seen in the other four histologic cell types, with a statistically significant increase of 3.7% (95% CI, 1.8 to 5.5) per year from 1990 through 2006 followed by a nonsignificant decrease of −8.3% per year from 2006 through 2010.
Overall, incidence rates in Korean men significantly declined for two of five histologic cell types from 1990 through 2010, with a −3.0% (95% CI, −4.7 to −1.2) annual decrease for unspecified types and a −4.8% (95% CI, −6.7 to −2.8) annual decrease for SCC.
During 1990-2010, Vietnamese men experienced marked declines in SCC (APC = −4.3; 95% CI, −7.9 to −0.7), SCLC (APC = −3.9; 95% CI, −6.5 to −1.2), and LC+OSC (APC = −7.3; 95% CI, −10 to −4.4). From 2006 through 2010, a large, nonsignificant increase in the incidence rate of adenocarcinoma was observed in Vietnamese men (APC = 11.0; 95% CI, −1.5 to 24.2). For native Hawaiian men, incidence rates for adenocarcinoma were stable. However, they experienced declining trends for unspecified types (APC 2003-2010 = −8.8; 95% CI, −15 to −2.1), SCC (APC 1990-2010 = −3.9; 95% CI, −5.8 to −2.0), and SCLC (APC 1990-2010 = −4.3; 95% CI, −6.7 to −1.9). Histologic data for Laotian men were sparse for all cell types and are not shown in Fig. 1 and Table 3, except for adenocarcinoma, whose rates showed a nonsignificant increase from 1990 through 2010. In contrast, Kampuchean men experienced slight decreasing trends in adenocarcinoma and unspecified types (albeit insignificant). Samoan men also experienced a nonsignificant decreasing trend in rates of adenocarcinoma, but stable rates in unspecified types and SCC. Although Asian Indian/Pakistani men had stable rates of unspecified types and SCLC, they experienced a nonsignificant annual decrease of −1.9% (95% CI, −8.7 to 5.3) in SCC, an annual decrease of −5.4% (95% CI, −21 to −13) in LC+OSC, and a nonsignificant annual increase of 2.2% (95% CI, −0.2 to 4.7) for adenocarcinoma from 1990 through 2010.
Lung cancer incidence trends in females
Among NHW women, adenocarcinoma and SCC were the only histologic cell types to show overall increasing trends from 1990 through 2010 (Fig. 2; Table 4). Incidence rates for adenocarcinoma increased 1.5% (95% CI, 1.0-2.1) per year from 1990 through 1998, appeared stable 1998–2004, then increased again from 2004 through 2010 (APC = 2.3%; 95% CI, 1.5-3.1). Rates for SCC decreased −2.5% (95% CI, −3.5 to −1.6) per year from 1996 through 2004 and increased 2.2% (95% CI, 0.9-3.4) per year from 2004 through 2010. Rates for unspecified types showed the largest decline of all histologic cell types, significantly decreasing −15.6% (95% CI, −20 to −10) per year from 2008 through 2010.
Table 4.
Female trends of lung cancer incidence rates and APC by race/ethnicity and histologic cell type, 1990–2010, United States
| SCLC | Adenocarcinoma | SCC | LC and OSC | Unspecified |
|---|---|---|---|---|
| NHW | ||||
| 1990–2010 −1.9a (−2.1 to −1.7) | 1990–1998 1.5a (1 to 2.1) | 1990–1996 0 (−1.2 to 1.2) | 1990–1997 −1 (−2 to 0) | 1990–2002 4.4a (4 to 4.8) |
| 1998–2004 −0.7 (−1.7 to 0.4) | 1996–2004 −2.5a (−3.5 to −1.6) | 1997–2003 −5.2a (−6.9 to −3.4) | 2002–2005 2.0 (−3.0 to 7.2) | |
| 2004–2010 2.3a (1.5 to 3.1) | 2004–2010 2.2a (0.9 to 3.4) | 2003–2010 −0.8 (−2 to 0.3) | 2005–2008 −5.4a (−10 to −0.5) | |
| 2008–2010 −15.6a (−20 to −10) | ||||
|
| ||||
| Chinese | ||||
| 1990–2008b −5.8a (−7.8 to −3.8) | 1990–2010 0.3 (−0.4 to 1.1) | 1990–2008b −4.3a (−6.8 to −1.8) | 1990–2008b −5.5a (−8.2 to −2.7) | 1990–2006 2.2 (−0.2 to 4.6) |
| 2006–2010 −13.1 (−24.7 to 0.2) | ||||
|
| ||||
| Filipino | ||||
| 1990–2008b −2.0 (−4.2 to 0.2) | 1990–2010 2.6a (1.7 to 3.5) | 1990−2008b −3.8a (−5.8 to −1.8) | 1990–2008b −1.9a (−3.5 to −0.3) | 1990–2005 4.0a (1.7 to 6.3) |
| 2005–2010 −5.1 (−11.9 to 2.3) | ||||
|
| ||||
| Japanese | ||||
| 1990–2010 0.9 (−1 to 2.9) | 1990–2010 1.0 (−0.1 to 2) | 1990–2010 2.4a (0.7 to 4.2) | 1990–2010b −1.4 (−4.5 to 1.9) | 1990–2005 6.6a (4.2 to 9) |
| 2005–2010 −8.2 (−16 to 0.1) | ||||
|
| ||||
| Korean | ||||
| 1990–2010c −4.7 (−1310 4.3) | 1990–2010b 3.0a (1.6 to 4.4) | 1990–2010c −2.8a (−3.7 to −1.9) | 1990–2010c 1.8 (−5.7 to 10) | |
|
| ||||
| Vietnamese | ||||
| 1990–2010c −8.7a (−11 to −6.4) | 1990–2010 0.5 (−1.1 to 2.1) | 1990–2010c −7.3a (−12 to −2.3) | 1990–2010c −6.8a (−11 to −2) | 1990–2004b 3.5 (−2.8 to 10) |
| 2004–2010 −18 (−40.7 to 13) | ||||
|
| ||||
| Native Hawaiian | ||||
| 1990–2010b −1.8 (−3.7 to 0.1) | 1990–2010 −1.7a (−3.1 to −0.2) | 1990–2002b 1.9 (−2.9 to 7.1) | 1990–2010c −3.0 (−16 to 12) | 1990–2007 −0.3 (−2.9 to 2.5) |
| 2002–2010 −10 (−20 to 1.2) | 2007–2010 −29.2 (−52 to 4.2) | |||
|
| ||||
| Kampuchean | ||||
| 1990–2010c −0.2 (−5.9 to 5.8) | ||||
|
| ||||
| Samoan | ||||
| 1990–2010c −0.5 (−13 to 14) | ||||
|
| ||||
| Asian Indian/Pakistani | ||||
| 1990–2010c −0.4 (−8.6, to 8.5) | ||||
The 95% CI for the APC does not include zero (P < 0.05).
Joinpoint and observed rates are based on 2-year groups.
Joinpoint and observed rates are based on 5-year groups.
From 1990 through 2010, Chinese women experienced statistically significant decreases in incidence rates of three histologic cell types: −5.8% (95% CI, −7.8 to −3.8) annual decrease for SCLC, −4.3% (95% CI, −6.8 to −1.8) annual decrease for SCC, and −5.5% (95% CI, −8.2 to −2.7) annual decrease for LC+OSC. Trends in rates for adenocarcinoma were relatively stable (APC = 0.3; 95% CI, −0.4 to 1.1).
For Filipino women, trends showed increasing rates for adenocarcinoma (APC 1990-2010 = 2.6; 95% CI, 1.7-3.5) and unspecified types (APC 1990-2005 = 4.0; 95% CI, 1.7-6.3), and decreasing rates for SCC (APC = −3.8; 95% CI, −5.8 to −1.8).
For Japanese women, slight increasing trends were observed for adenocarcinoma and SCLC, whereas decreasing trends were observed for LC+OSC (albeit insignificant). For unspecified types, rates significantly increased 6.6% (95% CI, 4.2 to −9) per year from 1990 through 2005 but showed a nonsignificant −8.2% (95% CI, −16 to 0.1) decrease per year from 2005 through 2010. Rates for SCC significantly increased 2.4% (95% CI, 0.7 to 4.2) per year from 1990 through 2010.
Like Filipino women, Korean women experienced a statistically significant increase in adenocarcinoma from 1990 through 2010 (APC = 3.0; 95% CI, 1.6 to 4.4) and decreasing rates for SCC (APC = −2.8; 95% CI, −3.7 to −1.9). For Vietnamese women, trends in incidence rates significantly declined for three histologic cell types from 1990 through 2010: SCLC (APC = −8.7; 95% CI, −11 to −6.4), SCC (APC = −7.3; 95% CI, −12 to −2.3), and LC+OSC (APC = −6.8; 95% CI, −11 to −2). Incidence rates for adenocarcinoma were otherwise stable (APC = 0.5; 95% CI, −1.1 to 2.1).
Native Hawaiian women were the only AANHPI population to experience a statistically significant decline in adenocarcinoma with a −1.7% decrease in incidence per year (95% CI, −3.1 to −0.2) from 1990 through 2010. Rates for all other histologic types showed nonsignificant declines during this period.
For Kampuchean, Samoan, and Asian Indian/Pakistani women, there were too few cases to show trends in incidence rates for most histologic cell types, except for adenocarcinoma, whose trends were stable for these populations from 1990 to 2010.
Summary of trends by histologic cell type
Overall, incidence trends of adenocarcinoma increased in Chinese men and in Filipino and Korean women, decreased in NH women, and remained stable in Chinese women.
For unspecified types, the trends in rates for men started increasing in 1990 but showed a marked decline in Filipinos, native Hawaiians, and NHWs in the years thereafter, with the decline beginning in 2000 for Filipino men, in 2003 for NH men, and in 2003 for NHW men. Korean men experienced a slow and steady decline in unspecified types during 19902010. Similar to men, trends in unspecified types for women significantly increased in Filipino, Japanese, and NHW women from 1990 through the mid-2000s, followed by a significant decline thereafter in NHW women.
For SCC, overall trends were declining or stable in men and women across all AANHPI populations, with the exception of increasing rates of SCC in Japanese women. SCLC trends were also declining or stable in men and women across all AANHPI groups and NHWs, with the greatest declines observed in Vietnamese women and NH men.
Finally, trends in LC+OSC showed modest declines among all histology cell types for almost all AANHPI populations in men and women.
Discussion
Using 21 years of cancer incidence data from SEER, this study is the first to present population-based data describing detailed trends in lung cancer incidence by histologic cell type across ten AANHPI populations. Although lung cancer incidence has declined overall in the United States and worldwide in men, with stable rates in women, findings from this study show marked differences in trends by histologic cell type across AANHPI populations. We observed notable significant increases in adenocarcinoma in Filipino and Korean women, similar to NHW women, and increases in SCC among Japanese women, whereas trends in rates for other histologic cell types (unspecified, SCC, SCLC, and LC+OCS) were declining or stable in most AANHPI populations and NHWs. More recently, rates of adenocarcinoma also appeared to be slightly increasing in Chinese and NHW men. These findings suggest the importance of disaggregating data among AANHPI populations to identify vulnerable groups who may experience disparate trends, thus aiding public health prevention efforts to target those groups in greatest need.
The steadily increased trend of adenocarcinoma among Chinese men since 1996, the dramatically increasing trend among Vietnamese men (albeit not statistically significant), the significantly increased rates among Filipino and Korean women, and the stable rates among Chinese women point to the growing burden of adenocarcinoma among these Asian American populations. Data on Asian countries from the International Agency for Research on Cancer (Volumes VII-Volume IX from 1988-2002) indicate large increases in the incidence trends of lung adenocarcinoma for Chinese men (APC = 7.0) and Filipino and Korean women (APC = 8.1 and 3.6, respectively), while a somewhat stable trend was estimated (APC = 1.0) for Chinese women (data not shown). These data from Asia also point to the growing burden of lung adenocarcinoma among these particular Asian populations.
Possible reasons underlying the trends demonstrated here may be due to the persistence of exposure to lung cancer carcinogens among these AANHPI populations. Adenocarcinoma is more weakly associated with smoking than other histologic cell types such as SCC and SCLC (27, 28), indicating that other exposures are particularly relevant for this cell type as well as genetic susceptibility (29-32). Interestingly, the increasing incidence rates of adenocarcinoma were seen among some of the more recent Asian American immigrants to the United States— Vietnamese, Laotian, and Asian Indian/Pakistani men and Filipino and Korean women. It is possible that higher prevalence of exposures found among immigrant groups (33, 34) that have been linked to adenocarcinoma such as preexisting tuberculosis infection (35) may play a role in the increasing rates of disease. On the basis of the 2002–2005 National Survey on Drug Use and Health, the prevalence of smoking varied greatly between Asian American men and women (12). For men, the prevalence of smoking within the past 30 days was 16.1% in Chinese men and 33.5% in Vietnamese men (12). For women, the prevalence of smoking within the past 30 days was highest in Korean women at 20.1% out of six Asian American populations followed by Filipinas at 10.4%, and was low among other groups (12). Acculturation, the process by which foreign-born individuals adopt values, customs, attitudes, and behaviors of the mainstream culture (36, 37), has been reported to differentially influence smoking behavior among Asian American men and women (38). This has important implications on the underlying factors affecting lung cancer development among these populations. In particular, a meta-analysis study reported that acculturated Asian American men were roughly 50% less likely to smoke than less acculturated men (38). In contrast, acculturated Asian American women were five times more likely to smoke than less acculturated women (38). Our previous work confirms this pattern as we have reported lower rates of smoking in U.S.-born Asian American men in comparison with foreign-born Asian American men, yet an opposite pattern by nativity was seen for Asian American women (39). This suggestion that acculturation may have beneficial effects on smoking behavior in Asian American men yet a harmful effect in Asian American women (38, 39) is an important consideration for future etiologic and tobacco prevention studies of lung cancer among Asian Americans.
The rise in adenocarcinoma rates may be due to other contributing factors such as second hand tobacco smoke, diet and cooking methods, and previous lung diseases as highlighted by studies conducted in Asia, and particularly in epidemiologic studies of Asian women given their lower rates of smoking and higher rates of lung cancer (40–42). Among never smoking Chinese women, environmental tobacco smoke from the home or workplace and the use of coal for household heating and cooking, which is a source of carcinogenic polycyclic aromatic hydrocarbons, were estimated to account for 25% and nearly 20% of lung cancer cases, respectively (43). Previous lung diseases contribute to a significant proportion of lung cancer in Chinese never-smokers, with tuberculosis accounting for a greater proportion of the burden of lung cancer in China (43). Although these studies in Asia provide important insight on the risk factors for lung cancer among Asian populations, the prevalence of these factors is likely considerably lower among the Asian American population in the United States. Previous work conducted among AANHPIs from Hawaii observed an increased risk of lung cancer unrelated to smoking among Chinese women, indicating that other etiologic factors may be responsible and additional investigation is needed (44). Conducting etiologic studies of lung cancer risk among AANHPIs is an important research priority given the growing Asian American population, our limited knowledge of the underlying risk factors, and as this report exemplifies, their steady and increasing trends of adenocarcinoma in specific populations.
The steady decline in rates of LC+OSC was consistent for men and women across nearly all AANHPI populations. The decline in LC+OSC was greatest among Vietnamese men, whose prevalence of smoking is among the highest of all racial/ethnic groups (12), and may be correlated with declining prevalence of smoking in California (45).
For unspecified types, incidence trends seemed to increase among most AANHPIs through the 1990s and 2000s, with a marked decline beginning around the early-to mid-2000s. Such a notable trend around a similar time-frame across most populations suggests the possibility of a systematic change in coding practices that may lead to improved specificity of unspecified types to being coded to more specific histologic cell types. Indeed, beginning with bevacizumab in 2005 (46), the development of lung cancer therapeutic agents restricted to specific histologies has led to an increased emphasis of histologic subtyping and a focused effort to reduce histologically unspecified diagnoses. However, this divergent trend was not observed for Vietnamese, Korean, Kampuchean, Samoan, and Asian Indian/Pakistani men or Korean women. Continued surveillance in trends of unspecified histologic cell types after 2010, given the new classification codes issued in 2011 (47), may provide further insight into explanations for these trends.
It has been suggested that these changes in histology practices and coding systems for unspecified types may result in artifactual trends in histologic specific cell types such as the increasing trends for adenocarcinoma and SCC starting around 2005 (48). In our study, the declining trends in unspecified types (~2000s) did not correspond with the increasing trends for adenocarcinoma and SCC (mostly 1990–2010), indicating our findings may not be largely biased by such changes in histologic diagnosis.
There are several caveats worth noting. First, because data on race/ethnicity are primarily derived from medical records (17), they may be misclassified; however, prior research shows low to moderate misclassification for AANHPIs, highest among Vietnamese and South Asians (sensitivity < 50%) but >75% sensitivity among other Asian American populations (17, 49–55). Second, rates may be underestimated because of exclusion of cases coded as “Asian, not otherwise specified.” Third, there may be errors associated with the inter-censal annual population estimates (56). Fourth, small case and denominator counts in some groups lead to unstable rates and potential trends that could not be detected. Finally, many of these patterns could be attributable to cohort changes in acculturation over time, which could not be assessed.
Despite these potential limitations, this report based on robust national data covering more than half of the Asian American population and two-thirds of the NHPI population, provides important insight on contemporary histologic cell-type–specific lung cancer trends among ten AANHPI populations in the United States, serving as a basis of critical evidence to inform future research and health policies. Although the declines in adenocarcinoma among most groups of AANHPI males mirror the trends seen among NHW males, the increasing trends among Filipino and Korean females and Chinese men are of particular concern. Also, the increase in SCC among Japanese females is of concern. These results point to areas where targeted preventive efforts for tobacco prevention and control can be undertaken now in public health, policy, and clinical arenas. Furthermore, given the low smoking prevalence among specific Asian American females and few known lung cancer risk factors in U.S. never-smoker populations, additional research of etiologic factors, focused especially on these ethnic groups of Asian American females, may elucidate knowledge and progress against lung cancer among never smokers.
Supplementary Material
Acknowledgments
The authors thank Dr. Clayton Schupp and Meg McKinley for their contributions.
Funding Support
This work was supported by the Stanford Cancer Institute (to S. Glaser). The collection of cancer incidence data used in this study was supported by the California Department of Health Services as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #1U58 DP000807-01 awarded to the Public Health Institute.
Footnotes
Disclosure of Potential Conflicts of Interest
S.L. Gomez reports receiving a commercial research grant from Genentech. No potential conflicts of interest were disclosed by the other authors.
Publisher's Disclaimer: Disclaimer
The ideas and opinions expressed herein are those of the authors, and endorsement by the State of California, the California Department of Health Services, the NCI, or the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
Authors’ Contributions
Conception and design: I. Cheng, R.W. Haile, H.A. Wakelee, S.L. Gomez
Development of methodology: S.L. Gomez
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): A.-M. Noone, S.L. Gomez
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): I. Cheng, G.M. Le, A.-M. Noone, K. Gali, M. Patel, R.W. Haile, H.A. Wakelee, S.L. Gomez
Writing, review, and/or revision of the manuscript: I. Cheng, G.M. Le, A.-M. Noone, K. Gali, M. Patel, R.W. Haile, H.A. Wakelee
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): G.M. Le, K. Gali, M. Patel, S.L. Gomez
Study supervision: I. Cheng, S.L. Gomez
Note: Supplementary data for this article are available at Cancer Epidemiology, Biomarkers & Prevention Online (http://cebp.aacrjournals.org/).
References
- 1.Borghouts LB, Keizer HA. Exercise and insulin sensitivity: a review. Int J Sports Med. 2000;21:1–12. doi: 10.1055/s-2000-8847. [DOI] [PubMed] [Google Scholar]
- 2.Pew Research Center. The Rise of Asian Americans. Washington, D.C.: Apr 4, 2013. [cited 2014]. Available from: http://www.pewsocial-trends.org/2012/06/19/the-rise-of-asian-americans/ [Google Scholar]
- 3.Murphy SLXJ, Kochanek KD. National vital statistics reports. Hyattsville, MD: National Center for Health Statistics; 2013. Deaths: final data for 2010. [PubMed] [Google Scholar]
- 4.Gomez SL, Noone AM, Lichtensztajn DY, Scoppa S, Gibson JT, Liu L, et al. Cancer incidence trends among Asian American populations in the United States, 1990–2008. J Natl Cancer Inst. 2013;105:1096–110. doi: 10.1093/jnci/djt157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humes KRJN, Ramierz RR. Overview of Race and Hispanic Origin: 2010. Census Briefs. 2011 issued March 2011. [Google Scholar]
- 6.Liu L, Noone AM, Gomez SL, Scoppa S, Gibson JT, Lichtensztajn D, et al. Cancer incidence trends among native Hawaiians and other Pacific Islanders in the United States, 1990–2008. J Natl Cancer Inst. 2013;105:1086–95. doi: 10.1093/jnci/djt156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117:294–9. doi: 10.1002/ijc.21183. [DOI] [PubMed] [Google Scholar]
- 8.Toyoda Y, Nakayama T, Ioka A, Tsukuma H. Trends in lung cancer incidence by histological type in Osaka, Japan. Jpn J Clin Oncol. 2008;38:534–9. doi: 10.1093/jjco/hyn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Au JS, Mang OW, Foo W, Law SC. Time trends of lung cancer incidence by histologic types and smoking prevalence in Hong Kong 1983–2000. Lung Cancer. 2004;45:143–52. doi: 10.1016/j.lungcan.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Janssen-Heijnen ML, Coebergh JW. The changing epidemiology of lung cancer in Europe. Lung Cancer. 2003;41:245–58. doi: 10.1016/s0169-5002(03)00230-7. [DOI] [PubMed] [Google Scholar]
- 11.Polednak AP. Lung cancer incidence trends by histologic type in areas of California vs. other areas in the Surveillance, Epidemiology and End Results Program. Cancer Epidemiol. 2009;33:319–24. doi: 10.1016/j.canep.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Caraballo RS, Yee SL, Gfroerer J, Mirza SA. Adult tobacco use among racial and ethnic groups living in the United States, 2002–2005. Prev Chronic Dis. 2008;5:A78. [PMC free article] [PubMed] [Google Scholar]
- 13.Mishra SI, Osann K, Luce PH. Prevalence and predictors of smoking behavior among Samoans in three geographical regions. Ethn Dis. 2005;15:305–15. [PubMed] [Google Scholar]
- 14.Kuang PKX. Lung cancer in asian women. North Am J of Med and Sci. 2009;2:69–73. [Google Scholar]
- 15.Lewis DRCD, Caporaso NE, Travis WD, Devesa SS. Lung cancer trends by histologic type cancer. 2014 doi: 10.1002/cncr.28749. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Surveillance Epidemiology and End Results web site. http://seer.cancer.gov/. SEER Registries. [internet]. [accessed May 2010]. Available from: http://seer.cancer.gov/registries/
- 17.Gomez SL, Le GM, West DW, Satariano WA, O’Connor L. Hospital policy and practice regarding the collection of data on race, ethnicity, and birthplace. Am J Public Health. 2003;93:1685–8. doi: 10.2105/ajph.93.10.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez SL, Satariano W, Le GM, Weeks P, McClure L, West DW. Variability among hospitals and staff in collection of race, ethnicity, birthplace, and socioeconomic information in the Greater San Francisco Bay Area. J Reg Manag. 2009;36:105–10. [PMC free article] [PubMed] [Google Scholar]
- 19.Adamo MB, Johnson CH, Ruhl JL, Dickie LA. 2010 SEER Program Coding and Staging Manual. Bethesda, MD: 2010. [Google Scholar]
- 20.U.S. Census Bureau. Public-Use Microdata Samples (PUMS) Census of Population and Housing. 1990 [accessed Jan 2011]. Available from URL: http://www.census.gov/main/www/pums.html.
- 21.American Cancer Society, Cancer Research Center of Hawai’i, Hawai’i State Department of Health. Hawai’i Cancer Fact & Figures. 2010 [Accessed Feb 4, 2013]. http://www.uhcancercenter.org/research/research-highlightsreports.
- 22.U.S. Census Bureau. Census 2000 Summary File 2 Technical Documentation. U.S. Census Bureau; 2001. Appendix H. Characteristic iterations. p H-1. [accessed Jan 2011]. Available from URL: www.census.gov/prod/cen2000/doc/sf2.pdf. [Google Scholar]
- 23.Brillinger DR. The natural variability of vital rates and associated statistics. Biometrics. 1986;42:693–734. [PubMed] [Google Scholar]
- 24.Devesa SS, Donaldson J, Fears T. Graphical presentation of trends in rates. Am J Epidemiol. 1995;141:300–4. doi: 10.1093/aje/141.4.300. [DOI] [PubMed] [Google Scholar]
- 25.Jatoi I, Anderson WF, Rao SR, Devesa SS. Breast cancer trends among black and white women in the United States. J Clin Oncol. 2005;23:7836–41. doi: 10.1200/JCO.2004.01.0421. [DOI] [PubMed] [Google Scholar]
- 26.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for join-point regression with applications to cancer rates. Stat Med. 2000;19:335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 27.Charloux A, Quoix E, Wolkove N, Small D, Pauli G, Kreisman H. The increasing incidence of lung adenocarcinoma: reality or artefact? A review of the epidemiology of lung adenocarcinoma. Int J Epidemiol. 1997;26:14–23. doi: 10.1093/ije/26.1.14. [DOI] [PubMed] [Google Scholar]
- 28.Kenfield SA, Wei EK, Stampfer MJ, Rosner BA, Colditz GA. Comparison of aspects of smoking among the four histological types of lung cancer. Tob Control. 2008;17:198–204. doi: 10.1136/tc.2007.022582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiraishi K, Kunitoh H, Daigo Y, Takahashi A, Goto K, Sakamoto H, et al. A genome-wide association study identifies two new susceptibility loci for lung adenocarcinoma in the Japanese population. Nat Genet. 2012;44:900–3. doi: 10.1038/ng.2353. [DOI] [PubMed] [Google Scholar]
- 30.Miki D, Kubo M, Takahashi A, Yoon KA, Kim J, Lee GK, et al. Variation in TP63 is associated with lung adenocarcinoma susceptibility in Japanese and Korean populations. Nat Genet. 2010;42:893–6. doi: 10.1038/ng.667. [DOI] [PubMed] [Google Scholar]
- 31.Hsiung CA, Lan Q, Hong YC, Chen CJ, Hosgood HD, Chang IS, et al. The 5p15.33 locus is associated with risk of lung adenocarcinoma in never-smoking females in Asia. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landi MT, Chatterjee N, Yu K, Goldin LR, Goldstein AM, Rotunno M, et al. A genome-wide association study of lung cancer identifies a region of chromosome 5p15 associated with risk for adenocarcinoma. Am J Hum Genet. 2009;85:679–91. doi: 10.1016/j.ajhg.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trends in tuberculosis–United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60:333–7. [PubMed] [Google Scholar]
- 34.Manangan LP, Salibay CJ, Wallace RM, Kammerer S, Pratt R, McAllister L, et al. Tuberculosis among persons born in the Philippines and living in the United States, 2000–2007. Am J Public Health. 2011;101:101–11. doi: 10.2105/AJPH.2009.175331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang HY, Li XL, Yu XS, Guan P, Yin ZH, He QC, et al. Facts and fiction of the relationship between preexisting tuberculosis and lung cancer risk: a systematic review. Int J Cancer. 2009;125:2936–44. doi: 10.1002/ijc.24636. [DOI] [PubMed] [Google Scholar]
- 36.Chen X, Unger JB, Johnson CA. Is acculturation a risk factor for early smoking initiation among Chinese American minors? A comparative perspective. Tob Control. 1999;8:402–10. doi: 10.1136/tc.8.4.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suinn RAC, Khoo G. The Suinn-Lew Asian Self-Identity Acculturation Scale: concurrent and factorial validation. Educ Psychol Meas. 1992;52:1041–6. [Google Scholar]
- 38.Choi S, Rankin S, Stewart A, Oka R. Effects of acculturation on smoking behavior in Asian Americans: a meta-analysis. J Cardiovasc Nurs. 2008;23:67–73. doi: 10.1097/01.JCN.0000305057.96247.f2. [DOI] [PubMed] [Google Scholar]
- 39.Raz DJ, Gomez SL, Chang ET, Kim JY, Keegan TH, Pham J, et al. Epidemiology of non-small cell lung cancer in Asian Americans: incidence patterns among six subgroups by nativity. J Thorac Oncol. 2008;3:1391–7. doi: 10.1097/JTO.0b013e31818ddff7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurian AW, Lichtensztajn DY, Keegan TH, Leung RW, Shema SJ, Hershman DL, et al. Patterns and predictors of breast cancer chemotherapy use in Kaiser Permanente Northern California, 2004–2007. Breast Cancer Res Treat. 2013;137:247–60. doi: 10.1007/s10549-012-2329-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torok S, Hegedus B, Laszlo V, Hoda MA, Ghanim B, Berger W, et al. Lung cancer in never smokers. Future Oncol. 2011;7:1195–211. doi: 10.2217/fon.11.100. [DOI] [PubMed] [Google Scholar]
- 42.Zhou W, Christiani DC. East meets West: ethnic differences in epidemiology and clinical behaviors of lung cancer between East Asians and Caucasians. Chin J Cancer. 2011;30:287–92. doi: 10.5732/cjc.011.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sisti J, Boffetta P. What proportion of lung cancer in never-smokers can be attributed to known risk factors? Int J Cancer. 2012;131:265–75. doi: 10.1002/ijc.27477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Marchand L, Wilkens LR, Kolonel LN. Ethnic differences in the lung cancer risk associated with smoking. Cancer Epidemiol Biomarkers Prev. 1992;1:103–7. [PubMed] [Google Scholar]
- 45.Goodman MT, Hankin JH, Wilkens LR, Lyu LC, McDuffie K, Liu LQ, et al. Diet, body size, physical activity, and the risk of endometrial cancer. Cancer Res. 1997;57:5077–85. [PubMed] [Google Scholar]
- 46.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 47.Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J Clin Oncol. 2013;31:992–1001. doi: 10.1200/JCO.2012.46.9270. [DOI] [PubMed] [Google Scholar]
- 48.Yu M, Feuer EJ, Cronin KA, Caporaso NE. Use of Multiple Imputation to Correct for Bias in Lung Cancer Incidence Trends by Histologic Subtype. Cancer Epidemiol Biomarkers Prev. 2014;23:1546–58. doi: 10.1158/1055-9965.EPI-14-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clegg LX, Reichman ME, Hankey BF, Miller BA, Lin YD, Johnson NJ, et al. Quality of race, Hispanic ethnicity, and immigrant status in population-based cancer registry data: implications for health disparity studies. Cancer Causes Control. 2007;18:177–87. doi: 10.1007/s10552-006-0089-4. [DOI] [PubMed] [Google Scholar]
- 50.Gomez SL, Glaser SL, Kelsey JL, Lee MM, Sidney S. Inconsistencies between self-reported ethnicity and ethnicity recorded in a health maintenance organization. Annals Epidemiol. 2005;15:71–9. doi: 10.1016/j.annepidem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Moscou S, Anderson MR, Kaplan JB, Valencia L. Validity of racial/ethnic classifications in medical records data: an exploratory study. Am J Public Health. 2003;93:1084–6. doi: 10.2105/ajph.93.7.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Polednak AP. Collecting information on race, Hispanic ethnicity, and birthplace of cancer patients: policies and practices in Connecticut hospitals. Ethn Dis. 2005;15:90–6. [PubMed] [Google Scholar]
- 53.West CN, Geiger AM, Greene SM, Harris EL, Liu IL, Barton MB, et al. Race and ethnicity: comparing medical records to self-reports. J Natl Cancer Inst Monogr. 2005:72–4. doi: 10.1093/jncimonographs/lgi041. [DOI] [PubMed] [Google Scholar]
- 54.Gomez SL, Glaser SL. Misclassification of race/ethnicity in a population-based cancer registry. Cancer Causes and Control. 2006;17:771–81. doi: 10.1007/s10552-006-0013-y. [DOI] [PubMed] [Google Scholar]
- 55.Liu L, Tanjasiri SP, Cockburn M. Challenges in identifying Native Hawaiians and Pacific Islanders in population-based cancer registries in the US. J Immigr Minor Health. 2011;13:860–6. doi: 10.1007/s10903-010-9381-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boscoe FP, Miller BA. Population estimation error and its impact on 1991–1999 cancer rates. Prof Geographer. 2004;54:516–29. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


