Abstract
Background
Tardive dyskinesia (TD) is an abnormal involuntary movement disorder caused by patients’ long-term use of antipsychotic medication. It diminishes the social functioning of patients with mental disorders, thereby affecting their compliance with antipsychotic medication. The cause and nosogenesis of TD remains unclear; furthermore, because the presentation differs greatly among individuals it often goes undiagnosed or can be easily misdiagnosed. The present study aims to evaluate the abnormal movement patterns in patients with TD, and analyze the differences among different TD patterns, in order to seek effective methods of preventing, diagnosing and treating TD.
Aims
To describe the movement patterns of patients with chronic schizophrenia with TD, and analyze their clinical characteristics and risk factors.
Methods
A cross-sectional study was carried out on a psychiatric unit of the Xuhui Mental Health Center with inpatients who had chronic schizophrenia as participants. Abnormal Involuntary Movement Scale (AIMS) was employed to screen for patients with schizophrenia who also had TD. These patients’ movement disorders were evaluated, and they were divided into groups based on their movement patterns. Positive and Negative Syndrome Scale (PANSS) was used to assess the psychotic symptoms of patients, collect clinical information, compare the differences between the two groups and analyze the clinical characteristics and risk factors of TD.
Results
(1) A total of 448 patients met the inclusion criteria for chronic schizophrenia with 46 in the TD group and 402 in the control group. After the TD group and the control group was compared, significant differences were seen between the two groups in gender, age, total duration of illness, age of onset, dosage of antipsychotic medication (daily chlorpromazine equivalent), factor scores of negative symptoms on PANSS and PANSS total scores. (2) It was possible that age, factor scores of negative symptoms in PANSS, the amount of antipsychotic medication used (daily chlorpromazine equivalent) and gender are correlated with the occurrence of TD. (3) There were significant differences among the number of TD patients with movement disorders in facial and oral areas (67.4%), limbs (58.7%) and torso (37%). The AIMS scores corresponding with movement disorders in different parts of the body were also significantly different. (4) Comparing TD patients with single affected area and those with multiple affected areas, we found that they had significant differences in gender, age of onset, AIMS total scores, severity scores of abnormal movements and loss of range due to abnormal movements.
Conclusion
(1) Compared to the control group, the TD group had more men, was older, had a longer duration of illness, later age of onset, generally took a higher dosage of antipsychotic medication and presented with more severe negative symptoms. It is possible that age, factor scores of negative symptoms on PANSS, dosage of antipsychotic medication (daily chlorpromazine equivalent) and gender are correlated with the occurrence of TD. (2) The occurrence of movement disorders in facial and oral areas for patients with chronic schizophrenia with TD was the most frequent, and the symptoms were the most severe. (3) Compared to TD patients with a single affected area, TD patients with multiple affected areas may have an earlier age of onset, more severe movement disorders, and more setbacks in their movement and functioning.
Key words: tardive dyskinesia, schizophrenia, clinical characteristics
Abstract
背景
迟发性运动障碍(Tardive Dyskinesia, TD)是由 于患者长期服用抗精神病药物后产生的一种异常不自 主运动障碍,它会加剧精神疾病患者的社会功能障碍, 并进一步影响患者对抗精神病药物的服药依从性。目 前对于TD 的病因及其发病机制尚不清楚,且TD 累及 部位多、个体差异性大,导致其极易被漏诊或误诊。 本研究旨在评估TD 患者的异常运动模式,进一步分析 TD 不同模式是否存在差异,为进一步寻找TD 预防和 早期诊断的方法,发现有效的治疗手段提供帮助。
目的
描述伴发TD 的慢性精神分裂症患者的运动模式, 分析其临床特征及危险因素。
方法
对徐汇区精神卫生中心精神科住院的慢性精神 分裂症患者进行横断面调查,运用异常不自主运动量 表(Abnormal Involuntary Movement Scale,AIMS) 筛 查出伴有TD 的慢性精神分裂症患者,对患者的运动 障碍进行评估,并按照疾病受累方式进行分组。采用 阳性和阴性症状量表(Positive and Negative Syndrome Scale,PANSS)对患者的精神症状进行评估,收集临床 资料,比较两组差异,分析TD 的临床特征和危险因素。
结果
(1) 共448 例患者符合慢性精神分裂症入组标准, TD 组46 例,对照组402 例。TD 组与对照组比较,性 别、年龄、精神分裂症总病程、精神分裂症起病年龄、 抗精神病药物剂量(每日氯丙嗪当量)、PANSS 量表 阴性症状因子分、PANSS 量表总分的差异均有统计学 意义。(2) 年龄、PANSS 量表阴性症状因子分、抗精神 病药物剂量(每日氯丙嗪当量)、性别等四个因素可 能与TD 的发生有关。(3)TD 患者中存在面部及口腔部 位运动障碍的有67.4%,肢体运动障碍的有58.7%,躯 干运动障碍的有37%,差异有统计学意义,不同部位 运动障碍相应的AIMS 分量表评分结果差异有统计学意 义。(4) 单一部位受累的TD 患者与多部位受累的TD 患者比较,性别、精神分裂症起病年龄、AIMS 量表总分、 异常运动的严重程度评分及由于异常运动导致的运动 能力丧失程度的差异有统计学意义。
结论
(1) 与对照组相比,TD 组中可能男性患者的比例 更高、年龄更大、总病程更长、起病年龄更晚、抗精 神病药物剂量更高、阴性症状更为突出。年龄、PANSS 量表阴性症状因子分、抗精神病药物剂量(每日氯丙 嗪当量)、性别等4 个因素可能是发生TD 的危险因素。 (2) 伴有TD 的慢性精神分裂症患者运动障碍以面部及 口腔部位障碍最为频发,异常运动的症状更严重。(3) 与单部位受累的TD 患者相比,多部位受累的TD 患者 可能起病年龄更早、运动障碍更加严重、对患者运动 功能的影响更大。
关键词: 迟发性运动障碍, 精神分裂症, 临床特征
1. Introduction
Tardive dyskinesia (TD) is a delayed and persistent abnormal involuntary movement disorder which occurs after patients’ long-term use of antipsychotic medication. Often TD begins to appear 1 to 2 years after patients begin antipsychotic medication. Among patients with schizophrenia, the life quality of patients with TD drops to 12.3%, compared to patients without TD.[1] This leads to a decrease in patients’ social functioning, and has been shown to affect quality of life and medication compliance significantly.[2] In addition secondary conditions such as TD can increase the difficulty in treating the primary illness, thereby increasing the financial burden on the patient’s family.
TD can present in a variety of ways. Early clinical symptoms are mainly disordered movements in the mouth, tongue and facial areas, which appear as repetitive and uncontrollable. TD symptoms can also affect movement of limbs, head, neck and torso, and patients with severe conditions may also suffer from unclear articulation, difficulty swallowing, and abnormal postures.[3, 4] In addition, some patients may have gastrointestinal tardive dyskinesia which causes symptoms, such as discomfort in the stomach, nausea and vomiting. TD can present with numerous different disordered movements involving many areas of the body and there can also be great variation in presentation among different individuals. It is not uncommon to see TD during the course of clinical work, nevertheless we do not have a deep understanding of this disorder due to its unclear etiology, numerous clinical symptoms, multiple affected areas and wide variation in presentation across individuals. In China and other low and middle income countries there is also a lack of mental health resources resulting in a higher medical staff to patient ratio. In clinical practice this can result in less time available for each individual patient and therefore a later recognition and diagnosis of TD. In the meantime, there has until now not been any effective treatment for TD found; so it affects patients’ life quality significantly and aggravates their economic and social burden. Hence, understanding the relationship between movement patterns, clinical characteristics, use of antipsychotic medications, psychotic symptoms and movement disorders is vital to recognizing TD in its early stages and providing timely interventions. The present study aims to explore the questions listed above and provide clinical data useful for the diagnosis and treatment of TD.
2. Methods
2.1 Participants
Participants were recruited from among psychiatric inpatients at the Xuhui Mental Health Center in Shanghai, China. Inclusion criteria were the following: (1) participants aged between 20 and 70; (2) diagnosed by two experienced psychiatrists as having schizophrenia according to the criteria of the International Classification of Diseases and Related Health Problems, 10th edition (ICD-10) [5]; (3) total duration of illness of schizophrenia being equal to or over one year; (4) duration of taking antipsychotic medication being equal to or over one year; (5) patients who were able to complete all the scales and sign written consent forms, or whose guardians could provide signed consent forms. Exclusion criteria: (1) patients with other comorbid severe medical conditions; (2) patients who had comorbid neurological diseases; (3) patients with incomplete medical records or scales; (4) patients who were not able to cooperate with the protocol of the study. We classified patients as having TD according to the the diagnostic criteria of TD developed by Schooler and Kane.[7] Those patients who did not have TD were classified as the non-TD group. Within the TD group, participants were divided into two groups based on their affected movement patterns (i.e. those with a single area movement disorder and those with movement disorder in multiple areas). All participants provided consent to participate in this study. This study was approved by the ethics committee of the Shanghai Xuhui Mental Health Center.
2.2 Research methods
2.2.1 Basic clinical information
Clinical information collected was the following: gender, age, date of the present hospitalization, total duration of illness, and antipsychotic medication use (i.e., types of medication used, treatment type such as single-drug or multiple-drug use, etc), and antipsychotic medication dosage (unified conversion to chlorpromazine equivalent). [6]
2.2.2 Evaluation of psychotic symptoms
Positive and Negative Syndrome Scale (PANSS) was used to carry out evaluation of the participants. The severity of patients’ psychotic symptoms were reflected by positive symptom factor scores, negative symptom factor scores, general psychopathological scale factor scores and the total scores of PANSS.
2.2.3 Diagnostic tools and evaluation of TD
According to the research diagnostic criteria of TD developed by Schooler and Kane,[7] patients whose symptoms had been present for over 3 months and had one sub-score of 3 or higher in the Abnormal Involuntary Movement Scale (AIMS), or two sub-scores of 2 or higher in AIMS met criteria for TD. In addition, patients whose abnormal involuntary movements were induced by infections, seizure, Parkinson’s disease and other diseases were excluded; and patients with brain diseases were also excluded. This scale has five rankings for the severity of abnormal movements and the loss of movement ability caused by abnormal movements: 0 = none; 1 = minimal; 2 = mild; 3 = moderate; 4 = severe. Patients’ self-awareness towards abnormal movement were also rated on a five point scale: 0 = not aware; 1 = aware, no distress; 2 = aware, mild distress; 3 = aware, moderate distress; 4 = aware, severe distress.
2.2.4 TD abnormal movement pattern analysis
For participants in this study, the areas affected and the characteristics of symptoms were recorded, and the severity of TD symptoms, loss of movement ability and self-awareness surrounding the disease were evaluated.
2.3 Statistics
SPSS 19.0 was used to analyze data. Count data were represented with percentage (%), and were analyzed using χ2 tests. Measurement data were represented with mean (standard deviation); data which met normal distribution were analyzed with ANOVA, while data which met non-normal distribution were analyzed with nonparametric tests. Ranked data were also analyzed with nonparametric tests. If the results of ANOVA were significant (p<0.05), the OR values of the factors were calculated with Logistic regression analyses to estimate the influences these factors had on TD. Two-sided tests were employed in all statistical tests, and the value of p being less than 0.05 indicated statistically significant differences.
3. Results
3.1 Comparisons between TD group and non-TD group
Six hundred and eighty-two inpatients were screened at the Xuhui Mental Health Center (Shanghai, China), and four hundred and forty-eight patients met the inclusion criteria for chronic schizophrenia (300 males and 148 females). Forty-six of the participants were classified as having TD (36 males and 10 females). There were 402 patients in the non-TD group (264 males and 138 females). There was a significant difference in gender between the TD group and the non-TD group (p=0.028, χ2=4.81). The mean (sd) age of the TD group was 57.8 (8.5) years old, while that of the non-TD group was 44.8 (13.0) years old; there was also a significant difference in age between the two groups (p<0.001, f=36.42). The mean total duration of illness of schizophrenia in the TD group was 32.8 (10.2) years, while that in the control group was 26.5 (6.1) years; the difference between two groups was significant (p<0.001, f=17.59). As for the age of onset, the mean (sd) age of the TD group was 18.0 (12.5) years old, while that of the non-TD group was 24.8 (6.0) years old; the two groups’ mean age of onset were significantly different from each other (p=0.001, f=11.99). In terms of the dose of antipsychotic medication (daily chlorpromazine equivalent), the mean (sd) dose for the TD group was 573.1 (228.6) mg/d, whereas that of the non-TD group was 447.4 (188.9) mg/d; the difference between two groups was significantly different (p=0.001, f=10.60). There were no significant differences between the two groups in the medication-taking method (i.e., single-drug or multiple-drug). As for the scores on the PANSS, the total scores and the negative symptom factor scores of TD group were significantly greater than those of the control group (the total score: p=0.009, f=6.98; the negative factor score: f=13.91). There were no significant differences in the positive symptom factor scores and the general psychopathological scale factor scores between two groups. See Table 1.
Table 1.
Comparisons between the TD group and the control group
| Variables | TD group (n=46) |
Control group (n=402) |
Statistics | p value | |
|---|---|---|---|---|---|
| Males (%) | 36(78.26°%) | 264(65.67%) | X2=4.811 | 0.028a | |
| Mean (sd) age | 57.83(8.538) | 44.78(12.989) | F=36.420 | <0.001c | |
| Mean (sd) duration of illness | 32.83(10.240) | 26.53(6.135) | F=17.594 | <0.001c | |
| Mean (sd) age of onset | 17.97(12.541) | 24.830(5.983) | F=11.989 | 0.001b | |
| Mean (sd) mg/d Chlorpromazine equivalent | 573.07(228.554) | 447.4(188.912) | F=10.597 | 0.001b | |
| Patient medication use (%) | Single-drug | 15(32.60%) | 166(41.30%) | X2=0.746 | 0.388 |
| Multiple-drug | 31(67.39%) | 236(58.70%) | |||
| Mean (sd) PANSS score | Total score | 94.93(16.432) | 85.36(20.834) | F=6.979 | 0.009b |
| Positive symptom factor score | 21.24(6.674) | 20.67(7.244) | F=0.184 | 0.669 | |
| Negative symptom factor score | 32.54(6.982) | 26.26(9.986) | F=13.905 | <0.001c | |
| General psychopathological scale factor score | 41.15(11.753) | 38.42(12.224) | F=1.447 | 0.231 | |
Notes: ap<0.05, bp<0.01, cp<0.001.
3.2 Comparisons of the affected areas in patients with TD
The movement disorder caused by TD in patients with chronic schizophrenia affected the following areas: facial and oral areas, limbs and torsos. There were 31 participants who were affected in the facial and oral areas (12 of them were affected in a single area, and 19 of them were affected in multiple areas). Twenty-seven participants were affected in their limbs (7 of them were affected in a single area, and 20 of them were affected in multiple areas). There were 17 participants who were affected in their torsos (2 of them were affected in a single area, and 15 of them were affected in multiple areas). There were significant differences among the movement disorder’s rates of occurrence in different body areas (p=0.011, χ2=9.11), and their corresponding AIMS sub-scale scores were also significantly different (p<0.001, χ2=9.98). See Table 3.
Table 3.
Comparisons of areas affected by movement disorders
| Variables | Face and mouth | Limbs | Torso | Statistics | p value |
|---|---|---|---|---|---|
| Occurrence of symptoms (n, %) | 31(67.4%) | 27(58.7%) | 17(37%) | X2=9.11 | 0.011a |
| AIMS sub-scale (points) | 4.88(2.579) | 3.10(1.599) | 2.56(0.964) | F=9.981 | <0.001c |
Notes: ap<0.05, cp<0.001.
3.3 Comparisons between TD patients with a single affected area and those with multiple affected areas
Within the TD group, there were 21 participants who had a single affected area, while 25 participants had multiple affected areas. There were 16 males and 5 females in the group of TD patients with a single affected area, and there were 20 males and 5 females in the group of TD patients with multiple affected areas. There was a significant difference in gender between these two TD groups (p=0.004, χ2=8.37). The mean (sd) age of onset of schizophrenia in TD patients with multiple affected areas (22.38 (4.72)) was significantly smaller than that in TD patients with a single affected area (26.88 (6.24)) (p=0.009, χ2=7.37). These two TD groups had no significant differences in age, total duration of illness, the medication-taking methods (single-drug or multiple-drug), and dose of antipsychotic medication (daily chlorpromazine equivalent).
According to the results of AIMS, the total scores of participants with a single affected area and multiple affected areas were significantly different from each other (p<0.001, χ2=19.62). Specifically, the severity of abnormal movement scores in TD patients with multiple affected areas were significantly greater than those in the other TD group (p<0.001, Z=-3.80). The loss of movement in these two groups was also significantly different (p<0.001, Z=-2.75). There were no significant differences in their self-awareness of abnormal movements.
According to the results of the PANSS, there were no significant differences in the positive symptom factor scores, negative symptom factor scores, general psychopathological scale factor scores and total scores between TD patients with a single affected area and those with multiple affected areas. See Table 4.
Table 4.
Comparisons of TD patients with a single affected area and those with multiple affected areas
| Variables | Single affected area group (n=21) | Multiple affected areas group (n=25) | Statistics | p value | |
|---|---|---|---|---|---|
| Males (%) | 16(76.2%) | 20(80%) | X2=8.37 | 0.004b | |
| Mean (sd) age | 57.10(8.596) | 58.44(8.617) | F=0.27 | 0.600 | |
| Mean (sd) duration of illness | 24.33(11.451) | 31.56(9.147) | F=0.834 | 0.366 | |
| Mean (sd) age of onset | 26.88(6.240) | 22.38(4.717) | F=7.368 | 0.009b | |
| Patient medication use (%) | Single-drug | 7(33.3%) | 8(32%) | X2=0.01 | 0.916 |
| Multiple-drug | 14(66.7) | 17(68%) | |||
| Mean (sd) mg/d Chlorpromazine equivalent | 555.14(256.732) | 588.12(206.158) | F=0.23 | 0.631 | |
| AIMS | Total score (points) | 4.33(1.197) | 8.20(3.841) | F=19.62 | <0.001 c |
| Severity | 15.82 | 29.96 | Z=-3.80 | <0.001 c | |
| Loss of movement ability | 17.83 | 28.26 | Z=-2.75 | 0.005 b | |
| Self-awareness | 23.21 | 23.74 | Z=-0.15 | 0.897 | |
| Mean (sd) PANSS score | Total score | 94.90(16.950) | 94.96(16.336) | F=0.000 | 0.991 |
| Positive symptom factor score | 20.80(5.728) | 21.50(6.865) | F=0.002 | 0.966 | |
| Negative symptom factor score | 37.94(11.956) | 38.52(13.493) | F=0.55 | 0.462 | |
| General psychopathological scale factor score | 40.24(11.912) | 41.92(11.807) | F=0.23 | 0.634 | |
Notes: The ranked data were recorded with the average rank sum; bp<0.01, cp<0.001.
4. Discussion
4.1 Main findings
Four hundred and forty-eight patients with chronic schizophrenia were investigated in the present study, and forty-six of them suffered from TD (comorbidity 10.3%). There were significant differences in gender, age, total duration of illness, age of onset, the dose of antipsychotic medication (daily chlorpromazine equivalent), negative symptom factor scores and the total PANSS scores between the TD group and non-TD group. It is possible that age, gender, negative symptom factor scores and the dose of antipsychotic medication (daily chlorpromazine equivalent) are correlated with the occurrence of TD. In terms of gender, the present study showed that the occurrence of disease in males was greater than that in females, which contradicted the results of previous studies, but some scholars disagreed with the previous results as well.[8,9] Due to the relatively small number of women in the present study, the sample size of future studies needs to be expanded to validate the results of the present study. As for age, the mean age of patients with TD was 57.8 years old (range: 31-69) with only 3 of them being below 40 years old. This is in accordance with the results of most previous studies which indicate that being older than 40 years old could be a risk factor for TD.[10,11] The reason for this phenomenon is probably correlated with neurological changes in the elderly and an increase of drug concentration in the blood due to slow metabolism.[9]
According to the results of the correlation analysis on risk factors, the duration of illness and the age of onset were not risk factors for TD. To our knowledge there have not been articles reporting on this finding. After comparing the TD group and the non-TD group, we found that patients in the TD group had significantly longer durations of illness and younger ages of onset. Since schizophrenia is a chronic illness, [12] complete remission of symptoms is not often seen and in most cases patients require some form of life-long treatment or follow-up. Longer durations of illness and younger ages of onset could mean that patients also have a longer amount of time taking antipsychotics, which in turn leads to an increased risk for TD. It is therefore important for clinicians to pay special attention to those elderly patients with a long history of taking antipsychotic medication as the risk for TD may be higher in this group. Also it is recommended that patients receive regular checkups for TD, especially after an extended period of time on antipsychotic medications or for older patients as it is possible that age is a risk factor for TD.
In terms of medication, significant differences were not found between participants with single-drug use and multiple-drug use in the TD and non-TD groups. In order to standardize different medications’ doses for the comparison, they were converted to chlorpromazine equivalent for all participants in the present study. After comparing both groups, we found that patients in the TD group took significantly more antipsychotic medications daily than those in the control group did. In previous studies, most scholars have written that in order to use medication safely and avoid TD, large doses of antipsychotic medication are not recommended;[13,14,15] however, there has been no conclusions drawn on the correlations between medication dosage and the occurrence of TD. The results of the present study show that high dosages require close attention as they may lead to TD in patients. However further study is need to look at which medications specifically increase the risk of TD, what the threshold of medication dosage is and how different medications are correlated with TD.
What is worth mentioning is that the results indicate that the negative symptom factor score in PANSS is a risk factor for TD, which means that chronic schizophrenia patients with negative symptoms as the main clinical symptoms are more likely to suffer from TD. According to the results of PANSS, the negative symptoms factor scores and the total scores of patients in the TD group are significantly greater than those in the non-TD group, which suggests that their psychotic symptoms are more severe. This result is in accordance with the studies of Miller, Gebhardt and colleagues.[16,17] Patients with negative symptoms as the main clinical symptoms show emotional indifference, lack of intimacy, attention deficit, lack of inner experience, lack of speech and social withdrawal. These negative symptoms in patients can cause avolition, decrease motivation to seek help and make them indifferent to reality. [18] In these cases, patients may ignore changes in their health, have delusions or hallucinations, have reduced medication compliance, and even refuse treatment. All of these can present an enormous challenge to treating TD or other illnesses. Our results also showed for those TD patients with abnormal movements only 1 of the 46 patients had awareness of their abnormal movements with moderate distress. 56% of those TD patients had no self-awareness surrounding their symptoms whereas 37% had awareness but no discomfort. This phenomenon is clearly not consistent with the actual severity of patients’ illness, and there is also strong evidence that chronic schizophrenia patients who suffer from TD have severe negative symptoms. Additionally, patients’ lack of self-awareness, low motivation to seek treatment and not receiving timely treatment could be some of the key reasons why TD is difficult to diagnose in its early stages.
Patients with TD have various clinical symptoms affecting many different areas. After comparing the areas affected by movement disorders, we found that symptoms were mostly seen in the facial and oral areas. This finding is consistent with previous reports that the abnormal involuntary movements in head and facial areas, whose classic symptom is the mouth-tongue-cheek triple sign, are seen the most in patients with TD.[3,4,19] Moreover, the AIMS sub-scale scores of patients with affected facial and oral movement disorders were greater than those of patients with other affected areas, and their symptoms were more severe as well. In comparison, patients with movement disorder in their limbs were the second most severe, and patients with movement disorders in torsos were the least severe. Further explorations are needed to investigate whether facial and oral movement disorders are the first symptoms to appear in TD.
The movement disorders of patients with TD can affect a single area, but mainly we found that they affected multiple areas. The present study showed that 54.3% of patients were affected in multiple areas. For patients who had TD that affected multiple areas the mean age of onset of illness was 22.4 years old. After evaluating TD patients’ abnormal movements with AIMS, we found that compared to TD patients with a single affected area, TD patients with multiple affected areas had more severe abnormal movements, which often led to a loss of mobility. Based on this, we speculate that TD patients with a young age of onset for schizophrenia are more likely to be affected in multiple areas with more severe symptoms. However, whether age of onset of schizophrenia is a risk factor for TD requires further exploration. Many scholars suggest that the age of onset is correlated with the prognosis for schizophrenia; specifically that the younger the age of onset is, the worse the prognosis is.[20] Hence, the correlation between the severity of TD in patients with schizophrenia and the prognosis of schizophrenia remains unclear for now.
After grouping TD patients according to their movement patterns we found that despite vastly different clinical symptoms, there were no significant differences in age, medication-taking methods, doses of antipsychotic medication, or the severity of psychotic symptoms between patients with a single affected area and patients with multiple affected areas. Additionally, even though patients with different affected patterns were significantly different in gender and the age of onset, their correlations were not statistically significant.
4.2 Limitations
The present paper tried to analyze the characteristics of movement disorders by analyzing and summarizing the clinical characteristics of patients with TD. There are several limitations to this study. (1) The sample size of the present study is relatively small, with an especially small number of women in the study. This could be expanded in the future. (2) Even though the present study discussed the two affected patterns of movement disorders in patients with TD, we did not find any relevant factors which could lead to different affected patterns. Even when patients were affected in the same area, they showed different symptoms. Therefore, in future studies with larger sample sizes we could further divide patients into groups according not only to the area affected by TD but also according to their clinical symptoms. (3) This study is cross sectional in nature and therefore no conclusions about cause and effect can be drawn. (4) In the present study, we did not find any significant differences in the methods of taking medication and the dose of antipsychotic medication between TD patients with a single affected area and those with multiple affected areas. Considering we compared different classifications of antipsychotic drugs by converting them to chlorpromazine equivalent, we were unable to discuss the correlations between the classifications of antipsychotic drugs and the different affected patterns of TD. Hence, future research could focus on the correlations between various classifications of antipsychotic drugs and the abnormal movement patterns of TD to explore the relationship between antipsychotic drug classifications and the clinical characteristics of TD.
4.3 Implications
In conclusion, the abnormal movements of patients with schizophrenia who have TD mainly occur in multiple affected areas; the severity of their abnormal movements may be greater, and their age of onset for schizophrenia may be younger. Age, the negative symptom factor score, the dose of antipsychotic medication (daily chlorpromazine equivalent) and gender are likely to be correlated with the occurrence of TD. This indicates that more caution is needed for patients with movement disorders in multiple body parts during the clinical evaluation of TD, especially for those with a young age of onset and severe negative symptoms.
Figure 1.
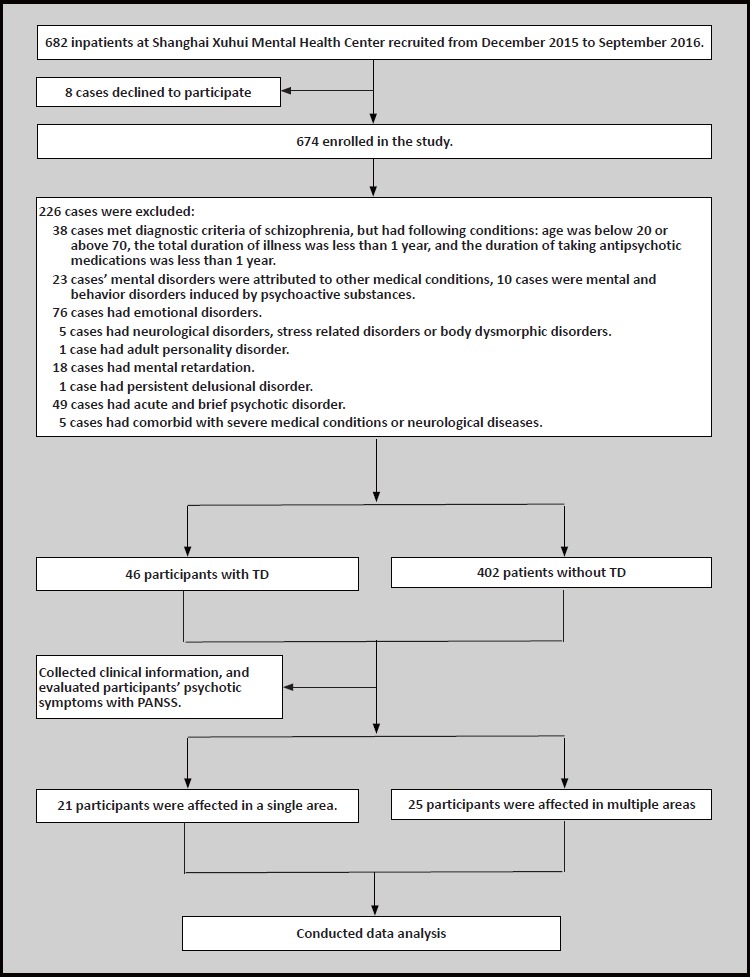
Flowchart of the study
Table 2.
Analysis of risk factors for Tardive Dyskinesia
| Variable | Partial regression coefficient | Standard error | Wald | p value | OR value | 95% CI |
|---|---|---|---|---|---|---|
| Age | -0.106 | 0.023 | 21.784 | <0.001c | 0.900 | 0.860-0.940 |
| Total duration of illness | -0.025 | 0.150 | 0.028 | 0.867 | 0.975 | 0.726-1.309 |
| Positive and negative symptom scores | -0.125 | 0.033 | 14.541 | <0.001c | 0.882 | 0.828-0.941 |
| Age of onset | -0.029 | 0.158 | 0.033 | 0.885 | 0.972 | 0.713-1.324 |
| Chlorpromazine equivalent | -0.004 | 0.001 | 8.294 | 0.004b | 0.996 | 0.993-0.999 |
| Total PANSS score | 0.014 | 0.021 | 0.468 | 0.494 | 1.014 | 0.974-1.057 |
| Gender | 1.787 | 0.443 | 16.258 | <0.001c | 5.970 | 2.505-14.228 |
Notes: bp<0.01, cp<0.001.
Biography

Yanan Huang graduated from Wannan Medical School in 2007. In September 2011, she began work on her master’s degree at Tongji Medical School (Shanghai, China) and in 2013 began working at the Xuhui Mental Health Center, where she is currently a resident physician. Her research interests include movement disorders and the psychological health of persons with schizophrenia.
Footnotes
Funding statement
None.
Conflicts of interest statement
The authors report no conflict of interest related to this manuscript
Ethical approval
The present study was approved by Shanghai Xuhui Mental Health Center ethical committee.
Informed consent
Informed consent was provided by all participants.
Authors’ contributions
Yanan Huang was responsible for writing up the paper, collecting, organizing and analyzing data.
Lizhen Pan, Fei Teng and Chenhu Li were responsible for providing guidance on study design, reviewing and editing the paper.
Geying Wang was responsible for designing the statistics plan and data analysis.
Lingjing Jin was responsible for reviewing and finalizing the paper.
References
- 1.Rana AQ, Chaudry ZM, Blanchet PJ. New and emerging treatments for symptomatic tardive dyskinesia. Drug Design Development & Therapy. 2013; 7(7): 1329-1340. doi: https://doi.org/10.2147/DDDT.S32328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Browne S, Roe M, Lane A, Gervin M, Morris M, Kinsella A, et al. Quality of life in schizophrenia: relationship to sociodemographic factors, symptomatology and tardive dyskinesia. Acta Psychiatr Scand. 1996; 94(2): 118-124. doi: https://doi.org/10.1111/j.1600-0447.1996.tb09835.x [DOI] [PubMed] [Google Scholar]
- 3.Stacy M, Jankovic J. Tardive tremor. Mov Disord. 1992; 7(1): 53-57. doi: https://doi.org/10.1002/mds.870070110 [DOI] [PubMed] [Google Scholar]
- 4.Tarsy D, Indorf G. Tardive tremor due to metoclopramide. Mov Disord. 2002; 17(3): 620-621. doi: https://doi.org/10.1002/mds.10227 [DOI] [PubMed] [Google Scholar]
- 5.Shekhar S, Benedetto S. The ICD-10 Classification of Mental and Behavioural Disorders. WHO; 1993 [Google Scholar]
- 6.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003; 64(6): 663667. doi: http://dx.doi.org/10.4088/JCP.v64n0607 [DOI] [PubMed] [Google Scholar]
- 7.Schooler NR, Kane JM. Research diagnoses for tardive dyskinesia (RD-TD). Arch Gen Psychiatry. 1982; 39(4): 486-487. doi: http://dx.doi.org/10.1001/archpsyc.1982.04290040080012 [DOI] [PubMed] [Google Scholar]
- 8.van Os J, Walsh E, van Horn E, Tattan T, Bale R, Thompson SG. Tardive dyskinesia in psychosis: are women really more at risk? Acta Psychiatr Scand. 1999; 99(4): 288-293. doi: http://dx.doi.org/10.1111/j.1600-0447.1999.tb07227.x [DOI] [PubMed] [Google Scholar]
- 9.Go CL, Rosales RL, Caraos RJ, Fernandez HH. The current prevalence and factors associated with tardive dyskinesia among Filipino schizophrenic patients. Parkinsonism Relat Disord. 2009; 15(9): 655-659. doi: http://dx.doi.org/10.1016/j.parkreldis.2009.02.015 [DOI] [PubMed] [Google Scholar]
- 10.Tenback D E, van Harten P N, Van O J. Non-therapeutic risk factors for onset of tardive dyskinesia in schizophrenia: a meta-analysis. Mov Disord. 2009; 24(16): 2309-2315. doi: http://dx.doi.org/10.1002/mds.22707 [DOI] [PubMed] [Google Scholar]
- 11.Johnson GFS, Hunt GE, Rey JM. Incidence and severity of tardive dyskinesia increase with age. Arch Gen Psychiatry. 1982; 39(4): 486-486. doi: http://dx.doi.org/10.1001/archpsyc.1982.04290040080012 [DOI] [PubMed] [Google Scholar]
- 12.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000 [Google Scholar]
- 13.Tarsy D. Tardive dyskinesia. Curr Treat Options Neurol. 2000; 2(3): 205-214. doi: http://dx.doi.org/10.1146/annurev.me.35.020184.003133 [DOI] [PubMed] [Google Scholar]
- 14.Morgenstern H, Glazer WM. Identifying risk factors for tardive dyskinesia among long-term outpatients maintained with neuroleptic medications. Arch Gen Psychiatry. 1993; 50(9):723-733. doi: http://dx.doi.org/10.1001/archpsyc.1993.01820210057007 [DOI] [PubMed] [Google Scholar]
- 15.Khouzam HR. Identification and management of tardive dyskinesia: A case series and literature review. Postgraduate Medicine. 2015; 127(7): 726-737. doi: http://dx.doi.org/10.1080/00325481.2015.1074031 [DOI] [PubMed] [Google Scholar]
- 16.Miller DD, McEvoy JP, Davis SM, Caroff SN, Saltz BL, Chakos MH, et al. Clinical correlates of tardive dyskinesia in schizophrenia: Baseline data from the CATIE schizophrenia trial. Schizophr Res. 2006; 80(1): 33-43. doi: http://dx.doi.org/10.1016/j.schres.2005.07.034 [DOI] [PubMed] [Google Scholar]
- 17.Gebhardt S, Härtling F, Hanke M, Theisen FM, von Georgi R, Grant P, et al. Relations between movement disorders and psychopathology under predominantly atypical antipsychotic treatment in adolescent patients with schizophrenia. Eur Child Adolesc Psychiatry. 2008; 17(1): 44-53. doi: http://dx.doi.org/10.1007/s00787-007-0633-0 [DOI] [PubMed] [Google Scholar]
- 18.Marder S R, Galderisi S. The current conceptualization of negative symptoms in schizophrenia. World Psychiatry. 2017; 16(1): 14. doi: http://dx.doi.org/10.1002/wps.20385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caroff SN, Hurford I, Lybrand J, Campbell EC. Movement disorders induced by antipsychotic drugs: Implications of the CATIE schizophrenia trial. Neurol Clin. 2011; 29(29): 127-148, viii. doi: http://dx.doi.org/10.1016/j.ncl.2010.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carbon M, Correll CU. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin Neurosci. 2014; 16(4): 505-524. doi: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4336920/ [DOI] [PMC free article] [PubMed] [Google Scholar]


