Abstract
An inflammatory tissue reaction around the electrode array of a cochlear implant (CI) is common, in particular at the electrode insertion region (cochleostomy) where mechanical trauma often occurs. However, the factors determining the amount and causes of fibrous reaction surrounding the stimulating electrode, especially medially near the perimodiolar location, are unclear. Temporal bone (TB) specimens from patients who had undergone cochlear implantation during life with either Advanced Bionics (AB) Clarion TM or HiRes90KTM (Sylmar, CA, USA) or Cochlear TM Nucleus (Sydney, Australia) devices were evaluated. The thickness of the fibrous tissue surrounding the electrode array of both types of CI devices at both the lower (LB) and upper (UB) basal turns of the cochlea was quantified at three locations: the medial, inferior, and superior aspects of the sheath. Fracture of the osseous spiral lamina and/or marked displacement of the basilar membrane were interpreted as evidence of intracochlear trauma. In addition, post-operative word recognition scores, duration of implantation, and post-operative programming data were evaluated.
Seven TBs from six patients implanted with AB devices and five TBs from five patients implanted with Nucleus devices were included. A fibrous capsule around the stimulating electrode array was present in all twelve specimens. TBs implanted with AB device had a significantly thicker fibrous capsule at the medial aspect than at the inferior or superior aspects at both locations (LB and UB) of the cochlea (Wilcoxon signed-ranks test, p<0.01). TBs implanted with a Nucleus device had no difference in the thickness of the fibrous capsule surrounding the track of the electrode array (Wilcoxon signed-ranks test, p>0.05). Nine of fourteen (64%) basal turns of the cochlea (LB and UB of seven TBs) implanted with AB devices demonstrated intracochlear trauma compared to two of ten (20%) basal turns of the cochlea (LB and UB of five TBs) with Nucleus devices, (Fisher exact test, p<0.05). There was no significant correlation between the thickness of the fibrous tissue and the duration of implantation or the word recognition scores (Spearman rho, p=0.06, p=0.4 respectively). Our outcomes demonstrated the development of a robust fibrous tissue sheath medially closest to the site of electric stimulation in cases implanted with the AB device electrode, but not in cases implanted with the Nucleus device. The cause of the asymmetric fibrous sheath may be multifactorial including insertional trauma, a foreign body response, and/or asymmetric current flow.
Keywords: Cochlear implant, Fibrous tissue, Advanced Bionics, Nucleus, Impedance, Temporal bone
1. Introduction
Cochlear implants (CI) have been considered a well-tolerated biocompatible device with a low rate of complications (Issa et al., 1983; Webb et al., 1988; Ray et al., 2004; Venail et al., 2008; Ding et al., 2009; Bennati et al., 2013). A chronic local inflammatory reaction, which may result in CI failure, explantation, or reimplantation has been reported in rare clinical CI cases. The causes for this chronic local inflammation might be a delayed hypersensitivity or a local tissue reaction to the CI electrode array (Beruleit et al., 1999; Ho et al., 2003; Puri et al., 2005; Kunda et al., 2006; Nadol et al., 2008; Lim et al., 2011; Neilan et al., 2012; Bennatti et al., 2013). Several temporal bone studies have demonstrated fibrosis and new bone formation adjacent to the electrode track (Nadol and Eddington, 2004; li et al., 2007; Somdas et al., 2007; Seyyedi and Nadol, 2014; Kamakura and Nadol, 2016), and have shown that this local inflammatory response is quite common. (Nadol and Eddington, 2004; Seyyedi and Nadol, 2014; Kamakura and Nadol, 2016). Seyyedi and Nadol (2014) demonstrated evidence of a chronic inflammatory reaction including inflammatory mediator cells, fibrous tissue and new bone formation in twenty-eight specimens (100%), from patients who during life underwent cochlear implantation.
They reported that these inflammatory effects were more severe at the basal turn of cochlea close to the cochleostomy, and suggested that trauma of electrode insertion (at the cochleostomy) and a foreign body response may initiate these inflammatory effects. However, determining factors of the amount and causes of fibrous reaction surrounding the stimulating electrode, especially medially at a perimodiolar location, are unclear. Moreover, fibrous tissue growth and new bone formation were inconclusively correlated with hearing performance (Li et al., 2007; Kamakura and Nadol. 2016).
We wished to systematically evaluate the pattern of fibrous tissue formation around different types of CI electrodes in an attempt to determine the potential causes of fibrous tissue, including trauma, inflammation, and the possible role of electrical stimulation. This study also examined whether there is a clinical correlation between the thickness of the fibrous tissue and hearing performance or duration of implantation.
2. Materials and Methods
2.1. Subjects
Temporal bone specimens were selected from the collection of the Otopathology Laboratory of the Massachusetts Eye and Ear from patients who in life had undergone cochlear implantation. These specimens were categorized by CI device in two groups: (1)Advanced Bionics (AB) Clarion TM or HiRes90KTM (Sylmar, CA, USA) devices, which have HiFocus perimodiolar partial banded electrode arrays, and (2) Cochlear TM Corporation Nucleus (Sydney, Australia) devices, which have full banded straight electrode arrays. Demographic and clinical data including sex and age, duration of implantation, cause of deafness, type of CI device and electrode array and post-implantation last-recorded word recognition scores [CNC (Consonant- Nucleus-Consonant Word Test score) or NU6 (Northwestern University Auditory Test No. 6) ] are presented in Table 1. When available, longitudinal CI impedance data from the manufacturer programming software were evaluated.
Table 1.
Clinical History of the Implanted Patients.
| No. | Case/Side | Sex | Age (yr.) | Diagnosis | C.I. usage (yr.) | Device | Electrode type | Electrode shape | C.I. WRS (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 L | F | 82 | PSNHL, SSNHL | 8 | AB Clarion CII | HF I | Perimodiolar | 44(CNC) |
| 2 | 2 L | F | 93 | PSNHL | 8 | AB HR90K | HF 1J | Perimodiolar | NA |
| 3 | 3 L | F | 89 | PSNHL | 9 | AB Clarion CII | HF I | Perimodiolar | 10(CNC) |
| 4 | 4 R | F | 94 | PSNHL | 12 | AB Clarion CII | HF II w/p | Perimodiolar | 10(CNC) |
| 5 | 5 R | M | 89 | PSNHL | 3 | AB HR90K | HF Helix | Perimodiolar | 74(CNC) |
| 6 | 6 R | M | 64 | SSNHL | 2 | AB HR90K | HF 1J | Perimodiolar | 60(CNC) |
| 7 | 6 L | M | 64 | SSNHL | 2 | AB HR90K | HF 1J | Perimodiolar | 50(CNC) |
| 8 | 7 R | M | 80 | Genetic | 24 | Nucleus 22 | Banded | Straight | 46(CNC) |
| 9 | 8 R | F | 84 | PSNHL | 13 | Nucleus 22 | Banded | Straight | 34(NU6) |
| 10 | 9 R | M | 83 | Meniere’s sd., PSNHL | 12 | Nucleus 24 | Banded | Straight | 57(CNC) |
| 11 | 10 L | M | 76 | PSNHL | 18 | Nucleus 22 | Banded | Straight | 0(NU6) |
| 12 | 11 L | M | 88 | Acoustic trauma | 11 | Nucleus 22 | Banded | Straight | NA |
No, number; L, left; R, right; M, male; F, female; yr., year; PSNHL, progressive sensorineural hearing loss.
SSNHL, sudden sensorineural hearing loss; sd, syndrome; AB, Advanced Bionics; HR, Hi Res.
HF, HiFocus; w/p, with positioner; NA, not available; C.I., cochlear implant.
WRS, word recognition score (monosyllabic).
2.2. Histological methods and 2-D reconstruction of the cochlea
The temporal bone specimens were removed and fixed in 10% buffered formalin solution. The specimens were scanned, using computerized tomography with the intra-cochlear electrodes left in place, and decalcified in ethylene diamine tetra-acetic acid (EDTA). Electrodes were removed from the specimens after decalcification, and the specimens were then embedded in celloidin. The specimens were sectioned at a thickness of 20 microns in an axial plane, and every tenth section was stained with hematoxylin and eosin (H&E), and mounted on a glass slide. Every tenth section was studied using light microscopy for 2-D reconstruction based on the method described by Schuknecht (1953) and Otte et al. (1978).
2.3. Quantification of intra cochlear fibrous sheath
The fibrous sheath enveloping the electrode array was studied at two locations of the cochlea: the lower and upper basal turns (LB and UB respectively). The most orthogonal section at each location was selected to avoid bias caused by oblique section angle (Figure 1A). Specimens in which a fibrous sheath was not seen at both locations of the cochlea were excluded.
Figure 1. Illustration of cochlear duct showing the locations in which the fibrous sheath was measured.
(A) An illustration of a 2-D reconstruction of the cochlear duct. Open circles represent the locations where the measurements were performed in the lower basal turn (LB), and upper basal turn (UB). The bold line indicates the location of the intracochlear electrode.
(B) Thickness (red lines) of the fibrous sheath capsule at each of three points around the electrode track: Medial (M), inferior (I) and superior (S). The bold line represents the osseous spiral lamina, which was the reference line parallel to which the measurements were performed. SV: scala vestibuli, ST: scala tympani, circles indicate spiral ganglion cells.
Image J software (http:/rsbweb.nih.gov/ij/) was used to measure the thickness of the fibrous sheath at locations medial, inferior, and superior to the track of the electrode array with reference to the plane of the osseous spiral lamina (Figure 1B). Lateral measurements were excluded in order to eliminate the possible influence of the juxtaposition of the lateral boney cochlear wall to the forming fibrous capsule.
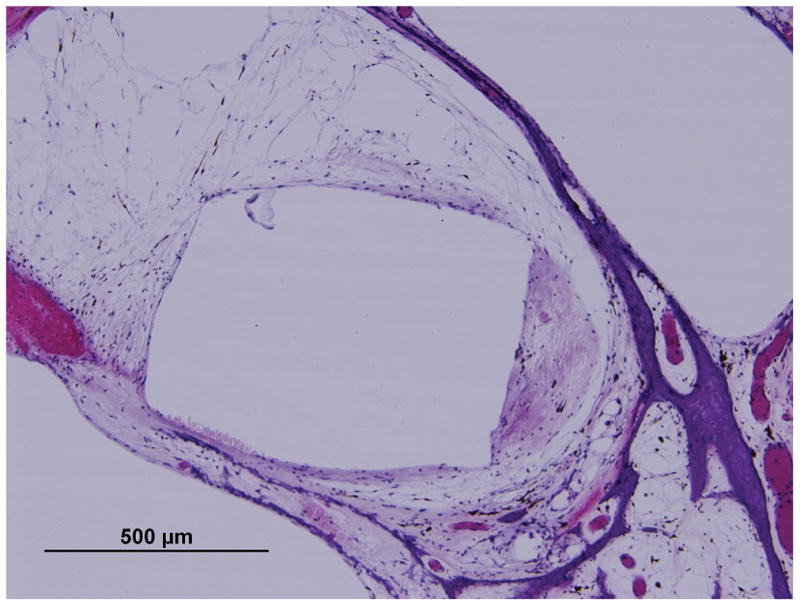
Furthermore, in two TBs (one AB and one Nucleus) the thickness of the medial aspect of the fibrous capsule was measured in sequential H&E stained sections (every 200 um) along a 2 mm length of the cochlear duct at a location orthogonal to the electrode array (Figure 2A, B). Since the electrode contacts ranged in size from 300 to 400 um, and the inter-electrode spaces ranged from 350 to 850 um, the 2 mm length included both areas directly adjacent to the electrode contact as well as adjacent to nonstimulating areas. These measurements were used to determine whether there was a variation of the thickness along the cochlear duct that corresponded to the location of stimulating electrode contacts and thus electric current flow.
Figure 2.
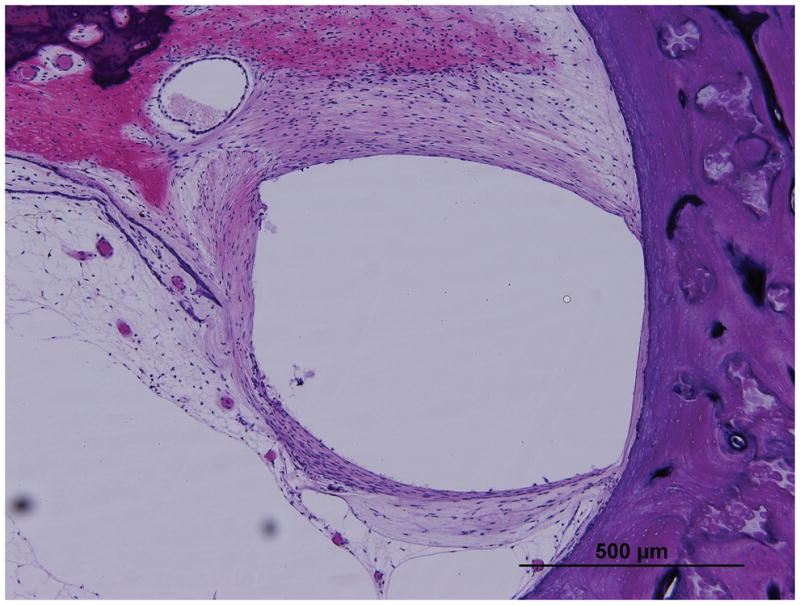
Illustration showing the measurements of fibrous sheath medially along a 2 mm length of the track of the cochlear duct (horizontal lines): (A) Cochlear Nucleus straight-banded electrode and with (B) Advanced Bionics HiFocus perimodiolar electrode.
2.4. Evaluation of intracochlear damage caused by electrode array insertion
Fracture of the osseous spiral lamina and/or marked displacement of the basilar membrane were assessed as markers for intracochlear trauma caused by the electrode.
Furthermore, translocation of the electrode track from the scala tympani to the scala vestibuli was identified and correlated with TB groups (AB vs. Nucleus).
3. Results
3.1. Clinical history
The average age of the subjects was 72 years (range from 56 to 86 years). All were postlingually deafened, and most of them had progressive severe to profound sensorineural hearing loss with unknown etiology. The duration of cochlear implantation ranged from 2 to 24 years with an average of 10 years. There were no cases with re-implantation. The last post-operative word performance score tested by CNC or NU6 word lists was available except in two patients (Case 2L and Case 11L). Word recognition scores ranged from 0% to 74% with an average of 42% (Table 1).
The post-operative course for all patients was unremarkable except in Case 1L and Case 11L.
In Case 1L, an 82-year-old woman implanted with a AB (Clarion TM CII) device initially did well as judged by word recognition score, until she had two attacks of severe vertigo, one and two years after implantation. These episodes were considered to be related to the cochlear implantation, and a diagnosis of delayed endolymphatic hydrops was made. The TB finding confirmed the clinical diagnosis and showed endolymphatic hydrops in all cochlear turns. In Case 11L, a 88-year-old man was implanted with a Nucleus 22 device and had minimal benefit by clinical report (word recognition scores were not available). An integrity check of the internal electrode eleven years following implantation indicated that seven electrodes were broken or shorted. Therefore, the defective electrodes were turned off; however, no hearing improvement was noted. Consequently, the device was abandoned with no further stimulation. Both cases showed the presence of a thick fibrous sheath.
3.2. Fibrous capsule around the electrode track
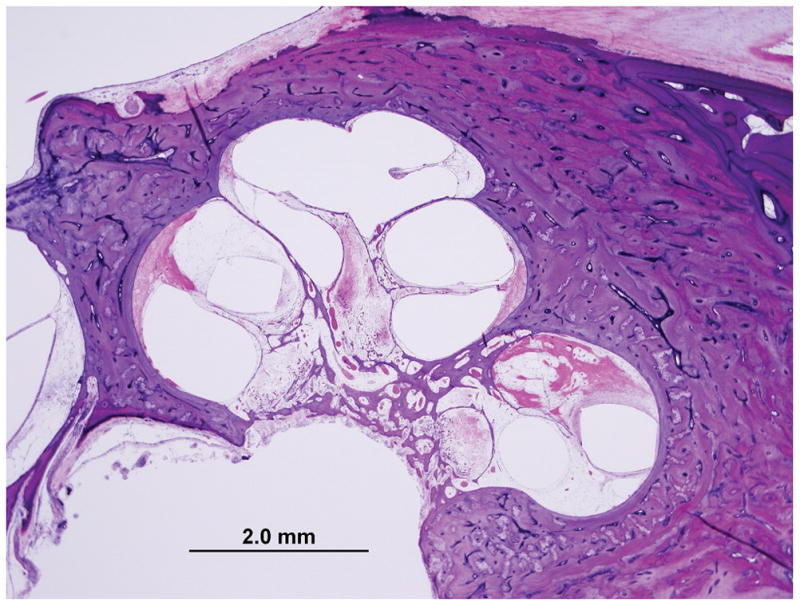
Figure 4 shows an example of a TB specimen implanted with an AB device, in which an asymmetric capsule has developed that is thicker on the medial side, abutting the exposed stimulating electrode surface. In Figure 5, a TB specimen implanted with a Nucleus device, with circumferential exposure of stimulating electrode contacts, resulted in a more circumferentially distributed fibrous capsule. The measurements of the fibrous capsule surrounding the electrode track for all twelve TB specimens with both CI devices (AB and Nucleus) are shown in Table 2. As shown Figure 3, in the TB specimens implanted with an AB device, the fibrous sheath at both locations of the cochlea (LB, UB) was significantly thicker medially as compared to the thickness at superior or inferior locations (paired t-test, p<0.01). In specimens implanted with Nucleus devices, the fibrous sheath showed a relatively symmetric thickness and there was no statistical difference between the medial, superior, and inferior measurements, (paired t- test, p>0.05). Overall, the thickness of the medial fibrous capsule in specimens that received AB device was significantly greater than the thickness of medial fibrous capsule in specimens that received Nucleus devices (paired t-test, p=0.004).
Figure 4. Fibrous capsule measurements as seen in Case 5R implanted with perimodiolar electrode of AB device.
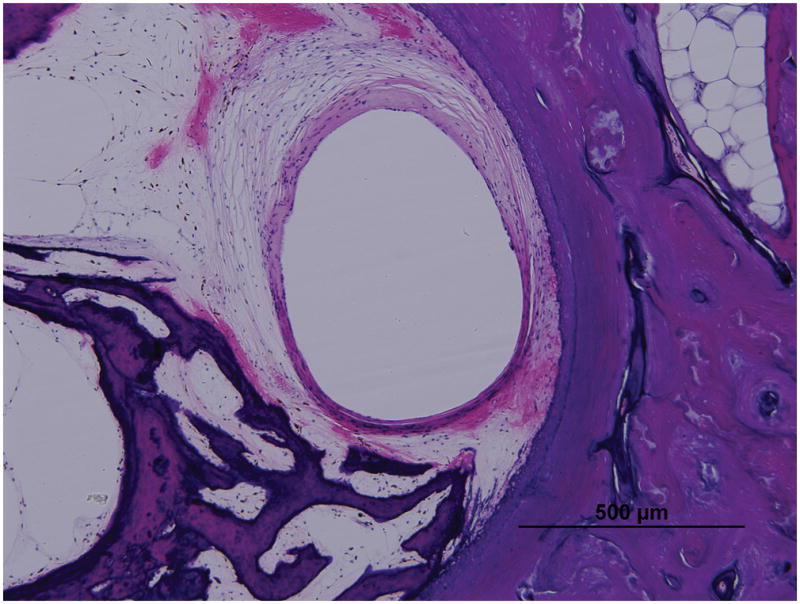
Low (A) and high (B) magnification view at lower basal turn of the cochlea (LB), (large arrow).
Low (C) and high (D) magnification view at upper basal turn of the cochlea (UB), (large arrow).
Asterisk indicates the electrode track; Lines indicate the thickness of fibrous tissue at each point: S, superior; M, medial; I, inferior; SV, scala vestibuli; ST, scala tympani; }, new bone formation; small arrow, osseous spiral lamina; arrowheads, organ of Corti ; IAC, internal acoustic canal
Figure 5. Fibrous capsule measurements as seen in Case 10L implanted with straight-banded electrode of Nucleus 22 device.
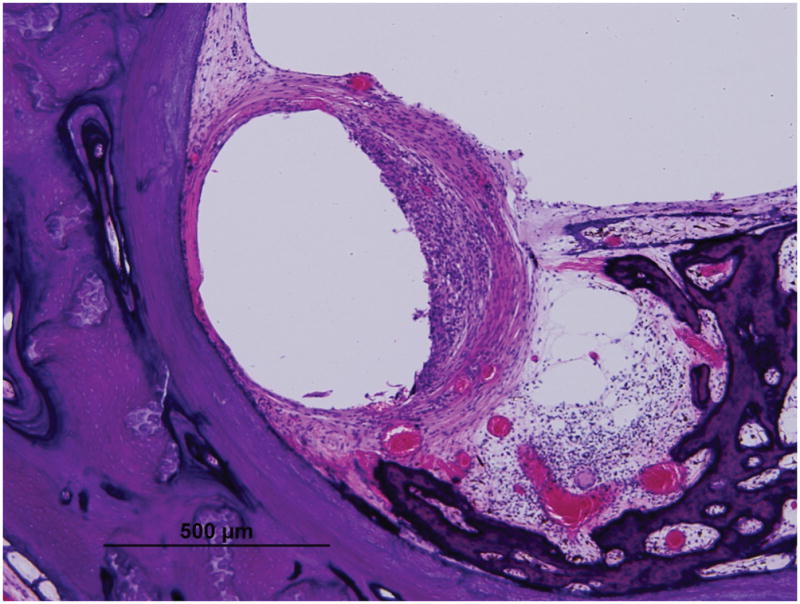
Low (A) and high (B) magnification view at LB (large arrow).
Low (C) and high (D) magnification view at UB (large arrow). Asterisk indicates the electrode track; Lines indicate the thickness of fibrous tissue at each point: S, superior; M, medial; I, inferior; SV, scala vestibuli; ST, scala tympani; }, new bone formation; small arrow, osseous spiral lamina; IAC, internal acoustic canal
TABLE 2.
Capsular Thickness
| Case/ Side | Device | Measurement location | Electrode position | Capsular thickness(μm) | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Medial | Superior | Inferior | ||||
| 1L | AB | LB | ST | 121 | 0 | 5 |
| 2L | AB | LB | ST | 151 | 8 | 32 |
| 3L | AB | LB | SV | 365 | 8 | 49 |
| 4R | AB | LB | ST | 245 | 61 | 46 |
| 5R | AB | LB | ST | 190 | 14 | 72 |
| 6R | AB | LB | ST | 127 | 18 | 14 |
| 6L | AB | LB | SV | 109 | 155 | 41 |
|
| ||||||
| 1L | AB | UB | SV | 54 | 11 | 0 |
| 2L | AB | UB | ST | 91 | 9 | 56 |
| 3L | AB | UB | SV | 248 | 90 | 89 |
| 4R | AB | UB | ST | 207 | 30 | 40 |
| 5R | AB | UB | ST | 178 | 67 | 49 |
| 6R | AB | UB | ST | 153 | 56 | 33 |
| 6L | AB | UB | SV | 171 | 17 | 24 |
| 7R | Nucleus | LB | ST | 27 | 82 | 43 |
| 8R | Nucleus | LB | ST | 93 | 12 | 34 |
| 9R | Nucleus | LB | ST | 54 | 22 | 19 |
| 10L | Nucleus | LB | ST | 119 | 82 | 62 |
| 11L | Nucleus | LB | ST | 41 | 60 | 35 |
| 7R | Nucleus | UB | SV | 19 | 70 | 31 |
| 8R | Nucleus | UB | ST | 65 | 9 | 47 |
| 9R | Nucleus | UB | ST | 6 | 15 | 0 |
| 10L | Nucleus | UB | ST | 133 | 124 | 134 |
| 11L | Nucleus | UB | SV | 128 | 101 | 111 |
L, left; R, right; AB; Advanced Bionics; LB, lower basal turn of the cochlea.
UB, upper basal turn of the cochlea; ST, scala tympani; SV, scala vestibuli.
Figure 3. Comparison of medial (M) fibrous tissue thickness with superior (S) and inferior (I) thickness in both locations of the cochlea.
(A) Upper basal turn (UB) in cases implanted with Advanced Bionics (AB) device.
(B) UB in cases implanted with Nucleus device.
(C) Lower basal turn (LB) in cases implanted with AB device.
(D) LB in cases implanted with Nucleus device.
NS: not significantly different, p>0.05
However, the thickness of superior and inferior capsule between TBs that received AB devices and TBs that received Nucleus devices were not significantly different (paired t- test, p>0.05).
As a separate analysis, the thickness of the fibrous capsule at medial aspect was measured in sequential orthogonal stained sections over a 2mm length(Figure 2A, B) in two TBs with a well-defined fibrous sheath (Case 5R, AB and Case 10 L, Nucleus device). The difference between medial thickness of one point to another consecutive point was not significantly different in both specimens along 2 mm of the cochlear duct (Figure 6), (Wilcoxon signed- rank test, p>0. 05).
Figure 6.
Consecutive measurement of medial capsular thickness in case 5R, which was implanted with an Advanced Bionics device, and case 10L, which was implanted with a Nucleus device. Statistical analysis was made from one to another consecutive measurement point in each case (Wilcoxon signed-rank test).
3.3. Intracochlear damage
The incidence of fracture of the osseous spiral lamina and/or marked displacement of the basilar membrane, which was interpreted as causative of intracochlear damage, was significantly higher among the specimens implanted with AB devices (9 out of 14 specimens (64%) at both locations, LB and UB, (Figure 7A, 7B), than the specimens implanted with Nucleus devices (2 out of 10(20%), LB and UB), (Figure 7C, 7D), (Fisher exact test, p<0.05). However, as shown in Table 2, the incidence of transposition of the electrode into the scala vestibuli (as another indicator for potential intracochlear damage) was not significantly different between the AB and the Nucleus devices (Fisher exact-test, p>0.05).
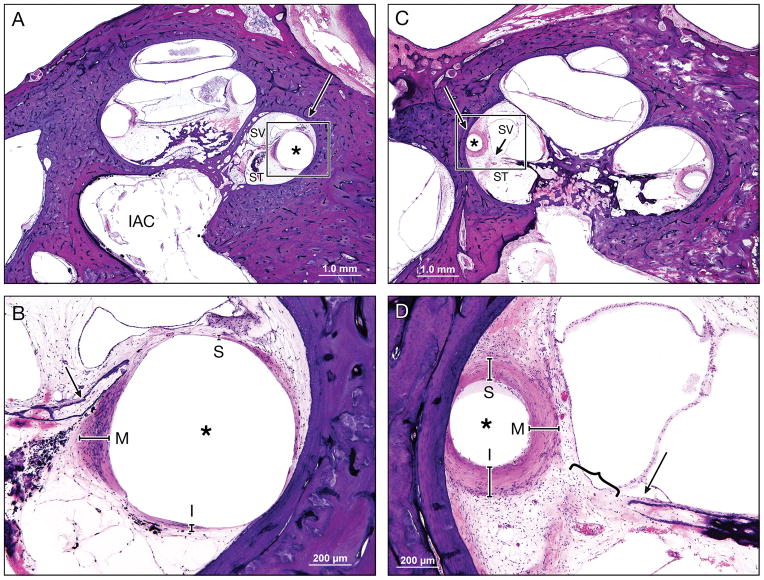
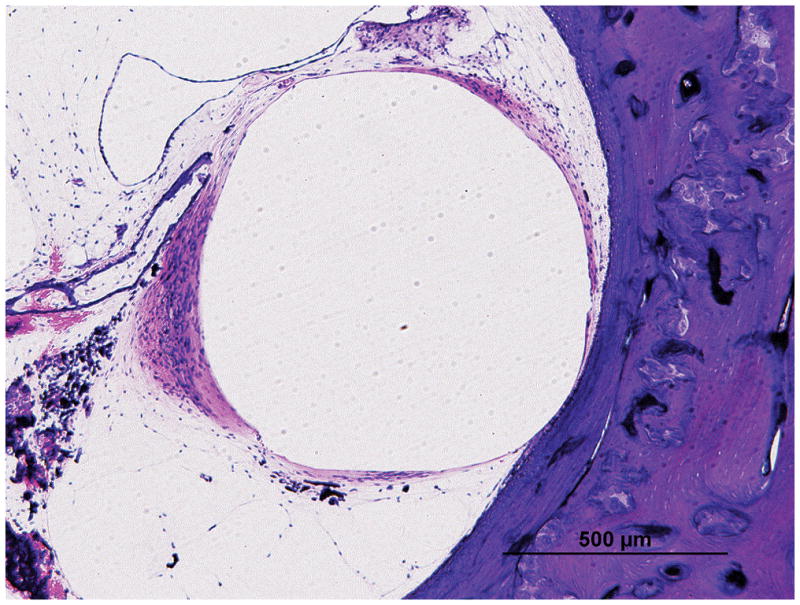
Figure 7. Intracochlear damage and fibrous capsule thickness.
An asymmetric fibrous tissue thickness in Case 2L (Low (A) and high (B) magnification view at lower basal turn of the cochlea), which was implanted with an AB perimodiolar electrode resulted in fracture of the osseous spiral lamina (arrow in Figure B) and marked displacement of the basilar membrane.
A symmetric fibrous tissue thickness in Case 10L (Low (C) and high (D) magnification view at lower basal turn of the cochlea), which was implanted with a Nucleus 22 straight electrode and resulted in marked displacement of the basilar membrane (}), but with an intact osseous spiral lamina (arrow).
Large arrow marks basal turn of cochlea; asterisk (*) indicates the electrode track; S, superior; M, medial; I, inferior: represent the thickness of fibrous tissue in each point; SV, scala vestibuli; ST, scala tympani; IAC, internal acoustic canal.
3.4. Electrode impedance level and fibrous sheath
Post-operative electrode impedance data was available in three patients:
Impedances for Case 4R (94-year-old woman implanted with AB device) were within normal ranges (0–30 kOhm) and were stable over time ranging from 3.5 kOhm to11.4 kOhm.
Electrode impedance data for Case 5R (89-year-old man implanted with AB device) was extracted from the programming software and plotted for each electrode as a function of the number of days following activation. Impedances were within normal levels ranging from 6.4 kOhm to 9 kOhm, but data was available only for 30 post-activation days.
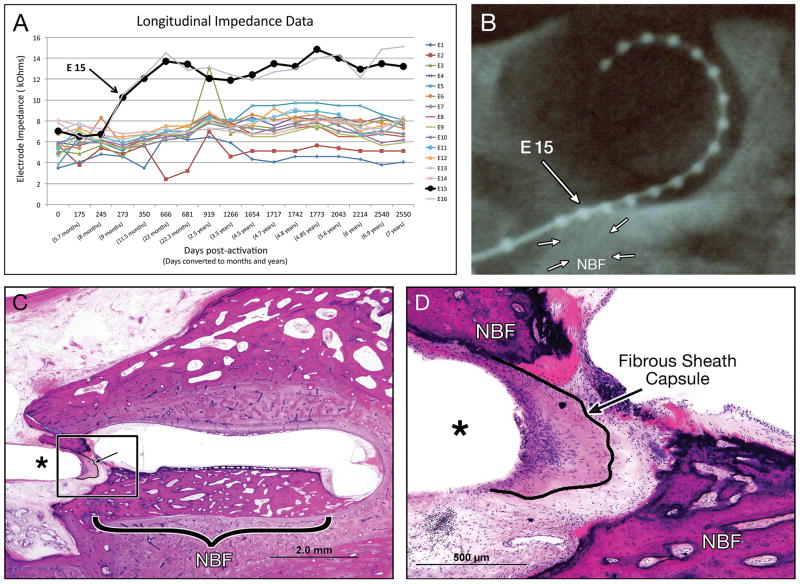
Electrode impedance data for Case 1L (82-year-old woman implanted with AB device) was plotted for each electrode for over 2500 days (approximately seven years) following activation. Impedance levels were within normal levels, but were relatively large for electrodes 15 and 16. As shown in Figure 8A, electrode 15 had a persistent fluctuant pattern, and its longitudinal impedance level was higher than the longitudinal impedance level of the other electrodes. Thus the impedance of electrode fifteen was higher compared to electrode seven (p<0.001, paired t-test). The post-mortem temporal X-ray (Figure 8B) helped us to identify this fifteenth electrode and assess its approximate track location in histological section (with H&E staining). There was pronounced fibrous tissue development and new bone formation (Figure 8C, D) surrounding its approximate track location, which was seen in the hook region of the cochlea.
Figure 8. Correlation between longitudinal impedance data and fibrous capsule in an 82-year-old woman (Case 1L) who underwent cochlear implantation with an AB perimodiolar HiFocus electrode.
(A) Longitudinal impedance of sixteen electrodes. The bold line represents the longitudinal impedance level of electrode fifteen (E15). (B) post-mortem x-ray showing AB perimodiolar HiFocus electrode.
The large arrow marks electrode fifteen. Adjacent to the electrode there is an area of relatively decreased opacity representing new bone formation (NBF, small arrows).
Low (C) and high (D) magnification view of histological H&E section corresponding the position of electrode 15 (*); arrow showing the outline of the fibrous sheath.
3.5. Correlation of clinical data and thickness of fibrous sheath
There was no significant correlation between the thickness of fibrous tissue and the number of years implanted or the word recognition score (Table 1) at both locations of the cochlea (Spearman’s rho, p=0.06, p=0.4 respectively).
4. Discussion
We hypothesized that electrical stimulation of the CI electrode array results in a thickened fibrous capsule, in addition to well-described causes of fibrosis, such as inflammation and trauma. This study was designed to evaluate whether patterns of fibrous capsule development correlated with the electrode array design, by comparing cochlear histopathology from implanted patients who received two fundamentally different types of electrodes (partially banded, perimodiolar arrays vs. full-banded lateral wall / straight arrays). From a clinical standpoint, we also wished to determine if the thickness of the fibrous sheath was related to the duration of implantation or to hearing performance. Our findings showed a thickened fibrous capsule at the medial aspect of the electrode array in individuals implanted with a partial banded perimodiolar AB device, which results in an asymmetric fibrous capsule. Individuals implanted with a Nucleus full-banded straight array device did not develop this asymmetric pattern. In contrast, there was a more symmetrical distribution of fibrous tissue surrounding the electrode track.
4.1. Electrode array design and development of the fibrous sheath
Nucleus and AB electrode arrays have different designs, which may in part explain differences in the thickness of the fibrous sheath.
First, the mode of the electric stimulation is different. The HiFocus electrode array of the AB device has 16 electrodes, which were designed to contact the medial side of the cochlea toward the modiolus and to stimulate the spiral ganglion cells (Skinner et al., 2007). This focused medial electric stimulation, may lead to a thicker fibrous sheath medially (Figure 4). On the other hand, the electrode array of the Cochlear TM Nucleus 22/24 device has 22 electrodes, which are banded in a circular fashion. As such, the electric current spreads centrifugally (Clark et al., 1983; Van Wermeskerken et al., 2006). Therefore, it is reasonable to believe that this “centrifugal” electric stimulation led to a relatively even distribution of fibrous tissue around the electrode array (Figure 5).
Second, the electric stimulation surface area the CI electrode design is different.
The large electrode contact surface area in the banded-electrode array of the Nucleus device results in a decreased charge density, which, has been shown to reduce the release of metal ions such as platinum from the electrode (Clark, 2003). In contrast, the smaller contact surface area in the HiFocus electrode array of the AB device results in an increased release of platinum resulting in an electro-chemical reaction with intracochlear toxic products (Brummer et al., 1977; Greatbatch et al., 1969; Agnew et al., 1977). These toxic products may lead to a chronic inflammatory reaction, including dense fibrous tissue around the electrode array (Clark et al., 2014, Nadol et. al., 2014). This may explain why specimens from individuals implanted with a banded electrode array (Nucleus device), which has a relatively large (from 0.4 to 0.6 mm2) electrode contact surface area, had significantly less fibrous tissue development around the electrode compared to smaller dimensions (with a width of the electrode is 0.4 mm) of surface contact facing only medially toward the modiolus of AB device electrodes (Hi Focus electrode), (Skinner et al., 2007).
4.2. Change in electrode impedance levels and growth of fibrous tissue
Electrode impedance reflects all the resistance in the pathway between the stimulating electrode in the scala tympani or scala vestibuli and the return electrode, which in monopolar stimulation is in the device case and in bipolar stimulation is in neighboring scala tympani/vestibuli electrodes. It would be expected that fibrous tissue or bone, which have more resistance than the pericochlear fluid (Peterson et al., 1978; Geddes et al., 1967; Grill et al., 1994), may contribute to an increased impedance level. Several studies have shown this correlation between high impedance electrode level and dense fibrous tissue around the electrode array (Clark et al., 1995; Ni et al., 1992; Tykocinsky et al., 2005; Shepherd et al., 1990; Shepherd et al., 1995). Persistent fluctuation of the electrode impedance level within the normal range may also be attributed to fibrous tissue (Wolfe at al., 2012), as demonstrated in the current study in Case 1L (Table 1). This woman was implanted with AB device and had longitudinal impedance data over 2500 post-activation days (approximately seven years). The data demonstrated normal impedance levels for all electrodes except electrode 15 and 16, which were significantly higher (upper range of normal limits) and fluctuated more than at the other electrodes (Figure 8B). As shown in the TB specimen (Figure 8C, D) there was a thick fibrous sheath and new bone adjacent to the location of electrode 15. A large impedance might also occur if an electrode is not functioning (e.g. open circuit) due to an electrical fault within the implant itself. In this case, the data suggests that electrode 15 and electrode 16 were functioning at least for the first days (245 post-activation days). Given the minimal amount of evidence with longitudinal impedance data, it is difficult to determine the true association between the impedance level (over time) and the development of fibrous tissue. In addition, medial thickness of fibrous sheath in both types of electrode (Case 5R, HiFocus Helix perimodiolar electrode of AB and Case 10L, banded-straight-electrode of Nucleus 22 device) along 2 mm length of cochlear duct (Figure 6) did not significantly differ. This may imply that there is significant - electric current spread between electrodes or that the biologic response that leads to fibrous capsule deposition may extend beyond just the immediately stimulated area. Electrode longitudinal impedance level data (which is missing in these cases) in these consecutive measurements may provide a more complete picture of the relationship between the stimulation spread and the thickness of fibrous tissue.
4.3. Association between intracochlear insertion and chronic inflammatory reaction
A chronic inflammatory reaction manifested by fibrous tissue growth may also be attributed to cochlear electrode insertion. Although the CI is generally considered clinically well tolerated and biocompatible (Issa et al., 1983; Webb et al., 1988; Ray et al., 2004; Venail et al., 2008; Ding et al., 2009), rare cases (0.55% in a Meta-analysis series of 7,132 cochlear implant recipients) showed severe local inflammation, which resulted in device failure and in some cases explantation, as reported by Bennati et al. (2013). It is hypothesized that this local inflammation has healed with fibrous tissue formation (Anderson et al., 2008; Kumar et al., 2010; Rubin et al., 2011; Franz et al., 2011).
Recent temporal bone studies (Nadol and Eddington, 2004; Seyyedi et al., 2014; Kamakura et al., 2016) have found that evidence of chronic inflammatory reaction including fibrous tissue formation was much more common than just that seen in the failed clinical cases (Bennati et al., 2013). Kamakura and Nadol (2016) demonstrated fibrous tissue formation within the cochlea of all seventeen specimens that received CI. It has been postulated that both insertion trauma and host tissue reaction to the electrode are responsible for this chronic inflammatory response (Li et al., 2007; Somdas et al., 2007; Fayad et al., 2009; Seyyedi and Nadol, 2014). DeSautel et al. (1999) demonstrated in the Mongolian gerbil formation of intracochlear fibrosis, neo-osteogenesis, and evidence of inflammatory cytokines following intrathecal injection of Streptococcus pneumonia. This animal study indicated that there can be delayed migratory chronic inflammatory reaction without electrode insertion. Li at al. (2007) reported significant fibrous tissue and new bone formation at the lateral cochlear wall, and Seyyedi and Nadol (2014) found a pronounced inflammatory response near the cochleostomy.
The current study confirms the above findings, and demonstrated fibrous tissue development, particularly surrounding the electrode, is quite common in specimens that received either type of electrode.
The common finding of intracochlear fibrous tissue around either electrode with and without mechanic trauma effect indicate that both inflammation and electrical stimulation may also contribute to the thickness of the fibrous tissue.
4.4. Correlation between intracochlear trauma and fibrous tissue growth
The AB electrode array was associated with severe intracochlear damage including trauma to the basilar membrane and the osseous spiral lamina (Richter et al., 2002; Clark et al., 2003, Aschendorff et al., 2003). A relatively large diameter of electrode array of the AB device (range: 0.6 to 1.1 mm in the HiFocus helix electrode and 0.6 to 0.8 mm in HiFocus 1J electrode), (Wackym et al., 2009), as compared to the diameter of the electrode array of the Nucleus device (range: 0.4 to 0.6 mm in the banded electrode), (Cochlear TM Nucleus, 2012), may explain the higher degree of intracochlear damage in the current study. The AB device has a perimodiolar electrode (pre-curve shape), which is designed to decrease the distance between electrode and spiral ganglion cells (Merzenich et al., 1974). The electrode array consists of sixteen rectangular electrodes, which may cause damage to the osseous spiral lamina and the basal membrane. This damage may result in a thickened fibrous tissue. The Nucleus 22/24 device has, on the other hand, a straight electrode array, which is placed away from the modiolus, and consists of twenty-two smooth and circular thin electrodes. Given the likelihood of osseous spiral lamina fracture and/or marked displacement of the basilar membrane in specimens that received AB electrode devices it is reasonable to presume that this significant trauma may be another important contributing factor to the development of thickened fibrous tissue surrounding the electrode, especially medially. However, specimens implanted with AB devices, in which there was no intracochlear damage (for e.g. case 5R, Figure 4) also demonstrate an asymmetric fibrous sheath, and interestingly, the specimens implanted with Nucleus devices with intracochlear trauma (for e.g. case 10L, Figure 7) demonstrate symmetric and not asymmetric growth of fibrous tissue. Furthermore, translocation of the electrode into the scala vestibuli was not more common in AB cases and was not correlated with the thickness of the fibrous sheath (Fisher exact test, p>0.05, Simple linear regression test, p=0.3). This may imply another contributing factor, such as the spread of electric current, as causative of the asymmetry.
Kamakura and Nadol (2016) assessed the cochlear damage based on fibrous tissue growth and new bone formation including at the lateral cochlear wall, osseous spiral lamina fracture, and basilar membrane injury. They did not find a positive correlation between fibrous tissue growth and intracochlear damage. The current study assessed the fibrous tissue development around the electrode, especially medial (perimodiolar), where the electric stimulation does occur and not at lateral cochlear wall, where the fibrous tissue is inconsistently developed perhaps because of abutment to the lateral cochlear wall. We found, however, a positive association between cochlear damage (manifested by osseous spiral lamina fracture and/or marked displacement of the basilar membrane) and fibrous tissue growth specifically around the electrode array. The results of the current study which failed to show significant correlation between the fibrous capsule and duration of implantation (Spearman rho, p=0.06) provides additional data supporting previous reports (Li et al., 2007; Fayad et al., 2009; Kamakura et al., 2016). We did not evaluate the new bone formation given its patchy development and the relative inaccuracy as assessed by 2-D cochlear reconstruction as compared to 3-D reconstruction (Kamakura and Nadol. 2016; Seyyedi and Nadol, 2014).
4.5. Correlation between CI performance assessed by word recognition and thickness of fibrous tissue
Word recognition score (either CNC or NU6) was not correlated with thickness of the fibrous tissue capsule, either in the AB device or in the Nucleus device at both locations of the cochlea (Spearman rho, p=0.4). These results support the findings of Li et al. (2007), and Kamakura and Nadol (2016). Kamakura and Nadol (2016), however, did report a positive correlation between new bone formation and word recognition score. Lack of significant correlation between fibrous tissue thickness and word recognition scores may be due to either that fibrous encapsulation of the electrode is not an important determinant factor, or that given the small sample size, there is insufficient data to demonstrate a significant effect. Although fibrous tissue thickness does not correlate with CI performance, fibrosis within the cochlea may play a role in loss of residual acoustic hearing with cochlear implantation (Quesnel et al., 2016, Bas et al., 2016). Therefore, understanding the etiology of fibrous capsule development may influence electrode array design as the indications for cochlear implantation expand to include those with more residual hearing.
5. Conclusion
A thicker fibrous sheath was found on the medial side of implanted AB electrode array. This finding cannot be fully explained by a single cause. Possible causes include a higher incidence of insertion trauma and/or asymmetric current flow. Fibrous tissue formation was not significantly correlated either with word recognition scores or with duration of implantation.
Acknowledgments
We thank Diane Jones, Barbara Burgess, Jennifer O’Malley and Meng Yu Zhu for their expertise in preparation of the temporal bone specimens, and Garyfallia Pagonis for assistance in preparing the figures. We also thank Charles C. Finley, PhD and Donald K. Eddington, PhD for their expertise in relevant cochlear implant bioengineering.
Footnotes
The authors have no conflicts of interest to disclose
Conflicts of interest
The authors disclose no conflicts of interest.
References
- Agnew WF, Yuen TG, Pudenz RH, Bullara LA. Neuropathological effects of intracerebral platinum salt injections. J Neuropathol Exp Neurol. 1977;36(3):533–46. doi: 10.1097/00005072-197705000-00010. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bas E, Bohorquez J, Goncalves S, Perez E, Dinh CT, Garnham C, Hessler R, Eshraghi AA, Van De Water TR. Electrode array-eluted dexamethasone protects against electrode insertion trauma induced hearing and hair cell losses, damage to neural elements, increases in impedance and fibrosis: A dose response study. Hear Res. 2016;337:12–24. doi: 10.1016/j.heares.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Bennatti A, Castiglione A, Trevisi P, et al. Endocochlear inflammation in cochlear implant users: case report and literature review. Int J Pediatr Otorhinolaryngol. 2013;77:885–93. doi: 10.1016/j.ijporl.2013.03.016. [DOI] [PubMed] [Google Scholar]
- Bertuleit H, Groden C, Schafer HJ, et al. Removal of a cochlear implant with chronic granulation labyrinthitis and foreign body reaction. Laryngo- Rhino-Otologie. 1999;78:304–6. doi: 10.1055/s-2007-996876. [DOI] [PubMed] [Google Scholar]
- Brummer SB, Turner MJ. Electrochemical considerations for safe electrical stimulation of the nervous system with platinum electrodes. IEEE Trans Biomed Eng. 1977;24(1):59–63. doi: 10.1109/TBME.1977.326218. [DOI] [PubMed] [Google Scholar]
- Byron JGM. Cochlear Implants. Fundamentals & Applications. 2003:105–6. [Google Scholar]
- Clark GM, Shepherd RK, Patrick JF, Black RC, Tong YC. Design and fabrication of the banded electrode array. Ann N Y Acad Sci. 1983;405:191–201. doi: 10.1111/j.1749-6632.1983.tb31632.x. [DOI] [PubMed] [Google Scholar]
- Clark GM, Tong YC, Black R, Forster IC, Patrick JF, Dewhurst DJ. A multiple electrode cochlear implant. J Laryngol Otol. 1977;91(11):935–45. doi: 10.1017/s0022215100084607. [DOI] [PubMed] [Google Scholar]
- Clark GM1, Shute SA, Shepherd RK, Carter TD. Cochlear implantation: osteoneogenesis, electrode-tissue impedance, and residual hearing. Ann Otol Rhinol Laryngol Suppl. 1995;166:40–2. [PubMed] [Google Scholar]
- Clark GM, Shepherd RK, Franz BK, Dowell RC, Tong YC, Blamey PJ, Webb RL, Pyman BC, McNaughtan J, Bloom DM, et al. The histopathology of the human temporal bone and auditory central nervous system following cochlear implantation in a patient. Correlation with psychophysics and speech perception results. Acta Otolaryngol Suppl. 1988;448:1–65. doi: 10.3109/00016488809098972. [DOI] [PubMed] [Google Scholar]
- Clark GM. Cochlear Implants. Fundamentals & Applications. 2003;Ch. 8:520. [Google Scholar]
- Clark GM. Cochlear Implants. Fundamentals & Applications. 2003:105–6. [Google Scholar]
- Cochlear™ Implant Electrode Comparison. 2012 Jul; Retrieved from http://www.cochlear.com/wps/wcm/connect/b29815ab-da8c-453c-a8f4-2041e6088459/FUN1142_ISS4_JUL12_Electrode_Comparison4.pdf?MOD=AJPERES&CACHEID=b29815ab-da8c-453c-a8f4-2041e6088459.
- Coleman DL, King RN, Andrade JD. The foreign body reaction: a chronic inflammatory response. J Biomed Mater Res. 1974;8(5):199–211. doi: 10.1002/jbm.820080503. [DOI] [PubMed] [Google Scholar]
- DeSautel MG1, Brodie HA. Effects of depletion of complement in the development of labyrinthitis ossificans. Laryngoscope. 1999;109(10):1674–8. doi: 10.1097/00005537-199910000-00023. [DOI] [PubMed] [Google Scholar]
- Ding X, Tian H, Wang W, et al. Cochlear implantation in China: review of 1237 cases with an emphasis on complications. ORL J Otorhinolaryngol Relat Spec. 2009;71:192–5. doi: 10.1159/000229297. [DOI] [PubMed] [Google Scholar]
- Fayad JN, Makarem AO, Linthicum FH., Jr Histopathologic assessment of fibrous and new bone formation in implanted human temporal bones using 3D reconstruction. Otolaryngol Head Neck Surg. 2009;141:247–52. doi: 10.1016/j.otohns.2009.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz S, Rammelt S, Scharnweber D, et al. Immune responses to to implants- a review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 2011;32:6692–709. doi: 10.1016/j.biomaterials.2011.05.078. [DOI] [PubMed] [Google Scholar]
- Geddes LA, Baker LE. The specific resistance of biological material--a compendium of data for the biomedical engineer and physiologist. Med Biol Eng. 1967;5(3):271–93. doi: 10.1007/BF02474537. [DOI] [PubMed] [Google Scholar]
- Grill WM1, Mortimer JT. Electrical properties of implant encapsulation tissue. Ann Biomed Eng. 1994;22(1):23–33. doi: 10.1007/BF02368219. [DOI] [PubMed] [Google Scholar]
- Ho EC, Dunn C, Proops D, et al. Case report: explantation of a cochlear implant secondary to chronic granulating labyrinthitis. Cochlear Implants Int. 2003;4:191–5. doi: 10.1179/cim.2003.4.4.191. [DOI] [PubMed] [Google Scholar]
- Issa TK, Bahgat MA, Linthicum FH., Jr Tissue reaction to prosthetic material in human temporal bones. Am J Otol. 1983;5:40–3. [PubMed] [Google Scholar]
- Kamakura T, Nadol JB., Jr Correlation between word recognition score and intracochlear new bone and fibrous tissue after cochlear implantation in the human. Ear Res. 2016;339(29):132–141. doi: 10.1016/j.heares.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano A, Seldon HL, Clark GM. Computer-aided three-dimensional reconstruction in human cochlear maps: measurement of the lengths of organ of Corti, outer wall, inner wall, and Rosenthal’s canal. Ann Otol Rhinol Laryngol. 1996;105(9):701–9. doi: 10.1177/000348949610500906. [DOI] [PubMed] [Google Scholar]
- Kawano A, Seldon HL, Clark GM, Ramsden RT, Raine CH. Intracochlear factors contributing to psychophysical percepts following cochlear implantation. Acta Otolaryngol. 1998;118(3):313–26. doi: 10.1080/00016489850183386. [DOI] [PubMed] [Google Scholar]
- Kawano A, Seldon HL, Pyman B, Clark GM. Intracochlear factors contributing to psychophysical percepts following cochlear implantation: a case study. Ann Otol Rhinol Laryngol Suppl. 1995;166:54–7. [PubMed] [Google Scholar]
- Kumar V, Abbas AK, Fausto N, et al. Pathologic Basis of Disease. Philadelphia, PA: Saunders/Elsevier; 2010. Robbins and Cotran; pp. 70–4. [Google Scholar]
- Kunda LD, Stidham KR, Inserra MM, et al. Silicone allergy: a new cause for cochlear implant extrusion and its management. Otol Neurotol. 2006;27:1078–82. doi: 10.1097/01.mao.0000235378.64654.4d. [DOI] [PubMed] [Google Scholar]
- Leake-Jones PA, Rebscher SJ. Cochlear pathology with chronically implanted scala tympani electrodes. Ann N Y Acad Sci. 1983;405:203–23. doi: 10.1111/j.1749-6632.1983.tb31634.x. [DOI] [PubMed] [Google Scholar]
- Li PM, Somdas MA, Eddington DK, Nadol JB., Jr Analysis of intracochlear new bone and fibrous tissue formation in human subjects with cochlear implants. Ann Otol Rhinol Laryngol. 2007 Oct;116(10):731–8. doi: 10.1177/000348940711601004. [DOI] [PubMed] [Google Scholar]
- Lim HJ, Lee ES, Park HY, et al. Foreign body reaction after cochlear implantation. Int J Pediatr Otorhinolaryngol. 2011;75:1455–8. doi: 10.1016/j.ijporl.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Linthicum FH, Jr, Fayad J, Otto S, et al. Inner ear morphologic changes resulting from cochlear implantation. Am J Otol. 1991;12:8–10. discussions 8–21. [PubMed] [Google Scholar]
- Mens LH1, Brokx JP, van den Broek P. Averaged electrode voltages: management of electrode failures in children, fluctuating threshold and comfort levels, and otosclerosis. Ann Otol Rhinol Laryngol Suppl. 1995 Sep;166:169–72. [PubMed] [Google Scholar]
- Merzenich MM, Reid MD. Representation of the cochlea within the inferior colliculus of the cat. Brain Res. 1974;77(3):397–415. doi: 10.1016/0006-8993(74)90630-1. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr, Eddington DK, Burgess BJ. Foreign body or hypersensitivity granuloma of the inner ear after cochlear implantation: one possible cause of a soft failure? Otol Neurotol. 2008;29:1076–84. doi: 10.1097/MAO.0b013e31818c33cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadol JB, Jr, Eddington DK. Histologic evaluation of the tissue seal and biologic response around cochlear implant electrodes in the human. Otol Neurotol. 2004;25:257–62. doi: 10.1097/00129492-200405000-00010. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr, O’Malley JT, Burgess BJ2, Galler D. Cellular immunologic responses to cochlear implantation in the human. Hear Res. 2014;318:11–7. doi: 10.1016/j.heares.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilan RE, Pawlowski K, Isaacson, et al. Cochlear implant device failure secondary to cholesterol granuloma -mediated cochlear erosion. Otol Neurotol. 2012;33:733–5. doi: 10.1097/MAO.0b013e3182544fff. [DOI] [PubMed] [Google Scholar]
- Ni D1, Shepherd RK, Seldon HL, Xu SA, Clark GM, Millard RE. Cochlear pathology following chronic electrical stimulation of the auditory nerve. I: Normal hearing kittens. Hear Res. 1992;62(1):63–81. doi: 10.1016/0378-5955(92)90203-y. [DOI] [PubMed] [Google Scholar]
- Otter J, Schuknecht HF, Kerr AG. Ganglion cell population in normal and pathological human cochleae. Implication for cochlear implantation. Laryngoscope. 1978;88:1231–46. doi: 10.1288/00005537-197808000-00004. [DOI] [PubMed] [Google Scholar]
- Peterson SK, Frishkopff LS, Lechène C, Oman CM, Weiss TF. Element composition of inner ear lymphs in cats, lizards, and skates determined by electron probe microanalysis of liquid samples. Journal of comparative physiology. 1978;126(1):1–14. [Google Scholar]
- Puri S, Dornhoffer JL, North PE. Contact dermatitis to silicone after cochlear implantation. Laryngoscope. 2005;115:1760–2. doi: 10.1097/01.mlg.0000172202.58968.41. [DOI] [PubMed] [Google Scholar]
- Quesnel AM, Nakajima HH, Rosowski JJ, Hansen MR, Gantz BJ, Nadol JB., Jr Delayed loss of hearing after hearing preservation cochlear implantation: Human temporal bone pathology and implications for etiology. Hear Res. 2016;333:225–34. doi: 10.1016/j.heares.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J, Gibson W, Sanli H. Surgical complications of 844 consecutive cochlear implantations and observations on large small incisions. Cochlear Implants Int. 2004;5:87–95. doi: 10.1179/cim.2004.5.3.87. [DOI] [PubMed] [Google Scholar]
- Richter B, Aschendorff A, Lohnstein P, Husstedt H, Nagursky H, Laszig R. J Laryngol Otol. 2002;116(7):507–13. doi: 10.1258/002221502760132584. [DOI] [PubMed] [Google Scholar]
- Rubin R, Strayer DS, Rubin E. Rubin’s Pathology : Clinicopathologic Foundation of Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2011. p. 1464. [Google Scholar]
- Schuknecht H. Temporal bone removal at autopsy. Preparation and uses. Arch Otolaryngol. 1968;87:129–37. doi: 10.1001/archotol.1968.00760060131007. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF. Techniques for study of cochlear function and pathology in experimental animals; development of the anatomical frequency scale for the cat. ANA Arch Otolaryngol. 1953;58:377–97. doi: 10.1001/archotol.1953.00710040399001. [DOI] [PubMed] [Google Scholar]
- Seyyedi M, Nadol JB., Jr Intracochlear inflammatory response to cochlear implant electrodes in humans. Otol Neurotol. 2014;35:1545–1551. doi: 10.1097/MAO.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Matsushima J, Martin RL, et al. Cochlear pathology following chronic electrical stimulation of the auditory nerve: II. Deafened kittens. Hear Res. 1994;81:150–66. doi: 10.1016/0378-5955(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Somdas MA, Li PM, Whiten DM, Eddington DK, Nadol JB., Jr Quantitative evaluation of new bone and fibrous tissue in cochlea following cochlear implantation in human. Audio Neurotol. 2007;12(5):277–84. doi: 10.1159/000103208. [DOI] [PubMed] [Google Scholar]
- Sutton D, Miller J. Intra cochlear implants: potential histopathology. Otolarhingol Clin North Am. 1983;16:227–32. [PubMed] [Google Scholar]
- Venail F, Sicard, Piron JP, et al. Reliability and complications of 500 consecutive cochlear implantations. Arch Otolaryngol Head Neck Surg. 2008;138:1276–81. doi: 10.1001/archoto.2008.504. [DOI] [PubMed] [Google Scholar]
- Wackym PA, Runge-Samuelson CL. Cochlear and auditory brainstem implantation. In: Snow JB Jr, Wackym PA, editors. Otolaryngology Head and Neck Surgery. 17. People Medical Publishing House; Shelton: 2009. p. 373. [Google Scholar]
- Wardrop P1, Whinney D, Rebscher SJ, Roland JT, Jr, Luxford W, Leake PA. A temporal bone study of insertion trauma and intracochlear position of cochlear implant electrodes. I: Comparison of Nucleus banded and Nucleus Contour electrodes. Hear Res. 2005;203(1–2):54–6. doi: 10.1016/j.heares.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Webb RL, Clark GM, Shepherd RK, et al. The biologic safety of the Cochlear Corporation multiple-electrode intracochlear implant. Am J Otol. 1988;9:8–13. [PubMed] [Google Scholar]
- Wolfe J, Schafer E. Programming Cohlear Implants. (2) 2014;Ch. 2:81–82. [Google Scholar]
- Wolfe J, Schafer E. Programming Cohlear Implants. (2) 2014;Ch. 2:42–43. [Google Scholar]