Abstract
Patellofemoral osteoarthritis (PFOA) can be associated with anterior knee pain, stiffness, and functional impairment. Some authors report that PFOA affects approximately 9% of patients older than 40 years with a greater prevalence in females. Etiology of PFOA is multifactorial and is related to the presence of abnormal stresses at the PF joint due to knee- and patient-related factors. The need for a joint preserving treatment by isolated replacement of the injured compartment of the knee led to the development of PF arthroplasty (PFA). When a correct PF replacement is performed, PFA preserves physiologic tibiofemoral joint, thus allowing patients for a rapid recovery with a high satisfaction. The outcomes for PFA are quite variable with a trend toward good to excellent results, mainly owing to the improvement in surgical techniques, patient selection, and implant design. The development of the second generation of PFA improved the outcomes, which is attributed to the different trochlear designs. Recently, encouraging results have been provided by the association of PFA and unicompartmental knee arthroplasty (UKA). In many studies, the main cause of PFA failure is progression of tibiofemoral OA. The aim of this brief review of literature is to summarize the clinical features, indications and contraindications, surgical techniques, complications, and outcomes of PFA.
Keywords: patellofemoral, osteoarthritis, replacement, arthroplasty, knee
Introduction
Patellofemoral (PF) osteoarthritis (OA) can be associated with anterior knee pain, stiffness, and functional impairment. 1 Some authors report that approximately 9% of patients older than 40 years are affected by PFOA. 2 Other authors have reported an incidence ranging from 2 to 11% in men and from 8 to 24% in women older than 55 years. 3 4 The higher incidence in female patients may be related to a higher incidence of PF malalignment and dysplasia in this population. 5 Furthermore, Kobayashi et al 6 in a recent systematic review, including 32 studies, concluded that the prevalence of PFOA was 25% in the asymptomatic population (age > 20 years) and 39% in the symptomatic population (age > 30 years).
The development of PFOA is related to the presence of abnormal PF joint stress, which can be due to excessive amount or inadequate dispersion of forces. The cause of these abnormal stresses is multifactorial, including both knee- and patient-related risk factors. 7
Among the patient-related risk factors, increased body mass index (BMI) surely increased the forces on the PF joint during weight-bearing activities. 8 9 Some authors outlined female gender and increased age to be possible risk factors for PFOA, as well as activities involving increased load on PF joint (i.e., descending stairs). 7 Previous trauma to the PF joint (i.e., patellar fracture or dislocation) has also been related to the development of PFOA. 10 11
Among knee-related risk factors, increased rate of PFOA has been reported in patients who underwent anterior cruciate ligament reconstruction (ACLR), with a median prevalence of up to 50% at 10 to 15 years after surgery. 12 Quadriceps and hip abductor weakness has also been related to PFOA, as well as ileotibial band and hamstring contracture. 7 However, the most important knee-related risk factor is the presence of PF malalignment or trochlear dysplasia, as well as valgus knee alignment and increased femoral anteversion. 7 13 14
Treatment of PFOA includes nonoperative and surgical options. The nonoperative treatments include exercise, physical therapy, taping, and injections and may result in short-term benefit. 14 The surgical options range from joint debridement to arthroplasty. However, joint-preserving procedures, including anterior tibial tubercle transfer or cartilage procedures, may lead to insufficient short-term improvement. Total knee arthroplasty (TKA) with patellar replacement is a well-established procedure to treat PFOA, but anterior knee pain may persist in 7 to 19% of the patients. 4 10
The need for replacing the PF joint while maintaining a joint kinematic closer to that of the native knee in comparison with TKA led to the development of PF arthroplasty (PFA). 4 PFA preserves physiologic tibiofemoral joint, thus allowing patients for a rapid recovery with good satisfaction. 15 Although PFA is considered a valid therapeutic option to treat isolated PFOA, it is indicated in a small and highly selected population.
The first PFA was proposed in 1955 by MacKeever. Despite the initial encouraging results, it was quickly abandoned due to excessive wear in the trochlear groove. 16 PFA witnessed a rebirth in the 1970s, when the first generation of Richards' prostheses (Smith & Nephew; Memphis, Tennessee, United States) was introduced and subsequently developed later in the 1990s with the introduction of the second generation of PFA. 17 18 Although the clinical outcomes after PFA depend primarily on implant design and surgical technique, careful patient selection with very strict inclusion and exclusion criteria is mandatory for long-term survivorship and satisfactory outcomes.
The aim of the this article is to summarize the clinical features, indications and contraindications, surgical techniques, and outcomes of PFA.
Patellofemoral Osteoarthritis
Patient History and Physical Examination
Patients with isolated PFOA report an anterior or retropatellar knee pain, usually associated with activities that load the PF joint. 4 These activities include squatting, ascending or descending stairs, rising from a seated position, or prolonged sitting with flexed knees. 4 Frequently, some activities, such as full squatting and kneeling, are almost impossible. Pain is less severe when walking on level ground and in standing position with the knee completely extended. 11 Frequently, patients affected by PFOA experience the worse pain in the early stages of the disease. 11
During physical examination, it is mandatory to evaluate the entire lower limb, including muscle strength and coronal and PF malalignment (i.e., an increased Q angle). Indeed, the presence of malalignment should be carefully evaluated because in many cases a realignment osteotomy before or in conjunction with PFA may be required. 4 Crepitus and effusion may be reported, and the patella compression test is often positive in the case of PFOA. The range of motion (ROM) must be investigated, and the examiner should focus on the localization of pain and its correlation to the degree of flexion. Normally, distal lesions (inferior pole) are painful at the first degree of flexion, while proximal lesions are painful at higher flexion. 19 It is also mandatory to evaluate patella tracking throughout the entire knee ROM to assess maltracking or instability (i.e., the presence of a J sign). 20 21 Any involvement of the tibiofemoral joint should be ruled out, as well as knee stability, to avoid progressive tibiofemoral OA and/or instability. Furthermore, the neurovascular status, previous scars, and hip and lumbar spine status should be carefully evaluated. 4
Imaging
A routine series of conventional X-ray views should be obtained, including standing anteroposterior (AP), flexed weight-bearing posterior–anterior (PA, Rosenberg), lateral, and sunrise (Merchant) views. Both the PF and tibiofemoral joints should be carefully evaluated on these films. Sunrise views at 30 degrees flexion allow for the best assessment, but in some cases, it is useful to take views in various degrees of flexion to better evaluate patellar tilt, subluxation, trochlear dysplasia, or a narrowed joint space. 22 Lateral view can reveal PF osteophytes, patella height, and joint space narrowing. AP and PA views can reveal a medial or lateral OA. A weight-bearing, long-leg view should also be obtained to evaluate lower limb alignment. Some authors suggest additional computed tomography (CT) scan and/or magnetic resonance imaging (MRI) to calculate the tibial tubercle–trochlear grove (TT–TG) distance and evaluate bone stock, rotational malalignment, trochlear dysplasia, and intra-articular structures (articular cartilage, menisci, and ligaments). 3 4 21
Patellofemoral Arthroplasty: Indications and Contraindications
A prerequisite for the success of PFA is a proper patient selection to avoid complications. 5 17 23 24 25 The most common indication for PFA is severe primary isolated PFOA that affects daily activities without relief from conservative treatment. PFA is also indicated in patients with posttraumatic PFOA, isolated trochlear dysplasia, patellar subluxation, or failure of previous extensor mechanism unloading procedures, such as Fulkerson or Maquet osteotomy. 5 11 25 26 27 Moreover, the ideal patient age ranges from 40 to 60 years. 28 29
Many contraindications exist to PFA that should always be kept in mind. The presence of tibiofemoral OA is the most common contraindication and the most common cause of PFA failure. 5 PF malalignment, with an increased Q angle, cannot be corrected by PFA alone; so, it has to be considered as a relative contraindication. On the contrary, mild patellar tilt or subluxation can be corrected at the time of PFA with lateral retinacular release, medialization of the patellar component, or partial facetectomy. 4 A PFA should preserve normal knee kinematics, joint stability with intact ligaments, and menisci has to be considered as a fundamental prerequisite for PFA. 4 Furthermore, PFA is contraindicated in the presence of severe uncorrected coronal plane deformity (valgus > 8 or varus > 5 degree alignment) or sagittal plane deformity (120 degree flexion with < 10 degree flexion contracture). 4 11 28 Active infection and evidence or high suspicion of chronic regional pain syndrome are also contraindications to PFA. 11 23 Quadriceps atrophy, patellar tendon scarring, patella baja, and excessive BMI (>30) represent relative contraindications to PFA, as have been correlated to poorer outcome. 11 23 Hence, the ideal candidate for PFA is a middle age patient affected by isolated, debilitating noninflammatory PFOA not responsive to conservative treatment and with normal limb alignment. 4 The main indications and contraindications are summarized in Table 1 .
Table 1. Indications and contraindications for PFA.
| Indications | Contraindications |
|---|---|
| Advanced primary isolated PFOA | Absolute contraindications |
| Posttraumatic PFOA | Tibiofemoral OA |
| Trochlear dysplasia | PF malalignment |
| Patellar subluxation or mild patellar tilt | Knee instability (ligaments and/or menisci injuries) |
| Failed extensor mechanism unloading procedures | Limb malalignment (Valgus deformity > 8 degrees or varus deformity > 5 degrees) |
| Age > 40 years | Acute infection or CRPS |
| Relative contraindications | |
| Quadriceps atrophy | |
| Patella baja | |
| BMI > 30 |
Abbreviations: BMI, body mass index; CRPS, complex regional pain syndrome ; OA, osteoarthritis; PF, patellofemoral; PFA, patellofemoral arthroplasty; PFOA, patellofemoral osteoarthritis.
Implant Design
The first PFA design was an isolated resurfacing of the patella with a screw-on Vitallium patellar shell. Despite initial encouraging results, it was quickly abandoned due to excessive wear in the trochlear groove. 16 30
The first generation of complete PFA (both sides) only replaced the trochlear cartilage leaving the subchondral bone intact (inlay design); so, the position of the PFA was related to the anatomy of the native trochlea. Furthermore, the trochlear component was positioned flush with the surrounding cartilage, the rotational alignment was parallel to the trochlear inclination, the mediolateral coverage was limited, and the surgical technique was mainly free hand. 31 First-generation PFA was successful in the short-term period, providing early pain relief, but the results were not maintained over time and more than 50% of the implants failed in the mid- to long-term follow-up with high reoperation rate. 32
The second-generation PFA was designed to improve clinical outcomes and address the limitations of the previous design, mainly related to maltracking and instability. 31 These implants used the same anterior femoral cuts as in TKA and completely replaced the anterior compartment of the knee (onlay design). Furthermore, these were characterized by a broad trochlear flange that narrowed distally, 18 a valgus tracking angle and a good congruity throughout the entire ROM, avoiding catching, snapping, or popping. 4
Both generations include symmetric and asymmetric implants. The second-generation designs can be used in all patients, regardless of anatomic variations, and are therefore more versatile and suitable. 21
The PF implants can also be sorted according to the morphology of each component. The patellar button can be shaped with facets or as a dome and can be symmetric or asymmetric. It is advisable that patellar button design match the trochlear shape of TKAs to allow for retention of the component, if a PFA revision is required. 33
Nevertheless, the evolution of PFA is mostly based on the design of the trochlear component. The sagittal radius of curvature, the mediolateral width, the distal–proximal extension, and the grade of constrain are the most important features of the trochlear design. 34 A less constrained design allows the patella for more freedom but may increase the risk of patellar instability. On the contrary, a more constrained design (deep trochlear groove) allows less patellar movement causing an increase in PF loads and a high risk of early loosening. 33 Consequently, the goal of implant design is to reproduce as close as possible the normal anatomy, thereby allowing smooth patellar tracking and minimizing the risk of subluxation or dislocation. 34 35 36 The sagittal radius of curvature and the proximal extension of the implant determine the point of engagement of the patellar button with the trochlear component. 37 These parameters can significantly influence patellar tracking. Furthermore, the point at which the patellar button engages the trochlear component is also influenced by the distal coverage of the implant. 33
Nowadays, inlay implants have been mostly abandoned and onlay implants are mainly used worldwide.
Surgical Technique (Onlay Design)
After a midline incision (from 1 cm proximal to the patella to just proximal to the tibial tubercle), the arthrotomy can be performed using surgeon's choice between medial parapatellar, midvastus, or subvastus approach. Care must be taken to avoid damaging the menisci, ligaments, or tibiofemoral articular cartilage. The patella can be either laterally dislocated or left in place and gently and partially everted. At this point, the surgeon should inspect the entire knee to confirm that the tibiofemoral joint is not involved and there are no ligamentous injuries.
The precise technique of implantation varies with the implant used. As a general rule, the trochlea is prepared with an anterior cutting guide, taking care not to damage the anterior cortex of the femur. 33
Evaluation of the correct rotational alignment is mandatory to obtain good outcomes, and it can be achieved using an intramedullary femoral guide, navigation, or robotic devices. 38 39
The anterior femoral cut can be either performed in an “anatomical” or “functional” fashion. The “anatomical” anterior femoral cut is performed perpendicular to the AP axis (Whiteside's line) or parallel to the transepicondylar axis and then the patella is recentered by freeing up the lateral retinaculum. Alternatively, the “functional” femoral cut can be performed with more external rotation relative to the patellar plane and can be combined with lateral translation of the femoral component to avoid additional procedures on the extensor mechanism. 33
Once the appropriate rotation is obtained, the anterior cut is performed and the appropriate size of the trochlear component is selected. The presence of the “grand piano” is the sign of a good external rotation of the component. The sign is an image, resembling a grand piano, that results on the anterior cut of the femur as a consequence of the asymmetric anterior cut. 11 To avoid maltracking, the cutting guide and then the definitive implant, should be placed slightly lateral. Once the femur has been prepared, the trial component is impacted to make sure there is no ACL impingement and no stepoff with the condylar cartilage.
The patellar cut is performed as in the standard TKA. It is important to leave at least 14 mm of bone thickness, and care must be taken to avoid asymmetric cut and overstuffed component. If it is not possible to leave an adequate amount of bone stock, a bone graft, and cement or patella augmentation button are viable solutions. 40 To prevent maltracking, the patellar component should be medialized and the lateral osteophytes should be removed to avoid bony impingement. 11
The patella tracking should be carefully checked with trial components. If it is not adequate, a lateral release can be performed. In case of more severe malalignment, advancement of the vastus medialis or tibial tubercle transfer can be performed. However, these procedures should be carefully planned preoperatively, and worse outcomes can be expected. 33 40 When the correct size and position of the implant are achieved, the trial components are removed and the definitive components are cemented. Surgeons should be careful to avoid thermal damage to the adjacent cartilage during cementation ( Fig. 1 ).
Fig. 1.
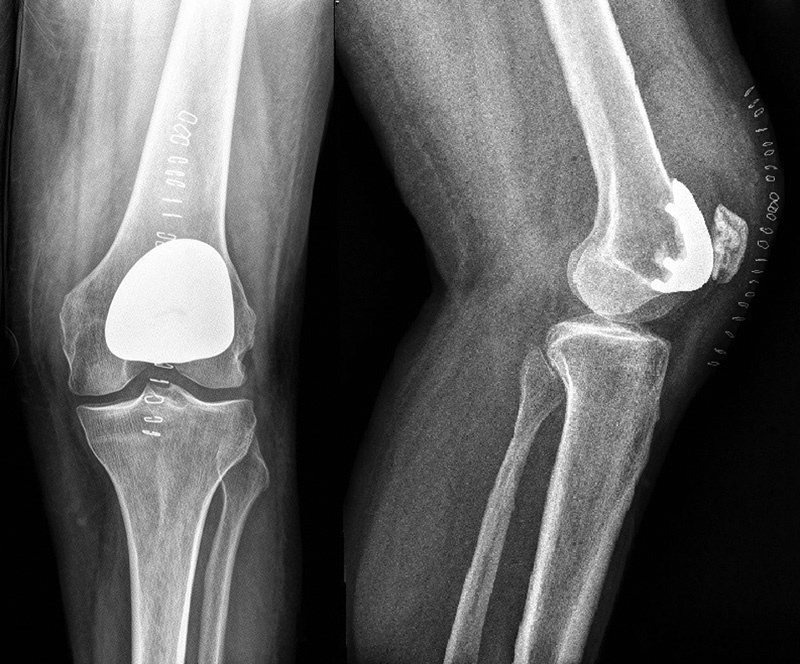
Anteroposterior and lateral X-ray view after left PFA implants. PFA, patellofemoral arthroplasty.
Postoperative Rehabilitation
Postoperative care follows the same indications as in the TKA. Full weight bearing is immediately allowed as tolerated. Rehabilitation focuses on active and passive ROM exercises along with gait training. In case of additional procedures, such as advancement of the vastus medialis oblique or tibial tubercle transfer, a slower and more cautious rehabilitation may be required. 11 40
Complications and Failure
PFA shares most of the complications with the TKA, such as polyethylene wear, arthrofibrosis, and persistent pain. Early complications, such as persistent anterior knee pain, patellar catching or snapping, intraoperative fracture, and extensor mechanism failure are more frequent in PFA than in TKA and are mostly related to malpositioning. 41 42 Conversely, loosening and infection occur at a lower rate compared with TKA. 43 44 However, there are some specific early complications related to PFA that should be mentioned. One specific complication is a lateral swelling due to a hematoma after bleeding in the lateral compartment when a lateral release during PFA is performed. Another common complication is patellar maltracking or patellar instability due to failure of a medial ligament repair or an inadequate tracking correction during surgery. Furthermore, peripatellar pain due to “overstuffing” of the patellar component can occur. Another specific complication is lateral catching due to wrong realignment of the extensor mechanism, or more frequently, to poor trochlear component placement, frequently related to a rotational malposition. 45
Late complications requiring revision usually occur in the setting of a well-functioning PFA. The most common cause of late PFA failure is progression of tibiofemoral OA, accounting for approximately 25% of the revision at 15 years of follow-up. 46 47 Some authors reported that tibiofemoral OA progression is more frequent in obese patients and when the indication is primary PFOA compared with those affected by trochlear dysplasia. 10 46 47 48 49 50 Aseptic loosening is another possible cause of revision and is more frequent in cementless PFA. 10 Van der List et al 42 published a systematic review, including 39 studies evaluating failures after PFA. The authors concluded that the first cause of failure was tibiofemoral OA progression (38%), pain (16%), aseptic loosening (14%), and patellar maltracking (10%). Furthermore, pain was recognized as the first cause (31%) of early failures (within 5 years), while OA progression was more common in late failures (46%).
Results: Literature Review
The outcomes for PFA were quite variable, mainly because of the improvement in surgical technique, patient selection, and implant design. 51 A recent systematic review published by van der List et al 42 concluded that the survivorship of PFA at 5-, 10-, 15-, and 20-year follow-up was 91.7, 83.3, 74.9, and 66.6%, respectively. There were few studies comparing inlay and onlay PFA. Van der List et al 42 found that studies published before 2010 reported a higher annual revision rate when compared with more recently published studies (2.33 vs. 1.93). Furthermore, the authors reported good to excellent results in 87.3% of the patients 5 years after surgery. Similar to other studies, the authors concluded that better long-term outcomes and survivorship could be expected with second-generation implants. 42 44
Dy et al 44 performed a meta-analysis to compare complication, reoperation, and revision rate following first- and second-generation PFA and between PFA versus TKA for PFOA. The authors concluded that there was a higher risk of reoperation (OR: 4.33) and revision (OR: 4.93) in first generation compared with second-generation PFA. 44
Inlay PFA
Tauro et al 32 reviewed 66 inlay PFAs with 65% of survivorship rate at 8-year follow-up and only 45% of good or excellent results. The most frequent cause of revision was patellar maltracking and progression of tibiofemoral OA. Argenson et al 10 reported 58% survival at 16-year follow-up, while Van Jonbergen et al 27 reported 69% survivorship at 20-year follow-up. However, also in these studies, the main reasons for revision were progression of tibiofemoral OA and patellofemoral complications. Other authors described an incidence of patellar maltracking after inlay PFA ranged between 17 and 36%. 17 32 35 52 The Australian National Joint Replacement Registry reported that more than 20% of the implants needed a revision at 5-year follow-up. 53 All the studies are summarized in Table 2 . In summary, results with the first generation of PFA have been disappointing, with high complication and failure rates. 33
Table 2. PFA inlay design results.
| Author | N cases (mean age) | Prosthesis design | Follow-up (y) | Revision rate (%) | Good or excellent results (%) |
|---|---|---|---|---|---|
| Blazina et al 17 | 57 (39) | Richards I & II | 2 | 35 | N/A |
| Arciero et al 24 | 25 (62) | Richards IICFS-Wright | 5.3 | 28 | 85 |
| Cartier et al 72 | 72 (65) | Richards I & II | 4 | 7 | 85 |
| Argenson et al 10 | 66 (57) | Autocentric | 5.5 | 15 | 84 |
| Krajca-Radcliffe and Coker 46 | 16 (64) | Richards I & II | 5.8 | 6 | 88 |
| Mertl et al 73 | 51 (60) | Spherocentric | 3 | 6 | 82 |
| Arnbjörnsson and Ryd 74 | 113 (56) | Multiple | 7 | 22 | 75 |
| De Cloedt et al 75 | 45 (51) | Autocentric | 6 | 18 | 63 |
| de Winter et al 52 | 26 (59) | Richards II | 11 | 19 | 76 |
| Tauro et al 32 | 62 (66) | Lubinus | 7.5 | 28 | 45 |
| Smith et al 76 | 45 (72) | Lubinus | 4 | 19 | 64 |
| Kooijman et al 47 | 45 (50) | Richards II | 17 | 22 | 86 |
| Board et al 77 | 17 (66) | Lubinus | 1.5 | 35 | 53 |
| Lonner 35 | 30 (38) | Lubinus | 4 | 33 | 84 |
| Merchant 78 | 16 (47) | LCS | 4.5 | 0 | 94 |
| Argenson et al 79 | 66 (57) | Autocentric | 16.2 | 42 | 84 |
| Cartier et al 36 | 79 (60) | Richards II & III | 10 | 25 | 77 |
| Sisto and Sarin 57 | 25 (45) | Kinematch | 6 | 0 | 100 |
| Jørgensen et al 80 | 31(N/A) | Richards II | 7.7 | 3 | 65 |
| Gadeyne et al 81 | 43(67) | Autocentric | 6 | 24 | 72 |
| van Wagenberg et al 48 | 24 (64) | Autocentric | 4.8 | 29 | 30 |
| van Jonbergen et al 27 | 185 (52) | Richards II | 13.3 | 25 | 84 |
| Charalambous et al 82 | 51 (64) | LCS | 2 | 33 | 33 |
| Hoogervorst et al 83 | 33 (47) | Richards II | 9.7 | 21 | 90 |
Abbreviations: LCS, low contact stress; N, number; PFA, patellofemoral arthroplasty; N/A, not available.
Onlay PFA
The second-generation PFA demonstrated significantly improved short- and mid-term outcomes due to a careful patient selection. The Australian National Joint Replacement Registry reported less than 10% revision rates for onlay PFAs. 53 The onlay design could reduce the incidence of patellar maltracking to 1% after PFA. 49 54 55 56 Therefore, when patellar tracking was satisfactory after PFA, revisions due to patellofemoral issues were reduced, leaving progression of tibiofemoral OA as the first cause of failure. Excellent results were reported by Ackroyd et al 54 with a 5-year survivorship of 96%. Sisto et al, 57 in a series of custom-made implants, reported 100% survivorship at 6 years with good to excellent results in all the patients. Also, Butler et al, 58 in a smaller series of custom-made implants, reported 91% survivorship at 5-year follow-up. Leadbetter et al, 49 in a series of 79 onlay PFAs, reported 94% survivorship at a mean 3-year follow-up with 84% good to excellent results. Similar results were reported by other authors at 5-year follow-up despite a 26% reoperation rate for lateral release, debridement, tibial tubercle transfer, or arthroscopic assessment. 59 All the studies are summarized in Table 3 .
Table 3. PFA onlay design results.
| Author | N cases (mean age) | Prosthesis design | Follow-up (y) | Revision rate (%) | Good or excellent results (%) |
|---|---|---|---|---|---|
| Lonner 35 | 25 (44) | Avon | 0.5 | 0 | 96 |
| Ackroyd and Chir 18 | 306 (62) | Avon | 2 | 3.6 | 80 |
| Cossey and Spriggins 39 | 4 (52) | Avon (Navigation) | 1 | 0 | 100 |
| Nicol et al 50 | 103 (68) | Avon | 7.1 | 14 | N/A |
| Ackroyd et al 54 | 109 (68) | Avon | 5.2 | 15 | 80 |
| Mohammed et al 59 | 101 (57) | Multiple | 4 | 4 | 72 |
| Butler and Shannon 58 | 22 (49) | Performa | 5 | 14 | N/A |
| Leadbetter et al 49 | 79 (58) | Avon | 3 | 16 | 84 |
| Starks et al 55 | 37 (66) | Avon | 2 | 0 | 86 |
| Odumenya et al 37 | 50 (66) | Avon | 5.3 | 4 | 94 |
| Gao et al 84 | 11 (54) | Avon | 2 | 0 | 100 |
| Sarda et al 85 | 44 (62) | Avon | 4.5 | 5 | 85 |
| Mont et al 56 | 43 (49) | Avon | 7 | 12 | N/A |
| Beitzel et al 86 | 22 (46) | Journey | 2 | 0 | N/A |
| Yadav et al 87 | 51 (54) | LCS | 4.2 | 20 | N/A |
| Davies et al 88 | 52 (61) | FPV | 2 | 13 | 79 |
| Hofmann et al 89 | 40 (61) | Natural Knee II | 2.5 | 10 | 95 |
| Morris et al 90 | 31 (55) | Multiple | 2.5 | 3 | N/A |
| Williams et al 91 | 48 (63) | FPV | 2 | 15 | N/A |
| Al-Hadity et al 92 | 53 (62) | FPV | 3 | 3 | 97 |
| Benazzo et al 93 | 25 (67) | Multiple | 4.7 | 12 | N/A |
| Dahm et al 94 | 59 (56) | Avon | 4 | 3 | N/A |
| Fink et al 95 | 53 (61) | Vanguard | 3.7 | 6 | N/A |
| Hernigou et al 96 | 85 (71) | Hermes | 12 | 5 | 82 |
| Akhbari et al 97 | 61 (66) | Avon | 5 | 7 | 80 |
| Liow et al 98 | 51 (53) | Sigma | 4 | 8 | 76 |
Abbreviations: FPV, Femoro Patella Vialla; LCS, low contact stress; N, number; PFA, patellofemoral arthroplasty; N/A, not available.
Patellofemoral Arthroplasty versus Total Knee Arthroplasty
Several studies reported successful results after TKA for isolated PFOA with good mid-term results in approximately 90% of patients. However, almost 19% of the patients still experienced anterior knee pain after surgery. 60 61 62 Dahm et al 41 compared 33 patients who underwent PFA versus 22 patients who underwent TKA for isolated PFOA with 2.5-year follow-up. The group reported similar functional and pain scores but higher activity scores in the PFA group. Furthermore, PFA group had fewer complications, shorter postoperative hospital stays, less blood loss, and a better postoperative flexion (127 vs. 118 degrees). Dy et al, 44 in a meta-analysis, compared the mid-term results and complications of PFA (inlay and onlay) versus TKA. The authors concluded higher reoperation and revision rates (odds ratio [OR] = 8.06 and 8.11, respectively) for PFA compared with TKA. However, when first- and second-generation PFA were grouped separately, first-generation PFA demonstrated a higher reoperation and revision rate (OR = 4.33 and 4.39, respectively) compared with second-generation PFA, and there were no differences between second-generation PFA and TKA regarding reoperation or revision rate, pain, or mechanical complications. 44 Lonner et al 63 and van Jonbergen et al 64 compared the outcomes of TKA after failed PFA to primary TKA. The studies reported similar outcomes in both the groups, and no significant technical problems were described in TKA after PFA. These findings were also confirmed by other studies. Several authors confirmed that PFA could potentially delay TKA, by 10 to 15 years in up to 80% of the patients, and revision to TKA can be performed without difficulty. 15 41 50 54 63 64 65
Patellofemoral Arthroplasty and Unicompartmental Knee Arthroplasty
Currently, the association of PFA and UKA is one of the most debated topics. Traditionally, PFA was contraindicated in patients with evidence of tibiofemoral OA. Nowadays, with recent implant design and improvement in surgical techniques, combined PFA and medial or lateral tibiofemoral UKA can be performed with good results. 66 67 Furthermore, the possibility to perform a UKA in cases of failure of PFA due to medial or lateral tibiofemoral OA progression may avoid the revision to TKA. 33 Indeed, the association between PFA and UKA allows for retaining of the medial pivot, with consequent better knee kinematic. 68 This procedure can be performed in young patients or in elderly patients with severe comorbidities because of the less invasiveness of the surgery compared with TKA. 23 Some authors have described promising results for PFA associated to UKA. Argenson et al 67 reported optimal ROM and no postoperative pain in 17 patients treated with unlinked PFA and UKA. Heyse et al 69 reported a 100% survivorship at 12-year follow-up in nine patients treated with UKA and PFA. Kamath et al 70 described an improvement in all 21 patients treated with PFA and UKA at 2-year follow-up and reported only one significant complication (knee instability). Parratte et al 71 reported a 54% survivorship at 17-year follow-up in 77 knees with a good satisfaction rate. Also, Sabatini et al 23 reported an improvement in functional and clinical scores at 18-month follow-up in a case series of six knees treated with isolated PFA and nine knees treated with combined PFA and UKA. However, few studies are available that have evaluated the association of PFA and UKA, with small numbers and short follow-ups.
Conclusion
PFA has shown to be a viable option for the treatment of isolated PFOA. The ideal candidate for a PFA is a middle-aged female with PFOA not responsive to the conservative treatment and without significant malalignment or tibiofemoral OA. Modern PFA design onlay style, strict patient selection, and improvement in surgical techniques have produced satisfactory results in the past decades in short- to mid-term follow-up, with 10-years of survivorship of almost 90%. The main cause of failure of second-generation PFA is progression of tibiofemoral OA. However, the introduction of the association of PFA and UKA may reduce the need for revision to TKA due to tibiofemoral OA progression. Despite the good mid-term outcomes after PFA, future research is warranted to evaluate the long-term results of the second-generation PFA, and eventually, the efficacy of combination of PFA and UKA in comparison with TKA.
References
- 1.Duncan R, Peat G, Thomas E, Wood L, Hay E, Croft P. Does isolated patellofemoral osteoarthritis matter? Osteoarthritis Cartilage. 2009;17(09):1151–1155. doi: 10.1016/j.joca.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Lüring C, Tingart M, Drescher W, Springorum H R, Kraft C N, Rath B. Therapy of isolated arthritis in the patellofemoral joint: are there evidence-based options? [Article in German] Orthopade. 2011;40(10):902–906. doi: 10.1007/s00132-011-1777-7. [DOI] [PubMed] [Google Scholar]
- 3.McAlindon T E, Snow S, Cooper C, Dieppe P A. Radiographic patterns of osteoarthritis of the knee joint in the community: the importance of the patellofemoral joint. Ann Rheum Dis. 1992;51(07):844–849. doi: 10.1136/ard.51.7.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker T, Perkinson B, Mihalko W M. Patellofemoral arthroplasty: the other unicompartmental knee replacement. J Bone Joint Surg Am. 2012;94(18):1712–1720. doi: 10.2106/JBJS.L.00539. [DOI] [PubMed] [Google Scholar]
- 5.Lonner J H. Patellofemoral arthroplasty. J Am Acad Orthop Surg. 2007;15(08):495–506. doi: 10.5435/00124635-200708000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi S, Pappas E, Fransen M, Refshauge K, Simic M. The prevalence of patellofemoral osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2016;24(10):1697–1707. doi: 10.1016/j.joca.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Mills K, Hunter D J. Patellofemoral joint osteoarthritis: an individualised pathomechanical approach to management. Best Pract Res Clin Rheumatol. 2014;28(01):73–91. doi: 10.1016/j.berh.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Grelsamer R P, Stein D A. Patellofemoral arthritis. J Bone Joint Surg Am. 2006;88(08):1849–1860. doi: 10.2106/JBJS.E.01394. [DOI] [PubMed] [Google Scholar]
- 9.Cooper C, McAlindon T, Snow S et al. Mechanical and constitutional risk factors for symptomatic knee osteoarthritis: differences between medial tibiofemoral and patellofemoral disease. J Rheumatol. 1994;21(02):307–313. [PubMed] [Google Scholar]
- 10.Argenson J N, Guillaume J M, Aubaniac J M. Is there a place for patellofemoral arthroplasty? Clin Orthop Relat Res. 1995;(321):162–167. [PubMed] [Google Scholar]
- 11.Oni J K, Hochfelder J, Dayan A. Isolated patellofemoral arthroplasty. Bull Hosp Jt Dis (2013) 2014;72(01):97–103. [PubMed] [Google Scholar]
- 12.Culvenor A G, Cook J L, Collins N J, Crossley K M. Is patellofemoral joint osteoarthritis an under-recognised outcome of anterior cruciate ligament reconstruction? A narrative literature review. Br J Sports Med. 2013;47(02):66–70. doi: 10.1136/bjsports-2012-091490. [DOI] [PubMed] [Google Scholar]
- 13.Minkowitz R B, Bosco J A., III Patellofemoral arthritis. Bull NYU Hosp Jt Dis. 2009;67(01):30–38. [PubMed] [Google Scholar]
- 14.van Jonbergen H P, Poolman R W, van Kampen A. Isolated patellofemoral osteoarthritis. Acta Orthop. 2010;81(02):199–205. doi: 10.3109/17453671003628756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta R R, Zywiel M G, Leadbetter W B, Bonutti P, Mont M A. Scientific evidence for the use of modern patellofemoral arthroplasty. Expert Rev Med Devices. 2010;7(01):51–66. doi: 10.1586/erd.09.53. [DOI] [PubMed] [Google Scholar]
- 16.Levitt R L. A long-term evaluation of patellar prostheses. Clin Orthop Relat Res. 1973;(97):153–157. doi: 10.1097/00003086-197311000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Blazina M E, Fox J M, Del Pizzo W, Broukhim B, Ivey F M. Patellofemoral replacement. Clin Orthop Relat Res. 1979;(144):98–102. [PubMed] [Google Scholar]
- 18.Ackroyd C E, Chir B. Development and early results of a new patellofemoral arthroplasty. Clin Orthop Relat Res. 2005;(436):7–13. doi: 10.1097/01.blo.0000171914.94503.d1. [DOI] [PubMed] [Google Scholar]
- 19.Senavongse W, Amis A A. The effects of articular, retinacular, or muscular deficiencies on patellofemoral joint stability: a biomechanical study in vitro. J Bone Joint Surg Br. 2005;87(04):577–582. doi: 10.1302/0301-620X.87B4.14768. [DOI] [PubMed] [Google Scholar]
- 20.Rauh M A, Parker R D. Philadelphia: Saunders; 2009. Patella. [Google Scholar]
- 21.Lonner J H, Bloomfield M R.The clinical outcome of patellofemoral arthroplasty Orthop Clin North Am 20134403271–280., vii [DOI] [PubMed] [Google Scholar]
- 22.Iwano T, Kurasawa H, Tokuyama H, Hoshikawa Y. Roentgenographic and clinical findings of patellofemoral osteoarthrosis. With special reference to its relationship to femorotibial osteoarthrosis and etiologic factors. Clin Orthop Relat Res. 1990;(252):190–197. [PubMed] [Google Scholar]
- 23.Sabatini L, Schirò M, Atzori F, Ferrero G, Massè A. Patellofemoral Joint Arthroplasty: our experience in isolated patellofemoral and bicompartmental arthritic knees. Clin Med Insights Arthritis Musculoskelet Disord. 2016;9:189–193. doi: 10.4137/CMAMD.S40498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arciero R A, Toomey H E. Patellofemoral arthroplasty. A three- to nine-year follow-up study. Clin Orthop Relat Res. 1988;(236):60–71. [PubMed] [Google Scholar]
- 25.Leadbetter W B, Seyler T M, Ragland P S, Mont M A. Indications, contraindications, and pitfalls of patellofemoral arthroplasty. J Bone Joint Surg Am. 2006;88 04:122–137. doi: 10.2106/JBJS.F.00856. [DOI] [PubMed] [Google Scholar]
- 26.Dejour D H. The patellofemoral joint and its historical roots: the Lyon School of Knee Surgery. Knee Surg Sports Traumatol Arthrosc. 2013;21(07):1482–1494. doi: 10.1007/s00167-012-2331-9. [DOI] [PubMed] [Google Scholar]
- 27.van Jonbergen H P, Werkman D M, Barnaart L F, van Kampen A. Long-term outcomes of patellofemoral arthroplasty. J Arthroplasty. 2010;25(07):1066–1071. doi: 10.1016/j.arth.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 28.Leadbetter W B, Ragland P S, Mont M A. The appropriate use of patellofemoral arthroplasty: an analysis of reported indications, contraindications, and failures. Clin Orthop Relat Res. 2005;(436):91–99. [PubMed] [Google Scholar]
- 29.Delanois R E, McGrath M S, Ulrich S Det al. Results of total knee replacement for isolated patellofemoral arthritis: when not to perform a patellofemoral arthroplasty Orthop Clin North Am 20083903381–388., vii [DOI] [PubMed] [Google Scholar]
- 30.Vermeulen H, De Doncker E, Watillon M. The Mac Keever patellar prosthesis in femoro-patellar arthrosis [Article in French] Acta Orthop Belg. 1973;39(01):79–90. [PubMed] [Google Scholar]
- 31.Borus T, Brilhault J, Confalonieri N, Johnson D, Thienpont E. Patellofemoral joint replacement, an evolving concept. Knee. 2014;21 01:S47–S50. doi: 10.1016/S0968-0160(14)50010-5. [DOI] [PubMed] [Google Scholar]
- 32.Tauro B, Ackroyd C E, Newman J H, Shah N A. The Lubinus patellofemoral arthroplasty. A five- to ten-year prospective study. J Bone Joint Surg Br. 2001;83(05):696–701. doi: 10.1302/0301-620x.83b5.11577. [DOI] [PubMed] [Google Scholar]
- 33.Lustig S.Patellofemoral arthroplasty Orthop Traumatol Surg Res 2014100(1, Suppl)S35–S43. [DOI] [PubMed] [Google Scholar]
- 34.Lonner J H.Patellofemoral arthroplasty: the impact of design on outcomes Orthop Clin North Am 20083903347–354., vi [DOI] [PubMed] [Google Scholar]
- 35.Lonner J H. Patellofemoral arthroplasty: pros, cons, and design considerations. Clin Orthop Relat Res. 2004;(428):158–165. [PubMed] [Google Scholar]
- 36.Cartier P, Sanouiller J L, Khefacha A. Long-term results with the first patellofemoral prosthesis. Clin Orthop Relat Res. 2005;(436):47–54. doi: 10.1097/01.blo.0000171918.24998.d1. [DOI] [PubMed] [Google Scholar]
- 37.Odumenya M, Costa M L, Parsons N, Achten J, Dhillon M, Krikler S J. The Avon patellofemoral joint replacement: five-year results from an independent centre. J Bone Joint Surg Br. 2010;92(01):56–60. doi: 10.1302/0301-620X.92B1.23135. [DOI] [PubMed] [Google Scholar]
- 38.Shakespeare D, Dikko B. A simple precise technique for making the anterior cut in patellofemoral resurfacing. Knee. 2005;12(06):454–455. doi: 10.1016/j.knee.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Cossey A J, Spriggins A J. Computer-assisted patellofemoral arthroplasty: a mechanism for optimizing rotation. J Arthroplasty. 2006;21(03):420–427. doi: 10.1016/j.arth.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Newman J. Italia: Springer-Verlag; 2013. Isolated patellofemoral replacement. [Google Scholar]
- 41.Dahm D L, Al-Rayashi W, Dajani K, Shah J P, Levy B A, Stuart M J. Patellofemoral arthroplasty versus total knee arthroplasty in patients with isolated patellofemoral osteoarthritis. Am J Orthop. 2010;39(10):487–491. [PubMed] [Google Scholar]
- 42.van der List J P, Chawla H, Villa J C, Pearle A D. Why do patellofemoral arthroplasties fail today? A systematic review. Knee. 2017;24(01):2–8. doi: 10.1016/j.knee.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Amanatullah D F, Jamali A A. Patellar polyethylene spinout after low-contact stress, high-congruity, mobile-bearing patellofemoral arthroplasty. Orthopedics. 2012;35(02):e272–e276. doi: 10.3928/01477447-20120123-27. [DOI] [PubMed] [Google Scholar]
- 44.Dy C J, Franco N, Ma Y, Mazumdar M, McCarthy M M, Gonzalez Della Valle A. Complications after patello-femoral versus total knee replacement in the treatment of isolated patello-femoral osteoarthritis. A meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2174–2190. doi: 10.1007/s00167-011-1677-8. [DOI] [PubMed] [Google Scholar]
- 45.Mulford J S, Eldridge J D, Porteous A J, Ackroyd C E, Newman J H. Revision of isolated patellofemoral arthroplasty to total knee replacement. Curr Orthop Pract. 2009;20(04):437–441. [Google Scholar]
- 46.Krajca-Radcliffe J B, Coker T P. Patellofemoral arthroplasty. A 2- to 18-year followup study. Clin Orthop Relat Res. 1996;(330):143–151. [PubMed] [Google Scholar]
- 47.Kooijman H J, Driessen A P, van Horn J R. Long-term results of patellofemoral arthroplasty. A report of 56 arthroplasties with 17 years of follow-up. J Bone Joint Surg Br. 2003;85(06):836–840. [PubMed] [Google Scholar]
- 48.van Wagenberg J M, Speigner B, Gosens T, de Waal Malefijt J. Midterm clinical results of the Autocentric II patellofemoral prosthesis. Int Orthop. 2009;33(06):1603–1608. doi: 10.1007/s00264-009-0719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leadbetter W B, Kolisek F R, Levitt R L et al. Patellofemoral arthroplasty: a multi-centre study with minimum 2-year follow-up. Int Orthop. 2009;33(06):1597–1601. doi: 10.1007/s00264-008-0692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nicol S G, Loveridge J M, Weale A E, Ackroyd C E, Newman J H. Arthritis progression after patellofemoral joint replacement. Knee. 2006;13(04):290–295. doi: 10.1016/j.knee.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Lonner J H. Patellofemoral arthroplasty. Instr Course Lect. 2010;59:67–84. [PubMed] [Google Scholar]
- 52.de Winter W E, Feith R, van Loon C J. The Richards type II patellofemoral arthroplasty: 26 cases followed for 1–20 years. Acta Orthop Scand. 2001;72(05):487–490. doi: 10.1080/000164701753532826. [DOI] [PubMed] [Google Scholar]
- 53.Registry Australian Oarthopaedic Association National Joint Replacement. Available at:https://aoanjrr.sahmri.com. Accessed 2016
- 54.Ackroyd C E, Newman J H, Evans R, Eldridge J D, Joslin C C. The Avon patellofemoral arthroplasty: five-year survivorship and functional results. J Bone Joint Surg Br. 2007;89(03):310–315. doi: 10.1302/0301-620X.89B3.18062. [DOI] [PubMed] [Google Scholar]
- 55.Starks I, Roberts S, White S H. The Avon patellofemoral joint replacement: independent assessment of early functional outcomes. J Bone Joint Surg Br. 2009;91(12):1579–1582. doi: 10.1302/0301-620X.91B12.23018. [DOI] [PubMed] [Google Scholar]
- 56.Mont M A, Johnson A J, Naziri Q, Kolisek F R, Leadbetter W B. Patellofemoral arthroplasty: 7-year mean follow-up. J Arthroplasty. 2012;27(03):358–361. doi: 10.1016/j.arth.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 57.Sisto D J, Sarin V K. Custom patellofemoral arthroplasty of the knee. J Bone Joint Surg Am. 2006;88(07):1475–1480. doi: 10.2106/JBJS.E.00382. [DOI] [PubMed] [Google Scholar]
- 58.Butler J E, Shannon R. Patellofemoral arthroplasty with a custom-fit femoral prosthesis. Orthopedics. 2009;32(02):81. [PubMed] [Google Scholar]
- 59.Mohammed R, Jimulia T, Durve K, Bansal M, Green M, Learmonth D. Medium-term results of patellofemoral joint arthroplasty. Acta Orthop Belg. 2008;74(04):472–477. [PubMed] [Google Scholar]
- 60.Parvizi J, Stuart M J, Pagnano M W, Hanssen A D. Total knee arthroplasty in patients with isolated patellofemoral arthritis. Clin Orthop Relat Res. 2001;(392):147–152. doi: 10.1097/00003086-200111000-00018. [DOI] [PubMed] [Google Scholar]
- 61.Laskin R S, van Steijn M. Total knee replacement for patients with patellofemoral arthritis. Clin Orthop Relat Res. 1999;(367):89–95. [PubMed] [Google Scholar]
- 62.Mont M A, Haas S, Mullick T, Hungerford D S. Total knee arthroplasty for patellofemoral arthritis. J Bone Joint Surg Am. 2002;84-A(11):1977–1981. doi: 10.2106/00004623-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 63.Lonner J H, Jasko J G, Booth R E., Jr Revision of a failed patellofemoral arthroplasty to a total knee arthroplasty. J Bone Joint Surg Am. 2006;88(11):2337–2342. doi: 10.2106/JBJS.F.00282. [DOI] [PubMed] [Google Scholar]
- 64.van Jonbergen H P, Werkman D M, van Kampen A. Conversion of patellofemoral arthroplasty to total knee arthroplasty: a matched case-control study of 13 patients. Acta Orthop. 2009;80(01):62–66. doi: 10.1080/17453670902805031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leadbetter W B.Patellofemoral arthroplasty in the treatment of patellofemoral arthritis: rationale and outcomes in younger patients Orthop Clin North Am 20083903363–380., vii [DOI] [PubMed] [Google Scholar]
- 66.Lonner J H.Modular bicompartmental knee arthroplasty with robotic arm assistance Am J Orthop 200938(2, Suppl)28–31. [PubMed] [Google Scholar]
- 67.Argenson J N, Parratte S, Bertani A et al. The new arthritic patient and arthroplasty treatment options. J Bone Joint Surg Am. 2009;91 05:43–48. doi: 10.2106/JBJS.I.00406. [DOI] [PubMed] [Google Scholar]
- 68.Leffler J, Scheys L, Planté-Bordeneuve T et al. Joint kinematics following bi-compartmental knee replacement during daily life motor tasks. Gait Posture. 2012;36(03):454–460. doi: 10.1016/j.gaitpost.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 69.Heyse T J, Khefacha A, Cartier P. UKA in combination with PFR at average 12-year follow-up. Arch Orthop Trauma Surg. 2010;130(10):1227–1230. doi: 10.1007/s00402-009-0997-3. [DOI] [PubMed] [Google Scholar]
- 70.Kamath A F, Levack A, John T, Thomas B S, Lonner J H. Minimum two-year outcomes of modular bicompartmental knee arthroplasty. J Arthroplasty. 2014;29(01):75–79. doi: 10.1016/j.arth.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 71.Parratte S, Ollivier M, Lunebourg A, Abdel M P, Argenson J N.Long-term results of compartmental arthroplasties of the knee: Long term results of partial knee arthroplasty Bone Joint J 201597-B(10, Suppl A)9–15. [DOI] [PubMed] [Google Scholar]
- 72.Cartier P, Sanouiller J L, Grelsamer R. Patellofemoral arthroplasty. 2-12-year follow-up study. J Arthroplasty. 1990;5(01):49–55. doi: 10.1016/s0883-5403(06)80009-4. [DOI] [PubMed] [Google Scholar]
- 73.Mertl P, Van F T, Bonhomme P, Vives P. Femoropatellar osteoarthritis treated by prosthesis. Retrospective study of 50 implants [Article in French] Rev Chir Orthop Repar Appar Mot. 1997;83(08):712–718. [PubMed] [Google Scholar]
- 74.Arnbjörnsson A H, Ryd L. The use of isolated patellar prostheses in Sweden 1977-1986. Int Orthop. 1998;22(03):141–144. doi: 10.1007/s002640050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Cloedt P, Legaye J, Lokietek W. Femoro-patellar prosthesis. A retrospective study of 45 consecutive cases with a follow-up of 3-12 years [Article in French] Acta Orthop Belg. 1999;65(02):170–175. [PubMed] [Google Scholar]
- 76.Smith A M, Peckett W R, Butler-Manuel P A, Venu K M, d'Arcy J C. Treatment of patello-femoral arthritis using the Lubinus patello-femoral arthroplasty: a retrospective review. Knee. 2002;9(01):27–30. doi: 10.1016/s0968-0160(01)00127-2. [DOI] [PubMed] [Google Scholar]
- 77.Board T N, Mahmood A, Ryan W G, Banks A J. The Lubinus patellofemoral arthroplasty: a series of 17 cases. Arch Orthop Trauma Surg. 2004;124(05):285–287. doi: 10.1007/s00402-004-0645-x. [DOI] [PubMed] [Google Scholar]
- 78.Merchant A C. A modular prosthesis for patellofemoral arthroplasty: design and initial results. Clin Orthop Relat Res. 2005;(436):40–46. doi: 10.1097/01.blo.0000171917.47869.6c. [DOI] [PubMed] [Google Scholar]
- 79.Argenson J N, Flecher X, Parratte S, Aubaniac J M. Patellofemoral arthroplasty: an update. Clin Orthop Relat Res. 2005;440(440):50–53. doi: 10.1097/01.blo.0000187061.27573.70. [DOI] [PubMed] [Google Scholar]
- 80.Jørgensen P S, Konradsen L A, Mati W B, Tørholm C. Treatment of patellofemoral arthritis with patello-femoral arthroplasties [Article in Danish] Ugeskr Laeger. 2007;169(23):2201–2204. [PubMed] [Google Scholar]
- 81.Gadeyne S, Besse J L, Galand-Desme S, Lerat J L, Moyen B. Results of self-centering patellofemoral prosthesis: a retrospective study of 57 implants [Article in French] Rev Chir Orthop Repar Appar Mot. 2008;94(03):228–240. doi: 10.1016/j.rco.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 82.Charalambous C P, Abiddin Z, Mills S P, Rogers S, Sutton P, Parkinson R. The low contact stress patellofemoral replacement: high early failure rate. J Bone Joint Surg Br. 2011;93(04):484–489. doi: 10.1302/0301-620X.93B4.25899. [DOI] [PubMed] [Google Scholar]
- 83.Hoogervorst P, de Jong R J, Hannink G, van Kampen A. A 21% conversion rate to total knee arthroplasty of a first-generation patellofemoral prosthesis at a mean follow-up of 9.7 years. Int Orthop. 2015;39(09):1857–1864. doi: 10.1007/s00264-015-2941-1. [DOI] [PubMed] [Google Scholar]
- 84.Gao X, Xu Z J, He R X, Yan S G, Wu L D. A preliminary report of patellofemoral arthroplasty in isolated patellofemoral arthritis. Chin Med J (Engl) 2010;123(21):3020–3023. [PubMed] [Google Scholar]
- 85.Sarda P K, Shetty A, Maheswaran S S. Medium term results of Avon patellofemoral joint replacement. Indian J Orthop. 2011;45(05):439–444. doi: 10.4103/0019-5413.83761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beitzel K, Schöttle P B, Cotic M, Dharmesh V, Imhoff A B. Prospective clinical and radiological two-year results after patellofemoral arthroplasty using an implant with an asymmetric trochlea design. Knee Surg Sports Traumatol Arthrosc. 2013;21(02):332–339. doi: 10.1007/s00167-012-2022-6. [DOI] [PubMed] [Google Scholar]
- 87.Yadav B, Shaw D, Radcliffe G, Dachepalli S, Kluge W. Mobile-bearing, congruent patellofemoral prosthesis: short-term results. J Orthop Surg (Hong Kong) 2012;20(03):348–352. doi: 10.1177/230949901202000317. [DOI] [PubMed] [Google Scholar]
- 88.Davies A P. High early revision rate with the FPV patello-femoral unicompartmental arthroplasty. Knee. 2013;20(06):482–484. doi: 10.1016/j.knee.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 89.Hofmann A A, McCandless J B, Shaeffer J F, Magee T H.Patellofemoral replacement: the third compartment Bone Joint J 201395-B(11, Suppl A)124–128. [DOI] [PubMed] [Google Scholar]
- 90.Morris M J, Lombardi A V, Jr, Berend K R, Hurst J M, Adams J B.Clinical results of patellofemoral arthroplasty J Arthroplasty 201328(9, Suppl)199–201. [DOI] [PubMed] [Google Scholar]
- 91.Williams D P, Pandit H G, Athanasou N A, Murray D W, Gibbons C L. Early revisions of the Femoro-Patella Vialla joint replacement. Bone Joint J. 2013;95-B(06):793–797. doi: 10.1302/0301-620X.95B6.31355. [DOI] [PubMed] [Google Scholar]
- 92.Al-Hadithy N, Patel R, Navadgi B, Deo S, Hollinghurst D, Satish V. Mid-term results of the FPV patellofemoral joint replacement. Knee. 2014;21(01):138–141. doi: 10.1016/j.knee.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 93.Benazzo F, Rossi S M, Ghiara M. Partial knee arthroplasty: patellofemoral arthroplasty and combined unicompartmental and patellofemoral arthroplasty implants--general considerations and indications, technique and clinical experience. Knee. 2014;21 01:S43–S46. doi: 10.1016/S0968-0160(14)50009-9. [DOI] [PubMed] [Google Scholar]
- 94.Dahm D L, Kalisvaart M M, Stuart M J, Slettedahl S W. Patellofemoral arthroplasty: outcomes and factors associated with early progression of tibiofemoral arthritis. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2554–2559. doi: 10.1007/s00167-014-3202-3. [DOI] [PubMed] [Google Scholar]
- 95.Fink B, Schwenninger C. Arthroplasty of the Femoropatellar Joint-What Data are Available? [Article in German] Z Orthop Unfall. 2014;152(02):182–187. doi: 10.1055/s-0033-1360353. [DOI] [PubMed] [Google Scholar]
- 96.Hernigou P, Caton J. Design, operative technique and ten-year results of the Hermes™ patellofemoral arthroplasty. Int Orthop. 2014;38(02):437–442. doi: 10.1007/s00264-013-2158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Akhbari P, Malak T, Dawson-Bowling S, East D, Miles K, Butler-Manuel P A. The avon patellofemoral joint replacement: mid-term prospective results from an independent centre. Clin Orthop Surg. 2015;7(02):171–176. doi: 10.4055/cios.2015.7.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liow M H, Goh G S, Tay D K, Chia S L, Lo N N, Yeo S J. Obesity and the absence of trochlear dysplasia increase the risk of revision in patellofemoral arthroplasty. Knee. 2016;23(02):331–337. doi: 10.1016/j.knee.2015.05.009. [DOI] [PubMed] [Google Scholar]


