Abstract
Background
Optimal dosing of oral tyrosine kinase inhibitor therapy is critical to treatment success and survival of patients with chronic myeloid leukemia (CML). Drug intolerance secondary to toxicities and nonadherence are significant factors in treatment failure.
Objective
The objective of this study was to develop and pilot-test the clinical feasibility and acceptability of a mobile health system (REMIND) to increase oral drug adherence and patient symptom self-management among people with CML (chronic phase).
Methods
A multifaceted intervention was iteratively developed using the intervention development framework by Schofield and Chambers, consisting of defining the patient problem and iteratively refining the intervention. The clinical feasibility and acceptability were examined via patient and intervention nurse interviews, which were audiotaped, transcribed, and deductively content analyzed.
Results
The intervention comprised 2 synergistically operating elements: (1) daily medication reminders and routine assessment of side effects with evidence-based self-care advice delivered in real time and (2) question prompt list (QPL) questions and routinely collected individual patient adherence and side effect profile data used to shape nurses’ consultations, which employed motivational interviewing to support adoption of self-management behaviors. A total of 4 consultations and daily alerts and advice were delivered over 10 weeks. In total, 58% (10/17) of patients and 2 nurses participated in the pilot study. Patients reported several benefits of the intervention: help in establishing medication routines, resolution of symptom uncertainty, increased awareness of self-care, and informed decision making. Nurses also endorsed the intervention: it assisted in establishing pill-taking routines and patients developing effective solutions to adherence challenges.
Conclusions
The REMIND system with nurse support was usable and acceptable to both patients and nurses. It has the potential to improve adherence and side-effect management and should be further evaluated.
Keywords: mobile phone, neoplasms, Internet, medication adherence
Introduction
Chronic myeloid leukemia (CML) is an uncommon clonal bone marrow stem cell disorder. The disease has a triphasic natural history—commencing in chronic phase, unless well controlled, progressing to accelerated phase, and ultimately to blastic phase with over 90% of patients being diagnosed in the chronic phase [1]. Oral tyrosine kinase inhibitor (TKI) therapy is the standard of care for patients with chronic-phase CML. Imatinib, nilotinib, and dasatinib, currently first-line oral TKI therapies, are highly successful in improving progression-free and overall survival [2-4]. However, optimal adherence to TKIs is critical to treatment success and survival, with continuous, daily dosing required for an indefinite period, often lifelong [4,5], unless complete deep molecular response has been achieved when a treatment-free period can be tested [6,7].
TKIs are associated with numerous potential toxicities, including myelosuppression, nausea, diarrhea, fatigue, and soft-tissue edema, especially in the face and lower legs [8-11]. Given these toxicities, it is not surprising that medication adherence is problematic; a recent review found that one-third to one-quarter of patients with CML have poor TKI adherence [12]. This is serious as treatment response is compromised for patients with less than 90% adherence [13,14]. Greater frequency of adverse events and higher levels of patient-reported symptoms predict lower levels of medication adherence [15,16]. Conversely, good knowledge of disease and treatment and confidence in medication self-management are linked to improved adherence [15,17], whereas forgetfulness is the most common reason for unintentional nonadherence [18]. These factors are potentially modifiable by educational and behavioral interventions. There is an urgent need to improve patient medication adherence among patients with CML [4,19,20].
Mobile health (mHealth), defined as employing mobile devices to support medical practice and public health, may improve TKI adherence using cellular phone apps and text messages. Internationally, phone text interventions have been tested to prompt oral drug adherence in a number of chronic conditions, including human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) [21-27], diabetes mellitus [28-31], asthma [32], and tuberculosis [33,34]. Studies reported short- [21,23,24,28,29,32] and long-term [26,29] improvements in adherence, with patients generally perceiving text reminders as beneficial [21,22,27,33,34]. Reviews of phone text reminder interventions have demonstrated efficacy in enhancing patients’ compliance to drug therapy [35-39]; however, only one study of cancer patients was identified, which was targeted at adolescents and young adults and phone text messages were not utilized [40]. A secondary in-depth analysis found no published trials that investigated a medication adherence intervention that integrates nurse-led phone consultations with mHealth systems in oncology [41].
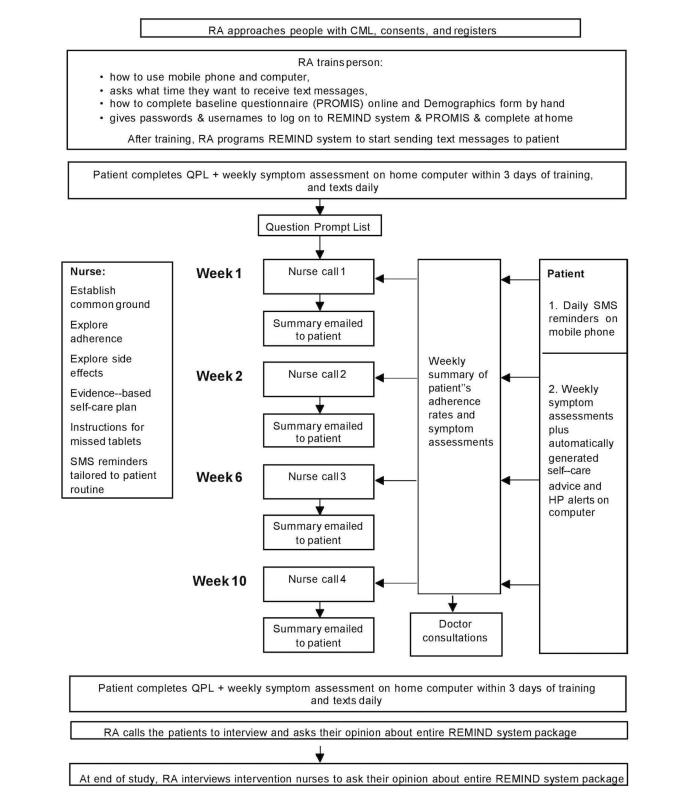
Oncology nurses are central to providing education and coaching in patient self-management of drug toxicities and medication adherence [16,42,43]. Patient and clinician acceptability of nurse-led mHealth interventions aimed at improving postchemotherapy symptom management has been demonstrated in solid tumors [44,45]. We conceived a multifaceted intervention package consisting of 2 integrated elements delivered over 10 weeks: (1) the REMIND mHealth system comprising daily medication reminder texts and individualized self-care advice based on self-reported side effects delivered in real time and (2) nurse telephone consultations to promote adherence to imatinib (Glivec) and coach patients in toxicity self-management (Figure 1). Briefly, after the patient was trained in using the REMIND system, he or she completed a Web-based symptom survey and question prompt list (QPL), which was used to guide the first nurse telephone consultation. Daily, the patient was asked to respond to a text reminder message for each dose based on his or her individual regimen.Once a week, a text message was sent to patients to remind them to complete an online symptom survey. For moderate to severe symptoms, self-care information was sent to the patient in real time.
Figure 1.
Flowchart of the nurse-mediated telehealth (including REMIND system) intervention package (CML, chronic myeloid leukaemia; HP, health professional; QPL, question prompt list; RA, research assistant; SMS, short message service).
Adherence and symptom profiles were available for clinician review and used to guide 3 more nurse telephone consultation. The aim of this paper is to describe the development of this intervention and its clinical feasibility and acceptability. The study is reported in accordance with the CONSORT eHealth checklist (V1.6.1) [46].
Methods
Phase 1: Development of the Intervention
The framework for the development of effective, clinically feasible, and sustainable interventions by Schofield and Chambers was used to guide intervention development [47]. This intervention framework represents an expansion of the Medical Research Council (MRC) framework for complex interventions development [48]. The MRC framework provides broad recommendations for intervention development, such as establishing the theoretical and evidence base and testing procedures. The intervention development framework by Schofield and Chambers specifies 7 features: (1) targeting cancer type and stage, (2) tailoring to unique individual needs, (3) promoting self-management, (4) efficient intervention delivery, (5) ensuring evidence-based and theoretical grounding, (6) specifying protocol training and adherence, and (7) confirming stakeholder acceptability.
The features of the framework by Schofield and Chambers [47] used to guide intervention development are described below.
Targeting
To understand the problem, a prior qualitative study by this team with 16 patients with CML prescribed a TKI and 10 health professionals was conducted to examine the nature, extent, and reasons for medication nonadherence [49]. Findings revealed that nonadherence was reported on at least one occasion among 75% of patients sampled with reasons for unintentional nonadherence including forgetfulness or misunderstanding medical instructions. Intentional nonadherence was related to side effects and insufficient health care support. Health professionals also experienced difficulty in accurately evaluating the medication adherence of their patients. These findings, in addition to those of others [17], indicate that intervention goals should be to increase depth of knowledge of disease, treatment regimen, and toxicities; increase confidence in self-management of side effects; and prompt TKI self-administration according to medical advice in relation to timing, dose, and frequency.
Tailoring
For the REMIND system, the timing of daily text-based medication reminders could be set to suit individual preferences. The evidence-based self-care advice was electronically delivered in real time, corresponding to the individual’s responses to weekly self-assessment of side effects. The structure and content of the nurse consultations were directed by a QPL administered electronically to the patient just before the first nurse consultation.
Promoting Self-Management
Successful self-management requires the ability to self-assess problems; marshal information, skills, or resources to problem-solve; set goals; and implement the planned solution. Key to this is self-efficacy, which is defined as a person’s beliefs in his or her ability to succeed in a given task. Although the REMIND system (medication reminders texts, side-effect assessment, and self-care advice) facilitated self-assessment of side effects and provided information and resources, coaching in self-management and behavior change was accomplished by the nurse-led phone consultations using motivational interviewing, which is a client-centered method that allows patients to explore and resolve their own ambivalence about adopting a new behavior, such as medication adherence, and to evoke self-motivation as opposed to traditional didactic health advice provision [50].
Efficiency
The combined delivery methods of this intervention were used to increase the efficiency of the intervention. Blending nurse phone consultations with the REMIND system increased the dose of the intervention, permitting the nurses to focus their expertise on coaching. Exclusive use of communication technologies for intervention delivery was intended to increase access to those living in rural communities or those who were too ill or had less time to attend face-to-face consultations.
Evidence and Theory
Both intervention content and delivery mechanism should be based on theory and available evidence. The list of relevant drug toxicities was generated using Monthly Index of Medical Specialties and expert clinician advice (JFS). Authors (PS and SA) updated their systematic review of self-care strategies for chemotherapy side effects [51] with additional literature searches to develop self-care recommendations for imatinib side effects in conjunction with an expert clinician (JFS). A consumer with CML (AD) iterative reviewed all content along with the multidisciplinary study team. Motivational interviewing, the central technique used the nurse delivery of content, arose from self-determination theory [52], which is a framework of intrinsic and extrinsic motives that facilitate or forestall behavior change. Motivational interviewing by telephone is significantly associated with improving medication adherence [53].
Protocolization
Interventions that layer digital technologies with targeted clinician contact rely on adequate training and supervision to ensure uniform delivery of content across intervention clinicians. Standardized manuals were developed specifying (1) the intervention content, including the resource manual describing the disease, treatments, side effects, and evidence-based, self-management advice; and (2) the training and supervision protocols. Protocolization of standardized content and training ensures a comprehensive knowledge base and attainment of core skills required by the intervention nurses and consistent, standardized, and reproducible delivery of the intervention content.
Stakeholder Acceptability
Using a codesign process involving the end users (clinicians and patients) throughout the development process optimizes stakeholder acceptability [54,55]. Our stakeholder qualitative research [49] directed the intervention goals and structure to ensure relevance. A clinical nurse specialist was engaged to write the nurse intervention manuals, detailing the content of each intervention session. A resource manual to support the provision of evidence-based advice was developed from booklets, fact sheets, and websites which were sourced from reputable peak cancer and other health bodies, in particular, the Leukaemia Foundation of Australia. The intervention development working party consisting of a consumer, hematologists, hematology nurse specialists, clinical psychologists, behavioral scientists, and an oncology pharmacist reviewed iterative revisions of intervention and resource manuals.
Phase 2: Pilot Testing
Setting
This study was conducted in a comprehensive cancer hospital in Melbourne, Australia.
Design
A qualitative research design was used to examine the clinical feasibility and acceptability of the intervention package to patients and nurses.
Participants
Participants were patients who received the intervention and nurses who delivered the intervention. Inclusion criteria for patients were as follows: age >18 years, proficiency in English, a confirmed diagnosis of chronic-phase CML with no signs of progression, and >3 months of continuous treatment with imatinib (Glivec) with no evidence of drug resistance. On the basis of feedback from the first 3 patients in this pilot testing phase, this last criterion was changed to treatment with imatinib and no evidence of drug resistance. Exclusion criteria were cognitive or psychological difficulties as assessed by the patient’s treatment team and being too unwell. In total, 2 clinical nurse consultants with postgraduate qualifications in cancer nursing and extensive experience in hematological oncology were trained to deliver the intervention.
Nurse Training
The nurses were trained to deliver the structured telephone consultations and use the REMIND system in a 1-day workshop, facilitated by a clinical communication expert (PS). The workshop comprised an overview of the project and intervention manual and training in (1) identifying and responding to emotional cues on the phone, (2) exploring concerns about medication adherence and providing evidence-based self-care advice, and (3) coaching patients using motivational interviewing techniques. Didactic instruction was complemented by 2 role-play sessions with a simulated patient and facilitator feedback: 1 in the workshop and 1 in a subsequent phone consultation, which was audiorecorded for self-appraisal and facilitator feedback.
Procedure
Potentially eligible participants were identified by clinician referral or pharmacy records of imatinib dispensing and examined for eligibility as per the eligibility criteria by the research assistant. After confirming eligibility and suitability with the treating clinician, patients were approached either face-to-face at outpatient clinic visits or over the telephone. An appointment was made with interested participants to review and sign the consent form, complete a baseline questionnaire, and be trained to use the REMIND system. All patients then received the intervention over 10 weeks and at the end of the intervention, completed a follow-up questionnaire. The patient-reported outcomes in both questionnaires covered quality of life [56], psychological morbidity [57], and self-management [58].
Patient and Intervention Nurse Interviews
Following completion of the intervention, consenting patients and intervention nurses participated in a semi-structured telephone interview of 20 to 40 min. Separate interview schedules were developed for patients and health professionals. Both interview schedules covered perceptions of the intervention package, including content, timing, and perceived utility of each component of the REMIND system and the nurse consultations, perceived impact of the intervention on adherence and self-management, overall satisfaction with intervention, and recommended changes. All interviews were audiorecorded and transcribed verbatim.
A content analysis was undertaken to identify themes [59]; 2 investigators (APS and JW) analyzed intervention patient data and 2 investigators (PS and JW) analyzed nurse data separately. The process involved iterative movement between transcripts, rereading to establish familiarity with content and reflection of ideas. Similar content was collated and summarized to form themes with codes assigned by each analyst. Following analysis completion, all data analysts met to discuss results of their respective data. Disagreements between coders were identified and discussed until agreement was reached. Coding themes were then agreed upon, and the data from each group were coded into these themes. Themes were then collapsed or divided through consultation and discussion. As the interviews covered similar topics, perspectives of the 2 groups were aggregated. Survey outcomes were not analyzed due to sample size.
Results
Phase 1: Development of the Intervention
The REMIND System
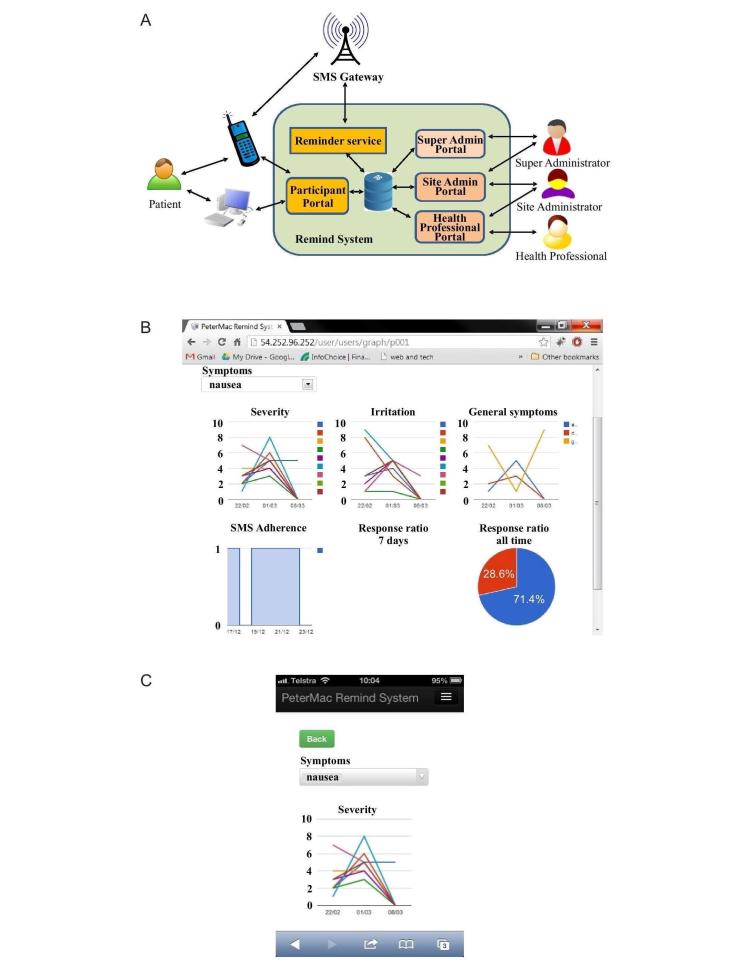
The REMIND system was a hosted Web application, deployed on the Amazon Web Services cloud platform as an EC2 instance, with multiple Web- and text-based interfaces provided for a variety of users and a range of services that control text messages sent to a third-party short message service (SMS) text messaging Gateway (Figure 2). It comprises the following:
Figure 2.
REMIND system representation. (A) Architecture of the REMIND system showing context of interaction with different types of users (patients, health care professionals, and administrators) and external SMS Gateway service. The patient receives reminders on his or her cellular phone, sent from the SMS Gateway, and can interact with the weekly symptoms survey via a browser on a standard computer or a smartphone. (B) Screenshot of patient dashboard, showing graphs (from left to right) of scores (for severity and irritation) for symptom (in this case, nausea) over time, bar chart indicating daily adherence (measured by response), and pie chart showing overall adherence rate. (C) Screenshot of specific symptoms—severity graph for nausea—as displayed on the cellular smartphone interface.
A database of all user information, including patient and health professionals’ log-in information; patient phone numbers and preferred notification time for medication reminders; and a log of all responses to daily reminders and answers to weekly survey questions
A Web-based application that allows patients and health professionals to view, and for health professionals to edit information regarding users, and patients to edit responses to reminders and symptom survey answers. The pages displayed by this application are designed for both laptop/desktop and mobile phone screens.
An automatic nightly service that pulls responses from the SMS Gateway, updates the database, creates SMS messages for the next day from information in the user database, and then forwards these messages to the SMS Gateway to be queued to be sent out at the preferred notification times. This program is sensitive to time zone and daylight saving and automatically adjusts the send-time of messages according to the local time of the patient’s location.
Daily or twice-daily cellular phone reminder message were sent via the REMIND system to each patient for each dose based on patients’ individual dosing regimen. Patients were asked to reply with a Yes message once the dose was taken or No if not taken. Adherence, patient compliance with imatinib, was considered to be a response of Yes received via a phone message from patients. If no reply was received within a designated time period (2 hours), this was interpreted as No.
Once a week, patients completed a side-effect assessment online via the REMIND system using a smart cellular phone or computer. For each of 11 potential side effects/symptoms patients may experience when taking imatinib, they reported severity and extent of bother on a scale of 1-10 for each side effect present. Automatically generated self-care advice was customized to the symptoms reported and sent to the patients in real time.
If 10% of scheduled doses in a week were missed or if side-effect severity was ≥4, this was included on a daily digest, which was forwarded to the nurse assigned to that patient via email. The nurse could decide to contact the patient outside of the scheduled consultations to discuss self-management strategies or address medication adherence barrier(s).
Phone-Based Nurse Consultation Sessions (4 Sessions in Weeks 1, 2, 6, and 10)
In the week before the first nurse consultation, the patient reported medication adherence daily, completed the weekly medication side-effect assessment, and selected questions from the CML-specific QPL. QPLs are structured list of commonly asked questions customized to disease type and have been found to improve clinician-patient communication and increase the amount of desired information received by patients [60]. The nurse accessed the REMIND system to view the patient’s responses to shape content of the first nurse consultation session. It covered the following: (1) building rapport; (2) addressing questions noted on the QPL; (3) establishing common ground by assessing patients’ understanding of their diagnosis, current symptom experience, and medication regimen; (4) discussing the patient’s medication adherence and explored adherence barriers; (5) coaching to address barriers; (6) discussing medication side effects; (7) coaching in evidence-based self-care strategies targeting each reported side effect; and (8) summarizing the consultation and assessing patient understanding. The subsequent consultations used the REMIND system’s patient-reported information to cover (4) to (8).
Phase 2: Pilot Testing
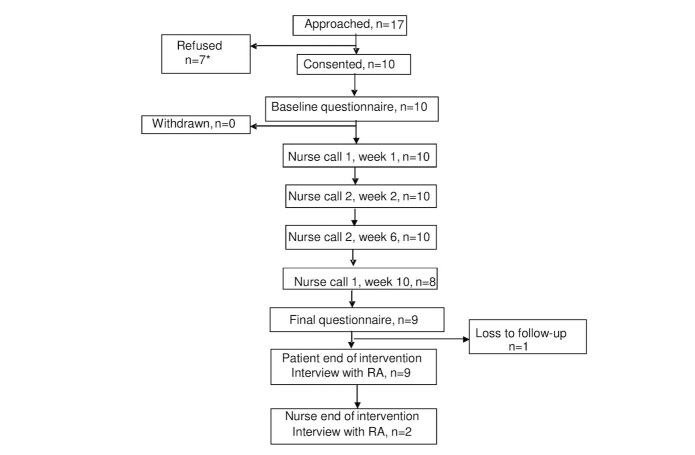
A total of 10 out of 17 patients agreed to participate, with a response rate of 58% (Figure 3). Of the participants, 9 completed all components of the intervention and 1 participant ceased before week 10 due to travel. Another participant was lost to follow-up. In total, 9 patients and 2 nurses completed interviews.
Figure 3.
CONSORT flowchart of participants completing each intervention component and data collection point (RA refers to research assistant).
Participant demographics are described in Table 1. Median age of patient participants was 54 years, ranging from 35 to 72 years; the majority of participants, that is, 6 out of 9, were male (67%). The 9 patients who participated in the pilot testing had received imatinib therapy for a median of 4 years before the start of the study, with a range from 15 days to 12 years.
Table 1.
Participant demographics.
| Characteristics | Patients (N=9) | |
| Age (years) | ||
| Median (interquartile range) | 54 (44.5-60.0) | |
| Range | 35-72 | |
| Sex, n (%) | ||
| Male | 6 (67) | |
| Female | 3 (33) | |
| Country of birth, n (%) | ||
| Australia | 7 (78) | |
| China | 1 (11) | |
| England | 1 (11) | |
| English as first language, n (%) | ||
| Yes | 8 (89) | |
| No | 1 (11) | |
| Education (highest level completed), n (%) | ||
| Secondary/high school | 3 (33) | |
| Trade/Technical and Further Education | 1 (11) | |
| Bachelor’s degree | 3 (33) | |
| Postgrad diploma/masters/PhD | 2 (22) | |
| Time since diagnosis | ||
| Median (interquartile range in years) | 4 (1-13) | |
| Range | 1 month-17 years | |
| Imatinib treatment durationa | ||
| Median (interquartile range in months) | 48 (16-123) | |
| Range | 15 days-12 years | |
| Employment status, n (%) | ||
| Full time | 4 (44) | |
| Part time | 2 (22) | |
| Home duties | 1 (11) | |
| Retired | 2 (22) | |
| Residence | ||
| Metropolitan | 4 (44) | |
| Rural | 5 (56) | |
aDuration on imatinib at the start of REMIND study: Patient 2 (9 years), Patient 3 (5 years), Patient 4 (4 years), Patient 5 (2.5 years), Patient 6 (11.5 years), Patient 7 (1 year), Patient 8 (1.6 years), Patient 9 (12 years), Patient 10 (2 weeks).
The system administrator as detected by the REMIND system monitored adherence (Table 2). The SMSs were sent on time (<1 hour) to patients on 671 out of 684 (98.0%) occasions, with a range of 0-7% participants experiencing SMS failure and a range of 0-22% participants not replying to SMS messages at all, over the total study period. In total, 2 patients reported that up to 40% of messages were not received by them by the appointed time due to problems with slow networks, particularly in rural areas.
Table 2.
System administrator details of SMS (short message service) texts sent and answered by patients.
| Patient | Total days | SMS failure, n (%) | Patient failure to answer SMS when sent, n (%) | |
| In 2 hours of SMS | Not at all | |||
| 2 | 74 | 5 (7) | 5 (7) | 5 (7) |
| 3 | 79 | 1 (1) | 3 (4) | 3 (4) |
| 4 | 62 | 0 (0) | 4 (7) | 3 (5) |
| 5 | 69 | 0 (0) | 14 (20) | 10 (15) |
| 6 | 120 | 0 (0) | 8 (7) | 6 (5) |
| 7 | 79 | 0 (0) | 4 (5) | 4 (5) |
| 8 | 80 | 1 (1) | 32 (41) | 17 (22) |
| 9 | 57 | 0 (0) | 2 (4) | 0 (0) |
| 10 | 75 | 4 (5) | 8 (11) | 8 (11) |
A number of common themes were identified across the nurses and patients.
Accountability and Routine
Patients and nurses reported that they found the intervention acceptable and helpful for improving adherence through enabling patient accountability. A newly diagnosed patient stated:
...it definitely helped reinforce habit when I was at the start of getting on, getting this medication into routine.
PT10
Longer-term patients with changed circumstances also benefited:
I’ve had overseas visitors for the last 2 weeks so I’m out of my usual routine…I’m sure I would have missed some of my drug taking…had I not had the reminders.
PT6
A nurse spoke about the intervention for modifying adherence behavior:
...we discussed the barriers that she had to taking her tablet regularly. And then over time she softened and realized that she could develop strategies to overcome the problems...
N2
Alleviating Psychological and Symptom Distress
Patients spoke about the benefits for alleviating psychological and symptom distress. The intervention was reported to be particularly useful for easing uncertainty about symptoms/side effects. One of the participants said:
It was good...if someone (nurse) noticed a change in symptoms I might not have picked up on it, and they were able to discuss it with me and find out why it might have happened.
PT3
Patients felt reassured by the intervention:
...the good part...was the personal touch...it’s just that really nice reminder that there’s someone out there that’s half looking out for you.
PT5
Access to expert advice was also valued.
You do talk about things with your partner but you don’t go down some of the tracks that you probably would with a nurse...they prompt information out of you from their knowledge.
PT5
Supporting Self-Efficacy
The intervention was identified as useful for uptake of self-management strategies and encouraging informed decision making. One patient commented:
Because I’ve been doing this, it makes me think about other things like diet.
PT2
Another patient explained self-care options and informed choice:
...we did discuss that I could take Imodium (diarrhoea medication) or something like that if I wanted to but...I didn’t want to take any more tablets...where I can’t get to a toilet and if I did need, like a wedding, I’d take Imodium to make sure that I didn’t have any accidents.
PT3
Functionality of Intervention
Overall, patients found the REMIND system highly usable. Although a patient (PT6) described the process as “pretty straightforward,” another mentioned:
I suppose the only bit I found a bit off-putting the most was the questions around depression and anxiety.
PT10
Patients routinely had access to a cellular phone; however, one patient admitted that they:
...struggled with that [the 2 hour window to respond].
PT 7
Most patients found the system reliable and received the messages at the appointed time, but there were exceptions:
There were maybe three times that the computer system either didn’t send a text or it was an hour or 2 late...
PT3
A rural patient had ongoing problems as he had an unreliable telephone network, and reported as many as 40% of the text messages delayed by 30-45 min [PT10]. The 2 participating nurses differed in their opinion about the REMIND system: one found it easy but the other needed time to become familiar with it. Both nurses found the initial QPL useful, comprehensive, and helpful in shaping the consultation content and facilitating rapport. The resource and intervention manuals supplied to the nurses were endorsed as comprehensive and very helpful, particularly the session checklist. Reorganization of the manual was suggested:
...I find these manuals just a bit fiddly to work around so that’s why I’ve got like post it notes everywhere...it’d be good if there was tabs or something...
N1
Training in motivational interviewing was perceived as beneficial:
I gained skills in the motivational interviewing techniques which I continue to use.
N2
Both nurses felt that the timing and number of interview sessions were appropriate with no recommendations for alterations. Both suggested that daily digest (or summary) of patient adherence and symptom responses should be emailed less frequently than daily because it was “too much.”
Overall, 3 patients fell below 90% reported adherence levels. The protocol stated patients with adherence below 90% should have been contacted outside to the scheduled calls. However, both nurses expressed that they were in a quandary when patients did not SMS Yes, but relied on their experience and decided not to make extra contact. One of the nurses commented:
My personal experiences on the phone calls has been that patients report that they are adhering to their medications even if they’re not responding to their SMS, so it’s kind of a bit tricky...I guess it’s a guide as to whether they’re taking their tablet but it’s not definitive...
N1
Optimal Intervention Target Group
In terms of timing, most patients thought the intervention would be most beneficial at the start of therapy or, even at diagnosis:
...if they’re just starting out on Glivec I reckon it would be invaluable.
PT2
The newly diagnosed participant (PT10) commented that it was “good timing” for him. Nurses agreed suggesting:
...A lot of people said it would have been helpful when they were first diagnosed.
N1
However, they also suggested that the intervention has broad applicability:
Not only in patients with CML but...there’s a whole lot of new oral medications on the market now for a whole variety of cancers and conditions.
N1
Discussion
Principal Findings: Intervention Development
The framework by Schofield and Chambers was successfully applied to the development process. Preliminary qualitative research [49] served to delineate the patient problem and aligned the intervention goals with patients’ needs. Relevant evidence and theoretical perspectives were investigated through literature reviews and combined with reputable patient education material to create appropriate evidence-based content and resources for the intervention. The automated features of the REMIND system permitted flexibility in intervention content and responsiveness to individualized concerns. Combining automated reminders, side-effect assessments, and self-care advice with personalized coaching fostered self-management. The dose of the intervention was maximized by using resource-intensive nursing expertise efficiently by basing the content of consultations on automated reminders and symptom assessments. While the standardized material supports reproducibility, the codesign processes promote acceptability. These approaches are consistent with the current literature that recognizes improved knowledge of disease course, medication, and management of side effects paired with expert clinician support as integral to improving medication adherence in patients with CML [15].
Principal Findings: Acceptability and Clinical Feasibility of Intervention
Pilot testing demonstrated that this intervention was able to be implemented and integrated into the clinical management of 10 patients with CML. It was highly acceptable to both patients and nurses. Most patients indicated that receiving and responding to the text reminders prompted medication adherence due to accountability. Nurses felt many long-term patients already had well-established routines. Although most patients agreed, those who changed their routine, for example, dining out, found the text reminder was useful to them. Nevertheless, both patients and nurses believed that this intervention would be most useful for patients at commencement of drug therapy. Patients found discussing their medication side effects with the nurse and receiving expert advice regarding self-management highly beneficial. Other benefits included increasing awareness of self-care, encouraging informed decision making and feeling reassured.
The usability of the intervention was high: most patients expressed ease with text reminders and the weekly symptoms survey. A small proportion of patients did experience difficulty either receiving or responding to the text within 2 hours either because they were not used to carrying a cellular phone or slow Internet speed. However, as the adoption of cellular phones/devices continues to rise and Internet speed and geographic coverage improve [61], this barrier is likely to diminish in future.
The nurses endorsed all facets of the system, including the administration website, QPL, resource and intervention manuals, session checklists, symptom surveys, automatically generated advice, and nurse phone consultations. They suggested improving the layout of the intervention manual and less frequently emailed daily digest (or summary) of patient adherence and symptom responses. One departure from the intervention protocol was nurses not calling patients when patients were less than 90% adherent. It is recommended that the application of the protocol is routinely monitored and departures are addressed through ongoing supervision and training. In particular, the importance of contacting patients who are less than 90% adherent immediately, not waiting until the next scheduled consultation, should be emphasized. Supervision could include role playing these scenarios to increase the skills to deal with issue.
Limitations
The limitations of this study include a relatively small sample of nurses and patients used to pilot test this intervention. Given the sample size, it is unlikely that saturation was reached. As CML is a relatively rare disease, it proved difficult and time-consuming to achieve this modest sample size. Furthermore, most patients had been diagnosed years before the study, hence possibly had higher levels of medication adherence than those newly diagnosed. Future trials may need to explore a different cancer type to test the impact of this intervention upon oral medication adherence. As high-cost oral therapeutics in oncology continue to become more prevalent, it will be critical to ensure that adherence is high to deliver the anticipated survival outcomes to ensure that the health care expenditure is justified [4].
Comparison With Prior Work
Despite phone text reminder interventions for patients’ with HIV [37], cardiac disease [35], and other chronic conditions [38] demonstrating improvements to medication adherence, not all studies included nurse or counselor supportive care in the intervention. In our study, an innovative medication intervention was developed and tested, which combined 2 features: (1) daily medication reminders and routine assessment of side effects with evidence-based self-care advice delivered in real time; and (2) QPL questions and routinely collected individual patient adherence and side-effect profile data used to shape nurses’ consultations, which employed motivational interviewing to support adoption of self-management behaviors.
A secondary analysis [41] identified 2 randomized controlled trials of phone text reminder adherence interventions combined with counseling sessions, 1 study in patients with HIV [62,63] and the other in patients with diabetes, which has since been retracted. Qualitative findings indicated beneficial experiences where patients with HIV emphasized emotional and mental support from health care providers in addition to medical care [63]. A similar finding has been described in patients with tuberculosis who reported feeling cared for and thankful that health professionals were available to answer their questions while reminders instilled a sense of responsibility and personal accountability for their treatment [33]. Comparably, patients receiving phone symptom monitoring and nurse support while undergoing chemotherapy for cancer have reported feeling reassured [45]. Although previous studies have recognized text reminders prompt adherence in the home environment as well as on vacation [21,34], some patients believed reminders may be more useful earlier in diagnosis before habits being formed [34]. Many of these sentiments were also expressed by patients in our study.
Patients in our study reported technological difficulties and an absence of cellular phones, whereas additional reasons for not responding have been reported in the literature. These include problems with cellular phones, out-of-phone credit, the phone being switched off, the phone’s battery running out, and losing the cellular phone [25,33,63]. It will be difficult for health services to overcome these types of practical problems and they may have to be accepted as a limitation of this type of intervention.
Conclusions
This paper contributes to the evolving knowledge base of how to develop an evidence-based, acceptable, and potentially effective complex intervention. We have provided examples of strategies that may assist researchers and clinicians in developing or trying out a new psychoeducational intervention. These strategies can enhance intervention methodological rigor for testing in a randomized controlled trial through standardized intervention content and training; and implementation considerations such as improved clinical acceptability through the codesign process. To our knowledge, this is the first cancer medication adherence intervention that integrates a mHealth platform with tailored phone consultations based on patient-provided questions, adherence rates, and side-effect profiles provided by nurses trained in motivational interviewing. The intervention was clinically feasible. Patients and nurses endorsed this intervention as acceptable and useful particularly for a newly diagnosed patient. The next step will be to conduct an appropriately powered randomized controlled trial targeting a wider range of cancer diagnoses that are currently managed by oral therapeutics to ensure adequate recruitment and assess broader applicability. This future research will focus on patients at diagnosis and assess adherence with an electronic pill monitoring device to examine short- and long-term adherence. In addition, because chronic illnesses are more prevalent among older citizens, many people will have 2 or more comorbidities requiring self-management with oral medications. Future iterations of the REMIND platform should cater for multiple medications for multiple comorbidities. Health care resource utilization and sustainability of the intervention should also be considered by integrating an economic evaluation into the randomized controlled trial.
Acknowledgments
This study was supported by a Peter MacCallum Cancer Foundation Grant and a seed funding grant from Australasian Leukaemia and Lymphoma Group. PS was supported by a National Health and Medical Research Council Career Development Award, ID 628563. JS has received honoraria as a member of the Novartis Advisory Board. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. This study has received institutional approval by the Peter MacCallum Ethics Committee, Ethics #E22-10. The authors would like to thank the patients who participated and Dhirendra Singh, Catherine Vassili, and Andrew Murnane for their assistance in the study.
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- CML
chronic myeloid leukemia
- HIV
human immunodeficiency virus
- mHealth
mobile health
- MRC
Medical Research Council
- QPL
question prompt list
- TKI
tyrosine kinase inhibitor
Footnotes
Authors' Contributions: PS, JFS, PB, LC, AU, SA, SB, and SK designed and lead the research program. AD provided patient feedback on the intervention design and research procedures. AP-S, JW, LR, CV, BO, and KO carried out the research program. PS, JFS, PB, SA, SB, AU, AD, CV, SK, and BO developed the content of the intervention. AP-S, JW, and PS analyzed the data. LC and KO designed and built the Web-based platform. All authors contributed to the writing and/or critical revision of the paper.
Conflicts of Interest: Janssen employs PS as an ad hoc consultant on medication adherence.
References
- 1.Baccarani M, Dreyling M, ESMO Guidelines Working Group Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010 May;21 Suppl 5:v165–7. doi: 10.1093/annonc/mdq201. [DOI] [PubMed] [Google Scholar]
- 2.Breccia M, Alimena G. How to treat CML patients in the tyrosine kinase inhibitors era? From imatinib standard dose to second generation drugs front-line: unmet needs, pitfalls and advantages. Cancer Lett. 2012 Sep 28;322(2):127–32. doi: 10.1016/j.canlet.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 3.Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, Baccarani M, Deininger MW, Cervantes F, Fujihara S, Ortmann CE, Menssen HD, Kantarjian H, O'Brien SG, Druker BJ, IRIS Investigators Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017 Dec 9;376(10):917–27. doi: 10.1056/NEJMoa1609324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel AB, Wilds BW, Deininger MW. Treating the chronic-phase chronic myeloid leukemia patient: which TKI, when to switch and when to stop? Expert Rev Hematol. 2017 Jul;10(7):659–74. doi: 10.1080/17474086.2017.1330144. [DOI] [PubMed] [Google Scholar]
- 5.Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, Cervantes F, Deininger M, Gratwohl A, Guilhot F, Hochhaus A, Horowitz M, Hughes T, Kantarjian H, Larson R, Radich J, Simonsson B, Silver RT, Goldman J, Hehlmann R, European LeukemiaNet Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009 Dec 10;27(35):6041–51. doi: 10.1200/JCO.2009.25.0779. http://europepmc.org/abstract/MED/19884523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahon FX, Réa D, Guilhot J, Guilhot F, Huguet F, Nicolini F, Legros L, Charbonnier A, Guerci A, Varet B, Etienne G, Reiffers J, Rousselot P, Intergroupe Français des Leucémies Myéloïdes Chroniques Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010 Nov;11(11):1029–35. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 7.Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Yeung DT, Dang P, Goyne JM, Slader C, Filshie RJ, Mills AK, Melo JV, White DL, Grigg AP, Hughes TP. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013 Jul 25;122(4):515–22. doi: 10.1182/blood-2013-02-483750. http://www.bloodjournal.org/cgi/pmidlookup?view=long&pmid=23704092. [DOI] [PubMed] [Google Scholar]
- 8.Bauer S, Buchanan S, Ryan I. Tyrosine kinase inhibitors for the treatment of chronic-phase chronic myeloid leukemia: long-term patient care and management. J Adv Pract Oncol. 2016;7(1):42–54. doi: 10.6004/jadpro.2016.7.1.3. http://europepmc.org/abstract/MED/27713843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douxfils J, Haguet H, Mullier F, Chatelain C, Graux C, Dogné JM. Association between BCR-ABL tyrosine kinase inhibitors for chronic myeloid leukemia and cardiovascular events, major molecular response, and overall survival: a systematic review and meta-analysis. JAMA Oncol. 2016 Feb 4; doi: 10.1001/jamaoncol.2015.5932. [DOI] [PubMed] [Google Scholar]
- 10.Mauro MJ, Deininger MW. Management of drug toxicities in chronic myeloid leukaemia. Best Pract Res Clin Haematol. 2009 Sep;22(3):409–29. doi: 10.1016/j.beha.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Moslehi JJ, Deininger M. Tyrosine kinase inhibitor-associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol. 2015 Dec 10;33(35):4210–8. doi: 10.1200/JCO.2015.62.4718. http://europepmc.org/abstract/MED/26371140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alrabiah Z, Alhossan A, Yun S, MacDonald K, Abraham I. Adherence to tyrosine kinase inhibitor therapy in patients with chronic myeloid leukemia: meta-analyses of prevalence rates by measurement method. Blood. 2016;128(22):3610. [Google Scholar]
- 13.Bazeos A, Khorashad J, Mahon FX, Eliasson LL, Milojkovic D, Bua M, Apperley J, Szydlo R, Kozlowski K, Paliompeis C, Desai R, Foroni L, Reid A, de Lavallade H, Rezvani K, Goldman J, Marin D. Long term adherence to imatinib therapy is the critical factor for achieving molecular responses in chronic myeloid leukemia patients. Blood. 2009;114(22):3290. [Google Scholar]
- 14.Marin D, Bazeos A, Mahon FX, Eliasson L, Milojkovic D, Bua M, Apperley JF, Szydlo R, Desai R, Kozlowski K, Paliompeis C, Latham V, Foroni L, Molimard M, Reid A, Rezvani K, de Lavallade H, Guallar C, Goldman J, Khorashad JS. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010 May 10;28(14):2381–8. doi: 10.1200/JCO.2009.26.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geissler J, Sharf G, Bombaci F, Daban M, De Jong J, Gavin T, Pelouchova J, Dziwinski E, Hasford J, Hoffmann VS. Factors influencing adherence in CML and ways to improvement: results of a patient-driven survey of 2546 patients in 63 countries. J Cancer Res Clin Oncol. 2017 Jul;143(7):1167–76. doi: 10.1007/s00432-017-2372-z. [DOI] [PubMed] [Google Scholar]
- 16.Noens L, Hensen M, Kucmin-Bemelmans I, Lofgren C, Gilloteau I, Vrijens B. Measurement of adherence to BCR-ABL inhibitor therapy in chronic myeloid leukemia: current situation and future challenges. Haematologica. 2014 Mar;99(3):437–47. doi: 10.3324/haematol.2012.082511. http://www.haematologica.org/cgi/pmidlookup?view=long&pmid=24598855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noens L, van Lierde MA, De Bock R, Verhoef G, Zachée P, Berneman Z, Martiat P, Mineur P, Van Eygen K, MacDonald K, De Geest S, Albrecht T, Abraham I. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood. 2009 May 28;113(22):5401–11. doi: 10.1182/blood-2008-12-196543. http://www.bloodjournal.org/cgi/pmidlookup?view=long&pmid=19349618. [DOI] [PubMed] [Google Scholar]
- 18.Eliasson L, Clifford S, Barber N, Marin D. Exploring chronic myeloid leukemia patients' reasons for not adhering to the oral anticancer drug imatinib as prescribed. Leuk Res. 2011 May;35(5):626–30. doi: 10.1016/j.leukres.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 19.de Almeida MH, Fogliatto L, Couto D. Importance of adherence to BCR-ABL tyrosine-kinase inhibitors in the treatment of chronic myeloid leukemia. Rev Bras Hematol Hemoter. 2014;36(1):54–9. doi: 10.5581/1516-8484.20140014. http://europepmc.org/abstract/MED/24624037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood L. A review on adherence management in patients on oral cancer therapies. Eur J Oncol Nurs. 2012 Sep;16(4):432–8. doi: 10.1016/j.ejon.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 21.da Costa TM, Barbosa BJ, Gomes e Costa DA, Sigulem D, de Fátima Marin H, Filho AC, Pisa IT. Results of a randomized controlled trial to assess the effects of a mobile SMS-based intervention on treatment adherence in HIV/AIDS-infected Brazilian women and impressions and satisfaction with respect to incoming messages. Int J Med Inform. 2012 Apr;81(4):257–69. doi: 10.1016/j.ijmedinf.2011.10.002. http://europepmc.org/abstract/MED/22296762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Georgette N, Siedner MJ, Zanoni B, Sibaya T, Petty CR, Carpenter S, Haberer JE. The acceptability and perceived usefulness of a weekly clinical SMS program to promote HIV antiretroviral medication adherence in KwaZulu-Natal, South Africa. AIDS Behav. 2016 Nov;20(11):2629–38. doi: 10.1007/s10461-016-1287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardy H, Kumar V, Doros G, Farmer E, Drainoni M, Rybin D, Myung D, Jackson J, Backman E, Stanic A, Skolnik PR. Randomized controlled trial of a personalized cellular phone reminder system to enhance adherence to antiretroviral therapy. AIDS Patient Care STDS. 2011 Mar;25(3):153–61. doi: 10.1089/apc.2010.0006. http://europepmc.org/abstract/MED/21323532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalichman SC, Kalichman MO, Cherry C, Eaton LA, Cruess D, Schinazi RF. Randomized factorial trial of phone-delivered support counseling and daily text message reminders for HIV treatment adherence. J Acquir Immune Defic Syndr. 2016 Sep 1;73(1):47–54. doi: 10.1097/QAI.0000000000001020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pop-Eleches C, Thirumurthy H, Habyarimana JP, Zivin JG, Goldstein MP, de Walque D, MacKeen L, Haberer J, Kimaiyo S, Sidle J, Ngare D, Bangsberg DR. Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: a randomized controlled trial of text message reminders. AIDS. 2011 Mar 27;25(6):825–34. doi: 10.1097/QAD.0b013e32834380c1. http://europepmc.org/abstract/MED/21252632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodrigues R, Shet A, Antony J, Sidney K, Arumugam K, Krishnamurthy S, D'Souza G, DeCosta A. Supporting adherence to antiretroviral therapy with mobile phone reminders: results from a cohort in South India. PLoS One. 2012;7(8):e40723. doi: 10.1371/journal.pone.0040723. http://dx.plos.org/10.1371/journal.pone.0040723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sidney K, Antony J, Rodrigues R, Arumugam K, Krishnamurthy S, D'souza G, De Costa A, Shet A. Supporting patient adherence to antiretrovirals using mobile phone reminders: patient responses from South India. AIDS Care. 2012;24(5):612–7. doi: 10.1080/09540121.2011.630357. [DOI] [PubMed] [Google Scholar]
- 28.Nelson LA, Mulvaney SA, Gebretsadik T, Johnson KB, Osborn CY. The MEssaging for Diabetes (MED) intervention improves short-term medication adherence among low-income adults with type 2 diabetes. J Behav Med. 2016 Dec;39(6):995–1000. doi: 10.1007/s10865-016-9774-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vervloet M, van Dijk L, de Bakker DH, Souverein PC, Santen-Reestman J, van Vlijmen B, van Aarle MC, van Der Hoek LS, Bouvy ML. Short- and long-term effects of real-time medication monitoring with short message service (SMS) reminders for missed doses on the refill adherence of people with Type 2 diabetes: evidence from a randomized controlled trial. Diabet Med. 2014 Jul;31(7):821–8. doi: 10.1111/dme.12439. [DOI] [PubMed] [Google Scholar]
- 30.Vervloet M, van Dijk L, Santen-Reestman J, van Vlijmen B, Bouvy ML, de Bakker DH. Improving medication adherence in diabetes type 2 patients through Real Time Medication Monitoring: a randomised controlled trial to evaluate the effect of monitoring patients' medication use combined with short message service (SMS) reminders. BMC Health Serv Res. 2011 Jan 10;11:5. doi: 10.1186/1472-6963-11-5. https://bmchealthservres.biomedcentral.com/articles/10.1186/1472-6963-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conway CM, Kelechi TJ. Digital health for medication adherence in adult diabetes or hypertension: an integrative review. JMIR Diabetes. 2017 Aug 16;2(2):e20. doi: 10.2196/diabetes.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strandbygaard U, Thomsen SF, Backer V. A daily SMS reminder increases adherence to asthma treatment: a three-month follow-up study. Respir Med. 2010 Feb;104(2):166–71. doi: 10.1016/j.rmed.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Iribarren S, Beck S, Pearce PF, Chirico C, Etchevarria M, Cardinale D, Rubinstein F. TextTB: a mixed method pilot study evaluating acceptance, feasibility, and exploring initial efficacy of a text messaging intervention to support TB treatment adherence. Tuberc Res Treat. 2013;2013:349394. doi: 10.1155/2013/349394. doi: 10.1155/2013/349394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohammed S, Siddiqi O, Ali O, Habib A, Haqqi F, Kausar M, Khan AJ. User engagement with and attitudes towards an interactive SMS reminder system for patients with tuberculosis. J Telemed Telecare. 2012 Oct;18(7):404–8. doi: 10.1258/jtt.2012.120311. [DOI] [PubMed] [Google Scholar]
- 35.Adler AJ, Martin N, Mariani J, Tajer CD, Owolabi OO, Free C, Serrano NC, Casas JP, Perel P. Mobile phone text messaging to improve medication adherence in secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2017 Apr 29;4:CD011851. doi: 10.1002/14651858.CD011851.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rathbone AL, Prescott J. Use of mobile apps and SMS messaging as physical and mental health interventions: systematic review. J Med Internet Res. 2017 Aug 24;19(8):e295. doi: 10.2196/jmir.7740. http://www.jmir.org/2017/8/e295/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horvath T, Azman H, Kennedy GE, Rutherford GW. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane Database Syst Rev. 2012 Mar 14;3:CD009756. doi: 10.1002/14651858.CD009756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thakkar J, Kurup R, Laba TL, Santo K, Thiagalingam A, Rodgers A, Woodward M, Redfern J, Chow CK. Mobile telephone text messaging for medication adherence in chronic disease: a meta-analysis. JAMA Intern Med. 2016 Mar;176(3):340–9. doi: 10.1001/jamainternmed.2015.7667. [DOI] [PubMed] [Google Scholar]
- 39.Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, Agoritsas T, Mistry N, Iorio A, Jack S, Sivaramalingam B, Iserman E, Mustafa RA, Jedraszewski D, Cotoi C, Haynes RB. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014 Nov 20;(11):CD000011. doi: 10.1002/14651858.CD000011.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kato PM, Cole SW, Bradlyn AS, Pollock BH. A video game improves behavioral outcomes in adolescents and young adults with cancer: a randomized trial. Pediatrics. 2008 Aug;122(2):e305–17. doi: 10.1542/peds.2007-3134. [DOI] [PubMed] [Google Scholar]
- 41.Mistry N, Keepanasseril A, Wilczynski NL, Nieuwlaat R, Ravall M, Haynes RB, Patient Adherence Review Team Technology-mediated interventions for enhancing medication adherence. J Am Med Inform Assoc. 2015 Apr;22(e1):e177–93. doi: 10.1093/jamia/ocu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Timmers L, Boons CC, Verbrugghe M, van den Bent BJ, Van Hecke A, Hugtenburg JG. Supporting adherence to oral anticancer agents: clinical practice and clues to improve care provided by physicians, nurse practitioners, nurses and pharmacists. BMC Cancer. 2017 Feb 10;17(1):122. doi: 10.1186/s12885-017-3110-2. https://bmccancer.biomedcentral.com/articles/10.1186/s12885-017-3110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winkeljohn DL. Oral chemotherapy medications: the need for a nurse's touch. Clin J Oncol Nurs. 2007 Dec;11(6):793–6. doi: 10.1188/07.CJON.793-796. [DOI] [PubMed] [Google Scholar]
- 44.Maguire R, McCann L, Miller M, Kearney N. Nurse's perceptions and experiences of using of a mobile-phone-based Advanced Symptom Management System (ASyMS) to monitor and manage chemotherapy-related toxicity. Eur J Oncol Nurs. 2008 Sep;12(4):380–6. doi: 10.1016/j.ejon.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 45.McCann L, Maguire R, Miller M, Kearney N. Patients' perceptions and experiences of using a mobile phone-based advanced symptom management system (ASyMS) to monitor and manage chemotherapy related toxicity. Eur J Cancer Care (Engl) 2009 Mar;18(2):156–64. doi: 10.1111/j.1365-2354.2008.00938.x. [DOI] [PubMed] [Google Scholar]
- 46.Eysenbach G, CONSORT-EHEALTH Group CONSORT-EHEALTH: improving and standardizing evaluation reports of Web-based and mobile health interventions. J Med Internet Res. 2011 Dec 31;13(4):e126. doi: 10.2196/jmir.1923. http://www.jmir.org/2011/4/e126/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schofield P, Chambers S. Effective, clinically feasible and sustainable: key design features of psycho-educational and supportive care interventions to promote individualised self-management in cancer care. Acta Oncol. 2015 May;54(5):805–12. doi: 10.3109/0284186X.2015.1010016. [DOI] [PubMed] [Google Scholar]
- 48.Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M, Medical Research Council Guidance Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008 Sep 29;337:a1655. doi: 10.1136/bmj.a1655. http://europepmc.org/abstract/MED/18824488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu S, Chee D, Ugalde A, Butow P, Seymour J, Schofield P. Lack of congruence between patients' and health professionals' perspectives of adherence to imatinib therapy in treatment of chronic myeloid leukemia: a qualitative study. Palliat Support Care. 2015 Apr;13(2):255–63. doi: 10.1017/S1478951513001260. [DOI] [PubMed] [Google Scholar]
- 50.Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. New York: Guilford Press; 1991. [Google Scholar]
- 51.Lotfi-Jam K, Carey M, Jefford M, Schofield P, Charleson C, Aranda S. Nonpharmacologic strategies for managing common chemotherapy adverse effects: a systematic review. J Clin Oncol. 2008 Dec 1;26(34):5618–29. doi: 10.1200/JCO.2007.15.9053. [DOI] [PubMed] [Google Scholar]
- 52.Deci EL, Ryan RM. Intrinsic Motivation and Self-Determination in Human Behavior. New York: Plenum; 1985. [Google Scholar]
- 53.Palacio A, Garay D, Langer B, Taylor J, Wood BA, Tamariz L. Motivational interviewing improves medication adherence: a systematic review and meta-analysis. J Gen Intern Med. 2016 Aug;31(8):929–40. doi: 10.1007/s11606-016-3685-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bate P, Robert G. Bringing User Experience to Healthcare Improvement: The Concepts, Methods and Practices of Experience-based Design. Oxford: Radcliffe Publishing; 2007. [Google Scholar]
- 55.Steen M, Manschot M, De Koning N. Benefits of co-design in service design projects. Int J Des. 2011;5(2):53–60. [Google Scholar]
- 56.Efficace F, Baccarani M, Breccia M, Saussele S, Abel G, Caocci G, Guilhot F, Cocks K, Naeem A, Sprangers M, Oerlemans S, Chie W, Castagnetti F, Bombaci F, Sharf G, Cardoni A, Noens L, Pallua S, Salvucci M, Nicolatou-Galitis O, Rosti G, Mandelli F. International development of an EORTC questionnaire for assessing health-related quality of life in chronic myeloid leukemia patients: the EORTC QLQ-CML24. Qual Life Res. 2014 Apr;23(3):825–36. doi: 10.1007/s11136-013-0523-5. [DOI] [PubMed] [Google Scholar]
- 57.Garcia SF, Cella D, Clauser SB, Flynn KE, Lad T, Lai JS, Reeve BB, Smith AW, Stone AA, Weinfurt K. Standardizing patient-reported outcomes assessment in cancer clinical trials: a patient-reported outcomes measurement information system initiative. J Clin Oncol. 2007 Nov 10;25(32):5106–12. doi: 10.1200/JCO.2007.12.2341. [DOI] [PubMed] [Google Scholar]
- 58.Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005 Dec;40(6 Pt 1):1918–30. doi: 10.1111/j.1475-6773.2005.00438.x. http://europepmc.org/abstract/MED/16336556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Green J, Thorogood N. Qualitative Methods for Health Research. London: SAGE Publications; 2009. [Google Scholar]
- 60.Dimoska A, Tattersall MH, Butow PN, Shepherd H, Kinnersley P. Can a “prompt list” empower cancer patients to ask relevant questions? Cancer. 2008 Jul 15;113(2):225–37. doi: 10.1002/cncr.23543. doi: 10.1002/cncr.23543. [DOI] [PubMed] [Google Scholar]
- 61.Ewing S, van der Nagel E, Thomas J, ARC Centre of Excellence for Creative Industries and Innovation Swinburne University of Technology . CCi Digital Futures 2014: The Internet in Australia. Hawthorn: Swinburne University of Technology; 2014. [Google Scholar]
- 62.Lester RT, Ritvo P, Mills EJ, Kariri A, Karanja S, Chung MH, Jack W, Habyarimana J, Sadatsafavi M, Najafzadeh M, Marra CA, Estambale B, Ngugi E, Ball TB, Thabane L, Gelmon LJ, Kimani J, Ackers M, Plummer FA. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010 Nov 27;376(9755):1838–45. doi: 10.1016/S0140-6736(10)61997-6. [DOI] [PubMed] [Google Scholar]
- 63.Smillie K, Van Borek N, Abaki J, Pick N, Maan EJ, Friesen K, Graham R, Levine S, van der Kop ML, Lester RT, Murray M. A qualitative study investigating the use of a mobile phone short message service designed to improve HIV adherence and retention in care in Canada (WelTel BC1) J Assoc Nurses AIDS Care. 2014;25(6):614–25. doi: 10.1016/j.jana.2014.01.008. http://linkinghub.elsevier.com/retrieve/pii/S1055-3290(14)00040-5. [DOI] [PubMed] [Google Scholar]