Abstract
Objective
To investigate the histopathological nature of myofascial trigger points (MTrPs) or spots (MTrSs) at different stages of recovery from injury in a rat model.
Methods
Forty Sprague–Dawley rats were randomly divided into two groups: a control group (CG) and experimental group (EG). The CG was further randomly subdivided into CG1 and CG2 subgroups. The CG2 was used for palpating the taut band and CG1 as a blank. EG was subdivided into three groups according to recovery times: 4 weeks (4W), 8 weeks (8W) and 12 weeks (12W); these groups consisted of eight rats each. All CG rats received no intervention, whereas the intervention in EG rats was by a blunt strike to the vastus medialis and eccentric exercise for 8 weeks. The taut bands with spontaneous electrical activity were then detected in the muscle to guide a muscle biopsy. The histopathological findings were investigated under optical and electron microscopes in all groups.
Results
Under optical microscopy, the differently augmented sizes of round fibres (contracture knots) with deep staining in the transverse section and fusiform shapes in a longitudinal view were clearly seen in CG2 and EGs with a large diameter; the number of contracture knots was significantly more in EGs than in CGs. Under an electron microscope, the mitochondria in EGs significantly decreased with abnormal structures. The sarcomeres were significantly shortened in the 8W and 12W EGs.
Conclusion
An injury can cause activation of MTrSs in a muscle and an activated level of MTrPs depending on the number of contracture knots in muscle with impaired energy production.
Keywords: animal models, histopathology, contracture knots, myofascial trigger points
Introduction
Skeletal muscles are among the largest organs in the human body, comprising 50% of its total weight. Any of these muscles may develop myofascial trigger points (MTrPs) with pain and dysfunction, which often affect the quality of daily life and work.1 2 Moreover, the prevalence of this condition increases with age.2 A clinical survey showed that 40% of musculoskeletal symptoms occur secondary to pain from active MTrPs, which are taut bands (TBs) characterised by distant referred pain, local twitch responses (LTRs) and spontaneous electrical activity (SEA).3 4 Therefore, Simons et al 5 defined an MTrP as ‘a hyperirritable spot,’ within a painful TB in a skeletal muscle that is, accompanied by characteristically referred pain, motor dysfunction and autonomic phenomena when compressed. Clinicians often use a referred pain pattern to make a correct diagnosis by precisely localising the TB of MTrPs.5 However, with respect to pain management, muscular and myofascial pain have generally received less attention as major sources of pain and dysfunction. Therefore, some clinicians have focused on treating only the area of referred pain when managing such patients, resulting in treatment that is not completely effective. The techniques used have included small needle-knife and silver needle treatment in China.6 This problem occurs because of a lack of understanding of MTrPs, and more basic knowledge is needed—for example, how the MTrP is formed and evolves throughout different stages after injury. Such information is unclear or unavailable in the literature.
In our previous study, MTrPs in a rat muscle were created by the combination of a blunt strike and eccentric exercise, and showed a stack of large circular and/or elliptical shapes in cross-section and continuous tapering of inflated fibres in longitudinal section.3 In this model, the shape of the established MTrPs when examined under a microscope was similar to the known structure of clinical MTrPs and confirmed as chronic MTrPs by electromyography (EMG) changes. Furthermore, we also found changes in spontaneous electrical activities.7 The activation of MTrPs was dependent on the firing rate of MTrP fibres, but most other studies used either an analogue model or latent MTrP (that is, a MTrP with a TB but no activation, thereby no spontaneous pain) for animal experiments on MTrPs, and earlier studies have obtained different and often contradictory results.8–10 Therefore, the aim of this study was to used our previously validated animal model to investigate the histopathology of the MTrPs under a transmission electron microscope (TEM) and an optical microscope.
Methods
Experimental animals
The experiment was conducted in the Laboratory of Exercise Molecular Biology, Shanghai University of Sport, in accordance with local guidelines for animal welfare consistent with the National Research Council’s ‘Guide for the Care and Use of Laboratory Animals’ (National Academies Press, Washington DC, USA). All experiments were approved by the laboratory animal ethics committee at the Shanghai University of Sport (reference no. 2014012) and all possible efforts were made to minimise the number of animals used and their suffering.
In 2016, a total of 40 adult male Sprague–Dawley rats (animal license no. SCXK 2007-0003, 7 weeks old, weighing 220–260 g) were purchased from the Shanghai Laboratory Animal Centre (Shanghai, China) and housed in a pathogen-free animal facility maintained at a controlled temperature of 20–22°C and air humidity of 45–55% with a 12/12-hour light/dark schedule. All rats were given 7 days of adaptive feeding to allow acclimatisation and were provided with free access to food and water. Subsequently, they were randomly divided into two groups according to a random number generator using the Statistical Package for the Social Sciences (SPSS) version 17.0 (SPSS Inc., Chicago, IL, USA): a control group (CG; n=16); and an experimental group (EG; n=24). The CG group was further randomly divided into two subgroups by computer (n=8 rats each): a blank control group (CG1); and a group that underwent palpation for possible latent myofascial trigger spots (MTrSs) in the vastus medialis (VM) and adductor femoris (AF) muscles (CG2). It should be noted that, here, we defined some spots with the lower prevalence and frequency of SEA as latent MTrSs, in contrast to the active MTrPs.7 The EG group was further divided into three subgroups (n=8 rats each) with different assigned recovery times of 4, 8, and 12 weeks (4W, 8W and 12W groups, respectively).
Experimental protocol
The experiment was conducted in three stages: (1) modelling; (2) biopsy and (3) morphological evaluation under both an optical microscope (OLYMPUS DP70 ×400, Japan) and a TEM (JEM-1200EX, Japan).
Stage 1: All EG rats were anaesthetised by injecting 10% chloral hydrate (0.4 mL/100 g) into the peritoneal cavity, before being fixed on the board of a home-made striking device in batches on the first day of every week.3 The proximal VM of the left hind limb was marked and struck at the marked position by a stick dropped from a height of 20 cm with a kinetic energy of 2.352 J once a week to induce muscle contusion.3 On the second day, all EG rats were made to run on a treadmill (DSPT-202, China) for 90 min at a −16° downhill angle and speed of 16 m/min.3 11 All EG rats were subjected to this modelling process for 8 weeks, while CG rats underwent no intervention. After modelling, EG rats were randomly reallocated to one of the three subgroups (4W, 8W and 12W) according to planned recovery time (n=8 rats each) and received no further interventions.
Stage 2: After the scheduled recovery time had elapsed (4, 8 or 12 weeks depending on subgroup allocation), EG and CG rats were both anaesthetised with intraperitoneal injections of 10% chloral hydrate (0.4 mL/100 g) in batches. The VM and AF of the left hind limb were surgically exposed by dissection of the skin and fascia. TBs were palpated, marked and counted by a skilled and experienced physician. A fine needle (Ф 0.3 mm) was inserted into each TB and, if a LTR was elicited by needling, the TB in question was considered to represent a possible MTrS. The needle was kept in situ at the location of the elicited LTR as one electrode and a second fine needle was longitudinally inserted into the TB roughly 2 mm from the first. A third needle was inserted into the right hind limb of the rat as a reference electrode, using a bipolar approach. All electrodes were connected to an EMG device (Z2J-NB-NCC08, NCC Medical Co., Ltd, Shanghai) to record the EMG. If the SEA recorded from the potential MTrS was 130 μV, it was considered to represent a verified MTrS.3 7 Finally, a muscle biopsy was taken at the site of the MTrS and either fixed in formalin solution, pending optical microscopy, or in a mixture of 4% paraformaldehyde and 1% osmic acid and stored at 4°C, pending TEM. Normal muscle fibres (without TBs) were collected from the CG1 group only.
Stage 3: Formalin-fixed muscle biopsy tissues were routinely embedded, sectioned, sliced and stained with haematoxylin and eosin (H&E), as previously described.3 12 The tissue slides were observed under an optical microscope. The number of contracture knots (CKs) and muscle cell diameters were quantified in a transverse section of each slide under light microscopy using a computer system. A series of electron microscopic staining methods were applied following tissue sampling, including fixation with glutaraldehyde (2.5%) and osmic acid (1%), dehydration, embedding, polymerisation, ultrathin sectioning and staining with uranyl acetate and lead citrate (3%).13 Under TEM, tissue samples and the Z-line (a cross-striation bisecting the I band of striated muscle myofibrils and serving as the anchoring point of actin filaments at either end of the sarcomere) were observed and sarcomere length was measured using image rulers.
Statistical analysis
Data are expressed as number, mean ±SD. Statistical analyses were performed by paired t test and one-way analysis of variance (ANOVA) followed by post hoc Tukey’s test using SPSS version 17.0. A value of p<0.05 was considered significant, and p<0.01 was considered highly significant.
Results
Identification and localisation of taut bands in myofascial trigger spots
TBs verified with LTRs and SEA by an EMG device were found in the CG2, EG 4W, 8W and 12W groups. The total number of TBs identified through palpation was 2, 24, 21 and 18 in the CG2, EG 4W, 8W and 12W groups, respectively (n=8 rats per group) (table 1). The taut bands in the CG2 group were palpated only in two rats (one in the VM of one rat, and one in the AF in another); these were verified by SEA using the EMG device as MTrS with only one or two contracture knots per field (p<0.01, table 1).
Table 1.
Myocyte diameter, sarcomere length and numbers of taut bands and contracture knots
| Groups | n | Numbers of TBs palpated | Numbers of CKs per field | Myocyte diameter (μm) | Sarcomere length (μm) |
| CG1 | 8 | NA | NA | 22.84±0.48 | 1.95±0.02 |
| CG2 | 8 | 2 (0.25±0.43) | 2 (0.5±0.89) | 40.43±0.55** | Missed |
| 4W | 8 | 24 (3.00±1.07)† | 7 (7.13±0.60)† | 44.73±9.50** | 1.92±0.08 |
| 8W | 8 | 21 (2.63±0.74)† | 6 (6.00±0.71)† | 45.77±8.70** | 1.53±0.06** |
| 12W | 8 | 18 (2.57±0.54)† | 8 (7.65±0.86)† | 47.81±5.84** | 1.48±0.07** |
**p<0.01 vs CG1.
†p<0.01 vs CG2.
Results are shown as total (mean±SD of eight samples) or mean±SD. NA, not applicable.
CG1 indicates normal control and CG2 indicates latent myofascial trigger spots; 4W, 8W, 12W, 4, 8, 12 weeks.
Sarcomere length was missed in CG2 because it is difficult to find the contracture knot.
Morphological changes under optical microscopy
In the biopsies of normal muscle fibres (without MTrSs) from the CG1 group, muscle cells were generally polygonal in shape and uniform in size (figure 1A). Single or multiple nuclei were located near the edge of the cells in the transverse section. In the longitudinal section, the muscle fibres showed a tight and ordered arrangement with multiple nuclei lined up along the edge of the fibre (figure 1B). However, in all EGs, more taut bands were palpated and 6–8 contracture knots were observed per field (table 1), which was significiantly more than that observed in the CG2 group (p<0.01).
Figure 1.
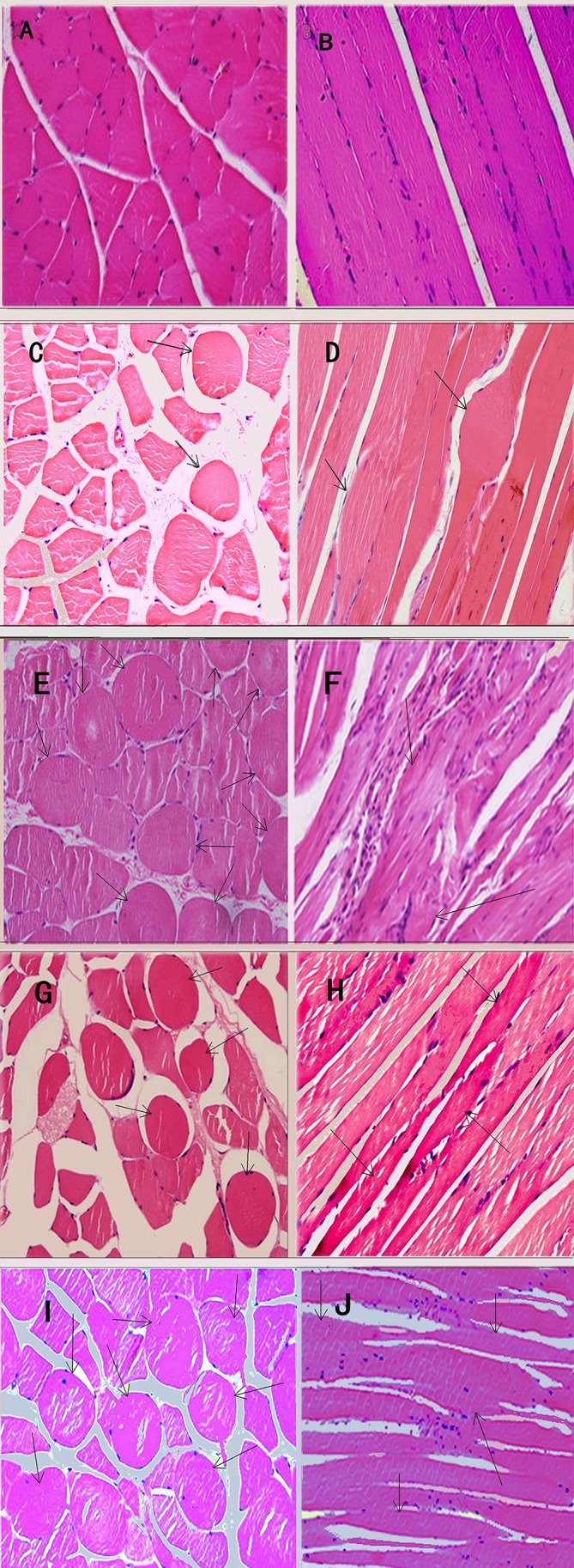
Light microscope views of muscle fibres with haematoxylin and eosin staining, ×400. In control group 1 (CG1), the muscle cells showed polygonal shapes of uniform size (A). Moreover, tight and ordered arrangement was found in the longitudinal section (B). In control group 2 (CG2), very few large round muscle cells could be seen in cross-section (C) except that a long thin fibre was observed connecting to a knot (D). In the 4-week (4W), 8W and 12W groups, several large and small hyperchromatic rounded muscle cells appeared (black arrows) in cross-section (E, G, I) and several fusiform muscle fibres (arrows) connecting thinning fibres matched with rounded muscle cells (F, H, J). In the 8W and 12W groups, continuous beaded muscular fibres with enlargement in the middle (black arrow) and attenuation at both ends and infiltration of slight inflammatory cells could be observed in longitudinal section (H and J). In both sections, spaces between fibres became the width in the 8W and 12W groups.
In the CG2 group, a few, large round muscle cells were observed in transverse section (figure 1C). Figure 1D shows an example of a long thin fibre connected to a knot. By enlarging the thin fibre on a single slide in a single animal, the transverse striation became bent in the middle by a contracture knot in longitudinal section. In the EGs, several large or small hyperchromatic rounded muscle cells were seen gathered together in transverse section (figure 1E, G and I) and internal nuclei and infiltration of inflammatory cells were observed. In longitudinal section, the muscle fibres were found to be disorganised shuttle-shaped muscle fibres, which were swollen centrally and thin at both ends (figure 1F, H and J). In the 4W group, in particular, infiltration of inflammatory cells was markedly increased, with evidence of adhesion near contracture knots (figure 1E), internal nuclei and narrow spaces in both transverse and longitudinal sections (figure 1E and F), and thickened, disarranged muscle fibres were seen. More cracks between muscle fibres appeared in the EG groups. In the 8W and 12W groups, continuous beaded, spindle-shaped muscle fibres were observed, gathered together in longitudinal section (figure 1H and I).
Under light microscopy, no CKs were observed in the biopsies of normal muscle fibres from the CG1 group (table 1). TBs were only palpable in two rats (in the VM of one rat and in the AF of another) in the CG2 group and biopsies of these latent MTrSs (verified by SEA using the EMG device) only showed one or two CKs per field. However, in all EGs, significantly more TBs (p<0.01) were palpated and 6–8 CKs were observed per field. The mean diameter of the muscle cells from the EGs was significantly greater than that of the CG1 group (p<0.01), while no significant difference was found between the CG2 and EG groups (p>0.05).
Morphological changes under transmission electron microscopy
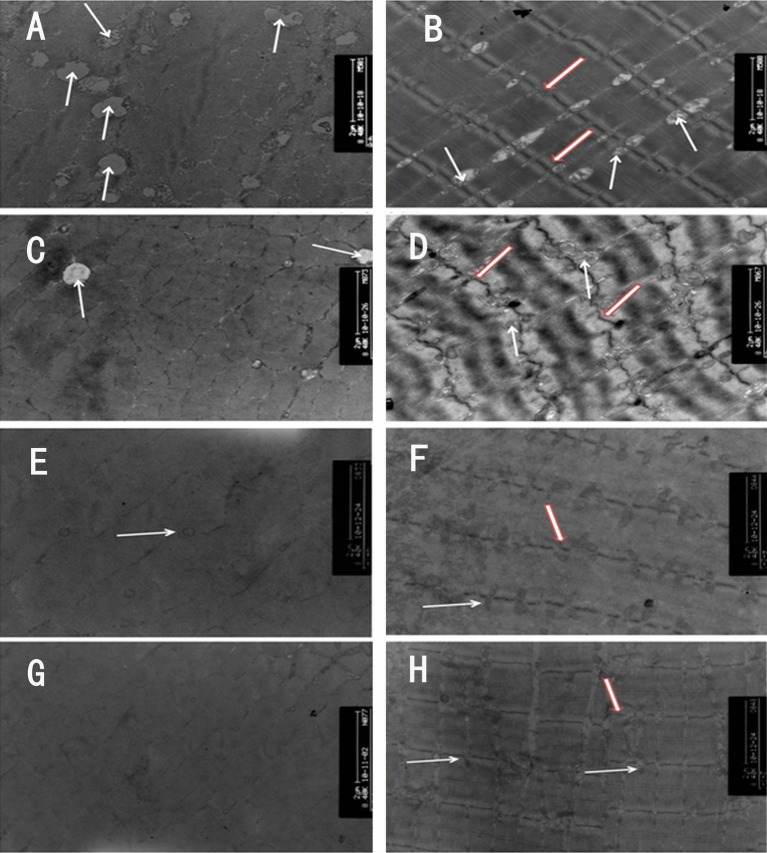
In the normal muscle fibre biopsies from the CG1 group, numerous ovate mitochondria with internal ridge-like structures and intact myofilaments in well-arranged and clean-cut sarcomeres were observed in transverse and longitudinal section (figure 2A and B). In the EG groups, the number of mitochondria appeared to be decreased or even absent in cross-section. They were also notably more circular with reduced or absent ridge-like structures (figure 2C, E and G). The arrangement of the sarcomeres was disordered in the 4W and 8W groups, and their myofilaments were disarranged and blurred in longitudinal section (figure 2D and F). The Z-line was diminished in comparison with the CG1 (figure 2B, D and F), with fewer surrounding mitochondria (figure 2C and E). Notably, the Z-line in the 4W group was wave-like in appearance (figure 2D). The arrangement of the myofilaments appeared relatively more organised with a greater prevalence of mitochondria in the 12W group, but the swollen appearance remained (figure 2G and H).
Figure 2.
Transmission electron microscope views of muscle fibres, ×8400. In control group 1 (CG1), large numbers of ovate mitochondria (white arrows) with ridge-like structures inside and intact myofilaments in well-arranged and clean-cut sarcomeres were observed either in cross-section or in longitudinal section (A, B). In the 4-week (4W), 8W and 12W groups, the number of mitochondria (white arrows) was reduced or even disappeared in cross-section. Their shapes were changed into a circle, and their ridge-like structures decreased or disappeared (C, E and G). The arrangement of sarcomeres was abnormal, and the myofilaments were disarranged and blurred in longitudinal section (D, F and H). The Z-line (hollow red arrow) became thinner than that in CG1 (D, F and H), around which fewer mitochondria were found. Moreover, the Z-line in the 4W rooked like a drifting wave-like line (D).
Compared with the CG1, sarcomere length in the 8W and 12W groups was significantly reduced (p<0.01; table 1), while no significant difference was found between the 4W and CG1 groups (p>0.05).
Discussion
On the basis of the rat model of MTrPs with features of TB, LTR and SEA, the histopathology of MTrPs demonstrated CKs (with adhesion and infiltration of inflammatory cells in the 4W group) under an optical microscope; fewer and more deformed mitochondria, the diminution or vanishing of ridge-like structures in mitochondria (and draft of the Z-line in muscle fibres in the 4W group) were observed under TEM. More CKs occurred among modelled rats in the EG but not in the CGs.
Inflammatory appearances in the rat model
In previous studies, this animal model of active MTrPs, which was built up via a combination of local strikes and eccentric exercises, was verified in the affected muscles by the characteristics of TB, CKs, LTR, SEA and abnormal electrical potentials.14–16 A previous study using EMG showed that more SEAs could be observed at certain stages according to different recovery times in rat models.6 In our study, the same model was used to further investigate the histopathological changes during different recovery phases at 4W, 8W and 12W after modelling. An active MTrP occurs at the beginning of a blunt injury with inflammation, which could be observed by the histopathological changes under optical and electron microscopes in the 4W group. The process persisted and gradually recovered at 8W and 12W, with a decrease in infiltration of inflammatory cells and an improvement in the disarrangement of muscle fibres and myofilaments. However, several histopathological changes remained, such as the presence of numerous CKs. In the 12W group, the mitochondria remained fewer in number and were deformed, which indicates that acute inflammation had recovered further; however, a local oxygen deficit still remained. Although repair was seen in the 8W and 12W groups, a slight infiltration of inflammatory cells could still be observed. On the basis of these features, it can be confirmed that the active MTrPs in rat models of blunt injury may gradually develop chronic pain with CKs.
An injury, especially a chronic injury, is considered a common cause of a MTrP in muscle, although other factors are also attributed to the MTrP, such as alimentary deficiency, retrogression, infection and gene mutation.5 14
Histopathological characteristics of myofascial trigger points
In our previous study, SEA frequency and amplitude varied at different stages of recovery after injury in this rat model and was relative to TBs in control rats.3 7 There is less activity and a low frequency of SEA in latent MTrPs, in contrast to greater activity and higher frequency in activated MTrPs.3 7 These findings led us to further investigate herein which morphological features match the activity of the EMG. Thus the CG2 group wasincluded for palpation of potentially latent MTrS of the VM, and additionally examined under the microscope.
In this study, very few CKs were observed in the CG2 group. Accompanied by less EMG activity,7 they may be considered to represent latent MTrPs. This suggests that a difference in the characteristics of latent and active MTrPs depends on the numbers of CKs. Several researchers have suggested that latent MTrPs might exist as a protective mechanism that prevents the area of the lesion from further damage because of the relatively strenuous activity of muscles resulting from pain.14 16
In the 1970s, Simons and Stolov17 found isolated, enlarged and hyperchromatic round muscle cells in MTrPs under light microscopy. Reitinger et al 18 found wider A-band and I-band loss under TEM when biopsies on MTrPs from fresh corpses were studied. On the basis of these studies, a simulated image of MTrPs in longitudinal section was proposed by Simons et al.5 In their image, a group of CKs appeared with increased diameter in transverse section and obviously thinner muscle fibres at both ends in longitudinal section.5
Recently, histopathological changes under light microscopy of MTrPs in rat models have been observed. The MTrPs consisted of a few abnormal muscle fibres gathered together with a group of large circular and/or elliptical shapes in cross-section and continuous inflated tapering fibres in longitudinal section.7 In our study, the appearance of MTrPs was similar to that of the aforementioned study, but the internal nuclei of >10% of MTrS muscle fibres and more cracks were seen in the 4W, 8W and 12W groups; these findings indicate that those muscle fibres were in the process of being repaired.19 However, numerous CKs were also present, which indicated that active MTrPs had been produced and were accompanied by a greater prevalence of SEA from the rat model in the 8W and 12W groups than in the CG groups in the previous EMG study.7 Therefore, fewer active trigger points with fewer CKs are analogous to latent trigger points, and the more active ones with more CKs are analogous to active trigger points.
Under TEM, the number of mitochondria decreased, and the round-shaped and ridge-like structures disappeared; these results are consistent with the ATP energy crisis found within the muscle fibre of MTrPs, as reported by Simons et al.5 In transverse section, the Z-line in the 4W group showed drifting and wave-like changes. Furthermore, the sarcomere demonstrated an irregular arrangement without obvious shortening. The results indicated that the tissues of MTrPs are in the early modelling period with only an inflammatory change from the injury. Data from other studies indicated that these injuries usually occur at the cytoskeletal level (eg, titin, nebulin and desmin), such as can be seen in disorders of A-band and wave-like changes of the Z-line.20 Compared with the 4W group, the tissue damage seen in the 8W and 12W groups was alleviated, with relatively well-arranged and clean-cut sarcomeres, which, however, became significantly shorter. Moreover, the number of mitochondria gradually increased, but the structure still maintained an abnormal status. It is likely that the abnormal mitochondria cannot normally complete metabolic processes and maintain muscle function owing to fewer ATP molecules. ATP is generated by carbohydrates, fatty acids and sometimes amino acids as their primary substrate.21 Carbohydrates are converted to glucose in the cell cytoplasm. Finally, pyruvate from this process is converted to acetyl CoA by pyruvate dehydrogenase in the mitochondria, which produces the energy in reducing equivalents to ATP for contraction. Muscles with higher energy demand have higher mitochondrial content.22 23
The results for the 8W and 12W groups suggests that the tissue injury had been recovering, and the main pathological changes of MTrPs (more CKs) appeared and/or started to enter the chronic stage. Investigation in humans showed that patients with trapezius myalgia had cytochrome oxidase deficiencies, reduced capillary numbers and a reduction in the microcirculation, which indicates that the pathogenesis of MTrPs is related to deficiency of energy supply and local ischaemia.24 25 This deficiency may be associated with abnormal mitochondria.24 25 Therefore, chronic MTrPs may represent a type of chronic myopathy caused by disturbances of local energy supplementation secondary to acute tissue damage.
According to the morphological changes in the MTrP muscle fibres at different stages of recovery, the formation of MTrPs can be classified into three steps. Firstly, a few CKs are already present as latent MTrPs, with a low frequency of SEAs due to the early stage of injury.5 7 Secondly, external factors, such as an injury, may induce local muscle inflammation while evoking an increased number of CKs with an increased prevalance of SEA.7 Thirdly, the existence of real CKs can be confirmed and their numbers counted under a light microscope. This finding shows the increased activation of MTrPs.
Conclusion
In the rat model of MTrPs induced by striking combined with eccentric exercise, the muscle fibres in MTrPs were found to be in the inflammatory stage of injury. As the recovery time increased, inflammation from injury gradually decreased and was associated with repair, however MTrP CKs were maintained at an advanced stage owing to an energy supply obstacle.
Compared with the CG2 group, a greater number of CKs appeared in the muscle fibres of the EGs (accompanied by increased prevalence of SEA7) with a slight inflammatory infiltrate. Therefore, increased activation of MTrPs may depend on how many CKs occur in the affected muscle. Moreover, a latent MTrP shows few CKs (accompanied by a low prevalence of SEA)6 and no inflammatory infiltrate.
Footnotes
Contributors: Q-MH thought of the idea and wrote the paper. J-JL and LL conducted the animal experiments and collectected the data. HZ and Q-GL analysed the results. O-AE was involved in the discussion and native English revision. All authors approved the final version of the manuscript accepted for publication.
Funding: This study was supported by the National Natural Science Foundation of China (grant no. 81470105).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Norman HR. Muscle pain syndromes. Am J Phys Med Rehabil 2007;86:S47–58. [DOI] [PubMed] [Google Scholar]
- 2. Zhuang X, Tan S, Huang Q. Understanding of myofascial trigger points. Chin Med J 2014;127:4271–7. [PubMed] [Google Scholar]
- 3. Huang QM, Ye G, Zhao ZY, et al. Myoelectrical activity and muscle morphology in a rat model of myofascial trigger points induced by blunt trauma to the vastus medialis. Acupunct Med 2013;31:65–73. 10.1136/acupmed-2012-010129 [DOI] [PubMed] [Google Scholar]
- 4. Partanen JV, Ojala TA, Arokoski JP. Myofascial syndrome and pain: a neurophysiological approach. Pathophysiology 2010;17:19–28. 10.1016/j.pathophys.2009.05.001 [DOI] [PubMed] [Google Scholar]
- 5. Simons DG, Travell TG, Simons LS, et al. Myofascial Pain and Dysfunction: The Trigger Point Manual. vol 1 2nd ed Baltimore: Lippincott, Williams & Wilkins, 1999. [Google Scholar]
- 6. Ling DY, Wang ZY, Zh L, et al. Medium-long term efficacy of dense silver needle therapy combined with intra-articular injection of ozone in patients with knee osteoarthritis [Chinese]. J Pain Med 2014;20:494–7. [Google Scholar]
- 7. Huang QM, Lv JJ, Ruanshi QM, et al. Spontaneous electrical activities at myofascial trigger points at different stages of recovery from injury in a rat model. Acupunct Med 2015;33:319–24. 10.1136/acupmed-2014-010666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernández-de-las-Peñas C, Cuadrado ML, Arendt-Nielsen L, et al. Myofascial trigger points and sensitization: an updated pain model for tension-type headache. Cephalalgia 2007;27:383–93. 10.1111/j.1468-2982.2007.01295.x [DOI] [PubMed] [Google Scholar]
- 9. Hong CZ, Simons DG. Pathophysiologic and electrophysiologic mechanisms of myofascial trigger points. Arch Phys Med Rehabil 1998;79:863–72. 10.1016/S0003-9993(98)90371-9 [DOI] [PubMed] [Google Scholar]
- 10. Itoh K, Okada K, Kawakita K. A proposed experimental model of myofascial trigger points in human muscle after slow eccentric exercise. Acupunct Med 2004;22:2–12. 10.1136/aim.22.1.2 [DOI] [PubMed] [Google Scholar]
- 11. Kawakita K, Itoh K, Okada K. Experimental model of trigger points using eccentric exercise. J Musculoskelet Pain 2008;16:29–35. 10.1080/10582450801960180 [DOI] [Google Scholar]
- 12. Carmo-Araújo EM, Dal-Pai-Silva M, Dal-Pai V, et al. Ischaemia and reperfusion effects on skeletal muscle tissue: morphological and histochemical studies. Int J Exp Pathol 2007;88:147–54. 10.1111/j.1365-2613.2007.00526.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toner E, Brennan GP, McConvery F, et al. A transmission electron microscope study on the route of entry of triclabendazole into the liver fluke, Fasciola hepatica. Parasitology 2010;137:855–70. 10.1017/S0031182009991247 [DOI] [PubMed] [Google Scholar]
- 14. Bron C, Dommerholt JD. Etiology of myofascial trigger points. Curr Pain Headache Rep 2012;16:439–44. 10.1007/s11916-012-0289-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hong CZ. New trends in myofascial pain syndrome. Zhonghua Yi Xue Za Zhi 2002;65:501–12. [PubMed] [Google Scholar]
- 16. Ge HY, Monterde S, Graven-Nielsen T, et al. Latent myofascial trigger points are associated with an increased intramuscular electromyographic activity during synergistic muscle activation. J Pain 2014;15:181–7. 10.1016/j.jpain.2013.10.009 [DOI] [PubMed] [Google Scholar]
- 17. Simons DG, Stolov WC. Microscopic features and transient contraction of palpable bands in canine muscle. Am J Phys Med 1976;55:65–88. [PubMed] [Google Scholar]
- 18. Reitinger A, Radner H, Tilscher H, et al. Investigations on the morphology of trigger points [Morphologische Untersuchung an Triggerpunkten]. Manuelle Medizin 1996;34:256–62. [Google Scholar]
- 19. Dubowitz V, Sewry CA, Oldfors A. Muscle biopsy: a practical approach. Beijing: Peking University Medical Press, 2009. [Google Scholar]
- 20. Koishi K, Zhang M, McLennan IS, et al. MyoD protein accumulates in satellite cells and is neurally regulated in regenerating myotubes and skeletal muscle fibers. Dev Dyn 1995;202:244–54. 10.1002/aja.1002020304 [DOI] [PubMed] [Google Scholar]
- 21. Jafri MS, Dudycha SJ, O’Rourke B. Cardiac energy metabolism: models of cellular respiration. Annu Rev Biomed Eng 2001;3:57–81. 10.1146/annurev.bioeng.3.1.57 [DOI] [PubMed] [Google Scholar]
- 22. Hoppeler H, Mathieu O, Krauer R, et al. Design of the mammalian respiratory system. VI Distribution of mitochondria and capillaries in various muscles. Respir Physiol 1981;44:87–111. 10.1016/0034-5687(81)90078-5 [DOI] [PubMed] [Google Scholar]
- 23. Schwerzmann K, Hoppeler H, Kayar SR, et al. Oxidative capacity of muscle and mitochondria: correlation of physiological, biochemical, and morphometric characteristics. Proc Natl Acad Sci U S A 1989;86:1583–7. 10.1073/pnas.86.5.1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saleet Jafri M. Mechanisms of myofascial pain. Int Sch Res Notices 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dommerholt J, Bron C, Franssen J. Myofascial trigger points: an evidence-informed review. Journal of Manual & Manipulative Therapy 2006;14:203–21. 10.1179/106698106790819991 [DOI] [PMC free article] [PubMed] [Google Scholar]