Conspectus
Brucellosis is a serious zoonotic bacterial disease that is ranked by the World Health Organization among the top seven “neglected zoonoses” that threaten human health and cause poverty. It is a costly, highly contagious disease that affects ruminants, cattle, sheep, goats, and other productive animals such as pigs. Symptoms include abortions, infertility, decreased milk production, weight loss, and lameness. Brucellosis is also the most common bacterial disease that is transmitted from animals to humans, with approximately 500 000 new human cases each year. Detection and slaughter of infected animals is required to eradicate the disease, as vaccination alone is currently insufficient. However, as the most protective vaccines compromise serodiagnosis, this creates policy dilemmas, and these often result in the failure of eradication and control programs. Detection of antibodies to the Brucella bacterial cell wall O-polysaccharide (OPS) component of smooth lipopolysaccharide is used in diagnosis of this disease, and the same molecule contributes important protective efficacy to currently deployed veterinary whole-cell vaccines. This has set up a long-standing paradox that while Brucella OPS confers protective efficacy to vaccines, its presence results in similar antibody profiles in infected and vaccinated animals. Consequently, differentiation of infected from vaccinated animals (DIVA) is not possible, and this limits efforts to combat the disease. Recent clarification of the chemical structure of Brucella OPS as a block copolymer of two oligosaccharide sequences has provided an opportunity to utilize unique oligosaccharides only available via chemical synthesis in serodiagnostic tests for the disease. These oligosaccharides show excellent sensitivity and specificity compared with the native polymer used in current commercial tests and have the added advantage of assisting discrimination between brucellosis and infections caused by several bacteria with OPS that share some structural features with those of Brucella. During synthesis and immunochemical evaluation of these synthetic antigens, it became apparent that an opportunity existed to create a polysaccharide–protein conjugate vaccine that would not create antibodies that give false positive results in diagnostic tests for infection. This objective was reduced to practice, and immunization of mice showed that antibodies to the Brucella A antigen could be developed without reacting in a diagnostic test based on the M antigen. A conjugate vaccine of this type could readily be developed for use in humans and animals. However, as chemical methods advance and modern methods of bacterial engineering mature, it is expected that the principles elucidated by these studies could be applied to the development of an inexpensive and cost-effective vaccine to combat endemic brucellosis in animals.
Introduction
Brucellosis is regarded by the World Health Organization as one of the most serious zoonotic bacterial diseases and ranks among the top seven “neglected zoonoses” that threaten human health and cause poverty.1 It is a costly, highly contagious disease that affects cattle, sheep, goats, pigs, camels, and other productive animals worldwide.2,3a Wildlife reservoirs of the disease are found in bison, elk, deer, caribou, and reindeer.4 Symptoms include abortions, infertility, decreased milk production, weight loss, and lameness. Brucellosis is also the most common bacterial disease that is transmitted from animals to humans,3b with approximately 500 000 new human cases each year. In humans, the disease presents symptoms similar to those of influenza or malaria and can be severely debilitating. Detection of antibodies to the bacterial cell wall O-polysaccharide (OPS) component of smooth lipopolysaccharide (sLPS) is used in diagnosis of this disease,2,5 and the same molecule contributes important protective efficacy to currently deployed veterinary live whole-cell vaccines.6Brucella OPS confers protective efficacy to vaccines, but its presence results in similar antibody profiles in infected and vaccinated animals.
Researchers have tried to resolve this issue by developing vaccines without OPS. These have included protein subunit, DNA, and vectored vaccines,7 but the only approach to result in a licensed vaccine has been the use of a rough Brucella abortus strain for use in cattle.8 However, the protective properties of this strain and approach are disputed,9 and new solutions are needed.10
Differentiation of infected from vaccinated animals (DIVA) is not possible with the most protective vaccines, and this limits efforts to combat the disease. Definitive structural studies of Brucella OPS11 in combination with chemical syntheses of diagnostic antigens12,13 and potential conjugate vaccines have identified an approach that facilitates DIVA.14,15 These developments suggest an approach that could break a decades-old scientific impasse for mass brucellosis vaccination in animals.
Vaccination of livestock can be a cost-effective way of controlling the disease and limiting its impact on human and animal health.1,2,6 Current live vaccines do not provide protection across different species of animal hosts, are unsafe for use in pregnant animals, and can harm humans, and the most protective make it difficult to effectively differentiate infected from vaccinated animals.16,17 A safe, low-cost, and efficacious vaccine would improve the economic circumstances of smallholder farmers, mitigate costly human infections, and avoid outbreaks, which can put millions of humans at risk18 and compromise livestock industries as well as international trade. Detection and slaughter of infected animals is required to eradicate the disease, as vaccination alone is currently insufficient. To bring the prevalence down to levels whereby slaughter is not prohibitively expensive, vaccination may be applied. However, as the most protective vaccines compromise serodiagnosis, this creates policy dilemmas, and these often result in the failure of eradication and control programs.19
Brucellosis is endemic in a number of countries. Across Africa, its prevalence in ruminants is estimated to be between 8.2% and 15.5%.20 In South Asia, its ruminant prevalence is estimated to be 16%. This equates to approximately 100 million animals in India, and the cost of this to the livestock industry alone is estimated to be 3.4 billion USD per year.21 The disease is widespread across Latin America. In Brazil it is estimated to cost $450 million USD per year.22 It is also re-emerging and becoming endemic in many regions of China.23 The toll on smallholder farmers is particularly devastating, since cattle and small ruminants such as goats and sheep are a crucial source of income and food.
Brucella Carbohydrate Antigens
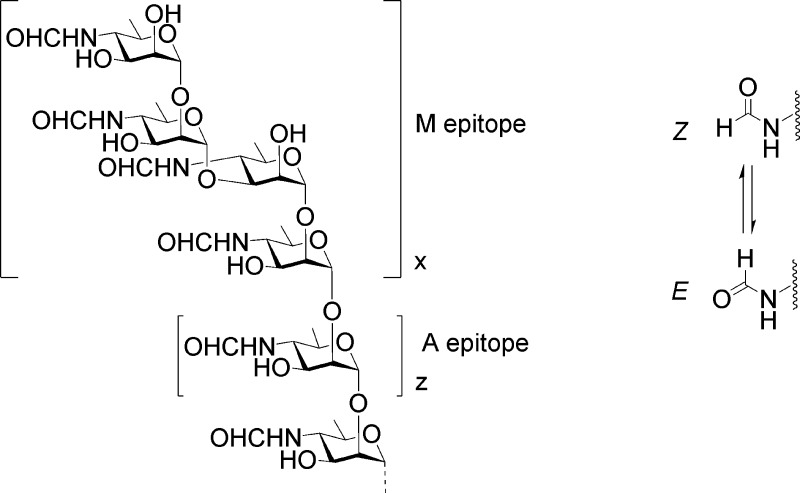
Brucellae are Gram-negative intracellular pathogens, although there are significant phases within the host where they are extracellular. The species of most significance to human and animal health, B. abortus (cattle), Brucella melitensis (sheep and goats), and Brucella suis (swine), all have an outer cell membrane that closely resembles that of other Gram-negative bacilli with a dominant sLPS component. This molecule consists of three structural domains, a lipid A to which is attached a core oligosaccharide, which in turn carries the most exposed OPS. On the basis of their reactivity with antibodies produced in rabbits, two carbohydrate antigens, A and M, were identified in the 1930s, and the antigenic phenotype of Brucella strains could be assigned to one of three groups: A+M–, A–M+, and A+M+. The two antigens were concluded to be inseparable.24 More recently they were identified as the components of OPS of Brucella sLPS.25,26 The chemical structure of each was only definitively established in 2013.11
Chemical Structures of the A and M Antigens
In 1939 the A and M antigens were found to contain a polyhydroxyamino compound and formate.27 This insight was confirmed when 4-formamido-4,6-dideoxy-α-d-mannopyrannose (d-Rha4NFo) was isolated as the sole monosaccharide component of the repeating portion of OPS from B. abortus.25 The formamido group populates both E and Z rotamers (Figure 1), and both forms are always present in solution. This creates two sets of 1H and 13C NMR resonances for each d-Rha4NFo residue and complicates structural analysis by this method. Initially the A antigen from B. abortus was erroneously concluded to be an exclusively α1,2-linked polymer of d-Rha4NFo,25 and the M antigen was mistakenly proposed to have a pentasaccharide repeating unit with one 1,3-linked and three 1,2-linked d-Rha4NFo residues.26 However, chemical and immunochemical studies of LPS samples from 13 distinct Brucella strains showed that the structures proposed for the A and M antigens were too simplistic.28,29 All Brucella strains classified serologically as A-dominant (A+M–) contained at least 2% of the 1,3-glycosidic linkage type that defines M character. The M strain, B. melitensis 16 M (A–M+), contained 21% of these linkages. All of the Brucella strains investigated had M character lying between these extremes. B. suis biovar 2 is an exception. Its OPS is devoid of 1,3-linked residues and is consequently a pure A-type antigen.30
Figure 1.
OPS structure of B. abortus, B. melitensis, and B. suis (except biovar 2) showing the M and A elements of the block copolymer.11 The capping tetrasaccharide containing a single 1,3 linkage defines the M epitope. The relative length of the M and A segments defined by the number of repeats, x and z, varies between strains. In solution, the Z rotamer of the formamido residue is the most abundant rotamer.
A definitive study has now identified Brucella OPS as a block copolymer of two distinct homopolysaccharide sequences (Figure 1). A longer inner sequence of α1,2-linked residues constitutes the A antigen. This is capped by a shorter sequence that creates the M antigen, which consists of one or more tetrasaccharide repeating units with the linkage sequence [α1,2;α1,3;α1,2] attached to additional copies of this tetrasaccharide or to the A segment by an α1,2 linkage (Figure 1). B. melitensis 16 M possesses several of these tetrasaccharide repeats, but other Brucella strains investigated had O-antigen capped by a single M tetrasaccharide.11
Immunochemistry of the A and M Antigens
Current serological tests for brucellosis depend upon the presence of OPS within the diagnostic antigen for their sensitivity. Since OPS contains A and M antigenic determinants, antibodies generated in response to infection are reactive with both antigens. Many monoclonal antibodies (mAbs) have been characterized by enzyme-linked immunosorbent assay (ELISA) screening against LPS of Yersinia enterocolitica O:9, A-dominant and M-dominant Brucella, and subsequently by inhibition with synthetic oligosaccharides.29,31,32 They possess high but not absolute selectivity for the A and M antigenic determinants.29,31−33
Whole-cell vaccine efficacy is improved by the presence of sLPS, but this results in A- and M-specific antibodies similar to those of infected animals, thereby defeating their differentiation. Another complication in the serodiagnosis of brucellosis is the existence of bacteria with OPS that contain N-acylated 4-amino-4,6-dideoxy-α-d-mannopyranose and in one case the gluco epimer (Table 1). Animals infected by these bacteria can produce antibodies that bind in diagnostic tests for brucellosis. Two Gram-negative bacteria possess OPS composed exclusively of α1,2-linked 4-amino-4,6-dideoxy-α-d-mannopyranose residues. One of these, Y. enterocolitica O:9, is N-formylated and therefore is identical to a pure Brucella A antigen.34 Infection with this organism inevitably results in false positive serological tests. The second, Vibrio cholera O1, is N-acylated by 2,4-dihydroxy (R) butyric acid and has also been reported to bind to antibodies generated in response to Brucella.2 Antibodies to several enteric bacteria that express O-antigens containing 4-acetamido-4,6-dideoxy-α-d-mannopyranose residues also bind to Brucella OPS (Table 1).2
Table 1. Gram-Negative Bacteria with OPS Containing N-Acylated 4-Amino-4,6-dideoxy-α-d-hexopyranose.
| bacterium | N-acyl group and linkage to Rha4N residue | ref(s) |
|---|---|---|
| Y. enterocolitica O:9 | N-formamido; α1,2 | (2), 34 |
| V. cholera O1 | N-hydroxybutylamido; α1,2 | (2), 35 |
| E. coli 0157 | N-acetamido; α1,2 | (2), 36 |
| E. hermanii | N-acetamido; α1,2 and α1,3 | (36) |
| P. maltophilia 555 | N-acetamido; α1,3 | (37) |
| S. urbana, S. godesberg | N-acetamido; α1,2 | (2), 38 |
| F. tularensis | N-formamido-4,6-dieoxy-d-glucose | (2), 39 |
Synthesis of Oligosaccharides Containing Brucella A and M Epitopes
Our initial work with mAbs and synthetic 1,2-linked tri-, tetra-, and pentasaccharide inhibitors suggested that the antigenic determinant of B. abortus A antigen is composed of an oligosaccharide, most likely a pentasaccharide of contiguous α1,2-linked d-Rha4NFo residues.28,29 The M antigen could in principle be an α1,3-linked d-Rha4NFo disaccharide, but it inhibited only one of several mAb generated against M-dominant B. melitensis 16M. Pentasaccharides with this linkage at either terminus showed weak M activity.29 This antigenic determinant seemed more likely to be defined as a larger oligosaccharide with the 1,3 linkage positioned as an internal rather than a terminal 1,3 linkage with a sufficiently short α1,2-linked d-Rha4NFo sequence to preclude recognition by A-specific antibodies.29
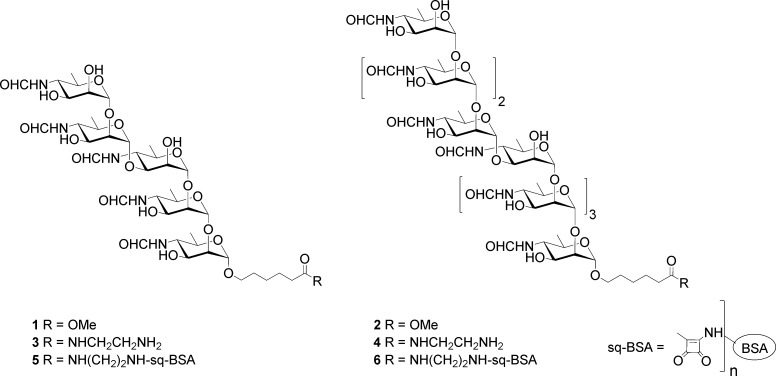
Our recent studies12 began prior to publication of the definitive clarification of the Brucella OPS structure reported by Kubler-Kielb and Vinogradov.11 We synthesized pentasaccharide 1 in which the 1,3 linkage occurs between Rh4NFo residues 2 and 3.12 The longest 1,2 oligomeric sequence in 1 is a trisaccharide that should exhibit weak A activity.29 Nonasaccharide 2, which can be regarded as an A tetrasaccharide linked via a 1,3 linkage to an A pentasaccharide, was expected to express both A and M epitopes. In order for the oligosaccharides to be useful in solid-phase binding assays (ELISA), synthetic oligosaccharides were assembled on a six-carbon tether and attached to proteins via homobifunctional reagents (Figure 2).12,13
Figure 2.
Glycoconjugate synthesis involved the reaction of esters 1 and 2 with ethylenediamine to give amides 3 and 4. Reaction of these with diethyl or dibutyl squarate gave stable squarate half-esters, which were reacted with lysine amino acids of bovine serum albumin (BSA) to give glycoconjugates 5 and 6.
When coated on ELISA microtiter plates, pentasaccharide–bovine serum albumin (BSA) glycoconjugate 5 showed preferential binding of M- over A-specific mAbs.12 As expected, both A- and M-specific mAbs bound with identical profiles to nonasaccharide conjugate 6. These data were consistent with our earlier inference that a 1,2-linked tetrasaccharide appears to be the smallest epitope that affords strong binding with Brucella A-specific mAbs. Pentasaccharide 1, while exhibiting M specificity, still provides too many 1,2-linked residues and retains modest to good binding to A-specific mAbs. We now know that 1 represents the terminal M tetrasaccharide that is α1,2-linked to the next d-Rha4NFo residue of Brucella OPS.11
The definitive structure of Brucella OPS shows the existence of a unique M tetrasaccharide capping sequence at the distal terminus of the polysaccharide.11 This suggested options for the synthesis of highly specific diagnostic antigens that could be used to define A and M epitopes (Figure 3).13 We also envisaged how this approach might facilitate a glycoconjugate vaccine that would not interfere with diagnostic tests for brucellosis.15
Figure 3.
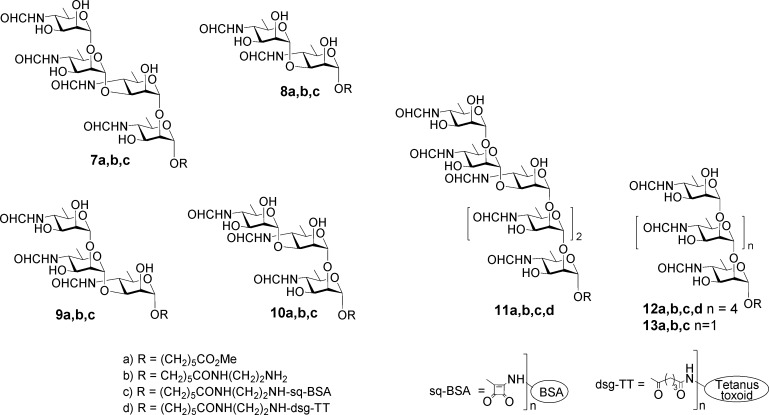
Synthetic targets used to probe antibodies present in infected cattle and in mice immunized with glycoconjugate vaccine 12d. M tetrasaccharide 7a, disaccharide 8a, and the two-component trisaccharides 9a and 10a found in the M repeating sequence. M and A hexasaccharides 11a and 12a represent larger epitopes. Trisaccharide 13a represents a small A-type epitope. Oligosaccharide amides 7b–13b were conjugated to BSA to provide glycoconjugates for use in ELISA. Hexasaccharide 12a was derivatized, conjugated to tetanus toxoid, and then used to immunize mice.
Tetrasaccharide 7 and disaccharide 8 were inferred to provide the largest and smallest M epitopes. For thoroughness, the component M trisaccharides 9a and 10a were also targeted.13 These structures are unique to Brucella OPS, and because they are the most exposed part of the bacterial surface, antibodies to them could be anticipated to be present in sera of infected animals. The detection of anti-M antibodies would indicate that an animal is infected by Brucella and not one of the other confounding bacteria that have OPS containing 1,2-linked Rh4NFo or Rh4NAc and are known to induce antibodies that are reactive in the serological test for brucellosis (Table 1). To examine whether a penultimate 1,2- or 1,3-linked d-Rha4NFo altered the specificity of the induced antibodies, hexasaccharides 11a and 12a were synthesized as potential conjugate vaccines.
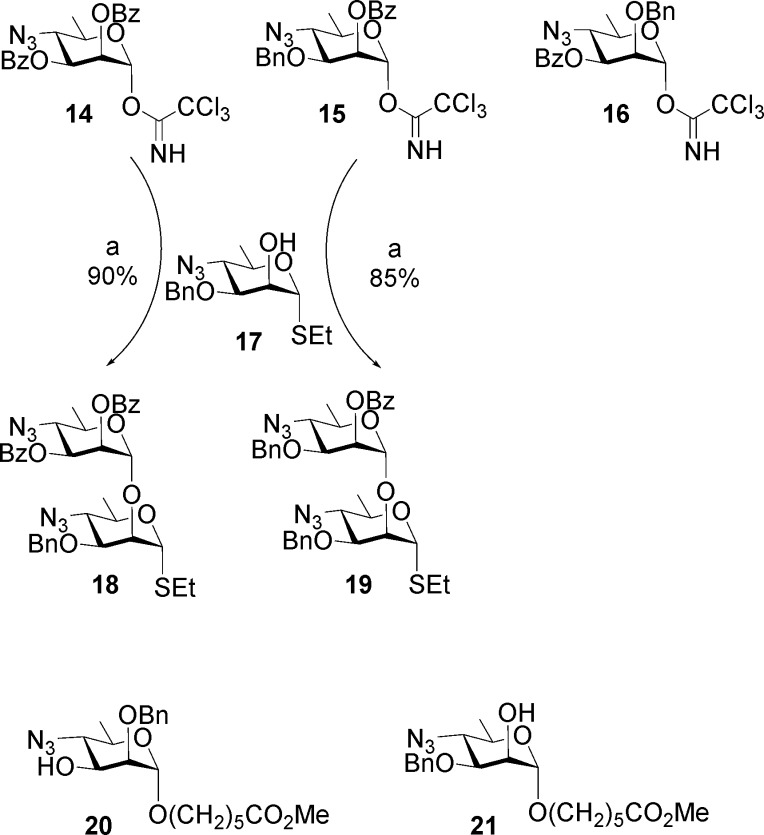
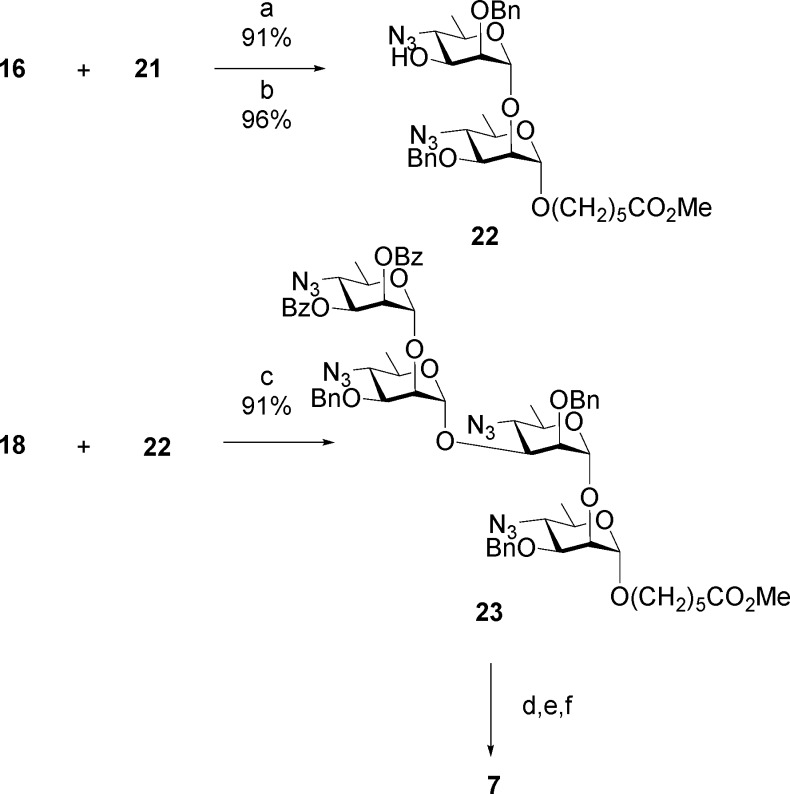
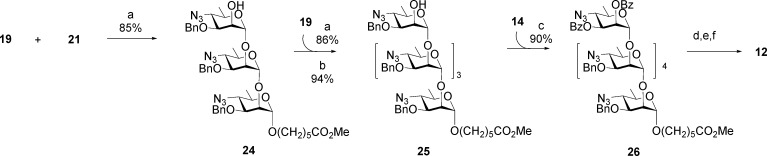
The synthesis of Brucella oligosaccharides utilized 4-azido-4,6-dideoxy-α-d-mannopyranose, which is accessible in nine steps from d-mannose.12,13,15,40,41 It was also used to synthesize oligosaccharides representing elements of V. cholera OPS.42 Trichloroacetimidate building blocks 14–16 were assembled from 4-azido-4,6-dideoxy-α-d-mannopyranose.13 Glycosyl donors 14 and 15 reacted with 17 to create two additional disaccharide donors as thioglycosides 18 and 19 (Scheme 1). Starting from glycosyl acceptors 20 and 21 and various combinations of donors 14–19, all six oligosaccharides 7–12 were synthesized.13 These were selected as having M-like epitope characteristics with increasing A-like epitope properties as the oligosaccharide length increases. The approach is illustrated for targets 7 and 12 (Schemes 2 and 3). Hexasaccharide 12 is an exclusively A-type antigen.
Scheme 1.
Reagents and conditions: TMSOTf, 3 Å MS, CH2Cl2.
Scheme 2.
Reagents and conditions: (a) TMSOTf, 3 Å MS, PhMe, 95 °C, 1 h; (b) NaOCH3, CH3OH, rt, 4 h; (c) MeOTf, 3 Å MS, CH2Cl2, rt, 48 h; (d) H2S, Py/H2O, 40 °C, 16 h; (e) (HCO)2O, MeOH, −20 °C, 3 h; (f) H2, Pd(OH)2, MeOH/H2O, rt, 16 h.
Scheme 3.
Reagents and conditions: (a) MeOTf, 3 Å MS, CH2Cl2, rt, 48 h; (b) NaOCH3, CH3OH, rt, 4 h; (c) TMSOTf, 3 Å MS, CH2Cl2, rt, 1 h; (d) H2S, Py/H2O, 40 °C, 16 h; (e) (HCO)2O, MeOH, −20 °C, 3 h; (f) H2, Pd(OH)2, MeOH/H2O, rt, 16 h.
The M repeating unit 7a that caps OPS was assembled in two glycosylation steps (Scheme 2). Tether glycoside 21 was glycosylated by imidate 16 bearing a persistent protecting group at O-2 and a benzoate ester at O-3, which was readily removed to provide disaccharide alcohol 22. Glycosylation by thioglycoside 18 afforded the protected target molecule 23. A uniform deprotection strategy was applied throughout these syntheses.13,15 Transesterification was used to remove benzoate esters. Then in three concerted steps, multiple azido groups were reduced to polyamines by H2S in pyridine, formic anhydride was the reagent of choice to unambiguously provide the formamido derivative, and a final hydrogenolysis step removed benzyl ethers to give 7.
A-type hexasaccharide 12 was assembled in three glycosylation steps (Scheme 3).13 Two iterative glycosylation steps employing disaccharide thioglycoside 19 converted monosaccharide 21 first to a trisaccharide, from which the benzoate group was removed to give trisaccharide alcohol 24. A second glycosylation/transesterification sequence provided pentasaccharide alcohol 25. Glycosylation by imidate 14 then gave hexasaccharide 26, from which hexasaccharide 12 was obtained by the same deprotection sequence (Scheme 3).
Diagnostic Antigens
International standard B. abortus serum prepared from cattle experimentally infected with an A-dominant strain of the organism bound strongly to M disaccharide–BSA conjugate 8c and M tetrasaccharide–BSA conjugate 7c.13 The same conjugates also exhibited strong binding to M-specific mAbs and weak to negligible binding with A-specific mAbs.13 This prompted more detailed studies with panels of cattle sera, including those falsely positive in conventional assays.
The diagnostic capabilities of six BSA conjugates 5, 6, and 7c–10c were tested for their ability to differentiate culture-positive and random noninfected samples. The nonasaccharide 6, pentasaccharide 5, and tetrasaccharide 7c antigens provided perfect discrimination (100% diagnostic sensitivity (DSn) and diagnostic specificity (DSp). The disaccharide 8c antigen was marginally less effective, with one false negative resulting in optimized DSn and DSp values of 97.78% and 100%. Assays developed with disaccharide conjugate 8c were the best at resolving false positive serological results. This was supported by additional tests of sera from cattle experimentally infected with B. abortus or Y. enterocolitica O:9.14 Here disaccharide 8c showed 87.5% specificity while B. abortus and B. melitensis sLPS (the standard antigens of current ELISA diagnostics) had specificities of 50 and 56%, respectively.14 This result suggested that the antibodies raised against exclusively 1,2-linked d-Rha4NFo (Y. enterocolitica O:9 OPS) did not bind well to the 1,3-linked disaccharide. A 1,2-linked antigen with such characteristics could form the basis of a DIVA vaccine.
Potential Role of Conjugates in Differentiation of Infected from Vaccinated Animals
We hypothesized that a synthetic M-diagnostic antigen should not react with antibodies induced by a synthetic Brucella A vaccine. If validated, this would allow unique identification of infected animals since these animal sera would test positive utilizing the M-diagnostic antigen whereas animals immunized with the synthetic vaccine would have exclusively anti-A antibodies that can bind Brucella OPS antigen and potentially afford protection against the disease.
However, when hexasaccharide 12a conjugated to tetanus toxoid was used to immunize mice, the antibody response not only showed strong cross-reactivity with B. abortus LPS and weaker reaction with the LPS of B. melitensis and Y. enterocolitica O:9 but also reacted strongly with the M disaccharide and tetrasaccharide antigens 7c and 8c (Figure 3). Substantial antibody titers against a d-Rha4NFo–BSA monosaccharide conjugate prompted consideration that a population of antibodies were able to bind the terminal monosaccharide. Tip-specific antibodies have been reported in other bacteria, e.g., V. cholera(35,42) and Francisella tularensis.39 Terminal-residue-specific antibodies generated by a glycoconjugate vaccine lacking a 1,3 linkage would bind 7c and 8c and preclude DIVA.
To avoid the induction of antibodies to the terminal capping residue of an A-type epitope, we adopted a seldom-used conjugation strategy. This located a tether at the nonreducing end, as shown in heptasaccharide 27, which is an A hexasaccharide capped by a d-rhamnopyranoside residue bearing a tether at O-4 (Figure 4).
Figure 4.
Heptasaccharide 27 was synthesized as the amine and conjugated to tetanus toxoid using disuccinimidyl glutarate (DSG) to obtain 28 and to BSA using diethyl squarate to obtain 29. Conjugate 28 was used to immunize mice and conjugate 29 to monitor antibody responses by ELISA.
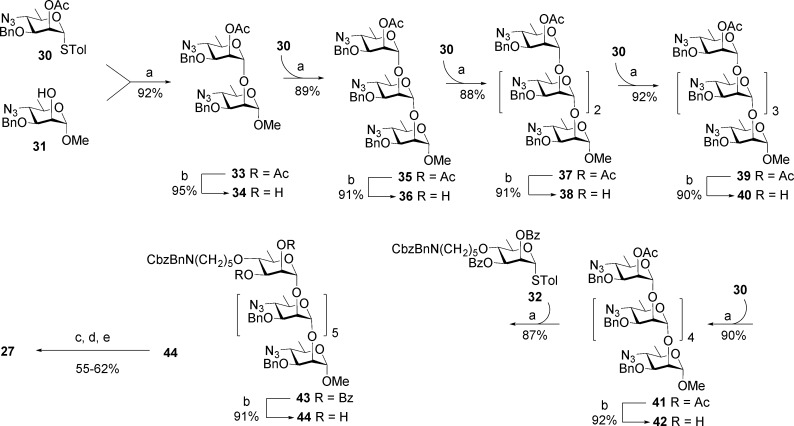
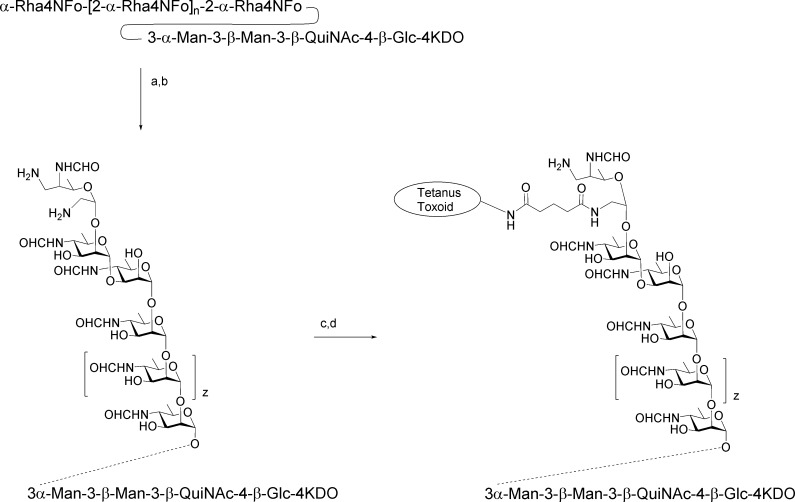
The synthesis of 27 and from it conjugates 28 and 29 employed three intermediates, 30–32 (Scheme 4). Thioglycoside 30 was the glycosyl donor for a series of iterative glycosylations that began with glycosylation of the known methyl glycoside 31(40) to afford disaccharide 33. Transesterification provided disaccharide alcohol 34, which is the acceptor for a second glycosylation reaction. Sequential glycosylation by 30 followed by de-O-acetylation of the resultant oligosaccharide was repeated four times to provide, in succession, fully protected oligosaccharides 36, 38, 40, and 42 (Scheme 4), all in yields of 89% or higher. Selectively protected hexasaccharide alcohol 42 was glycosylated by rhamnopyranosyl donor 32 equipped with a protected 5-aminopentanyl tether. The resulting heptasaccharide 44 was transformed to 27 by a series of familiar deprotection steps.9 Amine 27 was activated with disucinimidyl glutarate (DSG) and dibutyl squarate and covalently linked to tetanus toxoid and BSA to provide glycoconjugates 28 and 29, respectively.15
Scheme 4.
Reagents and conditions: (a) TMSOTf, NIS, 4 Å MS, CH2Cl2, −20 °C to rt, 3 h; (b) NaOMe, MeOH, rt, 6 h: (c) H2S, Py/H2O, 40 °C, 16 h; (d) (HCO)2O, MeOH, −20 °C, 3 h; (e) H2, Pd(OH)2, MeOH/H2O, rt, 16 h.
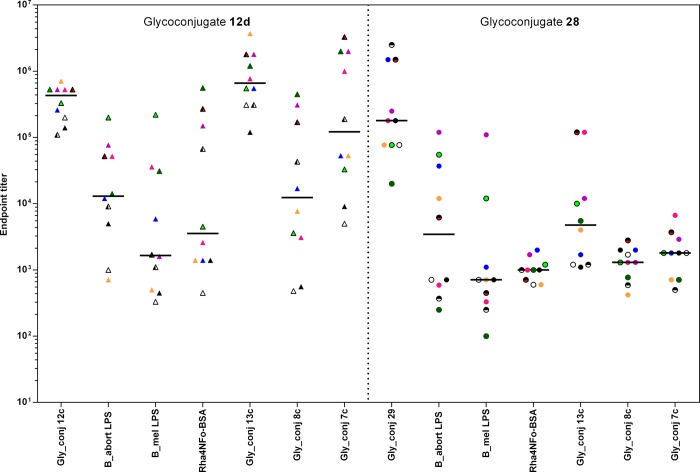
Mice immunized with 28 using a protocol identical to that employed with 12d produced high antibody titers when measured against the immunizing hapten, BSA conjugate 29 (Figure 5). A wide distribution of titers against the three LPS antigens (B. abortus, B. melitensis, and Y. enterocolitica O:9) was observed, similar to that seen with 12d, although the median titer for the 10 mice was 2–3-fold lower. Most striking was the markedly confined distribution of titers against the M disaccharide and tetrasaccharide glycoconjugates 7c and 8c. Whereas titers from sera of mice vaccinated with glycoconjugate 12d spanned a three-log range against 7c and 8c, sera of mice vaccinated with 28 were restricted to a single-log span and furthermore were 1–2 log units lower.15 This low titer against the M-diagnostic antigen compound 8c approaches but does not meet the objective for a DIVA vaccine. The modest to good level of antibodies specific for internal 1,2-linked Rh4NFo residues in the group immunized with 28 suggested that a similar glycoconjugate but with a larger A epitope should increase antibody levels against internal 1,2-linked Rh4NFo residues. If the terminal epitope were also eliminated from this structure, the presence of antibodies that bind chain-end residues present in the M antigen (7c and 8c) would be reduced further.
Figure 5.
Comparison of the glycoconjugate and Brucella LPS binding profiles with antibodies generated in mice immunized with glycoconjugates 12d and 28.
This idea was tested by conjugating the OPS of B. abortus strain S99, with 98% α1,2 and 2% α1,3 linkages, to tetanus toxoid.15 Conjugation was achieved via targeted alteration and activation by mild periodate oxidation of the OPS. Importantly, the terminal d-Rha4NFo residue was oxidized and the tip epitope was destroyed. Reductive amination of aldehydes created at this site followed by reaction with DSG allowed conjugation of the activated polysaccharide OPS-S99 to tetanus toxoid to yield OPS-TTS99 (Scheme 5).15 A second site between C7 and C8 of keto-deoxyoctulosonate (KDO) may also be oxidized, and conjugation may also occur via this site. A conjugate of this type would also have its terminal d-Rha4NFo residue destroyed by oxidation/reduction.
Scheme 5. Conjugation of B. abortus Strain S99 OPS to Tetanus Toxoid To Yield the Vaccine Conjugate OPS-TTS99.
Reagents and conditions: (a) 10 mM NaIO4, 50 mM NaOAc, pH 5.5, 4 °C, 1 h; (b) 0.5 M NH4Cl, 0.1 M NaCNBH3, 37 °C; (c) aminated OPS (5 mg/mL) in PBS containing 10% DMSO and 5 mg/mL disuccinimidal glutarate (dsg), 45 min, rt; (d) activated OPS, tetanus toxoid (2.5 mg/mL in PBS).
Mice were immunized three times with conjugate vaccine OPS-TTS99.15 All eight immunized mice produced antibodies against B. abortus S99 and B. melitensis 16 M sLPS, despite the fact that latter is M-dominant and has OPS with 20% α1,3 links (Figure 6). Binding to hexasaccharide antigen 12c compared with that to 1,3-linked hexasaccharide 11c showed that antibodies to the A epitope dominate. The lack of antibodies against the M epitope (as detected by the absence of binding to conjugates 7c and 8c), even though 2% of the linkages are α1,3, may be due to the occurrence of these linkages near the site of terminal conjugation, resulting in their reduced accessibility to the immune system.
Figure 6.
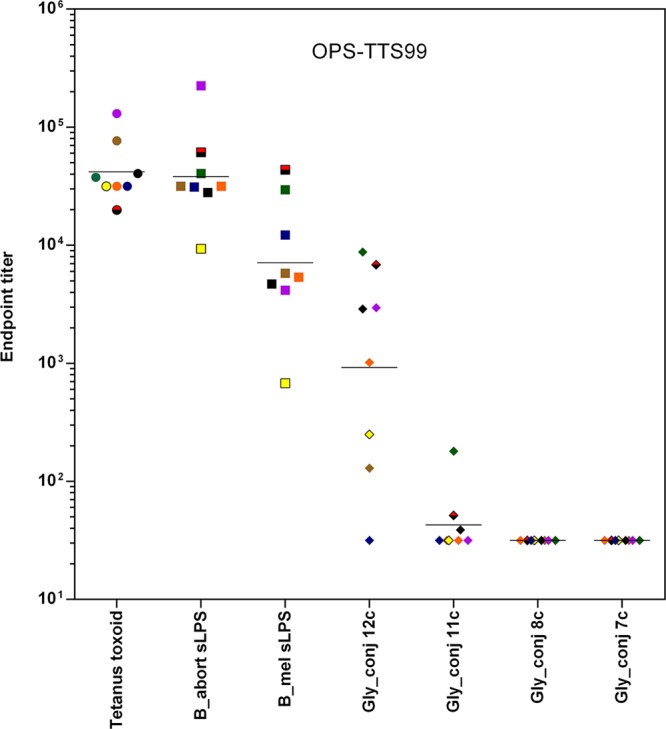
Final bleed titers from eight CD1 mice immunized with the modified B. abortus S99 conjugate OPS-TTS99. The antigens used for antibody detection are shown on the x axis.
Importantly, the results show that the OPS tetanus toxoid conjugate can induce antibodies that bind to M-dominant cells via the internal A epitope of their OPS. Antibodies to linear 1,2-linked d-Rha4NFo sequences can bind to these internal epitopes present in M-dominant OPS. The binding to the M-dominant OPS presumably occurs toward the reducing end of this block copolymer, where longer stretches of α1,2-linked d-Rha4NFo units exist. It has also been shown previously that antibodies with anti-A specificity can provide protection against challenge with M-dominant strains of Brucella.43
Polysaccharide conjugates such as the one as described here create a long-sought Brucella vaccine component that is able to produce the anti-O-antigen antibodies that are considered to be a vital element of the most protective vaccines. Unlike the various whole-cell live vaccines, these glycoconjugates produce a dominant immune response against only the α1,2-linked d-Rha4NFo units that make up the A epitope present in the Brucella OPS antigen. Thus, diagnostics based upon the M and terminal epitopes, including disaccharide and tetrasaccharide conjugates (7c and 8c), provide a universal diagnostic that can discriminate infected from vaccinated animals. In the ELISA format that we have reported, the assay is cheap, simple, and robust, and other assay formats are also possible
We have applied these antigens in a cheap, simple, and robust iELISA format to a panel of sera from naturally (n = 45) and experimentally (n = 4 with four postinfection bleed dates) B. abortus (A-dominant) infected cattle and noninfected cattle (n = 125) and demonstrated their excellent sensitivity and discriminating power.14 The existence of anti-Brucella OPS antibodies that do not bind these M- and tip-epitope-specific conjugates (7c and 8c) has been shown previously.32 We have now demonstrated the means to reliably and exclusively induce high titers of polyclonal antibodies with these particular characteristics, i.e., no binding to the M and terminal epitopes and the ability to bind to the α1,2-linked d-Rha4NFo units that make up the A epitope in OPS of both A and M serotypes.44 The combination of a diagnostic test and a vaccine that does not generate antibodies that bind to short M-type conjugates establishes the important principles of a viable DIVA.
Reduction of these design principles to practice will require several additional embodiments that enable a vaccine to be produced inexpensively, amenable to transport in adverse temperatures, and capable of conferring protection across a broad range of domestic ruminants and pigs.
One could envisage various scenarios for a successful, cost-effective vaccine. Combining anti-Brucella OPS antibody induction with an effective cell-mediated immune response will give the most effective protection against brucellosis.45 This could be achieved via OPS conjugation to or inclusion of a relevant Brucella protein, of which many have been reported,46 or by using the glycoconjugate in combination with rough Brucella vaccines. The glycoconjugate could be used in adult animals that have received smooth vaccines when young. This would boost immunity without incurring the safety and confounding serological issues that occur when live and smooth vaccines are used. Avoiding the growth of Brucella to harvest OPS, which mandates level-3 containment, could be achieved with conjugate vaccines derived from Y. enterocolitica O:9, which shares the same d-Rha4NFo polymer structure as B. suis biovar 228,32 but requires less stringent containment and likely represents the most cost-effective route to a vaccine. The synthetic and semisynthetic constructs described here could also guide biotechnology approaches for the creation of a genetically engineered live DIVA vaccine that exploits known OPS biosynthetic mechanisms.47−49
Conclusion
Brucellosis is a zoonotic infection that is passed to humans by contact with infected animals but is not spread by human-to-human contact. Eradication of the disease in humans can be achieved only by mass vaccination of animals combined with test and slaughter.50 The work summarized here is the first to establish a concept for an OPS-based brucellosis vaccine that can be applied in a DIVA format. Moreover, this work describes the main elements of a safe, viable, and thermostable glycoconjugate vaccine that could protect humans and animals against the disease.
Biographies
David R. Bundle was born in London, England, in 1946. He obtained his B.Sc. (Hons.) from the University of Nottingham and his Ph.D. in Microbiological Chemistry in the laboratory of Professor Sir James Baddiley at the University of Newcastle. After postdoctoral fellowships with H. J. Jennings at the National Research Council of Canada (NRC) and R. U. Lemieux at the University of Alberta, he joined the NRC as a research officer in 1975. In 1993 he moved to the Department of Chemistry at the University of Alberta, where he held the R. U. Lemieux Chair in Carbohydrate Chemistry until his retirement in 2015. He is currently an Emeritus Distinguished University Professor.
John McGiven was born in Surry, England, in 1973. He graduated from the University of Liverpool with a B.Sc. (Hons.) in Zoology in 1994 and has spent his professional career at the Animal & Plant Health Agency (APHA) in Weybridge, U.K.. During this time he obtained an M.Sc. in Immunology from the University of Surrey, a graduate diploma in statistics from the Open University, and most recently a Ph.D. in Biochemistry from Imperial College London. He is currently leading the research and development team at the APHA’s OIE (World Organization for Animal Health) Reference Laboratory for Brucellosis.
This work was funded in part by a grant from the Bill & Melinda Gates Foundation through the Grand Challenges Exploration Initiative. It was also supported by the Department for Environment, Food, and Rural Affairs (United Kingdom) under Contract SE0316 and by a Discovery Grant to D.R.B. from the Natural Science and Engineering Research Council of Canada (NSERC).
The authors declare no competing financial interest.
References
- Maudlin I.; Weber S.. The Control of Neglected Zoonotic Diseases: A Route to Poverty Alleviation; WHO/SDE/FOS/2006.1.; World Health Organization: Geneva, 2005; www.who.int/zoonoses/Report_Sept06.pdf. [Google Scholar]
- Corbel M. J.; Elberg S. S.; Cosivi O.. Brucellosis in Humans and Animals; WHO/CDS/EPR/2006.7; World Health Organization: Geneva, 2006; www.who.int/csr/resources/publications/Brucellosis.pdf. [Google Scholar]
- a Pappas G.; Akritidis N.; Bosilkovski M.; Tsianos E. Brucellosis. N. Engl. J. Med. 2005, 352, 2325–2336. 10.1056/NEJMra050570. [DOI] [PubMed] [Google Scholar]; b Pappas G.; Papadimitriou P.; Akritidis N.; Christou L.; Tsianos E. The new global map of human brucellosis. Lancet Infect. Dis. 2006, 6, 91–99. 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- Godfroid J. Brucellosis in wildlife. Rev. Sci. Tech. Off. Int. Epiz. 2002, 21, 277–286. 10.20506/rst.21.2.1333. [DOI] [PubMed] [Google Scholar]
- Nielsen K. Diagnosis of brucellosis by serology. Vet. Microbiol. 2002, 90, 447–459. 10.1016/S0378-1135(02)00229-8. [DOI] [PubMed] [Google Scholar]
- Goodwin Z. I.; Pascual D. W. Brucellosis vaccines for livestock. Vet. Immunol. Immunopathol. 2016, 181, 51–58. 10.1016/j.vetimm.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Carvalho T. F.; Haddad J. P. A.; Paixão T. A.; Santos R. L. Meta-Analysis and Advancement of Brucellosis Vaccinology. PLoS One 2016, 11, e0166582. 10.1371/journal.pone.0166582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurig G. G.; Roop R. M.; Bagchi T.; Boyle S.; Buhrman D.; Sriranganathan N. Biological properties of RB51; a stable rough strain of Brucella abortus. Vet. Microbiol. 1991, 28, 171–188. 10.1016/0378-1135(91)90091-S. [DOI] [PubMed] [Google Scholar]
- Moriyon I.; Grillo M. J.; Monreal D.; Gonzalez D.; Marin C.; Lopez-Goni I.; Mainar-Jaime R. C.; Moreno E.; Blasco J. M. Rough vaccines in animal brucellosis: Structural and genetic basis and present status. Vet. Res. 2004, 35, 1–38. 10.1051/vetres:2003037. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. Revisting Brucellosis in the Greater Yellowstone Area; The National Academies Press: Washington, DC, 2017; DOI: 10.17226/24750. [DOI] [PubMed] [Google Scholar]
- Kubler-Kielb J.; Vinogradov E. Reinvestigation of the structure of Brucella O-antigens. Carbohydr. Res. 2013, 378, 144–147. 10.1016/j.carres.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiard J.; Paszkiewicz E.; Sadowska J.M.; Bundle D. R. Design and synthesis of a universal antigen to detect brucellosis. Angew. Chem., Int. Ed. 2013, 52, 7181–7185. 10.1002/anie.201302303. [DOI] [PubMed] [Google Scholar]
- Ganesh V. N.; Sadowska J. M.; Sarkar S.; Howells L.; McGiven J.; Bundle D. R. Molecular Recognition of Brucella A and M Antigens Dissected by Synthetic Oligosaccharide Glycoconjugates Leads to a Disaccharide Diagnostic for Brucellosis. J. Am. Chem. Soc. 2014, 136, 16260–16269. 10.1021/ja5081184. [DOI] [PubMed] [Google Scholar]
- McGiven J.; Howells L.; Duncombe L.; Stack J.; Ganesh N.; Guiard J.; Bundle D. R. Improved serodiagnosis of bovine brucellosis by novel synthetic oligosaccharide antigens based on the capping M epitope elements of Brucella O-polysaccharide. J. Clin. Microbiol. 2015, 53, 1204–1210. 10.1128/JCM.03185-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal S. S.; Duncombe L.; Ganesh N. V.; Sarkar S.; Howells L.; Hogarth P.; Bundle D. R.; McGiven J. Synthetic Glycoconjugates Reveal Novel Solutions for Diagnostics and Vaccines to Combat Brucellosis. ACS Cent. Sci. 2017, 3, 224–231. 10.1021/acscentsci.7b00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin Z. I.; Pascual D. W. Brucellosis vaccines for livestock. Vet. Immunol. Immunopathol. 2016, 181, 51–58. 10.1016/j.vetimm.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Olsen S. C. Recent developments in livestock and wildlife brucellosis vaccination. Rev. Sci. Technol. 2013, 32, 207–217. 10.20506/rst.32.1.2201. [DOI] [PubMed] [Google Scholar]
- Lai S.; Zhou H.; Xiong W.; Gilbert M.; Huang Z.; Yu J.; Yin W.; Wang L.; Chen Q.; Li Y.; Mu D.; Zeng L.; Ren X.; Geng M.; Zhang Z.; Cui B.; Li T.; Wang D.; Li Z.; Wardrop N. A.; Tatem A. J.; Yu H. Changing Epidemiology of Human Brucellosis, China, 1955–2014. Emerging Infect. Dis. 2017, 23, 184–194. 10.3201/eid2302.151710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamri-Saad M.; Kamarudin M. I. Control of animal brucellosis: The Malaysian experience. Asian Pac. J. Trop. Med. 2016, 9, 1136–1140. 10.1016/j.apjtm.2016.11.007. [DOI] [PubMed] [Google Scholar]
- Grace D.; Mutua F.; Ochungo P.; Kruska R.; Jones K.; Brierley L.; Lapar L.; Said M.; Herrero M.; Phuc P. D.; Bich N.; Akuku I.; Ogutu F.. Mapping of Poverty and Likely Zoonoses Hotspots; Zoonoses Project 4, Report to Department for International Development, UK; Department for International Development, UK: London, 2012; https://cgspace.cgiar.org/bitstream/handle/10568/21161/ZooMap_July2012_final.pdf?sequence=4. [Google Scholar]
- Singh B. B.; Dhand N. K.; Gill J. P. S. Economic losses occurring due to brucellosis in Indian livestock populations. Prev. Vet. Med. 2015, 119, 211–215. 10.1016/j.prevetmed.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Santos R. L.; Martins T. M.; Borges A. M.; Paixão T. A. Economic losses due to bovine brucellosis in Brazil. Pesq. Vet. Bras. 2013, 33, 759–764. 10.1590/S0100-736X2013000600012. [DOI] [Google Scholar]
- Li J.-Y.; Li X.-L.; Liang S.; Fang L.-Q.; Cao W.-C. Epidemiological features and risk factors associated with the spatial and temporal distribution of human brucellosis in China. BMC Infect. Dis. 2013, 13, 547–559. 10.1186/1471-2334-13-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. S.; Miles A. A. The Serological Differentiation of Smooth Strains of the Brucella Group. Br. J. Exptl. Pathol. 1932, 13, 1–13. [Google Scholar]
- Caroff M.; Bundle D. R.; Perry M. B.; Cherwonogrodsky J. W.; Duncan J. R. Antigenic S-type lipopolysaccharide of Brucella abortus 1119–3. Infect. Immun. 1984, 46, 384–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundle D. R.; Cherwonogrodzky J. W.; Perry M. B. Structural elucidation of the Brucella melitensis M antigen by high resolution NMR at 500 MHz. Biochemistry 1987, 26, 8717–8726. 10.1021/bi00400a034. [DOI] [PubMed] [Google Scholar]
- Miles A. A.; Pirie N. W. The Properties of Antigenic Preparations from Brucella melitensis: III. The Biological Properties of the Antigen and the Products of Gentle Hydrolysis. Br. J. Exptl. Pathol. 1939, 20, 278–296. [Google Scholar]
- Meikle P. J.; Perry M. B.; Cherwonogrodzky J. W.; Bundle D. R. Fine structure of A and M antigens from Brucella biovars. Infect. Immun. 1989, 57, 2820–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundle D. R.; Cherwonogrodzky J. W.; Gidney M. A.; Meikle P. J.; Perry M. B.; Peters T. Definition of Brucella A and M epitopes by monoclonal typing reagents and synthetic oligosaccharides. Infect. Immun. 1989, 57, 2829–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccheus M. V.; Ali T.; Cloeckaert A.; Zygmunt M. S.; Weintraub A.; Iriarte M.; Moriyón I.; Widmalm G. The epitopic and structural characterization of Brucella suisbiovar 2 O-polysaccharide demonstrates the existence of a new M-negative C-negative smooth Brucella serovar. PLoS One 2013, 8, e53941. 10.1371/journal.pone.0053941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiser-Wilke I.; Moennig V. Monoclonal antibodies and characterization of epitopes of smooth Brucella lipopolysaccharides. Ann. Inst. Pasteur/Microbiol. 1987, 138, 549–560. 10.1016/0769-2609(87)90040-8. [DOI] [PubMed] [Google Scholar]
- Zygmunt M. S.; Bundle D. R.; Ganesh N. V.; Guiard J.; Cloeckaert A. Monoclonal Antibody-Defined Specific C Epitope of Brucella O-Polysaccharide Revisited. Clin. Vaccine Immunol. 2015, 22, 979–982. 10.1128/CVI.00225-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal S. S.; Ganesh N. V.; Sadowska J. M.; Bundle D. R. Synthetic Glycoconjugates Characterize the Fine Specificity of Brucella A and M Monoclonal Antibodies. Org. Biomol. Chem. 2017, 15, 3874–3883. 10.1039/C7OB00445A. [DOI] [PubMed] [Google Scholar]
- Caroff M.; Bundle D. R.; Perry M. B. Structure of the O-chain of the phenol-phase soluble cellular lipopolysaccharide of Yersinia enterocolitica serotype O:9. Eur. J. Biochem. 1984, 139, 195–200. 10.1111/j.1432-1033.1984.tb07994.x. [DOI] [PubMed] [Google Scholar]
- Kenne L.; Lindberg B.; Unger P.; Gustafsson B.; Holme T. Structural studies of the Vibrio cholerae O-antigen. Carbohydr. Res. 1982, 100, 341–349. 10.1016/S0008-6215(00)81047-2. [DOI] [PubMed] [Google Scholar]
- Perry M. B.; Bundle D. R. Antigenic relationships of the lipopolysaccharides of Escherichia hermannii strains with those of Escherichia coli O157:H7, Brucella melitensis, and Brucella abortus. Infect. Immun. 1990, 58, 1391–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fabio J. L.; Perry M. B.; Bundle D. R. Analysis of the lipopolysaccharide of Pseudomonas maltophilia 555. Biochem. Cell Biol. 1987, 65, 968–977. 10.1139/o87-126. [DOI] [PubMed] [Google Scholar]
- Perry M. B.; Bundle D. R.; MacLean L.; Perry J. A.; Griffith D. W. The structure of the antigenic lipopolysaccharide O-chains produced by Salmonella urbana and Salmonella godesberg. Carbohydr. Res. 1986, 156, 107–122. 10.1016/S0008-6215(00)90103-4. [DOI] [PubMed] [Google Scholar]
- Lu Z.; Rynkiewicz M. J.; Yang C. Y.; Madico G.; Perkins H. M.; Wang Q.; Costello C. E.; Zaia J.; Seaton B. A.; Sharon J. The binding sites of monoclonal antibodies to the non-reducing end of Francisella tularensis O-antigen accommodate mainly the terminal saccharide. Immunology 2013, 140, 374–389. 10.1111/imm.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundle D. R.; Gerken M.; Peters T. Synthesis of antigenic determinants of the Brucella A antigen utilizing methyl 4-azido-4,6-dideoxy-α-d-mannopyranoside efficiently derived from d-mannose. Carbohydr. Res. 1988, 174, 239–251. 10.1016/0008-6215(88)85094-8. [DOI] [PubMed] [Google Scholar]
- Eis M. J.; Ganem B. An improved synthesis of d-perosamine and some derivatives. Carbohydr. Res. 1988, 176, 316–323. 10.1016/0008-6215(88)80144-7. [DOI] [PubMed] [Google Scholar]
- Kováč P. In Protein–Carbohydrate Interactions in Infectious Diseases; Bewley C., Ed.; Royal Society of Chemistry: Cambridge, U.K., 2006; pp 175–220. [Google Scholar]
- Cloeckaert A.; Jacques I.; de Wergifosse P.; Dubray G.; Limet J. N. Protection against Brucella melitensis or Brucella abortus in mice with immunoglobulin G (IgG), IgA, and IgM monoclonal antibodies specific for a common epitope shared by the Brucella A and M smooth lipopolysaccharides. Infect. Immun. 1992, 60, 312–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloeckaert A.; Weynants V.; Godfroid J.; Verger J. M.; Grayon M.; Zygmunt M. S. O-polysaccharide epitopic heterogeneity at the surface of Brucella spp. studied by enzyme-linked immunosorbent assay and flow cytometry. Clin. Diagn. Lab. Immunol. 1998, 5, 862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilló M. J.; Manterola L.; de Miguel M. J.; Muñoz P. M.; Blasco J. M.; Moriyón I.; López-Goñi I. Increases of efficacy as vaccine against Brucella abortus infection in mice by simultaneous inoculation with avirulent smooth bvrS/bvrR and rough wbkA mutants. Vaccine 2006, 24, 2910–2916. 10.1016/j.vaccine.2005.12.038. [DOI] [PubMed] [Google Scholar]
- Oliveira S. C.; Giambartolomei G. H.; Cassataro J. Confronting the barriers to develop novel vaccines against brucellosis. Expert Rev. Vaccines 2011, 10, 1291–1305. 10.1586/erv.11.110. [DOI] [PubMed] [Google Scholar]
- Whitfield C.; Trent M. S. Biosynthesis and export of bacterial lipopolysaccharides. Annu. Rev. Biochem. 2014, 83, 99–128. 10.1146/annurev-biochem-060713-035600. [DOI] [PubMed] [Google Scholar]
- Feldman M. F.; Wacker M.; Hernandez M.; Hitchen P. G.; Marolda C. L.; Kowarik M.; Morris H. R.; Dell A.; Valvano M. A.; Aebi M. Engineering N-linked protein glycosylation with diverse O antigen lipopolysaccharide structures in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 3016–3021. 10.1073/pnas.0500044102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.; Valentine J. L.; Huang C. J.; Endicott C. E.; Moeller T. D.; Rasmussen J. A.; Fletcher J. R.; Boll J. M.; Rosenthal J. A.; Dobruchowska J.; Wang Z.; Heiss C.; Azadi P.; Putnam D.; Trent M. S.; Jones B. D.; DeLisa M. P. Outer membrane vesicles displaying engineered glycotopes elicit protective antibodies. Proc. Natl. Acad. Sci. U. S. A. 2016, 113, E3609–E3618. 10.1073/pnas.1518311113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth F.; Zinsstag J.; Orkhon D.; Chimed-Ochir G.; Hutton G.; Cosivi O.; Carrin G.; Otte J. Human health benefits from livestock vaccination for brucellosis: case study. Bull. W. H. O. 2003, 81, 867–876. [PMC free article] [PubMed] [Google Scholar]