Abstract
Bovine viral diarrhoea (BVD) is a significant drain on efficient and successful cattle production in both dairy and beef systems around the world. Several countries have achieved eradication of this disease, but always through the motivation of stakeholders who accept the benefits of eradication. These include increased cattle welfare and fitness of cattle to withstand other diseases, and decreased costs of production, the latter resulting from both decreased costs spent on managing the disease and decreased losses. This paper provides a systematic review of 31 papers, published between 1991 and 2015, that address the economic impact of BVD. Each paper takes a different approach, in either beef or dairy production or both. However with the breadth of work collated, a stakeholder engaged in BVD eradication should find an economic figure of most relevance to them. The reported economic impact ranges from £0 to £552 per cow per year (£2370 including outliers). This range represents endemic or subclinical disease situations seen in herds with stable BVD virus infection, and epidemic or severe acute situations, most often seen in naïve herds. The outcome of infection is therefore dependent on the immune status of the animal and severity of the strain. The variations in figures for the economic impact of BVD relate to these immune and pathogenicity factors, along with the variety of impacts monitored.
Keywords: bovine viral diarrhoea virus (bvdv), eradication, cost-benefit, economic impact, systematic review, bvd
Introduction
Bovine viral diarrhoea
Bovine viral diarrhoea (BVD) is a disease caused by BVD virus (BVDV), a pestivirus belonging to the Flaviviridae family. The disease can manifest as generalised immunosuppression, with evidence of synergistic effects with other pathogens, fertility problems in male and female cattle, and other often more variable signs such as decreased milk production and weight gain, fever, diarrhoea and respiratory dysfunction.1–5 The extent of disease appears to be dependent on the level of immunity of the animal and pathogenicity of the virus strain.6 7
Control of BVD depends on removal of persistently infected (PI) animals, and maintenance of biosecurity to ensure that no new PI individuals are born. BVD is currently endemic in the majority of countries of the world, with control schemes progressing in Germany, Scotland, Belgium, Northern Ireland and Ireland, as well as regional schemes throughout many European countries.8–12 The basis for seeking freedom from BVDV in these countries has been economic, as well as on welfare grounds, and to promote proactive disease control rather than reactive disease control with associated increased use of antibiotics.13
Economic incentives for eradication programmes have been used both as a direct reward for culling of PI cattle and through the promise of greater efficiency and reduced losses.12 14 One incentive for many farmers involved in national BVD eradication schemes is the hope that they can stop vaccinating. While some countries have achieved eradication without vaccination, advances in cost-effective diagnostic testing mean that maintenance of biosecurity through vaccination when eradicating BVD is an option, as seen in Germany, Ireland and Scotland.
Veterinary practitioners are key to decisions regarding disease control on farms, certainly in the UK.15 However, it is apparent that veterinary practitioners need to have more of an understanding of the economic impact of disease, not just welfare effects, because this often affects the willingness of a farmer to undertake an action.16 The economic assessments of national BVD control by Weldegebriel and others17 and Stott and others18 were integral to the implementation of the government-backed BVD eradication schemes in Scotland and Ireland, respectively. However, for voluntary schemes, such as those proposed for England and Wales, farmer and veterinary practitioner engagement will be essential to ensure the momentum to proceed to a compulsory phase (ref 19 and N. Paton, personal communication).
Economic impact
Economic impact (cost, C) of BVD is determined by production losses (L) (direct and indirect) and control expenditures (E):
With the aim of reducing L to 0, it may be beneficial to increase E in the short term on diagnostics, biosecurity and vaccination.20 So for the fixed period of an eradication scheme, it may appear the scheme is not cost beneficial; however, once freedom from the disease is achieved and maintained, it is cost-effective in the long term. The minimal, and therefore optimal, level of C may also be achieved over a defined period through use of an optimal level of E, which may not reduce L to 0.
Assessing the economic impact of BVD therefore needs an understanding and calculation of the losses and the expenditures of BVD being present in a herd, as well as an understanding of the objective of the assessment and whether it seeks to calculate an economic impact, avoidable loss or address a control choice or E. These figures can be assessed through looking at case histories of losses from outbreaks, cost and benefits of farm-based or regional-based eradication schemes, or quantitative modelling. Quantitative modelling techniques for disease control take the form of four options: mathematical programming, network or decision analysis, simulation and cost–benefit analysis.20 21 Mathematical programming is useful for structured decision problems, with various options to take into account and can involve linear or dynamic programming. Network analysis can contain qualitative and quantitative information, and is often a diagram that can be used to describe, explain and analyse systems or processes. Decision analysis is similar to network analysis and is useful for poorly structured decision problems where risk and associated judgement is required. Cost–benefit analysis is an overall term for a number of ways of analysing different courses of action, but essentially it tries to identify, quantify and analyse the costs and benefits of a specific resource allocation decision using a partial budget structure often in a spreadsheet model. For national-level decisions, often the costs and benefits to society are considered, producing social cost–benefit analysis.21 22 This is often given as a net present value or as a ratio (cost:benefit or benefit:cost). Simulation allows experimentation with a model of a system rather than the system itself, and can incorporate the probability of events happening. Monte Carlo simulations use random numbers to simulate random processes, to take account of random distributions in the real world, resulting in a ‘see what happens’ analysis. Monte Carlo simulations are useful when models which are deterministic, or input-defined, and stochastic, or possess-inherent randomness, have no analytical solutions or are difficult to obtain. Markov processes or chains use transitional probabilities between the states of a system, for example, infected and immune. These processes can be mingled into one analysis.21
Methods and materials
A publication search was performed in PubMed and Web of Science to gather papers that are concerned with BVDV and associated economic impact, following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.23 Additionally, Google Scholar also was used as a search resource. Language was limited to English. A search was made of the past 25 years (from 1991 to 2015) because this coincides with an increase in understanding of the disease and therefore an increase in publications on it.
Results that could not be accessed electronically or were repeats were removed. Papers were then submitted to one screening question: ‘Are numerical results produced that provide an assessment of the economic impact of BVD?’ Information used to provide the economic assessment was then assembled into a table (Microsoft Excel 2010; Microsoft, Redmond, USA).
An advanced search was made on PubMed (http://www.ncbi.nlm.nih.gov/pubmed/advanced) using the following search: ((bovine viral diarrh*[Title] OR bovine virus diarrh*[Title] OR bvd[Title] OR bvdv[Title])) AND (economic*[Title] OR financial[Title] OR cost*[Title]).
The results were initially filtered on PubMed by selecting the article type and publication dates.
article types: case reports, clinical trial, congresses, journal article, lectures, meta-analysis, observational study, review and systematic reviews
publication dates: from January 1, 1991 to December 31, 2015.
The 24 results were filtered to remove any that were concerned either primarily with diagnostics or were not relevant, leaving 20 results.
A second search was made in Google Scholar (https://scholar.google.co.uk/), using the following searches: allintitle: bvd economic OR economics OR financial OR cost OR costs, allintitle: bvdv economic OR economics OR financial OR cost OR costs, allintitle: bovine viral diarrhoea economic OR economics OR financial OR cost OR costs, allintitle: bovine viral diarrhea economic OR economics OR financial OR cost OR costs, allintitle: bovine virus diarrhea economic OR economics OR financial OR cost OR costs, allintitle: bovine virus diarrhoea economic OR economics OR financial OR cost OR costs.
The results were initially filtered on Google Scholar by selecting custom range and removing citations and patents.
Return articles dated between 1991 and 2015.
There were 53 articles returned, 16 repeats were removed, 5 were removed that were involved in diagnostics and 2 that were not relevant, leaving 30 papers.
A final search was on Web of Science (V.5.21) (http://apps.webofknowledge.com/UA_GeneralSearch_input.do?product=UA&search_mode=GeneralSearch&SID=W1Y13NkN8qgoWoHxoiI&preferencesSaved=) using the advanced search: TI=((bovine viral diarrh* OR bovine virus diarrh* OR bvd OR bvdv) AND (economic* OR financial OR cost*)).
Timespan: 1991–2015. Search language=auto
There were 41 results returned; 4 repeats were removed, 6 diagnostic papers were removed and 2 papers that were not relevant from the title were removed, leaving 29 papers.
All 79 articles were then reviewed and submitted to the screening question. Following analysis of the papers, seven further papers were then sourced. There were then 43 repeats, 2 editorial pieces, 1 model, 4 review articles and 1 comparing costs with and without vaccination that were removed. Four articles were not available, leaving 31 papers, which were copied to a Microsoft Word document (Microsoft Word 2010; Microsoft).
Papers included in the systematic review were from peer-reviewed journals unless otherwise stated.
Fig 1 shows the breakdown of the systematic search method.
FIG 1:
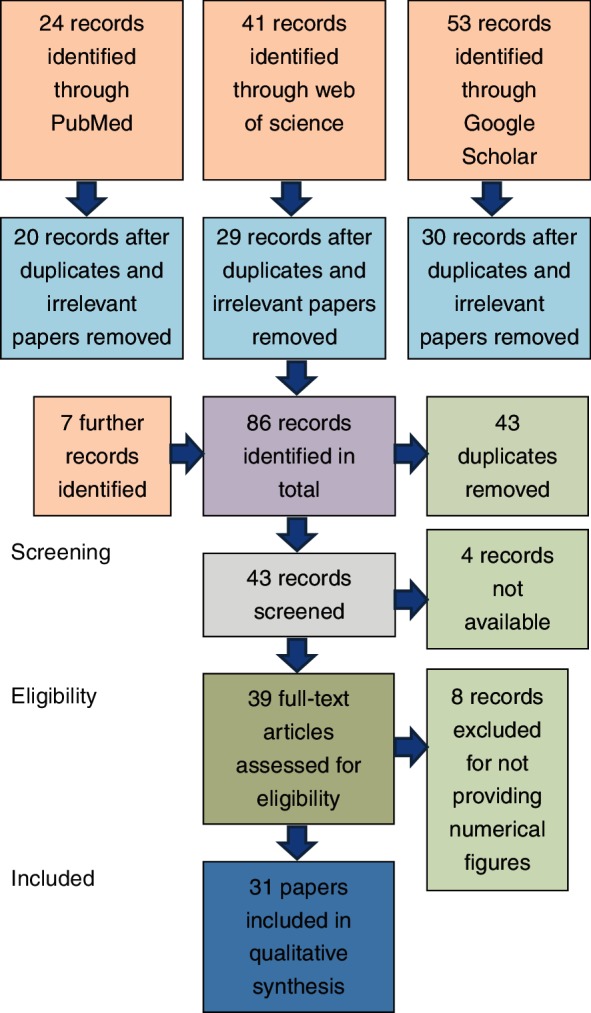
Recruitment and analysis of data through the different phases of the systematic review (from Moher and others23).
Historical figures from these papers were converted to current estimates, and this process can be illustrated by the equation below24:
Current value=Historical value × (1+inflation (%))number of years
However, this assumes a steady rate of inflation over many years. The Bank of England provides an online inflation calculator, which takes account of varying inflation rates over numbers of years, and this was used to produce the updated figures.25
Where results were given in a foreign currency, the figure was converted into pounds sterling before adjustment to present-day figures, using exchange rates at that time as provided by www.fxtop.com.26 Where economic impact figures were provided for the national herd in Great Britain, a figure per cow was calculated.27 28 The results were recorded to three significant figures.
Results
Table 1 displays the results of the systematic review of the economic impact of BVD from 1991 to 2015.
TABLE 1:
The results of the systematic review of the economic impact of BVD from 1991 to 2015
| Paper | Country | Dairy (D), beef (B) or beef fattening (F) | Endemic (End) or epidemic (Epi) | Standard (St) or severe (Se) | Method of economic assessment | Costs | Source of costs and losses | Losses | Figure produced per year per cow (year as per paper unless stated, and exchange rate if relevant) | Updated figure (£) |
| Bennett59 | UK | D | End/Epi | St | Decision analysis | Tx (TI), Tx (PI) | Literature | A, ML (TI), ML (PI) Im, TI, Inf, Con, M (PI), M (TI) YGC (PI), YGC (TI) | £13.12–£98.96 | 24.50–185 |
| Pasman and others30 | Netherlands | D | End/Epi | St | Markov chain (MC) simulation model | D, Dis | Literature, estimation, observation | M (TI), YGC (TI), M (PI), ML (PI), ML (TI), Inf, A, YGC (PI), Con, PC |
Year 1 cost – 49.55 Dfl = naive cost – 852.71 Dfl 2.77 NLG/£ =£17.9–£307 |
32.10–552 |
| Sørensen and others32 | Denmark | D | End/Epi | St | Stochastic simulation model | F, B | Literature | A, Inf, YGC (PI), Con, M (PI) | 0–10,000 DKr (50 cow herd)=200 DKr 9.73 DKr/£ (1993) £0–£20.6 |
0–37.80 |
| Carman and others29 | Canada | D | Epi | Se | Case study | NA | Farm data | M (PI), M (TI), ML (PI), ML (TI), A | $C40,000 – £100,000 per herd (40–191 cows) = $209–$2500. $C1.94/£ (1993) | 198–2370 |
| Bennett and others33 | Great Britain | D/B | End/Epi | St | Cost–benefit spreadsheet model | NA | Research, VLA | ML, A, M, PC | £5.2–£31.0 m (3.9 m cows, 1996) =£1.33–£7.95 |
2.25–13.50 |
| Houe13 | Denmark | D | End/Epi | St/Se | Cost–benefit spreadsheet model | NA | Field cases, literature | R, TI, ML (TI), M (TI), A, Inf, Con, YGC (PI), M (PI) | US$20–US$57 per calving $1.75/£ (1992) |
21.30–60.90 |
| Dufour and others60 | France | NA | NA | NA | Simulation model | NA | NA | NA | 25.5 F = €4.21 €1.75/£ |
3.76 |
| Bennett34 | UK | D | End/Epi | St | Decision analysis spreadsheet | NA | Bennett59 | M (PI), M (TI), Con, A, Inf, ML (TI), TI | £25.2–£90.7 (1999) | 39.50–142 |
| Chi and others35 | Canada | D | End/Epi | St | Partial budget, risk and sensitivity analyses | Vet, Tx, L, Rep | Research | ML (A), ML (TI), PC, M (PI), M (TI), A, YGC (PI), Inf | $C2422/50 cow herd =48.44 $C2.36/£ |
30.10 |
| Bennett38 | Great Britain | D/B | End/Epi | St | Cost–benefit spreadsheet model | NA | Bennett and others33 | ML, A, M, PC | £2–£12 m (3.7 m cows, 1999) =£0.54–£3.24 |
0.84–5.06 |
| Houe24 | Worldwide | D | End/Epi | St/Se | Review | NA | Review paper | NA | US$10–US$40 m/million calvings US$1.63/£ |
8.74–35.0 |
| Stott and others39 | Scotland | B | End/Epi | St | Linear programming | BS, Rep, L | Literature, SAC, vet interviews | Im, Con, YGC (TI), M (PI), A, Inf, PC | £20 status susceptible £22 status unknown |
28.50 31.40 |
| Gunn and others40 | UK | D | Epi | St | MC simulation model | NA | Literature | ML, M (PI) | £10,300 (low median) £10,400 (high median) £20.6–£20.8/cow/year |
29.40–29.70 (29.50) |
| Gunn and others41 | Scotland | B | End/Epi | St | MC simulation model | Vet, L, Dx, Tx, Rep | Literature, SAC, vet interviews | Im, Con, YGC (TI), A, M (PI), TI, Inf, PC | Transmission scenario low – £32.74, intermediate – £37.06, high – £40.53 | 45.30–56.10 (51.30) |
| Fourichon and others42 | France | D | Epi/End | St/Se | Partial budget, no stochasticity | Rep, Tx, | Literature, vet interviews | A, Inf, ML (A), M (PI), ML (TI), Mas, SCC, RP, M, TI | €75 (moderate) – 133 (severe) €1.46/£ (no milk quota) |
69.20–123 |
| Gunn and others43 | Europe | D | End | St | Stochastic simulation model | Vet, Tx, Rep | Expert opinion | Inf, PC, Mas, E, R, ML | 22% BVD-free annuity/farm >£4200/65 cow = £64.60 |
87.00 |
| Valle and others44 | Norway | D/B | End | St | Stochastic simulation model | Vet, Tx | Previous study on herd level effects | Inf, ML (TI), ML (PI), PC, M (TI), M (PI), TI | 40–50 m Norwegian krone/year = 77 Norwegian krone per calving 10.5 Norwegian krone/£ (1993) |
13.50 |
| Bennett and IJpelar22 | Great Britain | D/B | End | St | Cost–benefit spreadsheet model | D, Vac | Bennett,38 expert opinion | ML, Inf, PC, M, A | £25.4–£61.1 m (3.2 m cows) = £7.94–£19.1 |
10.70–25.70 |
| Compton and others45 | New Zealand | D | End | St | Case analysis | NA | Farm data | A, Inf, ML | NZ$90 NZ$2.83/£ |
41.50 |
| Heuer and others46 | New Zealand | D | End | St | Partial budget, retrospective case vs control | F | Farm data | Inf, A, (PR, 1st serve CR, CCI) PC, ML, M (PI) | NZ$87 NZ$2.72/£ |
40.00 |
| Barbudo and others48 | Scotland | B | End/Epi | St | MC and epidemiology model | B, F | Literature, Gunn and others41 | Inf, A | £22–£43 | 26.50–51.80 |
| Reichel and others47 | New Zealand | D | End/Epi | St/Se | Decision analysis | Separate costings | Voges and others61 | M (PI), ML (PI), Mas (PI), YGC (PI) | NZ$11,344 (322 cows/herd)=NZ$35.19 NZ$2.83/£ (2006) |
16.20 |
| Hessman and others3 | USA | F | End | St/Se | Partial budget, retrospective case vs control | Tx (TI), Tx (PI), F | Farm data | TI, R, Im, YGC (PI), YGC (TI), M (PI), M (TI), MD, PC | US$41.8–US$93.5 $1.56/£ |
32.40–72.50 |
| Stott and others49 | UK | B | End/Epi | St/Se | Simulation model | Rep, Vet, L (£1) | Literature, expert opinion | Im, Con, YGC (PI), YGC (TI), A, Inf, M (PI), PC | £0–£40 (2008) |
48.10 |
| Häsler and others50 | Switzerland | D/B | End | St | Partial budget spreadsheet model | Vet, Tx (TI), Tx (PI), D, Dis, L | Literature, expert opinion | M (PI), M (TI), PC, A, ML (PI), ML (TI), TI | 16.04 m CHF (1.5 m cows) =10.7 CHF 1.99CHF/£ (2008) |
6.46 |
| Stott and others18 | Ireland | D | End/Epi | St | Simulation model | Vet, Tx (PI), Tx (TI), Rep | Weldegebriel and others17 | ML (TI), ML (A) PC, Im, Mas, Inf, E, R, TI | €63 €1.23/£ = £51.2 |
54.50 |
| B | Simulation model | Vet, Rep Tx (TI), Tx (PI), L, | Stott and others,49 SAC, vet interviews | Im, PC, Con, YGC (PI) YGC (TI), A, Inf, M (PI) | €32 (€29 small – €38 large) €1.23/£ = £21.1 |
20.40–26.70 22.50 |
||||
| F | Partial budget MC spreadsheet | Vet, Tx (TI), L, Tx (PI) | Expert panel, Gunn and others41 | YGC (TI), YGC (PI) | €19 €1.23/£ = £15.4 |
16.40 | ||||
| Smith and others51 | USA | B | End | St | Stochastic model | NA | Literature, surveys, expert opinion | A, M (TI), M (PI), TI, YGC (TI), YGC (PI), Inf, Con | US$205,429 (460 cows/10 years) = $44.66 US$1.65/£ = £27.0 |
27.30 |
| Knific and Zgajnar52 | SIovenia | D | End | St | MC simulation | Rep, F, Vet, Tx, | Jeric (2011) | ML (TI), ML (PI), PC, YGC (TI), YGC (PI), A, M (TI), M (PI), Inf, Mas, RP | €189 €1.24/£ = £152.4 |
154 |
| Szabára and Ózsvári53 | Hungary | D | End | St | Partial budget estimations | NA | Own calculations | ML (TI), A, M (TI), M (PI), PC, | €13.7 €1.24/£ = £11.0 |
11.10 |
| Santman-Berends and others54 | Netherlands | D | End | St | Stochastic simulation model | Vac, D, Rep, Vet | Hogeveen55 | A, Con, YGC (PI), YGC (TI), ML, TI, PC, M (TI), M (PI), Inf | €30.8 m/year (1.6 m dairy cows) = $19.25 (2014) 1.24€/£ = £15.5 |
15.70 |
| Karabozhilova and others19 | England | D/B | End | St | Partial budget analysis | Tx (TI), Tx (PI), Dis, Rep, Vet, D, F, B | Literature, case reports, Häsler and others50 | M (PI), M (TI), PC, ML (PI), ML (TI), TI, Inf, A | Dairy – £21.32 and £42.63; beef – £26.78 and £53.56 | 31.50 40.20 |
Premature cull costs may include replacement costs minus slaughter value. TI losses may also be represented by treatment costs.
A, abortion; B, decreased bedding costs; BS, biosecurity costs; BVD, bovine viral diarrhoea; Cd, newborn calf death; Con, congenital defects; D, diagnostics; Dis, disposal costs; E, enteritis; F, decreased feed costs; Im, immunosuppression; Inf, infertility (days open, returns to service); L, increased labour costs; M (PI) (MD included), mortality of PIs; M (TI), mortality of acutely infected animals; Mas, mastitis; ML (A), milk loss following abortion; ML (PI), milk loss from PI cow; ML (TI), milk loss from acute infection; NA, (data) not available or applicable; PC, premature culling; R, respiratory disease; Rep, replacement costs; RP, retained placenta; SAC, Scottish Agricultural College; SCC, decreased milk quality; TI, acute infection; Tx (PI), PI treatment costs; Tx (TI), acute infection treatment costs; V, vaccination; Vet, veterinary cost; YGC (PI), youngstock growth check of PIs; YGC (TI), youngstock growth check of acute infected animals;
The majority of papers (19 out of 31) looked at the effects of BVD in dairy herds, with five papers looking at both dairy and beef cattle. There were seven papers that analysed a separate suckler beef figure, and two papers considered beef fattening systems. Indirect losses, such as poorer milk quality and immunosuppressive effects, are less well studied, compared with direct effects such as abortion.
The range of economic impacts ranged from £0 to £2370, although this does include a severe BVD type 2 outbreak.29 Removing this figure leaves a maximum figure of £552, which relates to the impact of reintroduction of BVD to a completely naïve herd after PIs have been removed.30 The mean economic impact of the 31 papers was £82.80. When adjusted by removing the severe type 2 outlier, this figure became £46.50.
Of the various searches performed, Google Scholar found 22 of the final 31 papers and 31 surplus papers. Web of Science found 22 of the final 31 papers and 7 surplus papers, and PubMed found 17 of the final 31 papers and 3 surplus papers. Google Scholar found the most results, but the search method is not simple to perform with multiple search terms, and inevitably it does return more repeated results. There were also more irrelevant results and more grey literature results.
Discussion
The variation seen in the outputs of the papers shows differences between the impact of endemic or subclinical BVD and epidemic or severe acute situations, which are usually associated with infection in a naïve herd. The impact of infection is therefore dependent on the immune status of the animal and severity of the strain. The range in economic impacts is also accounted for by differences in impact measurement.
Carman and others29 highlighted a range of impacts on dairy herds affected by the outbreak and discovery of BVDV type 2, and amounted to between £198 and £2370 in adjusted figures. There is little description of the calculation of this figure however. The Pasman and others30 paper addressing standard mixed endemic and epidemic infections in the Netherlands is interesting in that the authors assumed a lifelong immunity following infection. Scientific opinion more recently assumes only 12 months’ duration of immunity because of the nature of BVDV and the fact that true immunity from BVD is not about protection for the vaccinated dam, but actually concerns sterile immunity, or freedom from challenge, for the fetus within.31 The Pasman and others30 paper was published a year or so before the widespread availability of efficacious vaccines in Europe, and the authors state that under no circumstances should PIs be removed from a herd, lest the herd becomes naïve and then suffer such a costly breakdown. Sørensen and others32 looked at the impact of BVD in a standard, naïve Danish dairy herd, and showed that while there appeared to be a significant difference in annual net revenue between a ‘no risk’ and ‘risk of introduction’ situation over the first five years of virus introduction, there was no significant difference in the following five years, hence the lowest economic impact figure given as 0. Bennett and others33 also give a very low figure of between £2.25 and £13.50 (adjusted); however, it is worth noting that this assumes a national UK incidence of susceptible herds of only 5 per cent, and only losses in those herds, not in herds that have endemic disease. The non-peer-reviewed paper by Bennett34 examines the effect of acute infection in fully susceptible dairy herds, and this provides a relatively high figure of up to £142. This may be because, as well as addressing widespread acute infection, he assumes that all infection occurs during gestation. In an all-year-round calving herd this is unlikely; however, it does highlight the even higher economic risk to seasonal calving herds that suffer an outbreak in a naïve herd during the breeding season. Following the theme of immunity to BVDV, Chi and others35 assumed that 40 per cent of vaccinated herds suffered no effects of BVD. From an immunological point of view, many BVD vaccines only provide a reduction in clinical effects of the disease, and failure to prevent the birth of PI animals is still a risk factor. Furthermore, from a compliance point of view, it has been shown that the majority of vaccine is not used in a way that would provide the protection that is claimed.36 37 Bennett38 is a review of the Bennett and others33 paper, but with the impact of government subsidies removed, representing ‘border prices’. Inflation-adjusted, both the maximum and minimum values represent 37 per cent of the supported prices. Again, values are low as losses are assumed in only 5 per cent of UK herds that are naïve. Bennett also states that this variation in values reflects changes in the severity of the disease effects.
Houe24 is a review paper that collated a lot of the published information; however, there were no formal selection criteria, and so it did not constitute a systematic review or meta-analysis. Stott and others39 looked at disease prevention measures to reduce avoidable losses and whether they were cost-effective, showing that costs and losses of BVD including biosecurity in susceptible herds were on average lower than the costs and losses of BVD in unknown-status herds that spent less on biosecurity. The lack of knowledge made BVD biosecurity a less attractive risk management strategy with the constraint of a fixed income, which ultimately did not pay off. Gunn and others40 is a non-peer-reviewed poster that showed that small herds with low milk price and high death rate experienced less expensive outbreaks, but proportionally lost 20 per cent of income over 10 years, whereas outbreaks in larger herds with higher milk price and lower death rate were more expensive in the short term but only suffered 8 per cent income loss over 10 years. Gunn and others41 is a paper relating to beef cattle using a Monte Carlo state transition model over 10 years, which highlighted that 53 per cent of expected losses are due to reduced reproductive efficiency, with the estimated overall impact on a beef suckler cow being between £45.30 and £56.10 with a mean of £51.30. This highlights the ongoing impact of BVD in an endemic situation, due to the effect on seronegative animals within a herd. Fourichon and others42 looked in detail at the impact of BVD on dairy herds in France. The paper demonstrated two scenarios of an average case farm and a severe case farm, with the greatest impact being on milk yield, producing figures of between €75 and €133 (£69.20 and £123 updated), without considering effects of milk quota. Economic impact was less when milk yield was maintained with purchase of cows, and the highest cost was through increased mastitis. Gunn and others43 proposed a figure for the maximum annual investment in BVDV prevention in dairy herds, justified to ensure that no PI is acquired. This was £64.60 per cow, or £87.00 after adjustment. This represents the benefit for a naïve herd excluding BVD from the farm. However, the authors concede that even this figure is conservative due to the difficulties in taking account of depressed fertility and immunosuppression in acutely infected animals.
Towards the end of Norway’s successful eradication of BVD, Valle and others44 produced a retrospective cost–benefit analysis after 10 years of BVD control. This stochastic simulation model used figures for the health, production and fertility impact of BVD from herds that were seropositive at the start of the eradication scheme. This is important to note because it should take account of the widest range of impacts of BVD, even in herds that have not isolated active infection, so can be seen as a ‘baseline impact’ of endemic disease, albeit in a low cattle density environment. The largest financial effects of BVD were seen in reproduction (extra days open) and extra animals lost, representing 24 per cent and 28 per cent of the total financial loss, respectively. Prenatal infections represented 37 per cent of losses.
In the most recent update to the Reading model, Bennett and IJpelaar22 examined the welfare impact of endemic diseases including BVD. Although there is no economic value produced for the welfare impact, the increased BVD impact figure, when compared with the authors’ previous estimates of £10.70–£25.70, represents revised and updated estimates of key disease variables, as well as revised numbers of animals affected, with a mean of 10 per cent of breeding cows. Work from New Zealand45 46 looked at a similar data set of around 600 dairy herds, and analysed bulk tank milk BVDV antibodies and associations with production and health parameters. The Compton and others paper45 was a proceedings paper; however, the later peer-reviewed Heuer and others paper46 showed that there was a 2 per cent increase in abortion rates, an increase from calving to conception of 2.4 days, and 5.8 per cent decrease in total milk production with increasing bulk milk antibody level. This thorough data analysis produced partial budget losses of NZ€87 per cow, giving an adjusted figure of £40.00. However, the authors concede the figure to be conservative because there was no consideration of impact on calf health, mastitis or retained placentae. A later paper by Reichel and others47 produced a lower figure of £16.20; however, within this decision-tree analysis, there is no consideration of the effects of transient infection or immunosuppression. As mentioned above with regard to assumed vaccine efficacy, this paper used a figure of a maximum of 80 per cent when analysing cost-effectiveness of control options.
Barbudo and others48 calculated that reproductive failure could account for up to 23 per cent loss in gross margin for beef suckler herds suffering BVD effects over a 10-year period following an initial epidemic. These costs, however, were often hidden by an extended breeding season. Hessman and others3 looked at the impact of BVD in a feedlot situation by analysing data retrospectively from over 20,000 calves using a partial budget analysis. The varying levels of exposure to PIs showed performance losses of acutely infected animals amounted to between £32.40 and £72.50 (adjusted figures), corresponding to $41.84 and $93.52 from the original analysis. There was also a 55 per cent increase in feed conversion efficiency for those cattle not exposed to PIs (P=0.03), which, along with differences in fatalities, would have accounted for the greatest economic impact. This difference was only in the 66 days of the feeding period that was analysed, and the mean bodyweight of youngstock was 233.182 kg ± 1.7 kg (standard error of the mean) on arrival.
Stott and others49 again looked at beef herds, producing a figure of up to £48.10 (adjusted). The paper highlighted the risk of reintroduction of disease and showed that the higher the probability of further infection, the greater the cost of disease. Of note in this paper is that veterinary and labour costs were included; however, labour costs were put at an arbitrary level of £1 an hour, representing the low opportunity cost of family labour often used in those farms studied.
Häsler and others50 analysed the cost–benefit of the Swiss eradication scheme using a spreadsheet model, producing a figure of just over SFr16 million for the impact on the whole cattle population. Mortality and milk yield were the most significant contributors to losses. The adjusted figure of £6.46 seems low; however, as this was based on 2008 figures, the figure is affected by the strength of sterling compared with the present day, and may also represent differences in cattle production. In 2012, Stott and others produced economic impact figures to support the Irish BVD eradication scheme. In the paper they analysed dairy, beef suckler and beef finisher systems in stochastic, Markov chain, partial budget simulations. The impact in dairy herds was greater, at $63 per cow (£54.30 adjusted figure) compared with beef suckler herds at an average of $32 per cow (£27.70 adjusted). Smaller herds (<51 cows) were affected more per cow than larger herds at $38 compared with $29. Beef finisher units suffered an impact of $19 (£16.40 adjusted) per cow per year, mainly through loss of value and growth rate and increased treatment costs.
Smith and others51 looked at cost-effectiveness of BVD control measures, and produced a figure for the impact of BVD in beef suckler herds of £27.30 (adjusted). The figure was produced by bringing three Monte Carlo simulation models together, which each looked at annual risk of BVDV introduction, effects of BVDV over 10 years after introduction to a naïve herd and a model for the economic costs of BVDV infection. A non-peer-reviewed poster52 was produced on the impact of BVD on Slovenian dairy herds, based on a Monte Carlo simulation model. The main costs identified were lower milk yield and additional treatment costs, with a final adjusted figure of £155. There was an assumption in the simulation however of a PI animal incidence of 2 per cent, with 40 per cent naïve animals and 58 per cent acutely infected. There was also a paper53 that used the authors’ own calculations to produce a figure for the impact of BVD in the Hungarian dairy sector of £11.20 (adjusted); however, there was no effect of infertility, immunosuppression or other subclinical effects. Santman-Berends and others54 recently produced a stochastic model for the eradication of BVD from the Netherlands’ dairy industry. It was assumed that a herd would go from immune to susceptible when 50 per cent of the herd were seronegative through replacement only, not through waning of immunity. Furthermore, for vaccinated herds they assumed no losses due to BVD, and only 0.1 per cent probability of ineffective vaccination and 10 per cent of herds not vaccinating effectively. This was using the six-monthly BVD vaccine, Bovilis BVD (MSD). The paper based the impact figures on Hogeveen and others,55 which is in Dutch. Santman-Berends and others54 produced an average figure of €72 per milking cow, altered for inflation. Production losses in youngstock were not considered. The economic impact produced was £15.70, after adjustment.
The final paper19 that has been included is work from the Royal Veterinary College, London, which was commissioned by the AHDB Dairy (DairyCo) for the English BVD working group, and is at the time of writing unpublished. The partial budget analysis addressed costs and losses associated with BVD in beef and dairy herds, and calculated that BVD costs the dairy industry between £21.32 and £42.63 per cow, with the impact split with 37 per cent losses and 63 per cent costs. The impact split in the beef sector was 50/50, with a resulting range of £26.78 and £53.55.
Some papers of relevance were not included in this review because they were published before the set timeline, in a foreign language or were part of other papers. Wentink and Dijkhuizen56 looked at a case study of 14 Dutch dairy farms affected by BVD, and provided a figure of around 136 Dfl (£86) per dairy cow with herd variation of 42–285 Dfl (£26.60–£180). Bennett and others57 produced similar data to a paper already included.33 Also of interest is a recent paper by Gates and others58 looking at the impact of BVDV seropositivity on performance indicators in 255 Scottish beef suckler herds and 189 Scottish dairy herds. On average, calf mortality rates were 1.35 per cent higher in seropositive beef herds and 3.05 per cent higher in dairy herds. While no economic figure was provided, this paper is of relevance because farmers will appreciate the economic impact of this on farm.
In summary, the economic impact of BVD ranges from £0 to £552 per cow per year, with a mean impact of £46.50. Endemically infected herds would be experiencing an impact of between £6.46 and £87 per cow per year, with outbreaks in naïve herds ranging from £28.50 to £2370 with a severe outbreak of virulent virus. There appears to be no consistent differentiation between the level of impact in beef and dairy systems; however, the impact of BVD infecting a large proportion of calves in a tight calving beef system cannot be overestimated. Most losses occur through reproductive issues and most analyses, whether on-farm or otherwise, will underestimate impact of secondary issues such as immunosuppression. Potential losses can be reduced through use of effective vaccination; however, ultimately eradication of BVD needs to be viewed as an investment, with costs of diagnostic testing, PI removal, vaccination and monitoring being factored against reduced losses in the long term.
Acknowledgments
Alistair Stott, Jonathan Rushton, Peter Nettleton and George Gunn gave guidance and support with the dissertation, which formed the basis of this paper.
I finally thank my partner, now wife, Francesca, for tolerating the time that the dissertation and this paper has taken.
Footnotes
Twitter: @mattyarnall
Contributor: MVT provided overall supervision
Funding: The main author was funded for his Master’s degree in International Animal Health by Boehringer Ingelheim Animal Health UK Limited.
Competing interests: The main author was funded for his Master’s degree in International Animal Health by Boehringer Ingelheim Animal Health UK Limited.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Fulton RW, Purdy CW, Confer AW, et al. . Bovine viral diarrhea viral infections in feeder calves with respiratory disease: interactions with pasteurella spp., parainfluenza-3 virus, and bovine respiratory syncytial virus. Can J Vet Res 2000;64:151–9. [PMC free article] [PubMed] [Google Scholar]
- 2. Fray MD, Paton DJ, Alenius S. The effects of bovine viral diarrhoea virus on cattle reproduction in relation to disease control. Anim Reprod Sci 2000;60-61:615–27. 10.1016/S0378-4320(00)00082-8 [DOI] [PubMed] [Google Scholar]
- 3. Hessman BE, Fulton RW, Sjeklocha DB, et al. . Evaluation of economic effects and the health and performance of the general cattle population after exposure to cattle persistently infected with bovine viral diarrhea virus in a starter feedlot. Am J Vet Res 2009;70:73–85. 10.2460/ajvr.70.1.73 [DOI] [PubMed] [Google Scholar]
- 4. Wilhelmsen CL, Bolin SR, Ridpath JF, et al. . Experimental primary postnatal bovine viral diarrhea viral infections in six-month-old calves. Vet Pathol 1990;27:235–43. 10.1177/030098589002700404 [DOI] [PubMed] [Google Scholar]
- 5. Beaudeau F, Fourichon C, Robert A, et al. . Bulk milk somatic cell counts and bovine viral diarrhoea virus (BVDV) infection in 7252 dairy herds in Brittany (western France). Prev Vet Med 2005;72(1-2):163–7. 10.1016/j.prevetmed.2005.08.017 [DOI] [PubMed] [Google Scholar]
- 6. Strong R, La Rocca SA, Paton D, et al. . Viral Dose and Immunosuppression Modulate the Progression of Acute BVDV-1 Infection in Calves: Evidence of Long Term Persistence after Intra-Nasal Infection. PLoS One 2015;10:e0124689 10.1371/journal.pone.0124689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gethmann J, Homeier T, Holsteg M, et al. . BVD-2 outbreak leads to high losses in cattle farms in Western Germany. Heliyon 2015;1:e00019 10.1016/j.heliyon.2015.e00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schirrmeier H. Three years of mandatory BVD control in Germany – lessons to be learned. Cairns, Australia: World Buiatric Congress Proceedings, 2014:245–8. [Google Scholar]
- 9. ANON. Scotland launches a BVD eradication programme. Vet Rec 2010;167:505 10.1136/vr.c5352 [DOI] [PubMed] [Google Scholar]
- 10. Laureyns J. Challenges in the control of bovine viral diarrhoea virus – Implications for a Belgian eradication programme Ghent University; Belgium: 2014. [Google Scholar]
- 11. AHWNI. Northern Ireland BVD eradication programme. 2016. http://www.animalhealthni.com/BVD.aspx (accessed 22 May 2016).
- 12. Barrett DJ, More SJ, Graham DA, et al. . Considerations on BVD eradication for the Irish livestock industry. Ir Vet J 2011;64:12–10. 10.1186/2046-0481-64-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Houe H. Epidemiological features and economical importance of bovine virus diarrhoea virus (BVDV) infections. Vet Microbiol 1999;64:89–107. 10.1016/S0378-1135(98)00262-4 [DOI] [PubMed] [Google Scholar]
- 14. Truyers IG, Mellor DJ, Norquay R, et al. . Eradication programme for bovine viral diarrhoea virus in Orkney 2001 to 2008. Vet Rec 2010;167:566–70. 10.1136/vr.c4944 [DOI] [PubMed] [Google Scholar]
- 15. Richens IF, Hobson-West P, Brennan ML, et al. . Farmers' perception of the role of veterinary surgeons in vaccination strategies on British dairy farms. Vet Rec 2015;177:465 10.1136/vr.103415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mclnerney J. Old economics for new problems - livestock disease: presidential address. J Agric Econ 1996;47:295–314. 10.1111/j.1477-9552.1996.tb00695.x [DOI] [Google Scholar]
- 17. Weldegebriel HT, Gunn GJ, Stott AW. Evaluation of producer and consumer benefits resulting from eradication of bovine viral diarrhoea (BVD) in Scotland, United Kingdom. Prev Vet Med 2009;88:49–56. 10.1016/j.prevetmed.2008.07.001 [DOI] [PubMed] [Google Scholar]
- 18. Stott AW, Humphry RW, Gunn GJ, et al. . Predicted costs and benefits of eradicating BVDV from Ireland. Ir Vet J 2012;65:12 10.1186/2046-0481-65-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karabozhilova I, HÄSLER B, Booth R, et al. . Cost-Benefit Analysis of BVD Control in England. London, UK: RVC, 2015. [Google Scholar]
- 20. Otte MJ, Chilonda P. Animal health economics: an introduction animal information, sector analysis and policy branch, animal production and health division. Rome: Food and Agricultural Organization of the United Nations, 2011. [Google Scholar]
- 21. Bennett RM. The use of ‘economic’ quantitative modelling techniques in livestock health and disease-control decision making: a review. Prev Vet Med 1992;13:63–76. 10.1016/0167-5877(92)90037-G [DOI] [Google Scholar]
- 22. Bennett R, IJpelaar J. Updated estimates of the costs associated with thirty four endemic livestock diseases in Great Britain: a note. J Agric Econ 2005;56:135–44. 10.1111/j.1477-9552.2005.tb00126.x [DOI] [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, et al. . PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 24. Houe H. Economic impact of BVDV infection in dairies. Biologicals 2003;31:137–43. 10.1016/S1045-1056(03)00030-7 [DOI] [PubMed] [Google Scholar]
- 25. Bank of England (2016) Inflation calculator tool. www.bankofengland.co.uk/education/Pages/resources/inflationtools/calculator/flash/default.aspx (accessed Jan 2016).
- 26. Fxtop (2016) Historical currency conversion rates. http://fxtop.com/en/historical-exchange-rates.php?MA=1 (accessed 14 Jan 2016).
- 27. AHDB. Cattle number estimations for GB. 2016. a http://dairy.ahdb.org.uk/resources-library/market-information/farming-data/cow-numbers/#.Vycr4fkrLIU (accessed 22 Jan 2016).
- 28. AHDB. Average UK dairy herd size. 2016. b http://dairy.ahdb.org.uk/resources-library/market-information/farming-data/average-herd-size/#.VytL5IQrLIV (accessed 5th May 2016).
- 29. Carman S, van Dreumel T, Ridpath J, et al. . Severe acute bovine viral diarrhea in Ontario, 1993-1995. J Vet Diagn Invest 1998;10:27–35. 10.1177/104063879801000106 [DOI] [PubMed] [Google Scholar]
- 30. Pasman EJ, Dijkhuizen AA, Wentink GH. A state-transition model to stimulate the economics of bovine virus diarrhoea control. Prev Vet Med 1994;20:269–77. 10.1016/0167-5877(94)90060-4 [DOI] [Google Scholar]
- 31. Ridpath JF. Immunology of BVDV vaccines. Biologicals 2013;41:14–19. 10.1016/j.biologicals.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 32. Sørensen JT, Enevoldsen C, Houe H. A stochastic model for simulation of the economic consequences of bovine virus diarrhoea virus infection in a dairy herd. Prev Vet Med 1995;23:215–27. 10.1016/0167-5877(94)00436-M [DOI] [Google Scholar]
- 33. Bennett RM, Christiansen K, Clifton-Hadley RS. Estimating the costs associated with endemic diseases of dairy cattle. J Dairy Res 1999;66:455–9. 10.1017/S0022029999003684 [DOI] [PubMed] [Google Scholar]
- 34. Bennett R. Modelling the costs associated with BVD in dairy herds. Cattle Practice 2000;8:15–16. [Google Scholar]
- 35. Chi J, VanLeeuwen JA, Weersink A, et al. . Direct production losses and treatment costs from bovine viral diarrhoea virus, bovine leukosis virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum . Prev Vet Med 2002;55:137–53. 10.1016/S0167-5877(02)00094-6 [DOI] [PubMed] [Google Scholar]
- 36. Meadows D. A study to investigate the use and application of BVDV vaccine in UK cattle. Cattle Practice 2010;18:202–15. [Google Scholar]
- 37. Cresswell E, Brennan ML, Barkema HW, et al. . A questionnaire-based survey on the uptake and use of cattle vaccines in the UK. Vet Rec Open 2014;1:e000042 10.1136/vropen-2014-000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bennett R. The ’Direct Costs’of Livestock Disease: The Development of a System of Models for the Analysis of 30 Endemic Livestock Diseases in Great Britain. J Agric Econ 2003;54:55–71. 10.1111/j.1477-9552.2003.tb00048.x [DOI] [Google Scholar]
- 39. Stott AW, Lloyd J, Humphry RW, et al. . A linear programming approach to estimate the economic impact of bovine viral diarrhoea (BVD) at the whole-farm level in Scotland. Prev Vet Med 2003;59:51–66. 10.1016/S0167-5877(03)00062-X [DOI] [PubMed] [Google Scholar]
- 40. Gunn GJ, Humphry RW, Jones GJ, et al. . Estimating the economic losses associated with BVD infection in the UK dairy herd. Acta Vet Scand 2003;44:P54–229. 10.1186/1751-0147-44-S1-P54 [DOI] [Google Scholar]
- 41. Gunn GJ, Stott AW, Humphry RW. Modelling and costing BVD outbreaks in beef herds. Vet J 2004;167:143–9. 10.1016/S1090-0233(03)00112-6 [DOI] [PubMed] [Google Scholar]
- 42. Fourichon C, Beaudeau F, Bareille N, et al. . Quantification of economic losses consecutive to infection of a dairy herd with bovine viral diarrhoea virus. Prev Vet Med 2005;72:177–81. 10.1016/j.prevetmed.2005.08.018 [DOI] [PubMed] [Google Scholar]
- 43. Gunn GJ, Saatkamp HW, Humphry RW, et al. . Assessing economic and social pressure for the control of bovine viral diarrhoea virus. Prev Vet Med 2005;72:149–62. 10.1016/j.prevetmed.2005.08.012 [DOI] [PubMed] [Google Scholar]
- 44. Valle PS, Skjerve E, Martin SW, et al. . Ten years of bovine virus diarrhoea virus (BVDV) control in Norway: a cost-benefit analysis. Prev Vet Med 2005;72:189–207. 10.1016/j.prevetmed.2005.07.017 [DOI] [PubMed] [Google Scholar]
- 45. Compton CWR, Mcdougall S, Heuer C. Bovine viral diarrhoea virus in dairy cattle in New Zealand- studies on its prevalence, biologic and economic impact. Proceedings of the New Zealand Society of Animal Production 2006;66:162–7. [Google Scholar]
- 46. Heuer C, Healy A, Zerbini C. Economic effects of exposure to bovine viral diarrhea virus on dairy herds in New Zealand. J Dairy Sci 2007;90:5428–38. 10.3168/jds.2007-0258 [DOI] [PubMed] [Google Scholar]
- 47. Reichel MP, Hill FI, Voges H. Does control of bovine viral diarrhoea infection make economic sense? N Z Vet J 2008;56:60–6. 10.1080/00480169.2008.36809 [DOI] [PubMed] [Google Scholar]
- 48. Barbudo AV, Gunn GJ, Stott AW. Combining models to examine the financial impact of infertility caused by bovine viral diarrhoea in Scottish beef suckler herds. J Agric Sci 2008;146:621–32. 10.1017/S0021859608008113 [DOI] [Google Scholar]
- 49. Stott AW, Humphry RW, Gunn GJ. Modelling the effects of previous infection and re-infection on the costs of bovine viral diarrhoea outbreaks in beef herds. Vet J 2010;185:138–43. 10.1016/j.tvjl.2009.05.020 [DOI] [PubMed] [Google Scholar]
- 50. Häsler B, Howe KS, Presi P, et al. . An economic model to evaluate the mitigation programme for bovine viral diarrhoea in Switzerland. Prev Vet Med 2012;106 162–73. 10.1016/j.prevetmed.2012.01.022 [DOI] [PubMed] [Google Scholar]
- 51. Smith RL, Sanderson MW, Jones R, et al. . Economic risk analysis model for bovine viral diarrhea virus biosecurity in cow-calf herds. Prev Vet Med 2014;113:492–503. 10.1016/j.prevetmed.2013.11.013 [DOI] [PubMed] [Google Scholar]
- 52. Knific T, Zgajnar J, 2014. Modelling the economic impacts of bovine viral diarrhoea virus at dairy herd level; the case of Slovenia. International Congress, European Association of Agricultural Economists., Ljubljana, Slovenia [Google Scholar]
- 53. SZABÁRA A, ÓZSVÁRI L. Economic impacts, control and eradication of bovine viral diarrhoea virus. DUNAY A, In book: Challenges for the agricultural1. Agroinform Kiadó, Budapest, Hungary: sector in central and eastern Europe, 2014:247–58. [Google Scholar]
- 54. Santman-Berends IM, Mars MH, van Duijn L, et al. . Evaluation of the epidemiological and economic consequences of control scenarios for bovine viral diarrhea virus in dairy herds. J Dairy Sci 2015;98:7699–716. 10.3168/jds.2014-9255 [DOI] [PubMed] [Google Scholar]
- 55. Hogeveen H, HUIRNE RBM, Meeuwissen MPM. Verzekeren van diergezondheid in de melkveesector; een risicoanalyse [Ensuring animal health in the dairy sector; a risk assessment. Wageningen, the Netherlands: IRMA, 2003. [Google Scholar]
- 56. Wentink GH, Dijkhuizen AA. Economic effects of infection with the Bovine Virus Diarrhea Virus (BVD virus) on fourteen dairy farms (in Dutch). Tijdschrift voor diergeneeskune 1990;115:1031–40. [PubMed] [Google Scholar]
- 57. Bennett R, Christiansen K, Clifton-Hadley R. Preliminary estimates of the direct costs associated with endemic diseases of livestock in Great Britain. Prev Vet Med 1999;39:155–71. 10.1016/S0167-5877(99)00003-3 [DOI] [PubMed] [Google Scholar]
- 58. Gates MC, Humphry RW, Gunn GJ. Associations between bovine viral diarrhoea virus (BVDV) seropositivity and performance indicators in beef suckler and dairy herds. Vet J 2013;198:631–7. 10.1016/j.tvjl.2013.09.017 [DOI] [PubMed] [Google Scholar]
- 59. Bennett RM. Case-study of a simple decision support system to aid livestock disease control decisions. Agric Syst 1992;38:111–29. 10.1016/0308-521X(92)90036-N [DOI] [Google Scholar]
- 60. Dufour B, Repiquet D, Touratier A. [Role of economic studies in animal health decisions: example of the cost-benefit ratio of eradication of bovine viral diarrhea in France]. Rev Sci Tech 1999;18:520–32. [PubMed] [Google Scholar]
- 61. Voges H, Young S, Nash M. Direct adverse effects of persistent BVDv infection in dairy heifers – a retrospective case control study. VetScript 2006;XIX 8:22–5. [Google Scholar]


