Abstract
Infectious keratoconjunctivitis (IKC) is a contagious eye disease primarily caused by Mycoplasma conjunctivae in domestic and wild Caprinae. Chlamydophila species have also been detected in ruminants with IKC. The objectives of this study are to investigate the ocular infection of M. conjunctivae and Chlamydiaceae and assess its interaction in relation to IKC in sheep and goats from remote communities around the Central Karakoram National Park in Pakistan, performing a combination of cross-sectional and case–control study design. Mostly asymptomatic and endemic infections of M. conjunctivae and Chlamydiaceae were found in sheep (19.3 per cent and 4.5 per cent, respectively) and goats (9.5 per cent and 1.9 per cent, respectively) from all communities, assessed by qPCR. Prevalence significantly differed between species only for M. conjunctivae (P=0.0184), which was also more prevalent in younger sheep (P<0.01). Chlamydophila pecorum was identified by sequencing and was related with IKC only when coinfection with M. conjunctivae occurred, which suggest a synergic interaction. Cluster analysis of M. conjunctivae strains revealed higher diversity of strains than expected, evidenced interspecific transmission and suggested a higher local livestock trade than previously assumed. These results highlight the widespread occurrence of M conjunctivae in sheep worldwide and its implications for wildlife should be assessed from a conservation perspective.
Keywords: Keywords:, Chlamydophila pecorum, coinfection, goat, infectious keratoconjunctivitis, sheep, wildlife livestock interface
Infectious keratoconjunctivitis (IKC) is a common contagious ocular disease among ruminants.1 In small domestic ruminants, IKC is usually bilateral and produces ocular discharge, epiphora, mild conjunctivitis and/or corneal opacity, causing transitory blindness in most of the cases. However, IKC outbreaks may course with more severe clinical signs, including affection of the cornea leading to ulceration and perforation of the eye if no treatment is applied.2
Mycoplasma conjunctivae has been associated with most of the IKC outbreaks reported in small domestic ruminants and wild Caprinae worldwide and is considered the primary pathogen of this condition.1 3–5 However, M. conjunctivae is also commonly detected in the eyes of asymptomatic sheep and is eventually endemic in sheep flocks throughout Europe.6–8 M. conjunctivae infection is therefore not consistent with IKC in sheep, indicating that other factors may determine the development of clinical signs in non-epizootic conditions.8 Other infectious agents such as Moraxella (Branhamella) ovis, Chlamydophila species and Listeria monocytogenes have been isolated from the eyes of clinically affected sheep and may act opportunistically as secondary invaders and contribute to the onset of IKC.7 9 However, Chlamydophila ocular infections have been occasionally associated with ocular disease in small domestic ruminants10–13 and wild ruminants,14 15 and therefore their potential role as primary pathogens for IKC has been discussed.12 13 Although chlamydial infections are frequent in the eyes of diseased and asymptomatic sheep and goats,16 17 ocular coinfections with M. conjunctivae have not always been addressed in IKC control–case studies to properly assess a causal relationship. Neither did the finding of both infectious agents in clinical cases described in some studies provide conclusive aetiological information.18–20
In mountain ecosystems, sheep and goats share alpine pastures with other IKC-susceptible wild species such as chamois (Rupicapra species), Alpine ibex (Capra ibex) or Himalayan tahr (Hemitragus jemlahicus).1 21 IKC outbreaks in wild free-ranging ruminants can cause significant mortality and demographic impact on affected herds.20 22 Although host specificity of certain M conjunctivae strains or genotypes has been suggested,23 interspecific transmission from domestic to wild ruminants can occur,24 and domestic sheep may play a key role as an M. conjunctivae reservoir host for wild ruminants.1 6 8 Therefore, asymptomatic or mildly symptomatic small domestic ruminants can be at the origin of IKC outbreaks in wild ruminants, particularly if the latter have not previously been in contact with M. conjunctivae.
Furthermore, IKC outbreaks in livestock can cause occasional economic losses for farmers, as well as a detrimental impact on animal welfare.25 In developing countries, livestock production is an important economic income in rural areas. In Pakistan, there are 29.1 million sheep and 66.6 million goats. In 2013–2014, livestock production represented 55.9 per cent of the agriculture and 11.8 per cent of the Gross Domestic Product.26
The objectives of this study are to investigate the presence of M. conjunctivae and Chlamydiaceae in the eyes of sheep and goats from two isolated valleys in the buffer zone of the Central Karakoram National Park (CKNP) in Pakistan and to evaluate its health significance in relation to IKC. Factors affecting prevalence are explored and cluster analysis of the M. conjunctivae strains are also performed to establish epidemiological associations and evaluate strain diversity in this remote area.
Materials and methods
Study design and sample collection
This study was based on a combination of cross-sectional sampling strategy to estimate the apparent prevalences of M. conjunctivae and Chlamydiaceae and a case–control design to evaluate their influence on the clinical condition of IKC. From March 2013 to April 2014, eye swabs were collected from 334 small domestic ruminants (176 sheep and 158 goats) belonging to six communities at the boundaries of the CKNP (Fig 1). The minimum sample size required for pathogen prevalence estimation was 196, calculated with the WinEpiscope V.2.0 software27 with an expected prevalence of 15 per cent (95 per cent CI, 5 per cent accepted error), in unknown total population,8 and was achieved depending on the availability for sampling. In particular, a team of veterinarians (including three of the authors) and livestock assistants either operated at a congregation point where local sheep and goat owners had been invited via Muezzin’s announcement or freely moved across a village looking for owners’ consent to sampling. All individuals in available flocks were sampled.
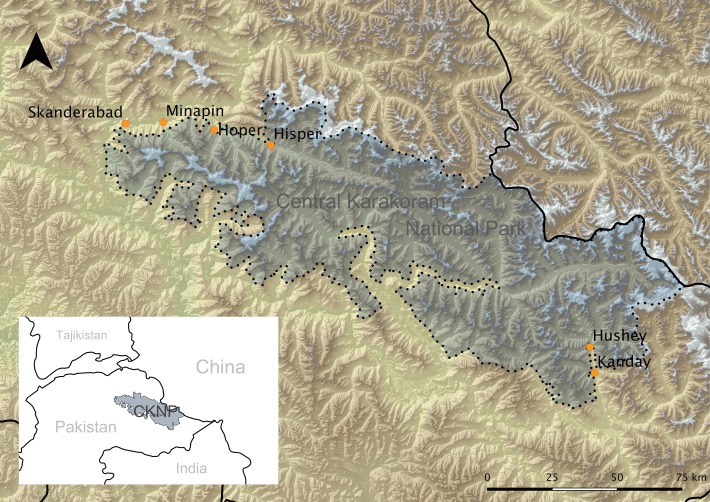
FIG 1:
Map showing the location of the Central Karakoram National Park (CKNP), in Pakistan, and the six communities were the study was performed, located in two main valleys, Hunza Nagar Valley in the northwest and the Hushe Valley in the southeast.
CKNP is the largest protected area of Pakistan and one of the largest worldwide, covering over 10,000 km2 in the Gilgit-Baltistan district (Fig 1). The sampled communities are located in two of the main valleys in the area, namely the Hunza Nagar Valley in the northwest (36°15′4.25′′N, 74°32′9.89′′E) and the Hushe Valley in the southeast (35°27′51.64′′N, 76°21′4.23′′E), at elevations ranging between 1900 m and 3500 m (Fig 1). Local climate is dry (rainfall<200 mm/year), with a relatively greater precipitation in winter and spring, arid continental climate in summer and sudden onsets of cold weather in early autumn. Winter snowfalls are not abundant but temperatures frequently reach −15°C.
Based on data provided by official veterinarians at the local Gilgit-Baltistan Livestock Department, there was a census of 7173 sheep and 6167 goats in the sampled communities at the beginning of the last decade. Typically, sheep and goats are kept in small (10–30 heads) mixed household flocks, which are seasonally merged into larger summer flocks of few hundred individuals. In the investigated area, small domestic ruminants share habitat with approximately 6000 cattle (cows, yaks and hybrids) and a limited number of horses and donkeys. Two wild Caprinae species are also found, the relatively common Asian ibex (Capra sibirica), and the rare and localised Flare-horned Markhor (Capra falconeri). Seasonal contacts between wild and domestic Caprinae occur mainly in summer, as shown to authors by members of the sampled communities. Sheep and goats in this study were sampled while kept in the surroundings of the investigated communities, before or just after the traditional summer transhumance to the high pastures.
Swabs were collected under the third eyelid from both eyes, transported refrigerated and stored frozen at −20°C until analysed. Ocular clinical signs were recorded at sampling considering the clinical condition of IKC whenever signs of ocular damage, inflammation or discharged occurred. Species, age, sex and body condition score (BCS) were also recorded.28 Age was determined in 141 of the 158 goats and in 166 of the 176 sheep by definitive incisor teeth eruption.29 All applicable institutional and/or national guidelines for the care and use of animals were followed.
Mycoplasma conjunctivae detection and LPPS sequencing
At the laboratory, eye swabs were placed into sterile tubes with 0.5 ml of lysis buffer (100mM Tris–HCl, pH 8.5, 0.05 Tween 20, 0.24 mg/ml proteinase K). After mixing with a vortex, cells were lysed for 60 minutes at 60°C and then heated to 97°C for 15 minutes in order to inactivate proteinase K.
The lysates obtained were tested for the presence of M. conjunctivae DNA with a TaqMan qPCR, using the primers LPPS-TM-L, LPPS-TM-R and the probe LPPS-TM-FT as described.30 For cluster analysis, a subtyping of the M conjunctivae strains based on the lppS gene was attempted with a selection of 26 samples which showed the lowest Ct values at the TaqMan qPCR. DNA was amplified by nested PCR according to the method described24 with minor modifications of the primers (online supplementary table s1). All PCR products were purified with the High Pure PCR Product Purification Kit (Roche Diagnostics, Rotkreuz, Switzerland) for subsequent DNA sequence analysis. DNA sequence determination was performed using the BigDye termination cycle sequencing kit (Applied Biosystems, Foster City, California, USA) with the sequencing primers Ser_start2, Ser_start0 and Ser_end0 (online supplementary table s1). The sequences obtained were trimmed to contain the variable part of gene lppS and flanking regions corresponding to the nucleotides 3935–5035 of lppS of the type strain HRC/581 of M. conjunctivae (accession number AJ318939). Cluster relationships between strains were assessed by generation of phylogenetic trees, based on the sequence of using the MEGA 6 software with the following parameters: gap opening penalty 15; gap extension penalty 6.6, DNA weight matrix IUB, transition weight 0.5 including the corresponding DNA sequence data from lppS of HRC/581 for comparison.
vetrec-2016-103948supp001.pdf (12.3KB, pdf)
Chlamydiaceae detection and identification
For the detection of Chlamydiaceae species in the eye swabs lysates, an SYBR green-based qPCR assay was performed using the primers Chuni-1F and Chuni-2R.31 Each reaction consisted of 2.5 µl of DNA sample, 12.5 µl of SYBRGreen PCR Master Mix 2x (Applied Biosystems, Warrington, UK), 400nM of each forward and reverse primer and nuclease-free water to a total volume of 25 µl. PCR was performed following reported cycling conditions.31 Samples were assayed per duplicate and were assayed with an exogenous Internal Positive Control (IPC; Applied Biosystems, USA) to detect eventual PCR inhibitors.
The positive samples were analysed further with a PCR that targets the Chlamydiales specific 298 bp signature of the 16S rRNA gene using the primers 16SIGR and 16SIGF.32 Each reaction consisted of 2.5 µl of test sample, 25 µl of AmpliTaq Gold 360 Master Mix (Applied Biosystems, UK), 400nM of each primer and nuclease-free water up to 50 µl. Amplifications were performed starting with an initial denaturation at 95°C for 10 minutes, 40 cycles that consisted in denaturation at 95°C for 30 seconds, annealing at 51°C for 30 seconds and extension at 72°C for 30 seconds, followed with a final extension step at 72°C for 7 minutes. All PCR reactions were run on an ABI 7500 instrument (Applied Biosystems, USA). Purified amplicons (Minelute Gel Extraction Kit, Qiagen, Hilden, Germany) were sequenced for its identification with Big Dye Terminator V.3.1 Kit and the ABI 3130xl Genetic Analyzer (Applied Biosystems, UK). The sequences obtained were introduced in the BLAST server from the National Centre for Biotechnology website (http://www.ncbi.nlm.nih.gov/blast/) to compare with the sequences available in GenBank.
Statistical analyses
A tree modelling approach was performed in order to identify factors that drive the M. conjunctivae and Chlamydiaceae infection. Conditional inference trees estimate relationship by binary recursive partitioning in which associations between variables are defined by P values. It is a robust statistical tool capable of dealing with variables of different nature and are suitable for complex epidemiological data.33 34 M. conjunctivae and Chlamydiaceae infection were considered as response variables (Bernoulli distribution) in two independent classification trees, whereas host (sex, age and BCS) and population variables (community and valley) were included as explanatory variables in each tree. The analyses were performed separately for sheep and goats. A conditional inference tree approach was also used to identify risk factors for IKC, including the occurrence of M. conjunctivae and Chlamydiaceae infection (ie, discrete nominal variables with two categories positive/negative) and individual host factors (sex, age and BCS) as explanatory variables. These analyses were also performed separately for each ruminant species. Differences of Ct values of the M. conjunctivae qPCR between asymptomatic and clinical sheep and goats were assessed by Wilcoxon signed-rank test. Prevalences of M conjunctivae and Chlamydiaceae were compared between species and communities using tests of proportions and setting statistical significance at 0.05. Statistical analyses were performed with R software,35 using the ‘party’ package for the trees and the ‘EpiR’ package to calculate the prevalence estimates.36
Results
M. conjunctivae had a 14.7 per cent prevalence (CI95 11.3 to 18.9, 49/334) in the sampled domestic ruminants. Prevalence was significantly (P=0.01842) higher in sheep (19.3 per cent, CI95 14.2 to 25.8, 34/176) than in goats (9.5 per cent, CI95 5.8 to 15.1, 15/158), both overall and for each sampling site (Tables 1 and 2). Chlamydiaceae prevalence was lower than M conjunctivae, both overall (3.3 per cent, CI95 1.8 to 5.8, 11/334) and for each species separately (sheep 4.5 per cent, CI95 2.3 to 8.7, 8/176 and goats 1.9 per cent, CI95 0.6 to 5.4, 3/158). Conversely to M conjunctivae, the prevalence of Chlamydiaceae did not significantly differ between species. M conjunctivae was detected in all investigated communities in sheep, and in four of them in goats, whereas Chlamydiaceae were detected in five communities in sheep and in three communities in goats (Tables 1 and 2).
TABLE 1:
Prevalence by species and ocular clinical signs.
| M. conjunctivae | Chlamydiaceae | ||||
| n | Positive | Prevalence (CI95) | Positive | Prevalence (CI95) | |
| Sheep | |||||
| With KC | 12 | 4 | 33.3% (13.8 to 60.9) | 2* | 16.7% (4.7 to 44.8) |
| Without KC | 164 | 30 | 18.3% (13.1 to 24.9) | 6 | 3.7% (1.7 to 7.7) |
| Total | 176 | 34 | 19.3% (14.2 to 25.8) | 8 | 4.5% (2.3 to 8.7) |
| Goats | |||||
| With KC | 2 | 1 | 50.0% (2.6 to 97.4) | 0 | 0% (0 to 65.8) |
| Without KC | 156 | 14 | 9.0% (5.4 to 14.5) | 3 | 1.9% (0.6 to 5.5) |
| Total | 158 | 15 | 9.5% (5.8 to 15.1) | 3 | 1.9% (0.6 to 5.4) |
| Total | 334 | 49 | 14.7% (11.3 to 18.9) | 11 | 3.3% (1.8 to 5.8) |
Summary of samples analysed (n), positives to qPCR (Positive) and prevalence (%) of M. conjunctivae and Chlamydiaceae in eye swabs of small domestic ruminants from the Central Karakoram National Park area, showed by ruminant species and by ocular clinical signs (KC)
*These two sheep were also positive to M. conjunctivae
KC, keratoconjunctivitis
TABLE 2:
Prevalence by species and communities.
| Community | Goats | Sheep | ||||||
| S | KC | M. conjunctivae | Chlamydiaceae | S | KC | M. conjunctivae | Chlamydiaceae | |
| Hisper | 23 | 0 | 0.0% (0)* | 0.0% (0) | 9 | 0 | 11.1% (1)* | 0.0% (0) |
| Hoper | 22 | 1 | 22.7% (5)* | 4.6% (1) | 10 | 2 | 30.0% (3)* | 10.0% (1) |
| Hushe | 36 | 0 | 5.6% (2)* | 2.8% (1) | 43 | 5 | 18.6% (8)* | 4.6% (2) |
| Kanday | 61 | 1 | 11.5% (7)* | 0.0% (0) | 73 | 5 | 17.8% (13)* | 4.1% (3) |
| Minapin | 2 | 0 | 0.0% (0)* | 0.0% (0) | 15 | 0 | 26.7% (4)* | 6.7% (1) |
| Skanderabad | 14 | 0 | 7.1% (1)* | 7.1% (1) | 26 | 0 | 19.2% (5)* | 3.9% (1) |
| Total | 158 | 2 | 9.5% (15)* | 1.9% (3) | 176 | 12 | 19.3% (34)* | 4.5% (8) |
Summary of samples (S), clinical signs (KC) and prevalence (percentage and number of positives) of M. conjunctivae and Chlamydiaceae bacteria in the eyes of small domestic ruminants from the Central Karakoram National Park area in Pakistan
* Mean M. conjunctivae prevalence was significantly (P=0.01842) higher in sheep than in goats both overall and for each sampling community
KC, keratoconjunctivitis
Among the 34 M. conjunctivae-positive sheep, 4 (11.8 per cent) had KC with ocular discharge, whereas the remaining 30 sheep (88.2 per cent) had no clinical signs at the time of sampling (Table 1). One of the 15 M conjunctivae-positive goats showed severe signs of KC (bilateral cornea perforation) associated with the ocular presence of M. conjunctivae (Fig 2), whereas the remaining 14 (93.3 per cent) M. conjunctivae-positive goats did not show any clinical signs. Median Ct values of the M. conjunctivae qPCR showed no statistical differences in sheep with IKC (median 30.2) and without (median 31.7), and in goats with IKC (26.3 in the only IKC case) and without (31.3). On the other hand, eight sheep (4.5 per cent, 8/176) and one goat (0.6 per cent, 1/158) with ocular clinical signs tested negative to the M. conjunctivae qPCR (Table 1). Also, 3 sheep of the 11 domestic small ruminants (8 sheep and 3 goats) positive to Chlamydiaceae were also positive to M. conjunctivae. KC clinical signs in the Chlamydiaceae-positive small ruminants were observed only when coinfection with M conjunctivae occurred, in two out of these three sheep.
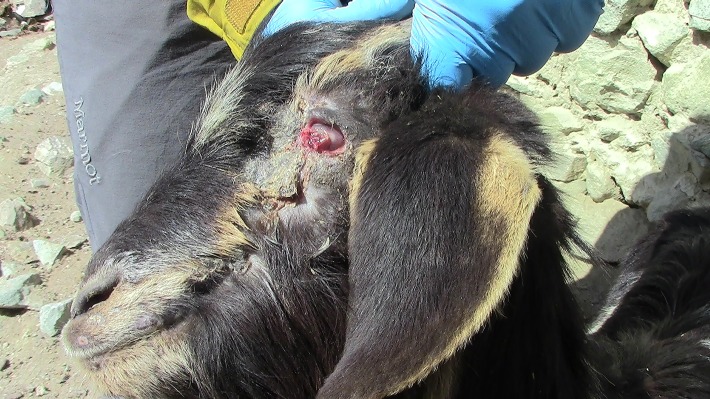
FIG 2:
Image of a goat exhibiting a severe stage of infectious keratoconjunctivitis, with bilateral panophthalmitis, lacrymation, hypopyon and perforation of the cornea. Mycoplasma conjunctivae was confirmed in eye swabs by qPCR.
Decision tree analyses indicated that M conjunctivae prevalence was significantly (P<0.01) higher in sheep younger than one year than in older sheep (Table 3). Differences by age category were not observed in goats and no other factors considered in the tree analysis were identified to influence significantly on M. conjunctivae prevalence. No age-related differences were observed in both hosts for Chlamydiaceae. On the other hand, no risk factors for IKC were identified among the explanatory variables considered in the tree, including M. conjunctivae and Chlamydiaceae occurrence in the eyes of sheep and goats.
TABLE 3.
Age-related differences in prevalence.
| Age category | ||
| 0–1 years | >1 year | |
| M. conjunctivae | ||
| Goats | 16.3% (8/49) | 3.3% (3/92) |
| Sheep | 30.0% (24/80)* | 10.5% (9/86)* |
| Total | 24.8% (32/129) | 6.7% (12/178) |
| Chlamydiaceae | ||
| Goats | 2.0% (1/49) | 2.2% (2/92) |
| Sheep | 5.0% (4/80) | 4.7% (4/86) |
| Total | 3.9% (5/129) | 3.4% (6/178) |
Results of M. conjunctivae and Chlamydiaceae qPCR (prevalence %; positive/tested) showed by age in 141 goats and 166 sheep in which age was determined
*Statistically significant differences (P<0.01)
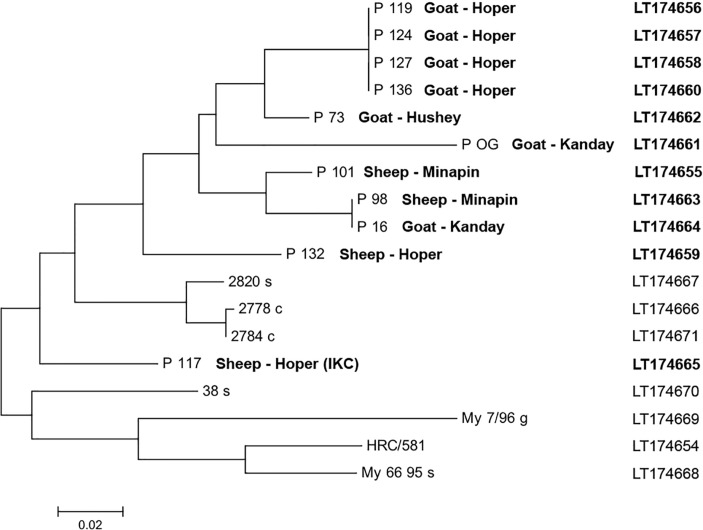
Eleven PCR-amplified fragments of the lppS gene of M. conjunctivae were sequenced from seven goats and from four sheep taken from four different communities. Sequence analyses allowed to differentiate seven M. conjunctivae strains that were all phylogenetically related to M. conjunctivae strains from sheep (2820s) and Alpine chamois (Rupicapra rupicapra) (2778c, 2784c) that were isolated in the Alps and related to IKC outbreaks in chamois and sheep24 (Fig 3). Among the strains sequenced, only one (P117) was associated with clinical KC in a sheep. In four goats (P119, P124, P122, P136) from Hoper community, a common strain was identified. Furthermore, a common strain was found in a sheep (P98) and a goat (P16) from Minapin and Kanday communities, respectively (Fig 3). The EMBL/GenBank accession numbers for the lppS gene fragments of the different strains are also shown in Fig 3.
FIG 3:
Phylogenetic representation of the M. conjunctivae strains sequenced from sheep and goats from the Central Karakoram National Park area (number starting with P) in comparison with the type strain of M conjunctivae (HRC/581) and strains from sheep in the Eastern Swiss Alps (38s and 2820s), from chamois in the Austrian Alps (2778c and 2784c), from sheep in Croatia imported from Australia (My6695) and from a goat imported to Croatia from the Southern French Alps (My7/96). The species and the community are specified next to the strain reference. Infectious keratoconjunctivitis (IKC) indicates the presence of ocular clinical signs at sampling. The EMBL/GenBank accession numbers for the lppS gene fragments of the different strains identified in the study are indicated in bold, whereas the accession numbers for the reference strains are indicated in plain text.
Sequences of the Chlamydiaceae 16S rRNA were obtained from five Chlamydiaceae-positive samples (four sheep and one goat from two different communities) and all had the highest similarity (96–100) with Chlamydophila pecorum 16S ribosomal RNA complete sequence (accession number NR_121750.1).
Discussion
M. conjunctivae and Chlamydiaceae were detected in asymptomatic and clinically affected eyes of sheep and goats from the CKNP, Gilgit-Baltistan district of Pakistan. These results agree with the previously reported sporadic IKC cases in sheep flocks with endemic M. conjunctivae infections and its capability to establish asymptomatic ocular infections.8 37 Reports of non-epidemic IKC in goats are scarce38 and most of the IKC descriptions refer to outbreaks.39 40 However, the results obtained in this study suggest that M conjunctivae is endemic in mixed sheep–goat flocks of the CKNP area. Although M conjunctivae has been previously reported in sheep from Pakistan41 and several other locations worldwide,18 42–44 its detection had not been reported in traditionally reared flocks from such remote and isolated communities. This finding confirms that M. conjunctivae is probably one of the most common and geographically widespread pathogens found in sheep.
M. conjunctivae has been traditionally studied as the main aetiological agent in IKC outbreaks characterised by high morbidity affecting all age classes and severe clinical signs.25 41 44 Asymptomatic carriers may have lower M. conjunctivae loads,45 which altogether with the fastidious nature of M. conjunctivae may have complicated its identification, resulting eventually in the underestimation of its frequency by traditional methods of isolation.7 40 Similar prevalence to those found in sheep and goats from the CKNP area has been reported in sheep from northern Spain (25.7–29.2 per cent) when assessed by qPCR, with also a similar percentage of asymptomatic M conjunctivae-positive sheep (87.3 per cent).8 20 Since M. conjunctivae-infected sheep from Pakistan were mostly asymptomatic, no statistically significant relationship could be established between M. conjunctivae infection and IKC. In these endemic and mostly subclinical infections, the development of clinical signs may depend on other factors, such as host immunity, strain virulence or concurrent infections.7 8 Ocular clinical signs were however not related with the Ct values of the M. conjunctivae qPCR and several asymptomatic infections exhibited low Ct values (ie, inverse to mycoplasmal loads), which suggests that a different host–mycoplasma interaction than the previously described in wild Caprinae occurred in sheep and goats.5 45 46
The higher prevalence of M. conjunctivae in sheep younger than one year compared with the older ones indicates that acquired immunity may influence the course of the infection in sheep. IKC typically causes more severe clinical signs in adult sheep than lambs.2 37 However, M. conjunctivae persistence in the eyes do not necessarily have to be related with clinical signs as broadly described in experimental infections.6 47 Higher prevalence of M. conjunctivae in young animals has been also described in ibex,5 45 which suggests that younger animals/lambs may be more important for the M. conjunctivae maintenance in the herd/population. Endemic and mostly asymptomatic M. conjunctivae infections had no apparent effect on BCS, which is probably influenced by other factors not assessed in this study. The small sample size and number of clinical cases might also have impaired the identification of risk factors associated with the disease.
The higher prevalence of M. conjunctivae in sheep compared with goats corresponds to previous reports from other geographic areas.8 This higher prevalence of M conjunctivae in sheep may be related to host specificity of the prevalent local strains, or to a higher density, aggregation and intraspecific contact among sheep than among goats within mixed flocks. Overall, the results of this study and previous descriptions suggest that sheep are better hosts than goats for the endemic and mostly asymptomatic maintenance of M. conjunctivae. However, high pathogenic strains of M. conjunctivae were isolated during IKC outbreaks in both ruminant species,25 39 and goats can also develop severe IKC in endemic infections as observed in this study (Fig 2).
Prevalence of Chlamydiaceae was lower than reported in previous studies in small domestic ruminants.16 17 The differences could be in part because different sampling and diagnostic methodology were used. Several Chlamydophila species, such as Chlamydophila abortus, C pecorum, Chlamydophila psittaci and Chlamydia suis have been identified in the eyes of livestock without correlation with ocular clinical signs.12 16 17 On the other hand, C. pecorum has been occasionally associated with KC and polyarthritis in sheep and goats48 and IKC was successfully induced experimentally with C. psittaci infection (as taxonomically considered at that time).11 Chlamydial KC outbreaks have also been reported both in domestic and wild ruminants.10 14 Since Chlamydiaceae and particularly C. pecorum infection in sheep and goats from CKNP area were exclusively associated with clinical signs of IKC in case of coinfection with M. conjunctivae, it is possible that they acted synergically with M conjunctivae as a secondary infectious agent for the development of clinical disease. However, the few number of coinfection cases found in this study does not allow inferring conclusions on virulence synergism with a statistical approach. The relative aetiological importance of Chlamydiaceae may also rely on other factors, such as the strain pathogenicity.48 Similar concurrent infections of M. conjunctivae and Chlamydiaceae have been reported in domestic sheep12 18 and free-ranging chamois19 20 in both diseased and asymptomatic individuals. According to these results, the interaction of Chlamydophila species with M conjunctivae should be considered in aetiological investigations of ocular disease in small domestic ruminants, although experimental evidence would probably be necessary to assess whether such coinfections determine the onset of IKC.
The diversity of the M. conjunctivae strains identified in the CKNP area suggests that M conjunctivae has been present for a long time in northern Pakistan. However, the relatively close relationship of the M conjunctivae strains found in this study with strains described in sheep and wild Caprinae from the Alps (Central Europe) suggests that M. conjunctivae might have been introduced in this area along with its hosts, as it was described in Croatia in the early 1990s.24 Furthermore, the molecular identification of the same strain infecting sheep and goats confirms interspecific transmission and the need to approach the epidemiological study of IKC considering all the susceptible hosts, taking into account the differences in host–pathogen interaction.8 Cluster analyses also revealed that the same M. conjunctivae strain was present in the communities of Kanday and Minapin, separated by 190-km straight-line distance and mountains ranging 5500–8000 m of altitude (Fig 1). Livestock trade in northern Pakistan is mainly on a local scale, and it can be assumed that trade movements between Kanday and Minapin are limited due to the great physical and cultural distance and the deriving lack of relationships. However, the molecular analyses of M. conjunctivae in sheep and goats mirror more complex trade habits and suggest a long endemic presence of M conjunctivae in the small domestic ruminants of the CKNP area.
The introduction of M. conjunctivae asymptomatic carriers into naive populations supposes a risk of severe IKC outbreaks in sheep flocks.25 44 Similarly, interspecific transmission of M conjunctivae from domestic to wild mountain ruminants may result into massive IKC outbreaks in wildlife as demonstrated in Alpine chamois.24 Therefore, domestic sheep and goat populations of the CKNP area, asymptomatically infected with M conjunctivae, may represent a potential source of infection and IKC outbreaks in sympatric wild hosts, namely the Asian ibex and the Flare-horned Markhor. To the authors’ knowledge, there is currently no robust information about IKC in wild ruminants from the CKNP. Syndromic surveillance of IKC in local flocks however would improve rapid detection of any major risk of spill over of virulent M. conjunctivae strains at the livestock–wildlife interface.
In conclusion, mostly asymptomatic and endemic infections of M. conjunctivae and Chlamydiaceae were found in sheep and goats populations of the CKNP area in the Gilgit-Baltistan district of Pakistan. Chlamydiaceae was associated with ocular clinical signs only when coinfection with M. conjunctivae occurred, but further studies are needed to better assess the effect of such coinfections in eye disease. Cluster analysis of M. conjunctivae strains revealed higher diversity of strains than expected, evidenced interspecific transmission and suggested a higher local livestock trade than previously assumed. M. conjunctivae is probably one of the most common pathogens found in sheep worldwide and its implications should be assessed from a conservation perspective if sheep are allowed to share pastures with endangered or vulnerable ruminant species.
Acknowledgments
Sampling would have not been possible without the enthusiastic compliance of local villagers and the precious collaboration of the staff members from the Central Karakoram National Park. Valuable support was also provided by official veterinarians and livestock assistants of the Livestock & Dairy Development Department, Gilgit Baltistan. The authors also acknowledge the invaluable technical assistance of Amandine Ruffieux, Institute of Veterinary Bacteriology, University of Bern.
Footnotes
Funding: This study benefited from the research project CGL2009 11631 of the Spanish MICINN, from the internal research fund #35539 of the Institute of Veterinary Bacteriology, University of Bern and the research project SEED (Social Economic Environmental Development in the Central Karakhoram National Park) of the Italian Government, implemented by Ev K2 CNR.
Competing interests: XFA was supported by the FI DGR programme from the Government of Catalonia.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Giacometti M, Janovsky M, Belloy L, et al. . Infectious keratoconjunctivitis of ibex, chamois and other Caprinae. Rev Sci Tech 2002;21:335–45. 10.20506/rst.21.2.1338 [DOI] [PubMed] [Google Scholar]
- 2. Hosie BD. Infectious Keratoconjunctivitis Aitken ID, Diseases of Sheep: Blackwell Publishing, 2007:342–5. [Google Scholar]
- 3. Trotter SL, Franklin RM, Baas EJ, et al. . Epidemic caprine keratoconjunctivitis: experimentally induced disease with a pure culture of Mycoplasma conjunctivae . Infect Immun 1977;18:816–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. ter Laak EA, Schreuder BE, Kimman TG, et al. . Ovine keratoconjunctivitis experimentally induced by instillation of Mycoplasma conjunctivae . Vet Q 1988;10:217–24. 10.1080/01652176.1988.9694175 [DOI] [PubMed] [Google Scholar]
- 5. FERNÁNDEZ-AGUILAR X, CABEZÓN O, Granados JE, et al. . Postepizootic persistence of asymptomatic Mycoplasma conjunctivae infection in Iberian ibex. Applied and Environmental Microbiology 2017;83:e00690–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Janovsky M, Frey J, Nicolet J, et al. . Mycoplasma conjunctivae infection is self-maintained in the swiss domestic sheep population. Vet Microbiol 2001;83:11–22. 10.1016/S0378-1135(01)00407-2 [DOI] [PubMed] [Google Scholar]
- 7. Akerstedt J, Hofshagen M. Bacteriological investigation of infectious keratoconjunctivitis in Norwegian sheep. Acta Vet Scand 2004;45:19–26. 10.1186/1751-0147-45-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernández-Aguilar X, Cabezón O, Marco I, et al. . Mycoplasma conjunctivae in domestic small ruminants from high mountain habitats in Northern Spain. BMC Vet Res 2013;9:253 10.1186/1746-6148-9-253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dagnall GJ. The role of Branhamella ovis, Mycoplasma conjunctivae and Chlamydia psittaci in conjunctivitis of sheep. Br Vet J 1994;150:65–71. 10.1016/S0007-1935(05)80097-1 [DOI] [PubMed] [Google Scholar]
- 10. Andrews AH, Goddard PC, Wilsmore AJ, et al. . A chlamydial keratoconjunctivitis in a British sheep flock. Vet Rec 1987;120:238–9. 10.1136/vr.120.10.238 [DOI] [PubMed] [Google Scholar]
- 11. Wilsmore AJ, Dagnall GJ, Woodland RM. Experimental conjunctival infection of lambs with a strain of Chlamydia psittaci isolated from the eyes of a sheep naturally affected with keratoconjunctivitis. Vet Rec 1990;127:229–31. [PubMed] [Google Scholar]
- 12. Gupta S, Chahota R, Bhardwaj B, et al. . Identification of chlamydiaeChlamydiae and Mycoplasma species in ruminants with ocular infections. Lett Appl Microbiol 2015;60:135–9. 10.1111/lam.12362 [DOI] [PubMed] [Google Scholar]
- 13. Walker E, Lee EJ, Timms P, et al. . Chlamydia pecorum infections in sheep and cattle: a common and under-recognised infectious disease with significant impact on animal health. Vet J 2015;206:252–60. 10.1016/j.tvjl.2015.09.022 [DOI] [PubMed] [Google Scholar]
- 14. Meagher M, Quinn WJ, Stackhouse L. Chlamydial-caused infectious keratoconjunctivitis in bighorn sheep of Yellowstone National Park. J Wildl Dis 1992;28:171–6. 10.7589/0090-3558-28.2.171 [DOI] [PubMed] [Google Scholar]
- 15. Taylor SK, Vieira VG, Williams ES, et al. . Infectious keratoconjunctivitis in free-ranging mule deer (Odocoileus hemionus) from Zion National Park, Utah. J Wildl Dis 1996;32:326–30. 10.7589/0090-3558-32.2.326 [DOI] [PubMed] [Google Scholar]
- 16. Polkinghorne A, Borel N, Becker A, et al. . Molecular evidence for chlamydial infections in the eyes of sheep. Vet Microbiol 2009;135:142–6. 10.1016/j.vetmic.2008.09.034 [DOI] [PubMed] [Google Scholar]
- 17. Osman KM, Ali HA, ElJakee JA, et al. . Prevalence of Chlamydophila psittaci infections in the eyes of cattle, buffaloes, sheep and goats in contact with a human population. Transbound Emerg Dis 2013;60:245–51. 10.1111/j.1865-1682.2012.01337.x [DOI] [PubMed] [Google Scholar]
- 18. Lysnyansky I, Levisohn S, Bernstein M, et al. . Diagnosis of Mycoplasma conjunctivae and Chlamydophila spp in an episode of conjunctivitis/keratoconjunctivitis in lambs. Israel Journal of Veterinary Medicine 2007;62:79–82. [Google Scholar]
- 19. Holzwarth N, Pospischil A, Mavrot F, et al. . Occurrence of Chlamydiaceae, Mycoplasma conjunctivae, and pestiviruses in Alpine chamois (Rupicapra r.)of Grisons, Switzerland. J Vet Diagn Invest 2011;23:333–7. 10.1177/104063871102300223 [DOI] [PubMed] [Google Scholar]
- 20. Arnal M, Herrero J, de la Fe C, et al. . Dynamics of an infectious keratoconjunctivitis outbreak by Mycoplasma conjunctivae on Pyrenean Chamois Rupicapra p. pyrenaica . PLoS One 2013;8:e61887 10.1371/journal.pone.0061887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ryser-Degiorgis M-P, Ingold P, Tenhu H, et al. . Encounters between Alpine Ibex, Alpine Chamois and Domestic Sheep in the Swiss Alps. Hystrix 2002;13:1–11. [Google Scholar]
- 22. Loison A, Gaillard J-M, Jullien J-M. Demographic patterns after an epizootic of Keratoconjunctivitis in a Chamois Population. J Wildl Manage 1996;60:517–27. 10.2307/3802069 [DOI] [Google Scholar]
- 23. Zimmermann L, Jambresic S, Giacometti M, et al. . Specificity of Mycoplasma conjunctivae strains for alpine chamois Rupicapra r. Rupicapra . Wildlife Biol 2008;14:118–24. doi: 10.2981/0909-6396(2008)14[118:SOMCSF]2.0.CO;2 [DOI] [Google Scholar]
- 24. Belloy L, Janovsky M, Vilei EM, et al. . Molecular epidemiology of Mycoplasma conjunctivae in Caprinae: transmission across species in natural outbreaks. Appl Environ Microbiol 2003;69:1913–9. 10.1128/AEM.69.4.1913-1919.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Naglić T, Hajsig D, Frey J, et al. . Epidemiological and microbiological study of an outbreak of infectious keratoconjunctivitis in sheep. Vet Rec 2000;147:72–5. 10.1136/vr.147.3.72 [DOI] [PubMed] [Google Scholar]
- 26. ANONYMOUS. Pakistan Economic Survey: Ministry of Finance, Goverment of Pakistan., 2014:23–41. [Google Scholar]
- 27. Thrusfield M, Ortega C, de Blas I, et al. . WIN EPISCOPE 2.0: improved epidemiological software for veterinary medicine. Vet Rec 2001;148:567–72. 10.1136/vr.148.18.567 [DOI] [PubMed] [Google Scholar]
- 28. Russel AJF, Doney JM, Gunn RG. Subjective assessment of body fat in live sheep. J Agric Sci 1969;72:451–4. 10.1017/S0021859600024874 [DOI] [Google Scholar]
- 29. Food and agriculture organization of the United Nations. How to age sheep, goats, cattle and buffalo A manual for primary animal health care worker: Animal Production and Health Division., 1994. http://www.fao.org/docrep/t0690e/t0690e05.htm#unit. [Google Scholar]
- 30. Vilei EM, Bonvin-Klotz L, Zimmermann L, et al. . Validation and diagnostic efficacy of a TaqMan real-time PCR for the detection of Mycoplasma conjunctivae in the eyes of infected Caprinae. J Microbiol Methods 2007;70:384–6. 10.1016/j.mimet.2007.05.007 [DOI] [PubMed] [Google Scholar]
- 31. Nordentoft S, Kabell S, Pedersen K. Real-time detection and identification of Chlamydophila species in veterinary specimens by using SYBR green-based PCR assays. Appl Environ Microbiol 2011;77:6323–30. 10.1128/AEM.00536-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Everett KD, Bush RM, Andersen AA. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int J Syst Bacteriol 1999;49:415–40. 10.1099/00207713-49-2-415 [DOI] [PubMed] [Google Scholar]
- 33. Friedman JH, Meulman JJ. Multiple additive regression trees with application in epidemiology. Stat Med 2003;22:1365–81. 10.1002/sim.1501 [DOI] [PubMed] [Google Scholar]
- 34. Hothorn T, Hornik K, Zeileis A. Unbiased recursive partitioning: a conditional Inference Framework. Journal of Computational and Graphical Statistics 2006;15:651–74. 10.1198/106186006X133933 [DOI] [Google Scholar]
- 35. R Development core team 3.1.3. A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2015. http://www.R-project.org. [Google Scholar]
- 36. Stevenson M, Nunes T, Heuer C. epiR: An R Package for the Analysis of Epidemiological Data. R package version 0.9 62 2012.
- 37. Jones GE, Foggie A, Sutherland A, et al. . Mycoplasmas and ovine keratoconjunctivitis. Vet Rec 1976;99:137–41. 10.1136/vr.99.8.137 [DOI] [PubMed] [Google Scholar]
- 38. Surman PG. Mycoplasma aetiology of keratoconjunctivitis ("pink-eye") in domestic ruminants. Aust J Exp Biol Med Sci 1973;51:589–607. 10.1038/icb.1973.56 [DOI] [PubMed] [Google Scholar]
- 39. Baas EJ, Trotter SL, Franklin RM, et al. . Epidemic caprine keratoconjunctivitis: recovery of Mycoplasma conjunctivae and its possible role in pathogenesis. Infect Immun 1977;18:806–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. ter Laak EA, Schreuder BE, Smith-Buys CM. The occurrence of Mycoplasma conjunctivae in The Netherlands and its association with infectious keratoconjunctivitis in sheep and goats. Vet Q 1988;10:73–83. 10.1080/01652176.1988.9694153 [DOI] [PubMed] [Google Scholar]
- 41. Shahzad W, Munir R, Rana MY, et al. . Prevalence, molecular diagnosis and treatment of Mycoplasma conjunctivae isolated from infectious keratoconjunctivitis affected Lohi sheep maintained at Livestock Experiment Station, Bahadurnagar, Okara, Pakistan. Trop Anim Health Prod 2013;45:737–42. 10.1007/s11250-012-0282-2 [DOI] [PubMed] [Google Scholar]
- 42. Barile MF, Del Giudice RA, Tully JG. Isolation and characterization of Mycoplasma conjunctivae sp. n. from sheep and goats with keratoconjunctivitis. Infect Immun 1972;5:70–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Van Halderen A, Van Rensburg WJ, Geyer A, et al. . The identification of Mycoplasma conjunctivae as an aetiological agent of infectious keratoconjunctivitis of sheep in South Africa. Onderstepoort J Vet Res 1994;61:231–7. [PubMed] [Google Scholar]
- 44. Motha MX, Frey J, Hansen MF, et al. . Detection of Mycoplasma conjunctivae in sheep affected with conjunctivitis and infectious keratoconjunctivitis. N Z Vet J 2003;51:186–90. 10.1080/00480169.2003.36362 [DOI] [PubMed] [Google Scholar]
- 45. Mavrot F, Vilei EM, Marreros N, et al. . Occurrence, quantification, and genotyping of Mycoplasma conjunctivae in wild caprinae with and without infectious keratoconjunctivitis. J Wildl Dis 2012;48:619–31. 10.7589/0090-3558-48.3.619 [DOI] [PubMed] [Google Scholar]
- 46. Ryser-Degiorgis MP, Bischof DF, Marreros N, et al. . Detection of Mycoplasma conjunctivae in the eyes of healthy, free-ranging Alpine ibex: possible involvement of alpine ibex as carriers for the main causing agent of infectious keratoconjunctivitis in wild Caprinae. Vet Microbiol 2009;134:368–74. 10.1016/j.vetmic.2008.08.005 [DOI] [PubMed] [Google Scholar]
- 47. Giacometti M, Nicolet J, Frey J, et al. . Susceptibility of alpine ibex to conjunctivitis caused by inoculation of a sheep-strain of Mycoplasma conjunctivae . Vet Microbiol 1998;61:279–88. 10.1016/S0378-1135(98)00186-2 [DOI] [PubMed] [Google Scholar]
- 48. Jelocnik M, Walker E, Pannekoek Y, et al. . Evaluation of the relationship between Chlamydia pecorum sequence types and disease using a species-specific multi-locus sequence typing scheme (MLST). Vet Microbiol 2014;174:214–22. 10.1016/j.vetmic.2014.08.018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
vetrec-2016-103948supp001.pdf (12.3KB, pdf)