Abstract
The human body is exposed to potentially pathogenic microorganisms at barrier sites such as the skin, lungs, and GI tract. To mount an effective response against these pathogens, the immune system must recruit the right cells with effector responses that are appropriate for the task at hand. Several types of CD4+ T cells can be recruited, including T helper cells known as Th1, Th2, and Th17, T follicular helper (Tfh) cells, and regulatory T cells (Tregs). These cells help to maintain normal immune homeostasis in the face of constantly changing microbes in the environment. As these cells differentiate from a common progenitor, the composition of their intracellular milieu of proteins changes to appropriately guide their effector function. One underappreciated process that impacts both levels and functions of effector fate-determining factors is ubiquitylation. This review will detail our current understanding of how ubiquitylation regulates CD4 T cell effector identity and function.
INTRODUCTION
Ubiquitylation is the post-translational addition of ubiquitin to a substrate protein. Ubiquitylation of a substrate requires the sequential action of three classes of enzymes: the E1 or ubiquitin activating enzyme, the E2 or ubiquitin conjugating enzyme, and the E3 ubiquitin ligase. E1s activate ubiquitin by the formation of a thiol ester with the carboxyl group of glycine 76 on ubiquitin1. The ubiquitin is then transferred to a catalytic cysteine on an E22, which then associates with an E3 ubiquitin ligase that is in a complex with a substrate. The E3 may serve as a scaffold to facilitate the transfer of ubiquitin from the E2 to the substrate, as is the case for RING (Really Interesting New Gene) type E3s3,4. Alternatively, HECT (Homologous to E6AP C-Terminus)5 and the RBR (RING-between-RING) type E3s6,7 first receive the ubiquitin onto a catalytic cysteine residue before transferring it to a lysine on the substrate. E3 ligases can thus identify the substrate as well as dictate the formation of ubiquitin linkages, driving the mono-, multi-mono-, or polyubiquitylation of the substrate. Ubiquitin has seven accessible lysines on its surface, K6, K11, K27, K29, K33, K48 or K63, each of which can be points of attachment for ubiquitin chains8–11. Furthermore, the amino terminal methionine of ubiquitin (M1) can also serve as a point of attachment for linear chains in a reaction catalyzed by an E3 ubiquitin ligase complex called the linear ubiquitination assembly complex (LUBAC)12. Ubiquitin chains can alter the fate of a substrate by changing its intercellular location, promoting its interactions with other proteins, or driving degradation. Ubiquitylation of protein substrates may be modified or reversed by enzymes called deubiquitinating enzymes (DUBs). The ~100 DUBs encoded by the human genome may be split into five main families based on their structural domains. These five families are the ubiquitin-specific proteases (USPs); the ubiquitin carboxy-terminal hydrolases (UCHs); the ovarian tumour-related proteases (OTUs); the Machado-Joseph disease domain proteases (MJDs) and the JAB1/MPN/Mov34 Metalloproteases (JAMMs)13. Given the capacity of ubiquitylation to alter protein levels and function, it is not surprising that ubiquitylation has the potential to influence the identity and function of CD4+ T cells.
When a naïve T cell encounters an antigen presenting cell (APC) expressing a peptide antigen displayed on its MHC (pMHC), the naïve T cell can get primed to become an effector T cell14,15. The sequence of events that leads to the formation of an effector requires that the T cell receive three signals; pMHC, co-stimulation, and cytokine16,17. Ubiquitylation can influence each of these signals within a T cell by altering the levels and functions of signaling intermediates or by influencing transcription factors that drive CD4+ T cell identity and function. Many E3 ligases have known roles in regulating T cell activation and co-stimulation. These include the RING E3 ligases: Cbl-b18–22, TRAC-123, Peli124 and the HECT E3 ligases, Itch and Nedd425–29, as reviewed30–33. This review will explore how ubiquitylation impacts the identity and lineage stability of CD4+ T helper/effector subsets.
TH1 CELLS
Th1 cells are important for the clearance of intracellular pathogens. They differentiate from naïve CD4+ T cells in response to pMHC and costimulation in a cytokine milieu containing IL-12 and IFNγ. IL-12 signaling induces the phosphorylation of Jak2 and Tyk2, leading to STAT4 activation which in turn drives IFNγ production34. In an amplification loop, IFNγ receptor signaling through STAT1 induces expression of the transcription factor T-Box Expressed In T Cells (T-bet)35. T-bet, transactivates the IFNγ gene to drive further IFNγ cytokine production35 and increases expression of the IL-12Rβ2 to promotes more IL-12 responsiveness36. Thus, STAT1, STAT4 and T-bet help to promote Th1 cell identity.
STAT1, STAT4, and T-bet are degraded via ubiquitin-mediated networks. An E3 ligase enzyme called STAT-interacting LIM (SLIM) protein (also known as PDLIM2 or mystique) drives rapid nuclear degradation of STAT 1 and STAT4 in response to IFNα or IL-12 signaling37. SLIM has been shown to aid in polyubiquitylation of STAT 4 in vivo and in vitro37, and STAT 1 in vitro38. The CD4+ T cells in SLIM −/− mice make increased IFNγ upon in vivo challenge with heat-killed Listeria monocytogenes37. It is however unclear whether the increased IFNγ production by these cells would offer enhanced resistance to pathogen or rather, enhanced immunopathology. Another E3 ligase, Smad ubiquitylation regulating factor 1 (Smurf1), has been shown to mediate K48 polyubiquitylation and degradation of STAT1 in transformed cell lines but it remains to be shown whether this happens in T cells as well39. STAT1 ubiquitylation is reversed by the DUB, USP1340. Furthermore, while T-bet can undergo ubiquitin-mediated degradation, the identity of the E3 ligase which drives this degradation is unknown41. However, a DUB, USP10, is known to reverse this ubiquitylation and stabilize T-bet42. Since the major driver of Th1 identity, Tbet, is regulated by ubiquitylation, it raises the question of how else ubiquitylation influences both epigenetic modifications and the stability of proteins that result in the decision to be a Th1 cell.
TH2 CELLS
Th2 cells mediate immunity against extracellular microbes, such as worms, and facilitate clearance of allergens and toxins. Th2 cells differentiate from naïve CD4+ T cells in response to pMHC and co-stimulation in the presence of the cytokine IL-4. Th2 cells may secrete a variety of cytokines including IL-4, IL-5 and IL-13. IL-4 drives Th2 cell generation in a positive feed-forward loop43. IL-4 binds to its receptor, resulting in the phosphorylation and activation of STAT6, which translocates to the nucleus and drives transcription of GATA3. GATA3 drives Th2 cell identity via both Notch-dependent and Notch-independent mechanisms44–47. While IL-4 and STAT6 may be dispensable for in vivo generation of Th2 cells46–48, GATA3 is required for the generation of Th2 cells44.
IL-4, GATA3, and STAT6 are regulated by ubiquitylation, either directly or indirectly. The catalytic ubiquitin ligase, Itch, regulates Th2 cell differentiation and identity by regulating IL-4 production. As with most catalytic E3 ligases, Itch enzymatic activity is restrained by a closed conformational state known as autoinhibition49. Upon T cell activation, a small membrane-bound adaptor known as Nedd4-family interacting protein 1 (Ndfip1) is expressed which activates Itch, allowing it to polyubiquitylate targets including JunB25,50 This results in JunB degradation and prevents its localization to the nucleus where it would otherwise pair with c-Maf to drive IL-4 transcription26,51,52. In mice lacking Ndfip1 or Itch, CD4 T cells accumulate high levels of JunB and produce excessive quantities of IL-4 resulting in a preponderance of Th2 cells25,26. Supporting this model, transgenic mice which overexpress JunB to levels found in Th2 cells, show a specific increase in Th2 cytokines such as IL-4 and IL-551.
STAT 6 levels are regulated by two different E3 ligases: gene related to anergy in lymphocytes (GRAIL) and Casitas B-lineage lymphoma b (Cbl-b). GRAIL drives polyubiquitylation and degradation of STAT6, and therefore limits the generation of Th2 cells. GRAIL is highly expressed in Th2 cells and its knockdown results in an increase in IL-4, IL-5 and IL-13 from T cells. GRAIL knockout animals are highly susceptible to allergic inflammation and their naïve CD4+ T cells fail to appropriately degrade STAT6 after in vitro TCR stimulation53. Cbl-b also drives STAT6 polyubiquitylation and degradation54. Similar to Grail−/− animals, Cbl-b−/− animals are highly susceptible to induced allergic inflammation due to a Th2 and Th9 bias in their T cells.
The E3 ligase, murine double minute 2 (Mdm2), drives GATA3 polyubiquitylation. Mdm2 is well known for its role in ubiquitin-driven degradation of the tumor suppressor p5355,56, but Mdm2 has several other substrates including NFAT2c57,58 and GATA359. Upon TCR stimulation, activation of the ERK-MAPK pathway leads to the association of Mdm2 with GATA3, and consequent polyubiquitylation and proteasomal degradation of GATA359. A DUB named USP15 deubiquitinates Mdm2 and prevents its proteasomal degradation57. Therefore ubiquitylation of GATA3 and STAT6, as well as of factors that regulate IL-4 production, all contribute to the decision of a CD4 naïve cell to become a Th2 cell.
TH17 CELLS
Th17 cells are important for the clearance of extracellular pathogens such as fungi. Th17 cells differentiate in the presence of a cytokine milieu containing transforming growth factor β (TGF-β) and IL-660–62. Other cytokines such as IL-1β and IL-23 drive differentiation and maintenance of Th17 cells and may even drive Th17s in the absence of TGF-β63. STAT3 is induced downstream of IL-6, IL-21 and IL-23, and cooperates with IRF4 (induced downstream of IL-1β) to elevate RORγT levels64,65. RAR-related orphan receptor gamma T (RORγT) and alpha (RORα) are sufficient to drive Th17 differentiation66,67.
Several enzymes in the ubiquitin conjugation pathway can influence the differentiation or maintenance of Th17 cells. For example, mice lacking Ndfip1 and Itch that, as discussed in the prior section, have increased frequencies of Th2 cells, also have increased frequencies of Th17 cells. While this may be due to cell-extrinsic effects of IL-4 mediated inflammation driving increased pro-inflammatory cytokines and tissue damage68, there may also be more direct roles for these factors in Th17 generation or function. Similarly, mice lacking a RING E3 ligase, Ro52/TRIM21, show increased production of inflammatory cytokines such as IL-23, IL-6 and IL-21, and these mice have increased frequencies of Th17 cells69. Crossing the Ro52−/− mice to IL-23p19−/− mice abrogates tissue damage, excessive cytokine production and lowers the frequencies of the Th17 cells, indicating that the increase in Th17 cells is indeed driven by the dysregulated IL-23/IL-17 axis. Ro52 acts downstream of IFN (predominantly Type II) signaling to polyubiquitylate IRF3, IRF5 and IRF8 and to target them for degradation. This limits the production of cytokines including IL-6 and IL-23 that would otherwise drive Th17 generation. A third example of an E3 ubiquitin ligase that influences the Th17 cell fate choice is SLIM. As described above, SLIM can drive degradation of STAT4 in Th1 cells, but can also limit Th17 differentiation by promoting the proteasomal degradation of STAT370,71. Supporting this, SLIM-deficient animals show increased frequencies of Th17 and Th1 cells and are very susceptible to Experimental Autoimmune Encephalitis (EAE), a mouse model of multiple sclerosis71.
Several DUBs are important for Th17 differentiation. USP4 regulates Th17s in two ways. First, USP4 deubiquitylates Ro52 in transformed cell lines72 and thus limits the expression of cytokines needed for Th17 generation69. Second, it directly interacts with RORγT in primary human Th17 cells and deubiquitylates RORγT in transformed cell lines73. USP17 (also known as DUB-3) is a second DUB that maintains RORγT stability74. Knockdown of USP17 in primary Th17 cells results in a decrease of endogenous RORγT levels74. Since IL-4 and IL-6 signaling can induce USP1775, it appears that in response to IL-6 signaling, two responses occur: first, RORγT levela are increased downstream of STAT3 and second, RORγT protein is stabilized downstream of USP17. How USP17 influences the timing and integration of these two events downstream of IL-6 signaling needs further evaluation in primary CD4 T cells.
A third DUB involved in Th17 differentiation is DUBA (also known as OTUD5), an OTU family DUB. DUBA facilitates the degradation of RORγT by removing a regulator of RORγT degradation known as UBR576. UBR5 polyubiquitylates and targets RORγT for proteasomal degradation. UBR5 itself is regulated via poylubiquitylation. When DUBA deubiquitylates UBR5 and rescues it from degradation, UBR5 is free to polyubiquitylate RORγT, driving its degradation and consequently limiting Th17 differentiation. T-cell specific loss of DUBA therefore results in reduced levels of UBR5, stabilization of RORγT, and increased IL-17A production in response to TCR stimulation76.
It is becoming clear that Th17 cells are heterogeneous; some are more pathogenic than others63,77,78. GM-CSF secretion, for example, which occurs downstream of IL-23 signaling, marks pathogenic Th17s which cause EAE79–82. Further work needs to be done to determine the extent to which ubiquitylation that occurs downstream of cytokines such as TGF-β or IL-23, or downstream of TCR signaling, may influence the pathogenic potential of a differentiating Th17 cell, as well as its ultimate function.
TFH CELLS
Tfh (T follicular helper) cells are CD4+ T cells that provide co-stimulatory help to B cells in germinal centers to enable B cell functions83–85. Tfh cells exist in an interdependent relationship with B cells, wherein B cells are required for appropriate Tfh differentiation and function, and, reciprocally, Tfh cells promote the generation of high affinity antibody producing B cells. Tfh cells differentiate from naïve precursors in response to pMHC interactions and under the influence of cytokines such as IL-6, ICOS and IL-2186–90. Tfh cells can secrete cytokines such as IL-4, and IL-21 as well as chemokines such as CXCL13, as reviewed91. The differentiation of a naïve CD4+ T cell into a Tfh cell occurs in a step-wise fashion, which requires the transcription factor, achaete-scute homologue 2 (Ascl2), to generate a BCl6lo CXCR5+ Tfh-intermediate cell. This intermediate undergoes maturation and complete differentiation in the presence of Bcl6 to generate a Bcl6hi CXCRhi Tfh cell92. Bcl6 is thus essential for Tfh identity93–95.
Several E3 ligases affect Tfh differentiation and function by regulating Bcl6 directly or indirectly. Four of these are RING type E3 ubiquitin ligases. First, Bcl6 may be repressed by interaction with Cul3 E3 ligase, a ligase known, among other things, to ubiquitinate histone proteins. In thymocytes, complexes of Cul3 and Bcl6 directly bind and lay down repressive epigenetic marks on two genes important for Tfh identity, namely Batf and Bcl696. Intriguingly, this repression is epigenetically carried over into the periphery when the T cells encounter antigen. Therefore, in mice which lack Cul3 in T cells, Tfh cells are increased in secondary lymphoid tissues and these cells drive germinal center B cell expansion97. Exactly how the Cul3 Ring Ligase complex represses Bcl6 expression remains unresolved. Does the complex directly ubiquitylate Bcl6 leading to its degradation? Or does the complex regulate Bcl6 indirectly by ubiquitylating other proteins such as histone modifiers that are associated with Bcl6? Second, the E3 ligase Roquin reduces expression of ICOS, upstream of Bcl6, to limit Tfh differentiation. Roquin is a RING E3 ligase with an RNA-binding domain that binds and silences target genes, including ICOS mRNA. Supporting this model, mice that bear a mutation in the gene encoding Roquin, (also called sanroque mice), have increased ICOS levels in both naïve and activated T cells. This leads to a T-cell-intrinsic increase in Tfh differentiation, large numbers of germinal centers, and increased serum antibodies of various IgG isotypes, among other defects98. Bcl6 is regulated by two other E3 ligases in diffuse large B cell lymphomas (DLBCLs)99,100. Pellino1 E3 ligase (PELI1) directs K63 (non-degradative) chains on Bcl6 leading to Bcl6 stabilization in transgenic mice overexpressing human PELI199. Furthermore, in these lymphoma cell lines, another E3 ligase, FBXO11, that normally marks Bcl6 for degradation, is found to undergo loss of function mutations100. Follow up of these observations in primary T cells will be crucial in determining whether PELI1 and FBXO11 have roles in regulating Bcl6 and consequently in Tfh differentiation in vivo.
The HECT-type E3 ligase Itch also regulates the differentiation of Tfh cells. Mice lacking Itch globally or only in their T cells are unable to generate Tfh cells following infection with vaccinia virus101. In Itch deficient T cells, Foxo1 is not appropriately degraded and Tfh development can be rescued in Itch-deficient animals by knockdown of Foxo1 or by forced expression of Bcl6, suggesting that the defect in Itch-deficient animals is upstream of Bcl6101. ICOS signaling converges on this pathway by transiently inactivating Foxo1 in order to relieve Foxo1 repression of Bcl6 and to allow Tfh differentiation downstream of short-term ICOS signals102. Surprisingly, complete knockout of Foxo1 in T cells prevents the formation of germinal centers or Tfh cells102. This suggests that Foxo1-mediated regulation of germinal centers and Tfh cells is complex, differentiation stage-dependent, and must be intricately regulated by precise timing of the expression of proteins such as Foxo1. Given that Foxo1 is important for regulatory T cell generation and function103 and Itch-deficient T cells have decreased Tfh cells, increased Foxo1 levels, and defective Treg numbers and function101,104, it will be important to determine whether Foxo1 levels are changed in Itch−/− Treg cells and to what extent the Tfh defects in Itch−/− mice are related to the Treg dysfunction. Further work will be needed to explore the role of ubiquitin-mediated pathways in other proteins that contribute to other factors in Tfh identity.
REGULATORY T CELLS (Tregs)
Tregs are a subset of CD4+ T cells that are capable of suppressing the actions and functions of other immune cell type types105,106. Several distinct subsets of Tregs have been described including the Foxp3- Tr1 cells (Type 1 regulatory T cells), Foxp3+ Th3 cells, and the Foxp3+ thymic-derived Tregs. Foxp3+ regulatory T cells develop in the thymus, when TCR/CD28 and IL-2 signaling drives expression of the transcription factor Foxp3107. Foxp3 is central to the function of this major subset of Tregs and mutations in foxp3 in both mice and men leads to non-functional Tregs and autoimmunity108. Foxp3+ Tregs may also be induced in the periphery through the action of TGF-β and IL-2. TGF-β signals through the complementary proteins, Smad2 and Smad3, to drive foxp3 transcription109. IL-2 binding to the IL-2R complex leads to JAK1 and JAK 3 recruitment and eventual recruitment and activation of STAT5 which in turn drives expression of Foxp3110,111.
Two E3 ligases can regulate Foxp3 stability: Stub1 and Cbl-b. Stub1 (also known as CHIP or carboxyl terminus of Hsc70-interacting protein), interacts with both Hsp70 and Foxp3 to drive K48-linked polyubiquitylation of Foxp3112. Exposure of the Jurkat T cell line to inflammatory cues such as LPS and IL-1β drives the translocation of Stub1 into the nucleus, where it interacts with Hsp70, and drives the ubiquitin-mediated proteasomal degradation of Foxp3. Overexpression of Stub1 in Tregs and subsequent cotransfer of these cells together with naïve T cells into a lymphoreplete host resulted in loss of Foxp3 in the Tregs and subsequent conversion of these cells into IFNγ+ Th-1 like effector cells. Cbl-b, has been shown to ubiquitylate Foxp3 by working together with Stub1. Specifically, Cbl-b binds ubiquitylated Foxp3 downstream of TCR/CD28 signaling, and recruits it to Stub1, allowing additional ubiquitylation of foxp3 and increased proteasomal degradation113.
E3 ligases may also influence Tregs by affecting their function. One such example is the regulation of Treg function by the E3 ligase von Hippel-Lindau, VHL114. Under normal oxygen levels (normoxia), an oxygen sensor named Hypoxia-inducible Factor alpha (HIF1α) is hydroxylated, recognized and then polyubiquitylated by VHL. This results in proteasomal degradation of HIF1α. Under conditions of low oxygen stress (hypoxia), HIF1α is not degraded by VHL, and is free to drive the transcription of genes necessary for surviving hypoxia. HIF1α also serves as a switch between Th17 and Treg fates115,116. HIF1α can directly bind RORγT and p300 to drive transcription of IL-17A and promote Th17 identity115 while simultaneously binding and targeting foxp3 for degradation to repress Treg differentiation115,116. Interestingly, mice lacking VHL only in their Tregs develop a disease characterized by large numbers of IFNγ+ Tregs which infiltrate tissue and fail to suppress conventional T cells or prevent colitis114. In VHL-deficient Tregs, stabilized HIF1α drives the IFNγ promoter resulting in large amounts of secreted IFNγ and poor in vivo function of these Tregs114.
The HECT-type E3 ubiquitin ligase Itch also regulates Treg differentiation and function. Mice encoding a Treg-specific deletion of the E3 ligase Itch develop a Th2-mediated disease characterized by infiltration of activated T cells into mucosal sites and show particularly severe inflammation in the airways117. The inability of Itch-deficient Tregs to suppress Th2-mediated inflammation supports other published studies showing that mice that lack Ndfip1, an important adaptor and activator of Itch, express an inactive form of Itch that fails to degrade JunB and limit IL-4 production25. Thus T cells from mice that lack Ndfip1 are defective in induced Treg (iTreg) generation due to high IL-4 production118. Two other E3 ligases, Smurf2119 and β-TrCP (FBXW1)120, regulate Treg function indirectly by mediating ubiquitylation and degradation of EZH2 in neurons and transformed cells respectively. EZH2 protein forms part of the polycomb repressive complex 2 (PRC2) that trimethylates histone H3 in order to repress gene transcription. EZH2-deficient Tregs are unable to suppress inflammation in vivo and show an increased ability to lose Foxp3 expression121. Whether these E3 ligases stabilize EZH2 and reinforce Treg identity in primary T cells will be interesting to explore in future studies.
Relatively little is known about the role of DUBs in Treg differentiation. The USP family member CYLD (cylindromatosis), plays a role in the generation of Tregs. In response to TGF-β signaling, K63-linked polyubiquitylation of Smad7 results in stabilized Smad7 which activates TAK1, increases binding of AP-1 to the foxp3 promoter, and increases Foxp3 transcription122. CYLD opposes this process by removing K63 chains on Smad7. In CYLD −/− T cells, unopposed K63 ubiquitination upon TGF-β signaling stabilizes Smad7 and drives increased differentiation of Tregs122. These examples show that in addition to regulating Treg abundance, ubiquitin enzymes can regulate Treg differentiation and function.
GUIDING CD4 T CELL EFFECTOR FATE AND LINEAGE IDENTITY
Maintaining CD4 T helper cell identity is important for proper immune function. On one hand, the flexibility to transition from an initial CD4 T effector cell into a more relevant effector cell may be important for quickly tailoring the immune response as infection progresses. However, recent work suggests that CD4 effector cells have the potential to lose stability and express transcription factors and cytokines that are typically ascribed to other lineages. While it remains possible that this helps to promote pathogen clearance, in many instances this is associated with an ineffective immune responses or correlates with inappropriate immune responses that are seen in autoimmune diseases.
Data from patients with Crohn’s disease suggests that dual Th1/Th17 cells may play a pathogenic role in disease123. These cells appear to retain aspects of both Th1 and Th17 identity, expressing T-bet and RORγT and secreting both IFNγ and IL-17A124,125. This co-expression of T-bet and RORγT is intriguing since T-bet is thought to repress Th17 identity by binding Runx1, to prevent Runx1 from transactivating Rorc126–128. Runx1 also represses the Th2 fate by binding directly to GATA3129. Runx1 is ubiquitylated and degraded by the E3 ligase Stub1130, placing ubiquitylation of Runx1 squarely at the center of decisions of CD4 effector fate. Furthermore, in vitro-derived Th1 cells bear activating H3K4me3 marks at the gene loci for IFNγ and T-bet, as expected, but also unexpectedly at the locus for GATA3131, suggesting that Th1 cells may be poised to take on other T helper identities. This corroborates data by other groups showing that Th1 cells may convert to IL-4 producing cells in response to infection with Nippostrongylus brasiliensis132. Conversely, in the absence of GATA3, CD4 T cells enter into the Th1 rather than Th2 cell lineage47,133. Continued research is needed to further elucidate the role of ubiquitylation in these Th1/Th2 cell fate decisions.
Th17 cells can exhibit plasticity in vivo. In a NOD/SCID autoimmune model of diabetes, transfer of Th17s cells led to acquisition of T-bet and IFNγ secretion by these cells in vivo134. Furthermore, in another model, Th17 cells could transdifferentiate into Tr1 cells via a TGF-β/Smad3 pathway, both at steady state and during immune responses to worms or bacterial infections135. Smad3 degradation in transformed cell lines is mediated by the RING E3 ligase, ROC1136 and may be reversed by the DUB, OTUB1137. Thus, ubiquitylation may be involved in plasticity of Th17 cells.
Bcl6 is crucial in driving Tfh lineage identity. Bcl6 levels may also be increased downstream of STAT1, STAT3 and STAT4138,139. During Th1 differentiation, there is a Tfh-like transition stage during which both Bcl6 and T-bet are expressed. However as T-bet expression increases, Bcl6 levels decrease, resulting in a bias towards Th1 cell identity138,139. Interestingly, although the E3 ligase, SLIM, degrades STAT1 and STAT4 in Th1 cells37 and STAT3 in Th17 cells71, a role for SLIM E3 ligase in regulating Tfh differentiation has not yet been reported. This connection remains to be explored.
Stability of Tregs continues to be a controversial topic. Some studies have shown remarkable stability of these cells in both lymphoreplete and lymphopenic hosts140,141. Other studies have shown that Tregs can lose foxp3 upon transfer into lymphopenic hosts and gain the capacity to express effector cytokines142,143. A consensus may be that while most thymically derived Tregs may be stable and committed to the Treg lineage, inflammatory conditions exist that can drive some loss of Foxp3 protein and thus Treg instability. Alternatively, there may be conditions in which a small subset of Tregs that are not fully committed to the Treg fate, may lose their Foxp3 expression144,145. In autoimmune arthritis, for example, “exTregs” which acquire the capacity to secrete IL-17A can play a pathogenic role in the disease146. Bcl6 −/− Tregs are also more likely to lose Foxp3 and to express GATA3, and to secrete Th2 cytokines and IL-17A147. Since Bcl6 may be degraded by ubiquitination, as discussed in the Tfh section, it will be interesting in future studies to determine how ubiquitylation and degradation of Bcl6 regulates Treg stability under steady state and under inflammatory conditions.
With continued use of modern genetic technology such as reporter mice for many of the known lineage-defining transcription factors and their key cytokines, future work may continue to reveal conditions under which Tregs may be unstable. Further work focusing on understanding how ubiquitylation regulates the human proteome, should also help shed more light on the role of ubiquitylation in regulating Treg stability.
CONCLUSIONS
With over 600 E3 ligase enzymes encoded in the human genome, our knowledge of the substrates and functions of all of these enzymes is in its infancy. To date, relatively little is known about the contribution of these ligases to CD4 effector fate and function (Figure 1). As gene targeting becomes more efficient, such as with the advances of CRISPR technology, our understanding of how these ligases function in vivo will be more fully explored. Additionally, as whole exome sequencing becomes more commonplace, mutations in E3 ligases are likely to be found to associate with immune-mediated disease, thus providing a more complete understanding of how these ligases regulate immune function. It is therefore easy to predict that our current knowledge is just the tip of the iceberg.
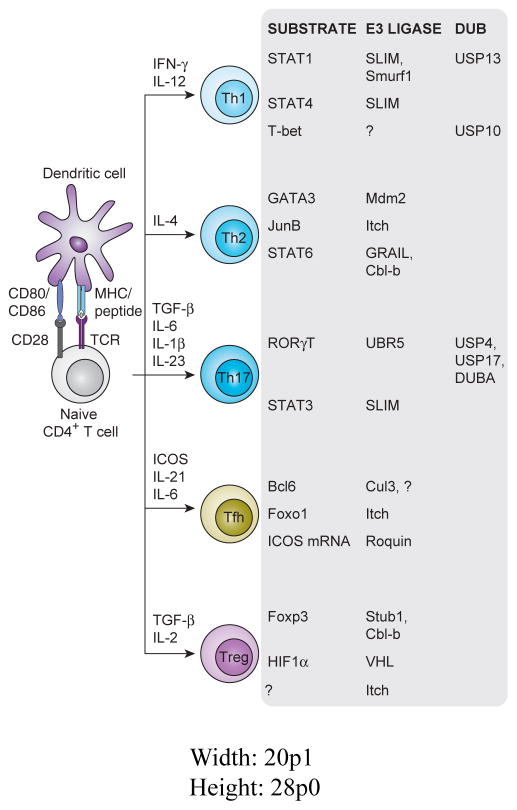
Figure 1.
E3 ubiquitin ligases and Deubiquitinating enzymes (DUBs) involved in CD4+ Tcell identity. After T cell activation by an activated APC, a naïve T cell can differentiate into any of the CD4+ effector cell fates diagramed. All of the key transcriptional factors that regulate these effector fates can be regulated directly or indirectly via ubiquitylation.
Acknowledgments
Funding: American Asthma Foundation 13-0020; NIH R01AI093566; NIH R01AI114515
References
- 1.Pickart CM, Kasperek EM, Beal R, Kim a. Substrate Properties of Site-Specific Mutant Ubiquitin Protein (G76A) Reveal Unexpected Mechanistic Features of Ubiquitin-Activating Enzyme (E1) J Biol Chem. 1994;269(10):7115–7123. [PubMed] [Google Scholar]
- 2.Sung P, Prakash S, Prakash L. Mutation of Cysteine-88 in the Saccharomyces Cerevisiae RAD6 Protein Abolishes Its Ubiquitin-Conjugating Activity and Its Various Biological Functions. Proc Natl Acad Sci U S A. 1990;87(7):2695–2699. doi: 10.1073/pnas.87.7.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freemont PS, I, Hanson M, Trowsdale J. A Novel Cysteine-Rich Sequence Motif. Cell. 1991;64(3):483–484. doi: 10.1016/0092-8674(91)90229-r. [DOI] [PubMed] [Google Scholar]
- 4.Deshaies RJ, Joazeiro CAP. RING Domain E3 Ubiquitin Ligases. Annu Rev Biochem. 2009;78(1):399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 5.Scheffner M, Nuber U, Huibregtse JM. Protein Ubiquitination Involving an E1-E2-E3 Enzyme Ubiquitin Thioester Cascade. Nature. 1995:81–83. doi: 10.1038/373081a0. [DOI] [PubMed] [Google Scholar]
- 6.Morett E, Bork P. A Novel Transactivation Domain in Parkin. Trends Biochem Sci. 1999;24(6):229–231. doi: 10.1016/s0968-0004(99)01381-x. [DOI] [PubMed] [Google Scholar]
- 7.van der Reijden BA, Erpelinck-Verschueren CA, Löwenberg B, Jansen JH. TRIADs: A New Class of Proteins with a Novel Cysteine-Rich Signature. Protein Sci. 1999;8(7):1557–1561. doi: 10.1110/ps.8.7.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim H, Kim K, Lledias F, Kisselev A, Scaglione K, Skowyra D, Gygi SP, Goldberg AL. Certain Pairs of Ubiquitin-Conjugating Enzymes (E2s) and Ubiquitin-Protein Ligases (E3s) Synthesize Nondegradable Forked Ubiquitin Chains Containing All Possible Isopeptide Linkages. J Biol Chem. 2007;282(24):17375–17386. doi: 10.1074/jbc.M609659200. [DOI] [PubMed] [Google Scholar]
- 9.Peng J, Schwartz D, Elias JE, Thoreen CC, Cheng D, Marsischky G, Roelofs J, Finley D, Gygi SP. A Proteomics Approach to Understanding Protein Ubiquitination. Nat Biotechnol. 2003;21(8):921–926. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- 10.Johnson ES, Ma PC, Ota IM, Varshavsky A. A Proteolytic Pathway That Recognizes Ubiquitin as a Degradation Signal. J Biol Chem. 1995;270(29):17442–17456. doi: 10.1074/jbc.270.29.17442. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda F, Dikic I. Atypical Ubiquitin Chains: New Molecular Signals. “Protein Modifications: Beyond the Usual Suspects” Review Series. EMBO Rep. 2008;9(6):536–542. doi: 10.1038/embor.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirisako T, Kamei K, Murata S, Kato M, Fukumoto H, Kanie M, Sano S, Tokunaga F, Tanaka K, Iwai K. A Ubiquitin Ligase Complex Assembles Linear Polyubiquitin Chains. EMBO J. 2006;25(20):4877–4887. doi: 10.1038/sj.emboj.7601360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun SC. Deubiquitylation and Regulation of the Immune Response. Nat Rev Immunol. 2008;8(7):501–511. doi: 10.1038/nri2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gowans JL, Knight EJ. The Route of Re-Circulation of Lymphocytes in the Rat. Proc R Soc B Biol Sci. 1964;159(975):257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- 15.Mempel TR, Henrickson SE, Von Andrian UH. T-Cell Priming by Dendritic Cells in Lymph Nodes Occurs in Three Distinct Phases. Nature. 2004;427(6970):154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 16.Kapsenberg ML. Dendritic-Cell Control of Pathogen-Driven T-Cell Polarization. Nat Rev Immunol. 2003;3(12):984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 17.Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory Cytokines Provide a Third Signal for Activation of Naive CD4+ and CD8+ T Cells. J Immunol. 1999;162(6):3256–3262. [PubMed] [Google Scholar]
- 18.Naramura M, Jang I-K, Kole H, Huang F, Haines D, Gu H. C-Cbl and Cbl-B Regulate T Cell Responsiveness by Promoting Ligand-Induced TCR down-Modulation. Nat Immunol. 2002;3(12):1192–1199. doi: 10.1038/ni855. [DOI] [PubMed] [Google Scholar]
- 19.Gay DL, Ramón H, Oliver PM. Cbl- and Nedd4-Family Ubiquitin Ligases: Balancing Tolerance and Immunity. Immunol Res. 2008;42(1–3):51–64. doi: 10.1007/s12026-008-8034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruber T, Hermann-Kleiter N, Hinterleitner R, Fresser F, Schneider R, Gastl G, Penninger JM, Baier G. PKC-Theta Modulates the Strength of T Cell Responses by Targeting Cbl-B for Ubiquitination and Degradation. Sci Signal. 2009;2(76):ra30. doi: 10.1126/scisignal.2000046. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Bárdos T, Li D, Gál I, Vermes C, Xu J, Mikecz K, Finnegan A, Lipkowitz S, Glant TT. Cutting Edge: Regulation of T Cell Activation Threshold by CD28 Costimulation through Targeting Cbl-B for Ubiquitination. J Immunol. 2002;169(5):2236–2240. doi: 10.4049/jimmunol.169.5.2236. [DOI] [PubMed] [Google Scholar]
- 22.Li D, Gál I, Vermes C, Alegre M-L, Chong ASF, Chen L, Shao Q, Adarichev V, Xu X, Koreny T, Mikecz K, Finnegan A, Glant TT, Zhang J. Cutting Edge: Cbl-B: One of the Key Molecules Tuning CD28- and CTLA-4-Mediated T Cell Costimulation. J Immunol. 2004;173(12):7135–7139. doi: 10.4049/jimmunol.173.12.7135. [DOI] [PubMed] [Google Scholar]
- 23.Zhao H, Li CC, Pardo J, Chu PC, Liao CX, Huang J, Dong JG, Zhou X, Huang Q, Huang B, Bennett MK, Molineaux SM, Lu H, Daniel-Issakani S, Payan DG, Masuda ES. A Novel E3 Ubiquitin Ligase TRAC-1 Positively Regulates T Cell Activation. J Immunol. 2005;174(9):5288–5297. doi: 10.4049/jimmunol.174.9.5288. [DOI] [PubMed] [Google Scholar]
- 24.Chang M, Jin W, Chang J, Xiao Y, Brittain GC, Yu J, Zhou X, Wang Y, Cheng X, Li P, Rabinovich BA, Hwu P, Sun S. The Ubiquitin Ligase Peli1 Negatively Regulates T Cell Activation and Prevents Autoimmunity. Nat Immunol. 2011;12(10):1002–1009. doi: 10.1038/ni.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliver PM, Cao X, Worthen GS, Shi P, Briones N, MacLeod M, White J, Kirby P, Kappler J, Marrack P, Yang B. Ndfip1 Protein Promotes the Function of Itch Ubiquitin Ligase to Prevent T Cell Activation and T Helper 2 Cell-Mediated Inflammation. Immunity. 2006;25(6):929–940. doi: 10.1016/j.immuni.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang D, Elly C, Gao B, Fang N, Altman Y, Joazeiro C, Hunter T, Copeland N, Jenkins N, Liu YC. Dysregulation of T Lymphocyte Function in Itchy Mice: A Role for Itch in TH2 Differentiation. Nat Immunol. 2002;3(3):281–287. doi: 10.1038/ni763. [DOI] [PubMed] [Google Scholar]
- 27.Liu YC. The E3 Ubiquitin Ligase Itch in T Cell Activation, Differentiation, and Tolerance. Semin Immunol. 2007;19(3):197–205. doi: 10.1016/j.smim.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang B, Gay DL, MacLeod MKL, Cao X, Hala T, Sweezer EM, Kappler J, Marrack P, Oliver PM. Nedd4 Augments the Adaptive Immune Response by Promoting Ubiquitin-Mediated Degradation of Cbl-B in Activated T Cells. Nat Immunol. 2008;9(12):1356–1363. doi: 10.1038/ni.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scharschmidt E, Wegener E, Heissmeyer V, Rao A, Krappmann D. Degradation of Bcl10 Induced by T-Cell Activation Negatively Regulates NF-Kappa B Signaling. Mol Cell Biol. 2004;24(9):3860–3873. doi: 10.1128/MCB.24.9.3860-3873.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friend SF, Deason-Towne F, Peterson LK, Berger AJ, Dragone LL. Regulation of T Cell Receptor Complex-Mediated Signaling by Ubiquitin and Ubiquitin-like Modifications. Am J Clin Exp Immunol. 2014;3(3):107–123. [PMC free article] [PubMed] [Google Scholar]
- 31.Paolino M, Penninger JM. E3 Ubiquitin Ligases in T-Cell Tolerance. Eur J Immunol. 2009;39(9):2337–2344. doi: 10.1002/eji.200939662. [DOI] [PubMed] [Google Scholar]
- 32.Park Y, Jin H, Aki D, Lee J, Liu Y-C. The Ubiquitin System in Immune Regulation. 1. Vol. 124. Elsevier Inc; 2014. [DOI] [PubMed] [Google Scholar]
- 33.O’Leary CE, Lewis EL, Oliver PM. Ubiquitylation as a Rheostat for TCR Signaling: From Targeted Approaches Toward Global Profiling. Front Immunol. 2015;6 doi: 10.3389/fimmu.2015.00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bacon CM. Interleukin 12 (IL-12) Induces Tyrosine Phosphorylation of JAK2 and TYK2: Differential Use of Janus Family Tyrosine Kinases by IL-2 and IL- 12. J Exp Med. 1995;181(1):399–404. doi: 10.1084/jem.181.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maldonado Ra, Soriano Ma, Perdomo LC, Sigrist K, Irvine DJ, Decker T, Glimcher LH. Control of T Helper Cell Differentiation through Cytokine Receptor Inclusion in the Immunological Synapse. J Exp Med. 2009;206(4):877–892. doi: 10.1084/jem.20082900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-Bet Is a STAT1-Induced Regulator of IL-12R Expression in Naïve CD4+ T Cells. Nat Immunol. 2002;3(6):549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka T, Soriano Ma, Grusby MJ. SLIM Is a Nuclear Ubiquitin E3 Ligase That Negatively Regulates STAT Signaling. Immunity. 2005;22(6):729–736. doi: 10.1016/j.immuni.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 38.Gao C, Guo H, Mi Z, Grusby MJ, Kuo PC. Osteopontin Induces Ubiquitin-Dependent Degradation of STAT1 in RAW264.7 Murine Macrophages. J Immunol. 2007;178(3):1870–1881. doi: 10.4049/jimmunol.178.3.1870. [DOI] [PubMed] [Google Scholar]
- 39.Yuan C, Qi J, Zhao X, Gao C. Smurf1 Protein Negatively Regulates Interferon-?? Signaling through Promoting STAT1 Protein Ubiquitination and Degradation. J Biol Chem. 2012;287(21):17006–17015. doi: 10.1074/jbc.M112.341198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeh HM, Yu CY, Yang HC, Ko SH, Liao CL, Lin YL. Ubiquitin-Specific Protease 13 Regulates IFN Signaling by Stabilizing STAT1. J Immunol. 2013;191(6):3328–3336. doi: 10.4049/jimmunol.1300225. [DOI] [PubMed] [Google Scholar]
- 41.Jang EJ, Park HR, Hong JH, Hwang ES. Lysine 313 of T-Box Is Crucial for Modulation of Protein Stability, DNA Binding, and Threonine Phosphorylation of T-Bet. J Immunol. 2013;190:5764–5770. doi: 10.4049/jimmunol.1203403. [DOI] [PubMed] [Google Scholar]
- 42.Pan L, Chen Z, Wang L, Chen C, Li D, Wan H, Li B, Shi G. Deubiquitination and Stabilization of T-Bet by USP10. Biochem Biophys Res Commun. 2014;449(3):289–294. doi: 10.1016/j.bbrc.2014.05.037. [DOI] [PubMed] [Google Scholar]
- 43.Brown MA. IL-4 Production by T Cells: You Need a Little to Get a Lot. J Immunol. 2008;181(5):2941–2942. doi: 10.4049/jimmunol.181.5.2941. [DOI] [PubMed] [Google Scholar]
- 44.Zheng W, Flavell RA. The Transcription Factor GATA-3 Is Necessary and Sufficient for Th2 Cytokine Gene Expression in CD4 T Cells. Cell. 1997;89(4):587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 45.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 Is Required for Mediating Responses to IL-4 and for the Development of Th2 Cells. Immunity. 1996;4(3):313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 46.Fang TC, Yashiro-Ohtani Y, Del Bianco C, Knoblock DM, Blacklow SC, Pear WS. Notch Directly Regulates Gata3 Expression during T Helper 2 Cell Differentiation. Immunity. 2007;27(1):100–110. doi: 10.1016/j.immuni.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amsen D, Antov A, Jankovic D, Sher A, Radtke F, Souabni A, Busslinger M, McCright B, Gridley T, Flavell RA. Direct Regulation of Gata3 Expression Determines the T Helper Differentiation Potential of Notch. Immunity. 2007;27(1):89–99. doi: 10.1016/j.immuni.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Panhuys N, Tang SC, Prout M, Camberis M, Scarlett D, Roberts J, Hu-Li J, Paul WE, Le Gros G. In Vivo Studies Fail to Reveal a Role for IL-4 or STAT6 Signaling in Th2 Lymphocyte Differentiation. Proc Natl Acad Sci U S A. 2008;105(34):12423–12428. doi: 10.1073/pnas.0806372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mund T, Pelham HRB. Control of the Activity of WW-HECT Domain E3 Ubiquitin Ligases by NDFIP Proteins. EMBO Rep. 2009;10(5):501–507. doi: 10.1038/embor.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riling C, Kamadurai H, Kumar S, O’Leary CE, Wu KP, Manion EE, Ying M, Schulman BA, Oliver PM. Itch WW Domains Inhibit Its E3 Ubiquitin Ligase Activity by Blocking E2–E3 Ligase Trans-Thiolation. J Biol Chem. 2015;290(39):23875–23887. doi: 10.1074/jbc.M115.649269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li B, Tournier C, Davis RJ, Flavell RA. Regulation of IL-4 Expression by the Transcription Factor JunB during T Helper Cell Differentiation. 1999;18(2):420–432. doi: 10.1093/emboj/18.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voice J, Donnelly S, Dorsam G, Dolganov G, Paul S, Goetzl EJ. C-Maf and JunB Mediation of Th2 Differentiation Induced by the Type 2 G Protein-Coupled Receptor (VPAC2) for Vasoactive Intestinal Peptide. J Immunol. 2004;172(12):7289–7296. doi: 10.4049/jimmunol.172.12.7289. [DOI] [PubMed] [Google Scholar]
- 53.Sahoo A, Alekseev A, Obertas L, Nurieva R. Grail Controls Th2 Cell Development by Targeting STAT6 for Degradation. Nat Commun. 2014;5:4732. doi: 10.1038/ncomms5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qiao G, Ying H, Zhao Y, Liang Y, Guo H, Shen H, Li Z, Solway J, Tao E, Chiang YJ, Lipkowitz S, Penninger JM, Langdon WY, Zhang J. E3 Ubiquitin Ligase Cbl-B Suppresses Proallergic T Cell Development and Allergic Airway Inflammation. Cell Rep. 2014;6(4):709–723. doi: 10.1016/j.celrep.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Momand J, Zambetti G, Olson D, George D, Levine A. The Mdm-2 Oncogene Product Forms a Complex with the p53 Protein and Inhi- Bits p53-Mediated Transactivation.pdf.crdownload. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 56.Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 Is a Ubiquitin Ligase E3 for Tumor Suppressor p53. FEBS Lett. 1997;420(1):25–27. doi: 10.1016/s0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]
- 57.Zou Q, Jin J, Hu H, Li HS, Romano S, Xiao Y, Nakaya M, Zhou X, Cheng X, Yang P, Lozano G, Zhu C, Watowich SS, Ullrich SE, Sun SC. USP15 Stabilizes MDM2 to Mediate Cancer-Cell Survival and Inhibit Antitumor T Cell Responses. Nat Immunol. 2014;15(6):562–570. doi: 10.1038/ni.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoeli-Lerner M, Yiu GK, Rabinovitz I, Erhardt P, Jauliac S, Toker A. Akt Blocks Breast Cancer Cell Motility and Invasion through the Transcription Factor NFAT. Mol Cell. 2005;20(4):539–550. doi: 10.1016/j.molcel.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 59.Yamashita M, Shinnakasu R, Asou H, Kimura M, Hasegawa A, Hashimoto K, Hatano N, Ogata M, Nakayama T. Ras-ERK MAPK Cascade Regulates GATA3 Stability and Th2 Differentiation through Ubiquitin-Proteasome Pathway. J Biol Chem. 2005;280(33):29409–29419. doi: 10.1074/jbc.M502333200. [DOI] [PubMed] [Google Scholar]
- 60.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the Context of an Inflammatory Cytokine Milieu Supports de Novo Differentiation of IL-17-Producing T Cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 61.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal Developmental Pathways for the Generation of Pathogenic Effector TH17 and Regulatory T Cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 62.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming Growth Factor-β Induces Development of the TH17 Lineage. Nature. 2006;441(7090):231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 63.Ghoreschi K, Laurence a, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, Grainger JR, Chen Q, Kanno Y, Watford WT, Sun HW, Eberl G, Shevach EM, Belkaid Y, Cua DJ, Chen W, O’Shea JJ. Generation of Pathogenic T(H)17 Cells in the Absence of TGF-Beta Signalling. Nature. 2010;467(7318):967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chung Y, Chang SH, Martinez GJ, Yang XO, Kang HS, Ma L, Watowich SS, Jetten A, Tian Q, Dong C. Critical Regulation of Early Th17 Cell Differentiation by IL-1 Signaling. Immunity. 2009;30(4):576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23–IL-17 Immune Axis: From Mechanisms to Therapeutic Testing. Nat Rev Immunol. 2014;14(9):585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The Orphan Nuclear Receptor RORgammat Directs the Differentiation Program of Proinflammatory IL-17+ T Helper Cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 67.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T Helper 17 Lineage Differentiation Is Programmed by Orphan Nuclear Receptors ROR Alpha and ROR Gamma. Immunity. 2008;28(1):29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramon HE, Beal AM, Liu Y, Worthen GS, Oliver PM. The E3 Ubiquitin Ligase Adaptor Ndfip1 Regulates Th17 Differentiation by Limiting the Production of Proinflammatory Cytokines. J Immunol. 2012;188(8):4023–4031. doi: 10.4049/jimmunol.1102779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Espinosa A, Dardalhon V, Brauner S, Ambrosi A, Higgs R, Quintana FJ, Sjöstrand M, Eloranta M-L, Ní Gabhann J, Winqvist O, Sundelin B, Jefferies Ca, Rozell B, Kuchroo VK, Wahren-Herlenius M. Loss of the Lupus Autoantigen Ro52/Trim21 Induces Tissue Inflammation and Systemic Autoimmunity by Disregulating the IL-23-Th17 Pathway. J Exp Med. 2009;206(8):1661–1671. doi: 10.1084/jem.20090585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tanaka T, Yamamoto Y, Muromoto R, Ikeda O, Sekine Y, Grusby MJ, Kaisho T, Matsuda T. PDLIM2 Inhibits T Helper 17 Cell Development and Granulomatous Inflammation through Degradation of STAT3. Sci Signal. 2011;4(202):ra85. doi: 10.1126/scisignal.2001637. [DOI] [PubMed] [Google Scholar]
- 71.Qu Z, Fu J, Ma H, Zhou J, Jin M, Mapara MY, Grusby MJ, Xiao G. PDLIM2 Restricts Th1 and Th17 Differentiation and Prevents Autoimmune Disease. Cell Biosci. 2012;2(1):23. doi: 10.1186/2045-3701-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wada K, Tanji K, Kamitani T. Oncogenic Protein UnpEL/Usp4 Deubiquitinates Ro52 by Its Isopeptidase Activity. Biochem Biophys Res Commun. 2006;339(3):731–736. doi: 10.1016/j.bbrc.2005.11.076. [DOI] [PubMed] [Google Scholar]
- 73.Yang J, Xu P, Han L, Guo Z, Wang X, Chen Z, Nie J, Yin S, Piccioni M, Tsun A, Lv L, Ge S, Li B. Cutting Edge: Ubiquitin-Specific Protease 4 Promotes Th17 Cell Function under Inflammation by Deubiquitinating and Stabilizing ROR T. J Immunol. 2015;194(9):4094–4097. doi: 10.4049/jimmunol.1401451. [DOI] [PubMed] [Google Scholar]
- 74.Han L, Yang J, Wang X, Wu Q, Yin S, Li Z, Zhang J, Xing Y, Chen Z, Tsun A, Li D, Piccioni M, Zhang Y, Guo Q, Jiang L, Bao L, Lv L, Li B. The E3 Deubiquitinase USP17 Is a Positive Regulator of Retinoic Acid-Related Orphan Nuclear Receptor γt (RORγt) in Th17 Cells. J Biol Chem. 2014;289(37):25546–25555. doi: 10.1074/jbc.M114.565291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burrows JF. DUB-3, a Cytokine-Inducible Deubiquitinating Enzyme That Blocks Proliferation. J Biol Chem. 2004;279(14):13993–14000. doi: 10.1074/jbc.M311291200. [DOI] [PubMed] [Google Scholar]
- 76.Rutz S, Kayagaki N, Phung QT, Eidenschenk C, Noubade R, Wang X, Lesch J, Lu R, Newton K, Huang OW, Cochran AG, Vasser M, Fauber BP, DeVoss J, Webster J, Diehl L, Modrusan Z, Kirkpatrick DS, Lill JR, Ouyang W, Dixit VM. Deubiquitinase DUBA Is a Post-Translational Brake on Interleukin-17 Production in T Cells. Nature. 2015;518(7539):417–421. doi: 10.1038/nature13979. [DOI] [PubMed] [Google Scholar]
- 77.Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, Sobel RA, Regev A, Kuchroo VK. Induction and Molecular Signature of Pathogenic TH17 Cells. Nat Immunol. 2012;13(10):991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Song X, Dai D, He X, Zhu S, Yao Y, Gao H, Wang J, Qu F, Qiu J, Wang H, Li X, Shen N, Qian Y. Growth Factor FGF2 Cooperates with Interleukin-17 to Repair Intestinal Epithelial Damage. Immunity. 2015;43(3):488–501. doi: 10.1016/j.immuni.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 79.Codarri L, Gyülvészi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORγt Drives Production of the Cytokine GM-CSF in Helper T Cells, Which Is Essential for the Effector Phase of Autoimmune Neuroinflammation. Nat Immunol. 2011;12(6):560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 80.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The Encephalitogenicity of T(H)17 Cells Is Dependent on IL-1- and IL-23-Induced Production of the Cytokine GM-CSF. Nat Immunol. 2011;12(6):568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McGeachy MJ. GM-CSF: The Secret Weapon in the T(H)17 Arsenal. Nat Immunol. 2011;12(6):521–522. doi: 10.1038/ni.2044. [DOI] [PubMed] [Google Scholar]
- 82.Lee Y, Peters A, Kuchroo VK. The Many Faces of Th17 Cells. Curr Opin Immunol. 2011;23(6):702–706. doi: 10.1016/j.coi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a Human Helper T Cell Population That Has Abundant Production of Interleukin 22 and Is Distinct from TH-17, TH1 and TH2 Cells. Nat Immunol. 2009;10(8):864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 84.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Förster R. Follicular B Helper T Cells Express CXC Chemokine Receptor 5, Localize to B Cell Follicles, and Support Immunoglobulin Production. J Exp Med. 2000;192(11):1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. Cxc Chemokine Receptor 5 Expression Defines Follicular Homing T Cells with B Cell Helper Function. J Exp Med. 2000;192(11):1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang Y, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T Follicular Helper Cells Is Mediated by Interleukin-21 but Independent of T Helper 1, 2, or 17 Cell Lineages. Immunity. 2008;29(1):138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C. Bcl6 Mediates the Development of T Follicular Helper Cells. Science. 2009;325(5943):1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goenka R, Barnett LG, Silver JS, O’Neill PJ, Hunter CA, Cancro MP, Laufer TM. Cutting Edge: Dendritic Cell-Restricted Antigen Presentation Initiates the Follicular Helper T Cell Program but Cannot Complete Ultimate Effector Differentiation. J Immunol. 2011;187(3):1091–1095. doi: 10.4049/jimmunol.1100853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pepper M, Pagán AJ, Igyártó BZ, Taylor JJ, Jenkins MK. Opposing Signals from the Bcl6 Transcription Factor and the Interleukin-2 Receptor Generate T Helper 1 Central and Effector Memory Cells. Immunity. 2011;35(4):583–595. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, Crotty S. ICOS Receptor Instructs T Follicular Helper Cell versus Effector Cell Differentiation via Induction of the Transcriptional Repressor Bcl6. Immunity. 2011;34(6):932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Crotty S. Follicular Helper CD4 T Cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 92.Liu X, Chen X, Zhong B, Wang A, Wang X, Chu F, Nurieva RI, Yan X, Chen P, van der Flier LG, Nakatsukasa H, Neelapu SS, Chen W, Clevers H, Tian Q, Qi H, Wei L, Dong C. Transcription Factor Achaete-Scute Homologue 2 Initiates Follicular T-Helper-Cell Development. Nature. 2014;507(7493):513–518. doi: 10.1038/nature12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 Are Reciprocal and Antagonistic Regulators of T Follicular Helper Cell Differentiation. Science. 2009;325(5943):1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, Ellyard JI, Parish IA, Ma CS, Li QJ, Parish CR, Mackay CR, Vinuesa CG. The Transcriptional Repressor Bcl-6 Directs T Follicular Helper Cell Lineage Commitment. Immunity. 2009;31(3):457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 95.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C. Bcl6 Mediates the Development of T Follicular Helper Cells. Science. 2009;325(5943):1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mathew R, Mao A, Chiang AH, Bertozzi-villa C, Bunker JJ, Scanlon ST, Mcdonald BD, Constantinides MG, Hollister K, Singer JD, Dent AL, Dinner AR, Bendelac A. A Negative Feedback Loop Mediated by the Bcl6 – Cullin 3 Complex Limits Tfh Cell Differentiation. 2014;211(6):1137–1151. doi: 10.1084/jem.20132267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mathew R, Seiler MP, Scanlon ST, Mao A, Constantinides MG, Bertozzi-Villa C, Singer JD, Bendelac A. BTB-ZF Factors Recruit the E3 Ligase Cullin 3 to Regulate Lymphoid Effector Programs. Nature. 2012;491(7425):618–621. doi: 10.1038/nature11548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, Yu D, Domaschenz H, Whittle B, Lambe T, Roberts IS, Copley RR, Bell JI, Cornall RJ, Goodnow CC. A RING-Type Ubiquitin Ligase Family Member Required to Repress Follicular Helper T Cells and Autoimmunity. Nature. 2005;435(7041):452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 99.Park H, Go H, Song HR, Kim S, Ha G, Jeon Y, Kim J, Lee H, Cho H, Kang HC, Chung H, Kim C, Chung DH, Lee C. Pellino 1 Promotes Lymphomagenesis by Deregulating BCL6 Polyubiquitination. 2014;124(11) doi: 10.1172/JCI75667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Duan S, Cermak L, Pagan JK, Rossi M, Martinengo C, di Celle PF, Chapuy B, Shipp M, Chiarle R, Pagano M. FBXO11 Targets BCL6 for Degradation and Is Inactivated in Diffuse Large B-Cell Lymphomas. Nature. 2012;481(7379):90–93. doi: 10.1038/nature10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xiao N, Eto D, Elly C, Peng G, Crotty S, Liu Y. The E3 Ubiquitin Ligase Itch Is Required for the Differentiation of Follicular Helper T Cells. Nat Immunol. 2014;15(7):657–666. doi: 10.1038/ni.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stone EL, Pepper M, Katayama CD, Kerdiles YM, Lai CY, Emslie E, Lin YC, Yang E, Goldrath AW, Li MO, Cantrell DA, Hedrick SM. ICOS Coreceptor Signaling Inactivates the Transcription Factor FOXO1 to Promote Tfh Cell Differentiation. Immunity. 2015;42(2):239–251. doi: 10.1016/j.immuni.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ouyang W, Beckett O, Ma Q, Paik J, DePinho Ra, Li MO. Foxo Proteins Cooperatively Control the Differentiation of Foxp3+ Regulatory T Cells. Nat Immunol. 2010;11(7):618–627. doi: 10.1038/ni.1884. [DOI] [PubMed] [Google Scholar]
- 104.Jin HS, Park Y, Elly C, Liu YC. Itch Expression by Treg Cells Controls Th2 Inflammatory Responses. J Clin Invest. 2013;123(11):4923–4934. doi: 10.1172/JCI69355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic Self-Tolerance Maintained by Activated T Cells Expressing IL-2 Receptor α-Chains (CD25). Breakdown of a Single Mechanism of Self-Tolerance Causes Various Autoimmune Diseases. J Immunol. 1995;155(3):1151–1164. [PubMed] [Google Scholar]
- 106.Sakaguchi S, Wing K, Miyara M. Regulatory T Cells - A Brief History and Perspective. Eur J Immunol. 2007;37(SUPPL 1):116–123. doi: 10.1002/eji.200737593. [DOI] [PubMed] [Google Scholar]
- 107.Lio CWJ, Hsieh CS. A Two-Step Process for Thymic Regulatory T Cell Development. Immunity. 2008;28(1):100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ramsdell F, Ziegler SF. FOXP3 and Scurfy: How It All Began. Nat Rev Immunol. 2014;14(5):343–349. doi: 10.1038/nri3650. [DOI] [PubMed] [Google Scholar]
- 109.Takimoto T, Wakabayashi Y, Sekiya T, Inoue N, Morita R, Ichiyama K, Takahashi R, Asakawa M, Muto G, Mori T, Hasegawa E, Shizuya S, Hara T, Nomura M, Yoshimura A. Smad2 and Smad3 Are Redundantly Essential for the TGF- -Mediated Regulation of Regulatory T Plasticity and Th1 Development. J Immunol. 2010;185(2):842–855. doi: 10.4049/jimmunol.0904100. [DOI] [PubMed] [Google Scholar]
- 110.Fujii H, Ogasawara K, Otsuka H, Suzuki M, Yamamura K, Yokochi T, Miyazaki T, Suzuki H, Mak TW, Taki S, Taniguchi T. Functional Dissection of the Cytoplasmic Subregions of the IL-2 Receptor Betac Chain in Primary Lymphocyte Populations. EMBO J. 1998;17(22):6551–6557. doi: 10.1093/emboj/17.22.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, Hennighausen L, Wu C, O’Shea JJ. Nonredundant Roles for Stat5a/b in Directly Regulating Foxp3. Blood. 2007;109(10):4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen Z, Barbi J, Bu S, Yang H, Li Z, Gao Y, Jinasena D, Fu J, Lin F, Chen C, Zhang J, Yu N, Li X, Shan Z, Nie J, Gao Z, Tian H, Li Y, Yao Z, Zheng Y, Park BV, Pan Z, Zhang J, Dang E, Li Z, Wang H, Luo W, Li L, Semenza GL, Zheng S, Loser K, Tsun A, Greene MI, Pardoll DM, Pan F, Li B. Article The Ubiquitin Ligase Stub1 Negatively Modulates Regulatory T Cell Suppressive Activity by Promoting Degradation of the Transcription Factor Foxp3. Immunity. 2013;39(2):272–285. doi: 10.1016/j.immuni.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhao Y, Guo H, Qiao G, Zucker M, Wallace Y. E3 Ubiquitin Ligase Cbl-B Regulates Thymic-Derived CD4 + CD25 + Regulatory T Cell Development by Targeting Foxp3 for Ubiquitination. J Immunol. 2015;194(4):1639–1645. doi: 10.4049/jimmunol.1402434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee JH, Elly C, Park Y, Lee JH, Elly C, Park Y, Liu Y. E3 Ubiquitin Ligase VHL Regulates Hypoxia-Inducible Factor-1 a to Maintain Regulatory T Cell Stability and Suppressive Capacity. Immunity. 2015;42(6):1062–1074. doi: 10.1016/j.immuni.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, Luo W, Zeller K, Shimoda L, Topalian SL, Semenza GL, Dang CV, Pardoll DM, Pan F. Control of T(H)17/T(reg) Balance by Hypoxia-Inducible Factor 1. Cell. 2011;146(5):772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1alpha-Dependent Glycolytic Pathway Orchestrates a Metabolic Checkpoint for the Differentiation of TH17 and Treg Cells. J Exp Med. 2011;208(7):1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jin HS, Park Y, Elly C, Liu YC. Itch Expression by Treg Cells Controls Th2 Inflammatory Responses. J Clin Invest. 2013;123(11):4923–4934. doi: 10.1172/JCI69355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Beal AM, Ramos-Hernández N, Riling CR, Nowelsky Ea, Oliver PM. TGF-β Induces the Expression of the Adaptor Ndfip1 to Silence IL-4 Production during iTreg Cell Differentiation. Nat Immunol. 2011;13(1):77–85. doi: 10.1038/ni.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yu YL, Chou RH, Shyu WC, Hsieh SC, Wu CS, Chiang SY, Chang WJ, Chen JN, Tseng YJ, Lin YH, Lee W, Yeh SP, Hsu JL, Yang CC, Hung SC, Hung MC. Smurf2-Mediated Degradation of EZH2 Enhances Neuron Differentiation and Improves Functional Recovery after Ischaemic Stroke. EMBO Mol Med. 2013;5(4):531–547. doi: 10.1002/emmm.201201783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sahasrabuddhe aa, Chen X, Chung F, Velusamy T, Lim MS, Elenitoba-Johnson KSJ. Oncogenic Y641 Mutations in EZH2 Prevent Jak2/β-TrCP-Mediated Degradation. Oncogene. 2014;34(August 2013):1–10. doi: 10.1038/onc.2013.571. [DOI] [PubMed] [Google Scholar]
- 121.DuPage M, Chopra G, Quiros J, Rosenthal WL, Morar MM, Holohan D, Zhang R, Turka L, Marson A, Bluestone JA. The Chromatin-Modifying Enzyme Ezh2 Is Critical for the Maintenance of Regulatory T Cell Identity after Activation. Immunity. 2015;42(2):227–238. doi: 10.1016/j.immuni.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhao Y, Thornton AM, Kinney MC, Ma CA, Spinner JJ, Fuss IJ, Shevach EM, Jain A. The Deubiquitinase CYLD Targets Smad7 Protein to Regulate Transforming Growth Factor Beta (TGF- Beta) Signaling and the Development of Regulatory T Cells. J Biol Chem. 2011;286(47):40520–40530. doi: 10.1074/jbc.M111.292961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Filì L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and Functional Features of Human Th17 Cells. J Exp Med. 2007;204(8):1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lexberg MH, Taubner A, Albrecht I, Lepenies I, Richter A, Kamradt T, Radbruch A, Chang HD. IFN-γ and IL-12 Synergize to Convert in Vivo Generated Th17 into Th1/Th17 Cells. Eur J Immunol. 2010;40(11):3017–3027. doi: 10.1002/eji.201040539. [DOI] [PubMed] [Google Scholar]
- 125.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Filì L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and Functional Features of Human Th17 Cells. J Exp Med. 2007;204(8):1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lazarevic V, Chen X, Shim JH, Hwang ES, Jang E, Bolm AN, Oukka M, Kuchroo VK, Glimcher LH. T-Bet Represses TH17 Differentiation by Preventing Runx1-Mediated Activation of the Gene Encoding RORγt. Nat Immunol. 2010;12(1):96–104. doi: 10.1038/ni.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brown CC, Esterhazy D, Sarde A, London M, Pullabhatla V, Osma-Garcia I, al-Bader R, Ortiz C, Elgueta R, Arno M, deRinaldis E, Mucida D, Lord GM, Noelle RJ. Retinoic Acid Is Essential for th1 Cell Lineage Stability and Prevents Transition to a Th17 Cell Program. Immunity. 2015;42(3):499–511. doi: 10.1016/j.immuni.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang F, Meng G, Strober W. Interactions among the Transcription Factors Runx1, RORγτ and Foxp3 Regulate the Differentiation of Interleukin 17-Producing T Cells. Nat Immunol. 2008;9(11):1297–1306. doi: 10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Komine O, Hayashi K, Natsume W, Watanabe T, Seki Y, Seki N, Yagi R, Sukzuki W, Tamauchi H, Hozumi K, Habu S, Kubo M, Satake M. The Runx1 Transcription Factor Inhibits the Differentiation of Naive CD4+ T Cells into the Th2 Lineage by Repressing GATA3 Expression. J Exp Med. 2003;198(1):51–61. doi: 10.1084/jem.20021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shang Y, Zhao X, Xu X, Xin H, Li X, Zhai Y, He D, Jia B, Chen W, Chang Z. CHIP Functions an E3 Ubiquitin Ligase of Runx1. Biochem Biophys Res Commun. 2009;386(1):242–246. doi: 10.1016/j.bbrc.2009.06.043. [DOI] [PubMed] [Google Scholar]
- 131.Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, Schones DE, Peng W, wei Sun H, Paul WE, O’Shea JJ, Zhao K. Global Mapping of H3K4me3 and H3K27me3 Reveals Specificity and Plasticity in Lineage Fate Determination of Differentiating CD4+ T Cells. Immunity. 2009;30(1):155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Panzer M, Sitte S, Wirth S, Drexler I, Sparwasser T, Voehringer D. Rapid in Vivo Conversion of Effector T Cells into Th2 Cells during Helminth Infection. J Immunol. 2012;188(2):615–623. doi: 10.4049/jimmunol.1101164. [DOI] [PubMed] [Google Scholar]
- 133.Zhu J, Min B, Hu-Li J, Watson CJ, Grinberg A, Wang Q, Killeen N, Urban JF, Guo L, Paul WE. Conditional Deletion of Gata3 Shows Its Essential Function in T(H)1–T(H)2 Responses. Nat Immunol. 2004;5(11):1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 134.Bending D, La Peña HD, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, Cooke A. Highly Purified Th17 from BDC2.5NOD/SCID. Conflict. 2009;119(3):1–8. doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gagliani N, Vesely MCA, Iseppon A, Brockmann L, Xu H, Palm NW, de Zoete MR, Licona-Limón P, Paiva RS, Ching T, Weaver C, Zi X, Pan X, Fan R, Garmire LX, Cotton MJ, Drier Y, Bernstein B, Geginat J, Stockinger B, Esplugues E, Huber S, Flavell RA. Th17 Cells Transdifferentiate into Regulatory T Cells during Resolution of Inflammation. Nature. 2015;523(7559):221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fukuchi M, Imamura T, Chiba T, Ebisawa T, Kawabata M, Tanaka K, Miyazono K. Ligand-Dependent Degradation of Smad3 by a Ubiquitin Ligase Complex of ROC1 and Associated Proteins. Mol Biol Cell. 2001;12(May):1431–1443. doi: 10.1091/mbc.12.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Herhaus L, Al-Salihi M, Macartney T, Weidlich S, Sapkota GP. OTUB1 Enhances TGFβ Signalling by Inhibiting the Ubiquitylation and Degradation of Active SMAD2/3. Nat Commun. 2013;4:2519. doi: 10.1038/ncomms3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Choi YS, Eto D, Yang JA, Lao C, Crotty S. Cutting Edge: STAT1 Is Required for IL-6-Mediated Bcl6 Induction for Early Follicular Helper Cell Differentiation. J Immunol. 2013;190(7):3049–3053. doi: 10.4049/jimmunol.1203032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nakayamada S, Kanno Y, Takahashi H, Jankovic D, Lu KT, Johnson TA, Sun H, Vahedi G, Hakim O, Handon R, Schwartzberg PL, Hager GL, O’Shea JJ. Early Th1 Cell Differentiation Is Marked by a Tfh Cell-like Transition. Immunity. 2011;35(6):919–931. doi: 10.1016/j.immuni.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, Benoist C, Rudensky AY. Stability of the Regulatory T Cell Lineage in Vivo. Science. 2010;329(5999):1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Miyao T, Floess S, Setoguchi R, Luche H, Fehling HJ, Waldmann H, Huehn J, Hori S. Plasticity of Foxp3+ T Cells Reflects Promiscuous Foxp3 Expression in Conventional T Cells but Not Reprogramming of Regulatory T Cells. Immunity. 2012;36(2):262–275. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 142.Komatsu N, Mariotti-Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S. Heterogeneity of Natural Foxp3+ T Cells: A Committed Regulatory T-Cell Lineage and an Uncommitted Minor Population Retaining Plasticity. Proc Natl Acad Sci U S A. 2009;106(6):1903–1908. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Duarte JH, Zelenay S, Bergman ML, Martins AC, Demengeot J. Natural Treg Cells Spontaneously Differentiate into Pathogenic Helper Cells in Lymphopenic Conditions. Eur J Immunol. 2009;39(4):948–955. doi: 10.1002/eji.200839196. [DOI] [PubMed] [Google Scholar]
- 144.Sakaguchi S, Vignali Daa, Rudensky AY, Niec RE, Waldmann H. The Plasticity and Stability of Regulatory T Cells. Nat Rev Immunol. 2013;13(6):461–467. doi: 10.1038/nri3464. [DOI] [PubMed] [Google Scholar]
- 145.Hori S. Regulatory T Cell Plasticity: Beyond the Controversies. Trends Immunol. 2011;32(7):295–300. doi: 10.1016/j.it.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 146.Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, Tanaka S, Bluestone Ja, Takayanagi H. Pathogenic Conversion of Foxp3+ T Cells into TH17 Cells in Autoimmune Arthritis. Nat Med. 2014;20(1):62–68. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- 147.Sawant DV, Wu H, Yao W, Sehra S, Kaplan MH, Dent AL. The Transcriptional Repressor Bcl6 Controls the Stability of Regulatory T Cells by Intrinsic and Extrinsic Pathways. Immunology. 2014;7592 doi: 10.1111/imm.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]