Introduction
Pili torti is a term that originates from Latin (pili meaning hair, torti meaning twisted) used to describe the irregularly flattened and twisted appearance of hair shafts on microscopic hair mount evaluation. Clinically, affected patients have fragile, coarse, and sparse hair. The 180° rotation along the hair shaft axis is thought to be caused by perifollicular fibrosis and abnormalities of the inner root sheath, with subsequent irregular formation of the hair shaft.1 The characteristic narrow and numerous twists distinguish pili torti from other types of hair shaft disorders.2
Congenital pili torti has been associated with a number of syndromes, including Björnstad syndrome, Menkes syndrome, and others.2 Björnstad syndrome is known to be caused by a missense mutation in the BCS1L gene on chromosome 2q34-36.3 BCS1L encodes an ATPase that has a central role in the proper functioning of the electron transport chain. In Björnstad syndrome, BSC1L mutations cause variable degrees of pili torti and sensorineural hearing loss, shown to be due to disrupted mitochondrial respirasome assembly and increased production of reactive oxygen species.3
Reports of acquired pili torti are few and have been associated with the use of oral retinoids and erlotinib and, in some patients, cicatricial alopecia. In these cases, however, the pili torti is localized, and medication-induced cases tend to resolve after discontinuation of the offending agent.4, 5, 6 Herein we describe 2 women with chronic graft-vs-host disease (cGVHD) with long-standing, diffuse alopecia and pili torti.
Case
Two female patients who underwent allogenic hematopoietic stem cell transplant (HSCT) with subsequent cGVHD sought treatment at the alopecia specialty clinic at Massachusetts General Hospital for abnormal regrowth of scalp hair >6 months after HSCT and induction treatment. They both lost 100% of scalp hair at the time of HSCT. The details of their induction regimens, HSCT, and demographics are listed in Table I. Both patients were given diagnoses of cGVHD by their respective oncologists on the basis of the National Institutes of Health consensus criteria for cGVHD. In addition to hair findings, they each had systemic involvement related to their cGVHD. Clinical findings for patient 1 included moderate dry eyes, transaminitis, mild respiratory symptoms, and moderate joint involvement with limited range of motion. Clinical findings for patient 2 included skin involvement with sclerotic features encompassing >50% of the body surface area, severe mouth involvement with major limitation of oral intake, severe dry eye with partial loss of vision, mild gastrointestinal involvement, elevated bilirubin >3 μmol/L, and mild joint involvement with limited range of motion. Regarding hair loss, both patients complained of hair sparseness, thinning, breakage, and failure to grow to prior length.
Table I.
Clinical characteristics of patients 1 and 2 with pili torti and chronic graft-vs-host disease
| HSCT indication | Induction regimen | Donor characteristics | Age at presentation, y | HSCT date | Time from HSCT to clinical presentation, month |
|---|---|---|---|---|---|
| AML | Cytarabine, daunorubicin | Matched related donor (brother, sex discordant) | 47 | August 27, 2015 | 20 |
| AML | RIC busulfan, fludarabine | Matched related donor (sister, sex concordant) | 66 | January 12, 2015 | 56 |
AML, Acute myeloid leukemia; HSCT, hematopoietic stem cell transplant; RIC, reduced intensity conditioning.
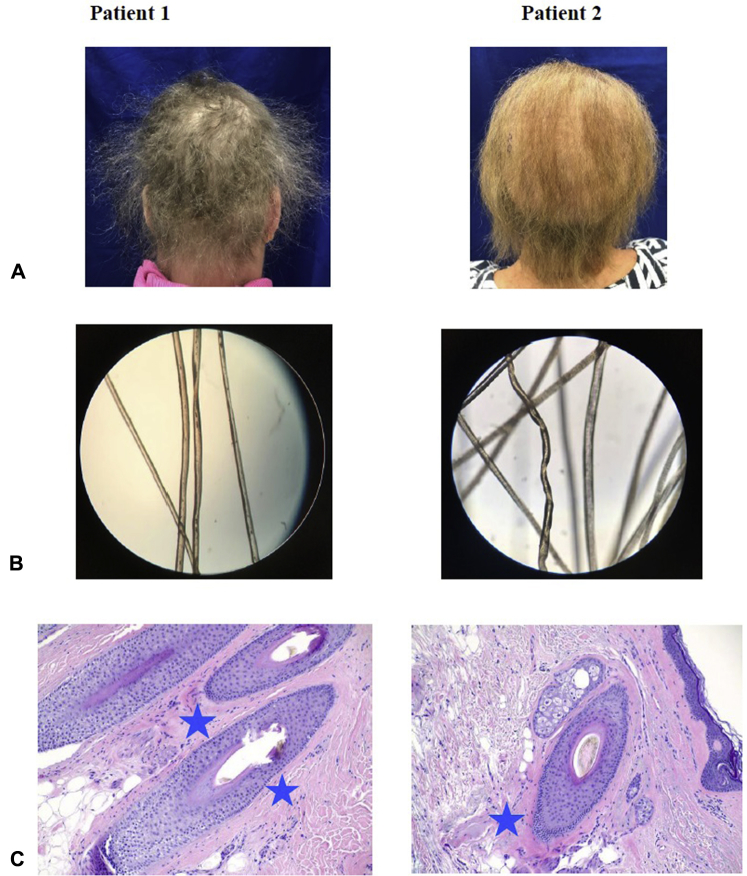
Both patients underwent hair and scalp evaluation, scalp biopsy, and microscopic hair mount evaluation. Clinical examination revealed diffusely coarse, sparse, and brittle hair (Fig 1, A). Both also underwent a hair pull test, in which the clinician grasps 50-60 hairs at the root and pulls firmly away from the scalp. Hair pull tests were normal, with <10% of hairs being removed from the root, but hair shafts were noted to break easily. Microscopic evaluation of the hair shafts revealed numerous hairs with pili torti (Fig 1, B). Histology revealed perifollicular fibrosis (Fig 1, C).
Fig 1.
Pili torti hair findings in patients 1 and 2. A, Clinical photographs of patient 1 and 2 reveal sparse, brittle hair. B, Hair mount microscopies of patient 1 and 2 reveal hair twisting at its axis. C, Histology of scalp biopsy from patient 1 and 2. Blue stars indicate areas of perifollicular fibrosis. (C, Hematoxylin-eosin stain; original magnification: ×20.)
Discussion
To our knowledge, this is the first report of acquired pili torti in patients with cGVHD of the scalp. The cause for long-standing hair loss in these patients is unclear but understandably caused a great deal of psychologic distress in these affected patients. One explanation for these findings is the presence of cGVHD affecting the scalp, given the notable perifollicular fibrosis seen on histology. Perifollicular fibrosis has been reported to disrupt the normal formation of the hair follicle by applying disruptive rotational forces on the inner root sheath in some acquired cases of pili torti. This mechanism is thought to lead to abnormal molding and twisting of the hair shaft.2 In these 2 cases, scalp biopsy revealed perifollicular fibrosis. Unfortunately, there is a lack of reports in the literature that describe histology of the scalp in patients with pili torti. Interestingly, in a case report of a patient treated with erlotinib found to have pili torti, perifollicular fibrosis with disintegration of the inner root sheath was noted on scalp biopsy histology, as well as an irregularly thinned outer root sheath.6 Further study is required to evaluate if chemotherapeutic agents or changes in host immunology that occur with cGVHD might lead to mutations in genes or aberrant functioning of the mitochondrial respiratory chain or if an accumulation of reactive oxygen species that target rapidly dividing hair follicle cells play a role in acquired pili torti.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Maruyama T., Toyoda M., Kanei A., Morohashi M. Pathogenesis in pili torti: morphological study. J Dermatol Sci. 1994;7(Suppl):S5–S12. doi: 10.1016/0923-1811(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 2.Mirmirani P., Samimi S.S., Mostow E. Pili torti: clinical findings, associated disorders, and new insights into mechanisms of hair twisting. Cutis. 2009;84(3):143–147. [PubMed] [Google Scholar]
- 3.Hinson J.T., Fantin V.R., Schonberger J. Missense mutations in the BCS1L gene as a cause of the Björnstad syndrome. N Engl J Med. 2007;356(8):809–819. doi: 10.1056/NEJMoa055262. [DOI] [PubMed] [Google Scholar]
- 4.Sakamoto F., Ito M., Saito R. Ultrastructural study of acquired pili torti-like hair defects accompanying pseudopelade. J Dermatol. 2002;29(4):197–201. doi: 10.1111/j.1346-8138.2002.tb00249.x. [DOI] [PubMed] [Google Scholar]
- 5.Hays S.B., Camisa C. Acquired pili torti in two patients treated with synthetic retinoids. Cutis. 1985;35(5):466–468. [PubMed] [Google Scholar]
- 6.Pirmez R., Pineiro-Maceira J., Gonzalez C.G., Miteva M. Loose anchoring of anagen hairs and pili torti due to erlotinib. Int J Trichol. 2016;8(4):186–187. doi: 10.4103/ijt.ijt_16_16. [DOI] [PMC free article] [PubMed] [Google Scholar]