Abstract
Background:
Population control of domestic, wild, invasive, and captive animal species is a global issue of importance to public health, animal welfare and the economy. There is pressing need for effective, safe, and inexpensive contraceptive technologies to ad-dress this problem. Contraceptive vaccines, designed to stimulate the immune system in order to block critical reproductive events and suppress fertility, may provide a solution. Fil-amentous bacteriophages can be used as platforms for development of such vaccines.
Objective:
In this review authors highlight structural and immunogenic properties of fila-mentous phages, and discuss applications of phage-peptide vaccines for advancement of immunocontraception technology in animals.
Results:
Phages can be engineered to display fusion (non-phage) peptides as coat proteins. Such modifications can be accomplished via genetic manipulation of phage DNA, or by chemical conjugation of synthetic peptides to phage surface proteins. Phage fusions with antigenic determinants induce humoral as well as cell-mediated immune responses in ani-mals, making them attractive as vaccines. Additional advantages of the phage platform include environmental stability, low cost, and safety for immunized animals and those ad-ministering the vaccines.
Conclusion:
Filamentous phages are viable platforms for vaccine development that can be engineered with molecular and organismal specificity. Phage-based vaccines can be pro-duced in abundance at low cost, are environmentally stable, and are immunogenic when administered via multiple routes. These features are essential for a contraceptive vaccine to be operationally practical in animal applications. Adaptability of the phage platform also makes it attractive for design of human immunocontraceptive agents.
Keywords: Animal population control, bacteriophage, contraception, phage-peptide fusions, phage immuno-genicity, vaccine
1. INTRODUCTION
Overpopulation of particular groups and species of animals is an expanding, global, economic and public health problem. Effective, safe population control of animals including stray dogs, feral cats and wild or invasive animal species (deer, horses, pigs, reptiles) is needed. Overabundance of wildlife species may also occur in zoos and other captive environments. Many mammalian species (elephants, lions, wolves, primates, etc.) breed well in captivity, often resulting in un-wanted animals that are euthanized. Controlling animal fertility with contraceptive vaccines is a humane option for managing their population. Such vaccines should stimulate adaptive immune responses (humoral and/or cell-mediated) against one or more reproductive targets that regulate gamete production and/or function. Administration of a contraceptive vaccine with the goal of suppressing or blocking reproduction via induction of an immune response is defined as immunocontraception.
Contraceptive vaccines can be effective tools for animal population control [1-4] if advanced to fulfill specific requirements for individual species and their settings. Ideally, contraceptives for wild and feral animals must cause permanent infertility after a single administration since, short of baiting, the probability of repeated delivery of contraceptives to animals in these groups is extremely low. Differently, contraceptives for animals in captivity are required to have a reversible effect on their fertility. Because the scale of the problem is vast (hundreds of millions of feral cats, dozens of millions of stray dogs, millions of wild pigs and horses, hundreds of thousands of zoo and captive wildlife species worldwide), contraceptive agents must be of low cost. Additionally, such contraceptives should be stable under varying and dynamic environmental conditions, defined on a global scale. They must also be safe for people who produce and deliver them, for treated animals, for non-target species and for the environment. Given these demanding criteria, filamentous bacteriophages (phages) represent an attractive platform for the development of contraceptive vaccines for use in wild, feral and zoo animals.
Bacteriophages are viruses that infect and replicate in bacteria and, as such, are not pathogenic for animals, including humans. Filamentous phages comprise a group of thread-like bacterial viruses that belong to the genus Inovirus of the Inoviridae family [5]. They are broadly utilized as vectors for display of various antigenic determinants for vaccine development. The most investigated in this group are phages of the Ff class (M13, fd, and f1). These phages are closely related structurally and are composed of a single stranded DNA enclosed in a protein coat. To act as vaccines, phage particles can be re-engineered genetically or modified chemically to carry desirable antigenic domains. Due to their natural immunogenicity and fewer endogenous B cell epitopes that can redirect the antibody response from its anticipated target, phages embody alternative carrier systems to traditional proteins [6]. Important for animal contraception, immune responses against filamentous phages can persist in immunized animals for months without re-administration.
Cloning and purification protocols required for the construction of recombinant phages are straightforward. Phages can be easily obtained in large quantities from bacterial cultures and their production does not require uniquely specialized equipment or facilities. In a laboratory setting, phage yields of ~2 × 1014 virions/L can be achieved, providing many vaccine doses. The phage production protocol can be scaled up easily using a fermenter that allows for programmed control of oxygen consumption, temperature, rotation speed, and pH [7]. This makes the cost of phage-based preparations low. In addition, phage preparations are very thermostable [8] and, by consequence, well suited for shipping, storage, delivery, and use under variable conditions. Filamentous phages can also withstand a wide range of pH (3-11), which is an essential property for vaccines administered orally, providing the preferable vaccine delivery route for wild and feral animals (Box 1).
The list of applications for phage-based vaccines is impressive and continues to grow. Phage-based vaccines were developed for the treatment of cancers [9, 10], HIV [11, 12], Alzheimer’s disease [13], candidiasis [14], rabies [15], and influenza [16]. The goal of this review is to highlight structural and immunogenic properties of filamentous phages as a platform for vaccine development and discuss applications of phage-peptide vaccines for advancement of contraception in animals.
2. Phage structure and phage vectors
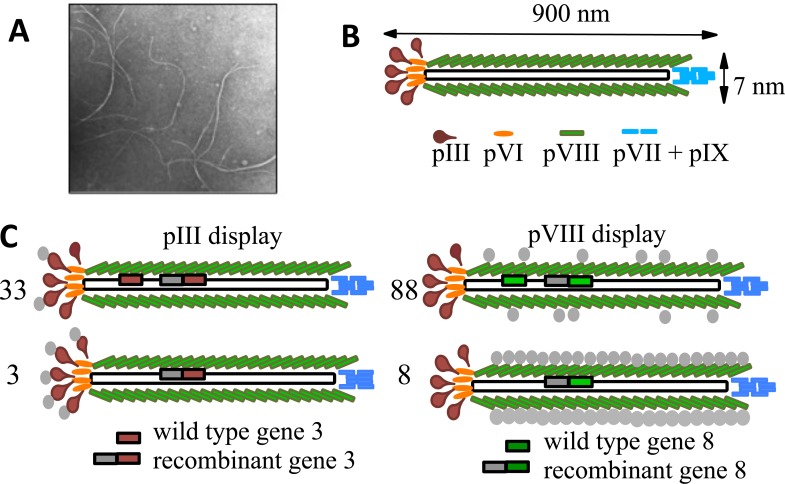
Filamentous phages that belong to the Ff class are long (~1µm), thin (~ 7nm), rod-shaped particles (Fig. F1A). They consist of a tubular protein coat surrounding a single stranded circular DNA (Fig. 1). The genome sizes of Ff phages differ slightly and are close to 6400 nucleotides. Ff phages contain eleven genes, five of which (genes 3, 6, 7, 8, and 9) encode phage coat proteins (pIII, pVI, pVII, pVIII, and pIX). Proteins III (406 aa), VI (112 aa), VII (33 aa), and IX (32 aa), present in low copy numbers (five copies each) constitute the minor coat proteins. Proteins III and VI form a cap on one end of the phage virion, whereas proteins pVII and pIX cap the other end of the particle. Protein III is composed of three domains (N1, N2, and CT). The N-terminal (N1 and N2) domains mediate infection of bacteria. The C-terminal (CT) domain is necessary for the formation of a stable phage particle. Other minor coat proteins are required for assembly and structural stability of the virion. Gene 8 encodes the major phage coat protein pVIII. This protein, present in high copy numbers (2700 in wild type phage), surrounds the phage DNA and forms the tubular body of the phage particle. The number of pVIII copies per virion varies depending on the size of the DNA and can reach 4000 copies in some recombinant phages [17]. Protein pVIII consists of 50 amino acids, approximately one third of which are exposed on the surface of the phage. Additional information about the structure of filamentous phages and phage biology is presented elsewhere [18].
Fig. (1).
Structure of filamentous phage. (A) Microphotograph of filamentous phage particles. (B) Schematic structure of Ff wild type phage particle. Ff phages consist of a tubular protein coat surrounding a single stranded circular DNA. Phage coat is composed of five proteins (pIII, pVI, pVII, pVIII, and pIX). Proteins III, VI, VII, and IX, present in low copy numbers, constitute the minor coat proteins. Proteins III and VI form a cap on one end of the phage virion, whereas proteins pVII and pIX cap the other end of the particle. Protein VIII is the major phage coat protein. It is present in high copy numbers (2700 in wild type phage). Protein VIII surrounds the phage DNA and forms the tubular body of the particle. (C) Major types of Ff filamentous phage vectors used for peptide display. Phage vectors 33 and 88 contain two respective genes, one encoding a recombinant protein and one encoding wild type protein. Therefore, the resulting phage particles display fusion proteins interspersed with wild type proteins. Phage vectors 3 and 8 contain gene encoding a fusion protein, but no gene for the wild type protein. As a result, all copies of their corresponding proteins are modified with fusion peptides.
While any of the genes that encode phage coat proteins can be engineered for the display of fusion peptides, genes 3 and 8 are the most popular due to specific exposure of pIII and pVIII on the phage surface. Inserts in gene 3 most often produce peptides fused to the N-terminus of pIII. Inserts in gene 8 produce fusions at the N-terminus of pVIII. The number of copies of fusion proteins displayed on individual phage particles depends on phage vector design (Fig. 1C). Phage vectors of the 33 or 88 type contain two genes. One of these genes can encode a recombinant protein, while the other encodes the wild-type protein. As a result, phage constructs based on 33 or 88 vectors display fusion proteins interspersed with wild type proteins. Consequently, these vectors provide reasonable flexibility with respect to the size and sequence composition of the insert. Type 33 phage particles can carry up to five copies of fusion proteins. The number of fusion proteins per virion that can be incorporated in type 88 phages varies depending on the fusion protein size and, to a large extent, protein composition [19]. Typically, type 88 phages display between 150 and 300 fusion proteins per virion, although displays of as many as 810 copies per virion can be achieved [20]. Theoretically, proteins composed of hundreds of amino acids can be displayed on pVIII using 88 type vectors [21]. In fact, fusion peptides of up to 15 amino acids and small proteins (ex. zinc fingers) have been displayed in phage vectors of this type [22-24].
Unlike phage vectors 33 and 88, phage vectors 3 and 8 contain a recombinant gene only (no wild type gene) (Fig. 1C). Therefore, all copies of their corresponding proteins are modified with fusion peptides. There are no particular constraints for the generation of phage-peptide fusions in vector 3, since pIII is a relatively large protein with a low number of copies located at the tip of a phage particle that do not interact with each other. In contrast, pVIII copies are located very close to each other and interact to form the phage protein capsid. This creates significant constraints for the use of vector 8. Phages of this type can house inserts of up to six amino acids [25]. Peptides that are longer, or those of certain amino acid composition, can interfere with phage assembly and physical stability. Even if such recombinant phage particles are able to get assembled they can be genetically unstable, mutating after amplification, or have low propagation rates. However, this type of phage virion has advantages for immunological applications. Each phage particle created using the type 8 vector displays 4000 copies of the fusion peptide [17]. Such densely displayed epitopes are presented in a highly organized manner and are properly spaced for binding to B cell receptors. Repetitive, highly organized epitope patterns usually permit cross-linking activation of B-cell receptors, which provides robust, long-lasting immune responses [26]. Characteristics of the major Ff phage display vectors are summarized in Table T1. Detailed information on the design of these and additional phage vectors and their applications can be found elsewhere [27].
Table 1.
Ff phage vectors and their characteristics.
| Vector Type | Number of Genes | Coat Protein of Display | Size of Fusion Peptide | Peptide Copies Per Virion |
|---|---|---|---|---|
| 33 | One recombinant & one wild type gene | pIII | From short peptides to large proteins | Less than 5 |
| 88 | One recombinant & one wild type gene | pVIII | From short peptides to large proteins | Usually 150-300 |
| 3 | One recombinant gene | pIII | From short peptides to large proteins | 5 |
| 8 | One recombinant gene | pVIII | Up to 6 amino acids | 4000 |
3. Approaches to engineering phage-based vaccines
Phage-based vaccines can be generated via cloning of an oligonucleotide encoding a desired antigenic peptide in a phage vector. Alternatively, such phages can be selected from a library of pre-engineered phages displaying random peptides. Both approaches have their advantages and limitations (Table T2). Obviously, phage modification via cloning can be done only when the fusion peptide sequence is known. This is not a requirement for identification of target-specific phages through affinity selection from a phage library. Conversely, cloning does not depend on availability and quality of the selection target. Such targets (ex. antigen-specific antibodies) must be on hand and well characterized when phage isolation is performed using affinity selection from a phage display library. In addition, an appropriate phage display library must be available, whether acquired from a commercial source (ex. New England BioLabs, Inc.) or constructed in-house.
Table 2.
Methods for generation of phage-peptide fusions.
| Method | Advantages | Disadvantages |
|---|---|---|
| Cloning in phage vector | Uses standard cloning techniques Does not require a selection target or a phage display library Phage clones are well defined molecularly Phage clones remain viable for extended periods when properly stored |
Nucleotide sequence encoding fusion peptide must be known Size and composition of fusion peptide depend on phage vector Phage clones can be genetically unstable and/or have low propagation rate, depending on phage vector, size and composition of fusion peptide |
| Selection from phage display library | Selection procedure is relatively simple Phage clones are genetically stable and propagate well Phage clones are well defined molecularly Phage clones remain viable for extended periods when properly stored |
Selection target has to be available and well characterized Appropriate phage display library has to be available |
| Chemical conjugation to phage coat proteins | Uses standard conjugation chemistries Flexible as to size and composition of fusion peptides Relatively chemically stable if stored properly |
Number of fusion peptide copies per phage particle varies between batches Conjugation sites of fusion peptides are not specific |
Phage display libraries are mixtures of billions of different phage clones, each displaying different fusion peptides. Libraries differ by the type of phage vector used for their construction and the size of fusion peptides. Owing to restrictions applied in the construction process, all phages present in phage libraries should be genetically stable and have good propagation characteristics. Phage libraries with more variants are more desirable since they provide additional possibilities for identification of target-specific phages. The selection procedure is straightforward and includes: (1) incubation of the phage library with a specific target; (2) removal of phages that do not bind to the target; and (3) recovery of phages bound to the target specifically. These steps, which comprise one selection round, are repeated several times (typically three to five) to enrich for specific target-binding phage. At completion of the selection process, phage DNAs are sequenced and translated to determine sequences of fusion peptides [28]. Fusion peptides on selected phages can be identical, show some sequence similarities to the desired antigenic epitope [29], or can be structural or functional mimotopes of the antigen [30].
Next generation sequencing (NGS) is a powerful tool that has potential to accelerate and improve isolation of target-specific phages from phage display libraries. Traditionally, when the selection process is completed, identification and analysis of fusion peptide sequences is achieved by picking of random phage-containing bacterial colonies manually, followed by Sanger sequencing of that population of phage DNAs. This time-consuming process is inefficient and limits screening to examination of a few hundred phage clones, leaving thousands of potentially valuable clones uncharacterized. By contrast, deep-sequencing technology allows characterization of the entire selection output (105-106 phage clones), enabling efficient discovery of rare, high quality clones that would otherwise be lost [31-33]. Use of NGS in this way can reduce the number of selection rounds to one or two. This, for example, might prevent isolation of false-positive phage clones that have high propagation rates or that bind to non-target components of the selection system (plastic or proteins added to blocking buffers).
A third method for generation of phage-peptide fusions is chemical conjugation of synthetic peptides to phage surface proteins. Advantages and disadvantages of this methodology are summarized in Table T2. Conjugation can be achieved by employing traditional chemistries such as acylation of amines using N-hydroxysuccinimide esters, modification of residues containing carboxylic acid by means of carbodiimide compounds, and modification of tyrosine using diazonium salts [34]. Most studies on phage-peptide chemical conjugates were performed to advance phage-assisted drug delivery [35, 36]. Few reports describe development of phage-peptide conjugates for use as vaccines.
Van Houten et al. [6] conjugated a peptide binding specifically to human immunodeficiency virus type 1 antibody b12 to two carriers, f1.K filamentous phage or ovalbumin, and compared corresponding antigenic responses. Calculations of peptide-to-carrier antibody ratios indicated that, when used as a carrier, phage can better focus the antibody response against antigenic peptides. Roehnisch et al. [37] demonstrated superior immunogenicity of a chemically linked idiotype protein when compared to a genetically engineered idiotype-phage construct using the murine B cell lymphoma 1 model. This group also reported that a chemically linked phage-based idiotype vaccine stimulated tumor-specific immune responses in a subset of patients with advanced multiple myeloma [38]. Thus, chemical conjugation could be a valid alternative to phage-based vaccines obtained via genetic manipulations, which have limitations defined by phage biology (peptide length, copy number, sequence composition, phage stability and propagation rate). A disadvantage of phage-peptide chemical conjugates is that they are less well defined molecularly. When compared to phages modified genetically, the number of peptides per chemically conjugated phage particle may vary from one batch of conjugates to another. The conjugation sites of fusion peptides may also vary.
4. Phage as immunogen
Filamentous phages possess a number of beneficial characteristics that support their utility as vaccine carriers. Inoculations of animals with whole phage particles, which started soon after phage display technology was introduced in 1985 [39], provided evidence of the ability of filamentous phages to stimulate a broad range of immune responses. Phages can stimulate strong humoral [40] as well as cell-mediated immune responses [41] and are highly immunogenic without adjuvants [42, 43]. Experiments in multiple mammalian species (mice, pigs, goats, sheep, monkeys) showed that phage-based preparations do not cause adverse effects even with high phage doses and repeated phage administrations [44, 45]. In the majority of applications phage particles were viable; however, if required, phage can be used in “killed” form since deactivated phages retain immunogenicity [46, 47]. Moreover, phage-based preparations are immunogenic after oral administration [48, 50], highlighting a realistic path for development of contraceptive vaccines that are practical for use in wild and feral animals.
To stimulate potent immune responses, phage particles must first be internalized and processed by antigen presenting cells (APCs). As bacterial viruses, wild type phages have no tropism to mammalian cells. They can be internalized by phagocytic APCs like dendritic cells and macrophages due to the particulate nature of phage virions, but not by other cell types. However, phages can enter many types of mammalian cells if modified to display ligands specific to cell-surface receptors. For example, Hart et al. [51] modified filamentous phage particles to display integrin-binding RGD peptides. The recombinant phage bound cell-surface integrin receptors on HEp-2 cells, a human laryngeal epithelial cell line and, after incubation, was localized inside the cells. In another study, RGD-displaying phages were internalized by and recovered from cells in an infective form [52]. RGD modification also enabled internalization of recombinant M13 phages carrying a segment of the polymorphic membrane protein D from Chlamydia trachomatis (Ct). These recombinant phages blocked Ct infection in HeLa and primary endocervical cells, serving as an anti-microbial therapeutic [53]. Interestingly, a non-phage viral vector modified with RGD peptides transduced dendritic cells more efficiently than the corresponding wild-type vector [54]. This is important from an immunological perspective, since targeting vaccines to dendritic cells is thought to improve their efficacy [55]. Cellular internalization of phage can also be achieved using cell-penetrating peptides. For example, phage particles modified with cell-penetrating 3D8 VL transbody or TAT peptide were internalized into several types of cultured mammalian cells by endocytosis via interaction with cell surface receptors [56].
Mechanisms of processing and presentation of filamentous phage virions in B lymphocytes were investigated by Gaubin et al. [57]. Wild type virions were fluorescently labeled, incubated with lymphocytes and subjected to confocal microscopy to trace virion fate. Results indicated that virions were internalized efficiently by B lymphocytes and degraded in the endosomal-lysosomal compartments, and that derived peptides were directed to MHC class I and II peptide loading compartments. The same study showed that recombinant phage particles displaying a cytotoxic T cell epitope from the reverse transcriptase of human immunodeficiency virus were processed similarly to wild type particles. Formation of recombinant phage-derived peptide:MHC class I complexes in macrophages was also reported by Wan et al. [58]. In that study, MHC class I peptide complexes were co-localized with class II molecules and molecular markers for the endocytic system, suggesting cross-presentation of endocytosed phages. Hashiguchi et al. [59] examined antibody response against M13 in wild type and knockout mice. Strong anti-M13 IgG response was induced in wild type C57BL/6 mice by a single administration of phage without an adjuvant. Induction of antibody responses to M13 phage was also analyzed in MyD88-knockout mice with the same background. MyD88-deficient mice did not produce antibody responses while wild type mice did, suggesting essential involvement of MyD88 in antibody induction.
The ability of filamentous phages to stimulate specific humoral and cellular immune responses against various B and T cell epitopes displayed on phage was demonstrated in multiple studies. Mascolo et al. [60] showed that display of a single cytotoxic epitope on the surface of phage virion was sufficient to induce priming and sustain long-term MHC class I-restricted cytotoxic T lymphocyte response in vivo. Strong, specific anti-tumor CTL responses activated by filamentous phage displaying MAGE-A10 or MAGE-A3-derived peptides were reported by Sartorius et al. [10]. In a study by Ulivieri et al. [61], fd recombinant phages displaying different human cytomegalovirus (HCMV) MHC II restricted epitopes were processed by human antigen presenting cells and activated HCMV-specific CD4+ T cells. A phage-displayed epitope originating from the Candida albicans heat shock protein 90 induced both antibody and CTL immune responses [14]. Looking further into the mechanisms of fd phage immunogenicity, Del Pozzo et al. [62] studied the ability of a CTL-displaying phage to mediate a delayed type hypersensitivity (DTH) reaction. In that study, recombinant bacteriophage elicited DTH in the absence of adjuvants. Additionally, authors showed that the presence of CD4+ T cells is required to elicit a long-term memory response against antigens delivered by phage.
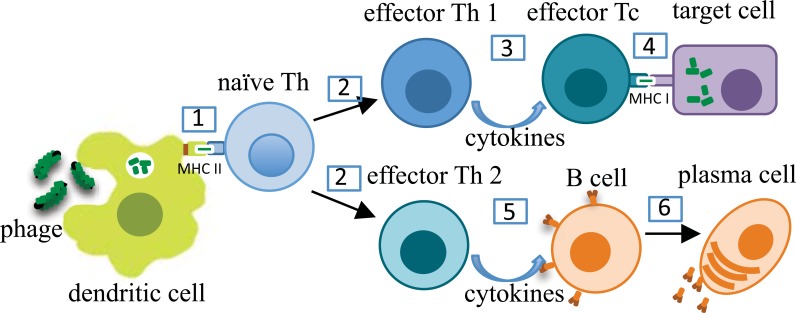
Mechanisms of filamentous phage internalization and processing by antigen presenting cells, as well as induction of corresponding humoral and cellular immune responses by phage particles, are summarized schematically in Fig. (2). The first step is uptake of the phage by DC at the immunization site followed by phage processing via MHC class I and II pathways. Consequently, activated DCs migrate to a local draining lymph node to present MHC II:phage-derived peptide complexes to naïve antigen-specific T helper (Th) cells. In turn, these cells develop into either Th1 or Th2 lymphocytes and become activated. Once activated, they produce cytokines leading either to antibody production by B cells (Th2 pathways) or cytotoxic T cell responses and macrophage upregulation (Th1 pathways). Th-independent B cell responses (not shown in Fig. 2) are also possible. They are initiated via binding and, ultimately, cross-linking of repeating antigenic epitopes displayed on the phage surface to antigen-specific B cell receptors (IgM antibodies), re-sulting in B cell activation and IgM secretion. Stimula-tory signals can also come from bacterial lipopolysac-charide molecules associated with phage coat proteins [63] and, possibly, from CpG sequences present in phage DNA binding with TLR on sentinel cells [64].
Fig. (2).
Immune cell responses to filamentous phage. (1) Dendritic cells uptake phage particles, degrade them, and present phage-derived peptides as complexes with MHC II molecules to naïve antigen-specific T helper (Th) cells. (2) Th cells become activated and undergo transformation into Th1 or Th2 effector cells. (3) Activated Th1 cells secrete Th1-type cytokines to upregulate cell-mediated immune responses such as activation of macrophages or cytotoxic T (Tc) cells. (4) Antigen-specific activated (effector) Tc recognize MHC I:phage-derived peptide complexes presented on target cells and destroy these cells. (5) Th2-type cytokines induce antigen-specific B cells, which (6) undergo transformation into antibody secreting plasma cells. Antibodies secreted by plasma cells neutralize antigens similar to B cell epitopes on phage particles.
5. Increasing potency of phage-based vaccines
High phage dose, different administration routes, and the use of adjuvants were explored to improve efficacy of phage-based vaccines. High phage doses were shown to be safe for animals. For example, beyond an expected immune response, sheep and goats inoculated with 1x1014 virions per animal of phage displaying cathepsin L1 mimotopes showed no adverse effects [45]. Safe doses in piglets were reported at 2.5x1013 virions per immunization [65]. No serious adverse events were observed in human patients vaccinated with 1x1011-2.5x1012 bacteriophages [38]. The highest reported phage dose of 2x1014 virions per animal was administered to rhesus macaques without adverse side reactions [44]. Mascolo et al. [60] reported that the strength of the cytotoxic activity of a CTL epitope-carrying phage in mice correlated directly with the dose of recombinant phage administered. Roehnisch et al. [37] showed that anti-phage antibody responses reached their peak at the maximum tested dose of 5x1011 virions per mouse. Immunization of mice with a dose of 2x1012 virions per animal resulted in a significant increase in serum antibodies against peptides displayed on phage as compared to the response of mice inoculated with 5x1011 virions each [66].
Supplementation of phage with adjuvants did not result in improved immune responses. Presence or absence of Freund's complete adjuvant indicated no difference in the immune response in mice immunized with phage displaying antigenic peptides of the circumsporozoite protein of the malaria parasite [42]. Similarly, the presence of saponin adjuvant did not increase the efficacy of a phage-based vaccine against Leishmania amazonensis parasite in mice when compared to the vaccine alone [43]. Also a possibility is that phage immunogenicity can be improved via optimization of vaccine administration routes. Only a few studies compared delivery routes for particular phage applications [50, 66]. Extensive side-by-side studies are needed to draw objective conclusions about preferable administration routes for phage-based vaccines.
Enhancement of humoral and cytotoxic immune responses can be achieved by phage targeting to cells and tissues of the immune system. Trepel et al. [67] demonstrated increased serum anti-phage antibody titers in mice vaccinated with phage carrying lymph node-targeting peptides compared to mice inoculated with control phage. Sartorius et al. [68] reported enhanced receptor-mediated uptake of phages displaying scFv against DC surface molecular marker DEC-205 by these cells. Moreover, phage hybrid particles displaying the DEC-205 targeting moiety and a CTL antigenic peptide induced potent antigen-specific immune responses leading to inhibition of tumor growth in mice.
Attempts to optimize antigenic properties of phage-displayed peptides by using mixtures of phages each displaying a separate epitope from the same antigen resulted in mixed outcomes. Villa-Mancera et al. [45] tested immunogenicity of individual phages displaying cathepsin L mimotopes of Fasciola hepatica as well as a combination of these phages. While mean F. hepatica worm burdens were reduced in sheep vaccinated with two individual clones, no improved effect was observed in animals inoculated with the mixture of seven clones. Using phage display Prudencio et al. [69] developed specific immunogens that mimic Rhipicephalus microplus tick antigen. Use of a mixture of nine clones did not stimulate increased immune responses in mice as compared to the individual phage clones. In contrast, Costa et al. [43] demonstrated that a combination of two phage clones improved the efficacy of a phage-based vaccine against Leishmania infantum. Wu et al. [70] showed that combining four phages stimulated a higher immune response against the antigen than when the same phages were tested individually. Such effects are likely to be antigen-specific. Phage mixtures might be useful in applications where broad immune responses are needed.
Focusing immune responses on phage-displayed peptides rather than on phage coat proteins by reducing complexity of phage coat proteins is another strategy used to enhance immune responses to displayed peptides. For example, antibody response against displayed peptides can be improved by removal or alteration of immunodominant B-cell epitopes on the minor phage coat protein III [71]. Henry et al. [72] provided a comprehensive review on engineering of filamentous phage vectors aimed at effective delivery of antigenic peptides in order to enhance humoral responses.
6. Phage-based vaccines for animal contraception
Molecules being explored as targets for development of contraceptive vaccines for animals can be divided broadly into two groups: (1) regulators of gamete production; and (2) regulators of gamete function [73]. The first group of targets includes gonadotropin-releasing hormone (GnRH), follicle-stimulating hormone (FSH), luteinizing hormone (LH) and their receptors (GnRHR, FSHR, and LHR). The second group of targets involves sperm-specific antigens and zona pellucida (ZP) proteins participating in sperm-oocyte interactions and fertilization. Reproductive targets from both groups were explored in development of immunocontraceptive vaccines based on the filamentous phage platform (Table T3).
Table 3.
Antigens and selection targets used for development of contraceptive vaccines based on filamentous phage.
| Antigen or Selection Target | Effect on Reproductive Characteristics | Test Animal or Cell System | References |
|---|---|---|---|
| GnRH | Suppression of testosterone | Mice | [66] |
| LHR | Reversible infertility Inhibition of vitellogenesis in females |
Mice Rainbow trout |
[76] [80] |
| FSHR | Reversible infertility Impaired fertility Inhibition of ovulation Delayed sexual maturation Reduced progeny size Delayed onset of puberty Azoospermia, reversible infertility Delayed sperm production in prepubertal males |
Mice Mice Sheep Mice Mice Male goats Bonnet monkeys Rainbow trout |
[76] [77] [77] [78] [78] [78] [79] [80] |
| Combination of LHR & FSHR | Inhibition of spermatogenesis, steroidogenesis | Rainbow trout | [80] |
| ZP-binding peptides | Anti-sperm antibodies Inhibition of sperm-oocyte binding, less cleaved embryos |
Pigs Porcine IVF |
[87] [86] |
| Sperm-binding peptides or antibodies | Inhibition of sperm-oocyte binding and fusion Inhibition of sperm-oocyte fusion Anti-sperm antibodies |
Hamster egg penetration test Sperm-oocyte fusion Human sperm binding |
[81] [84] [83] |
| FA-1 | Anti-sperm antibodies, inhibition of sperm acrosome reaction | Sperm acrosome reaction | [82] |
Samoylov et al. [66] developed multiple phage constructs displaying GnRH-like peptides and tested them in mice. Recombinant phages stimulated production of neutralizing anti-GnRH antibodies resulting in suppressed serum testosterone. Phage-GnRH constructs in this study were identified using different types of GnRH antibodies as selection targets, including antibodies raised in dog, cat, or rabbit. Interestingly, the core peptide sequence (XXXXHWSX) identified using GnRH antibodies raised in dog, was similar to a sequence (XHWSXXLXXX) identified by phage selection on GnRH antibodies from the same species by others [74]. Additionally, peptide sequences identified using antibodies raised in cat or rabbit contained an amino acid core sequence GLRP that was similar to a partial GnRH peptide GLRPG that had contraceptive effects in mice [75].
Jean-Jacques Remy's group produced recombinant filamentous phages displaying three overlapping N-terminal decapeptides of FSHR. When administered to animals they induced neutralizing antibodies against FSHR, blocking FSH-FSHR interaction [76, 77]. The constructs were tested in several mammalian species and in animal groups of different sexual maturity. Phage-FSHR constructs were employed for immunization of male mice and goats at the prepubertal stage [78]. Immunization of three-week old mice delayed their sexual maturation by one week. Once sexually mature, litter sizes produced by mating immunized males to untreated females were reduced by up to 60%. In goats, phage-FSHR vaccine administration resulted in low, prepubertal circulating levels of testosterone for several months, suggesting potential to delay onset of puberty. These vaccines induced anti-FSHR peptide antibody responses with 300 peptide copies per phage particle, stimulating both systemic and mucosal immunity.
In adult male bonnet monkeys, monthly subcutaneous phage-FSHR immunizations caused azoospermia. Breeding trials with proven fertile females (carried out between days 90 and 225) revealed that all the immunized males were infertile [79]. The sperm count in immunized males gradually returned to normal and the fertility was restored when immunizations were stopped. The results demonstrated that active immunization against FSHR using phage as a vaccine carrier can induce complete and reversible infertility in adult male monkeys. Such vaccines could be used in zoo animals, where temporary contraception is a highly desirable feature. Also important is that targeting FSHR with phage-based vaccines did not affect testosterone levels in immunized individuals, opening a possibility for the use of phage-FSHR vaccines in male human patients.
Sambroni et al. [80] studied the effect of phage displaying LHR- or FSHR-derived decapeptides on trout sexual maturation. Recombinant phages were used as vaccines against the receptors. The anti-FSHR phage delayed sperm production in prepubertal males. The anti-LHR phage blocked vitellogenesis in females. Immunization against LHR or FSHR simultaneously inhibited spermatogenesis and reduced plasma steroid hormone levels in maturing males. The use of phage-based vaccines in controlling the onset of puberty in aquaculture species is one of the most obvious applications resulting from this study.
Using total RNA from lymphocytes obtained from individuals with anti-sperm antibodies, Clayton et al. [81] constructed an “anti-sperm-biased” library of phages displaying fragments of antibodies (Fabs) against human sperm. Selection of the library against human spermatozoa led to the isolation of sperm-specific phages that inhibited sperm-egg binding and fusion in vitro, indicating their potential use for contraceptive purposes. Using a similar approach, Samuel and Naz [82] identified phages displaying Fabs reactive with fertilization antigen 1 (FA-1) on the surface of human sperm that inhibited the sperm acrosome reaction. Fiszer et al. [83] constructed a phage display library of Fabs recognizing sperm surface antigens and screened these against whole unfixed sperm cells. Selected Fabs showed similarities to antibodies generated against proteins involved in sperm motility and cell fusion. Authors suggested that such Fabs could cause infertility and, therefore, have value for development of contraceptive vaccines. Eidne et al. [84] identified a number of fusion peptides that bind to spermatozoa specifically and with high affinity, one of which had high sequence homology with a dominant epitope of human ZP3. These peptides were tested in a sperm-oocyte interaction assay and impaired sperm-oocyte fusion in vitro.
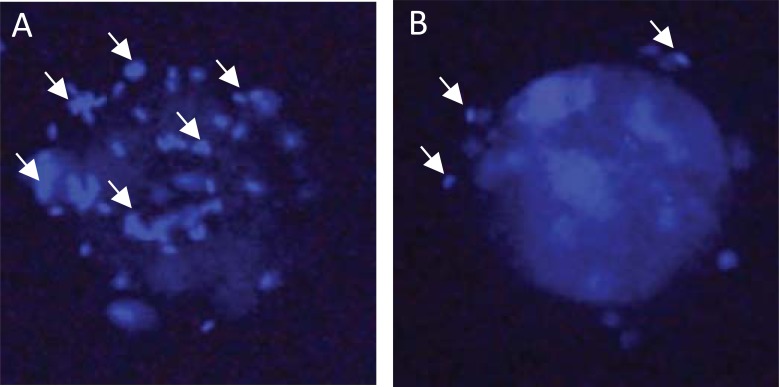
Phages displaying peptides for potential contraceptive uses were also isolated from phage display libraries selected against intact dog oocytes surrounded by ZP [85]. When administered to dogs, these peptides stimulated production of anti-sperm antibodies. Theoretically, this approach enables identification of peptides that mimic sperm surface molecules binding ZP at fertilization. Anti-sperm antibodies have potential to reduce fertility by disrupting events associated with fertilization, including sperm motility, the acrosome reaction, and/or aspects of sperm-egg interaction. Indeed, while preliminary, incubation of porcine oocytes with sera collected from pigs immunized with phage-peptide fusions developed to bind porcine ZP decreased sperm binding to oocytes (Fig. 3), percent embryo cleavage, and percent cleaved embryos that developed to blastocysts in vitro [86]. Phage-peptide constructs used in these studies were selected following a protocol designed to favor species specificity [87]. Prior to reaction with porcine oocytes, the phage library was preabsorbed with oocytes of non-target species (cat, dog, and cow) that possess close homology with porcine ZP proteins. When administered to pigs, these phage antigens induced production of anti-sperm antibodies. Phage-peptide constructs with pig specificity were identified by staining of semen samples collected from different species, including pig, cat, dog, bull, and mouse, with anti-sera from pigs immunized with ZP-binding phage [87]. Species-specificity is a valuable characteristic of contraceptive vaccines for oral administration to be used in control of wild/feral animal populations to limit negative effects on non-target species.
Fig. (3).
Sperm-oocyte interaction in in vitro fertilization system in the presence of porcine serum. (A) Serum collected from a control pig (not immunized). (B) Serum collected from a pig immunized with phage particles that stimulated production of anti-sperm antibodies. Dim circles are pronuclei in oocytes. Small bright elongated dots (indicated with arrows) are sperm cells. Cellular DNA is blue (staining with Hoechst dye). (The color version of the figure is available in the electronic copy of the article).
Non-filamentous phages have been investigated as platforms for contraceptive vaccines. One study reported cloning of RNA bacteriophage AP205 fusions for display of a GnRH peptide [88]. Immunization of mice with AP205-GnRH fusions resulted in production of anti-GnRH antibodies and suppression of testo-sterone. In another instance [89], RNA bacteriophage PP7 was modified to display peptides derived from human chorionic gonadotropin (hCG). The resulting phages induced a substantial anti-hCG response and reduced uterine weight gain in immunized mice.
7. Other types of biological nano-particles currently used in vaccine formulations
Due to their nature, structure and size, bacteriophages are considered to be biological nanoparticles. Nanoparticles that closely resemble phages with respect to their origin, structural and immunologic characteristics are virus-like particles (VLPs). VLPs are defined as particles composed of a single or multiple viral coat proteins without genetic material. Depending on their structural complexity, VLPs require prokaryotic or eukaryotic expression systems for protein production. The particles are then formed from proteins via a self-assembling process. Simple VLPs (composed of a single protein) can be assembled in a cell-free environment, while assembly of complex VLPs can be accomplished using yeast, insect or plant cells [90]. VLPs can derive from a variety of viruses. Similar to phage-peptide fusions, they can be modified genetically or via chemical conjugation to display peptide epitopes and serve as vaccines. The diversity and adaptability of VLPs supports a wide range of applications, including vaccination against viral infections [91]. Several human VLP-based vaccines (ex. anti-papilloma virus vaccines Gardasil® and Cervarix®) are licensed, and many are in clinical trials. The use of VLPs for contraceptive purposes is currently undocumented. Major limitations of VLPs relate to their cost of production [90].
Conclusion
Filamentous phages constitute an attractive platform for vaccine development that can be engineered efficiently, effectively and with remarkable specificity at both molecular and organismal levels. Next generation sequencing provides a mechanism for efficient identification of novel phages of unique utility in this domain. Phage-based vaccines can be produced in abundance at low cost, they are stable in extreme environments and are immunogenic when administered parenterally or orally. These features are essential for a contraceptive vaccine to be effective and practical in animal applications. Contraceptive vaccines based on filamentous phage might be applicable to various species and groups of animals, including wild, feral, and captive animals. The adaptability of this platform also makes it attractive for design of human immunocontraceptive agents.
Box 1. Advantages of filamentous phage as a vaccine platform.
| • Very immunogenic; stimulates humoral and cytotoxic immune responses. • Stable in various environmental conditions (temperature, pH, buffers). • Low cost to produce; requires no special equipment for production. • Safe in multiple mammalian species, including human. • Retains immunogenicity when inactivated (killed) by certain methods. • Holds potential for development of species-specific vaccines. • Methods to engineering of phage-peptide fusions are well established and relatively easy. |
ACKNOWLEDGEMENTS
Experimental studies conducted in the Samoylova laboratory and cited in this review were supported by the Found Animals Foundation, Michelson Grant in Reproductive Biology (grant number DO910-S10), Alabama Farmers Federation, the Animal Health and Disease Research program and the Scott-Ritchey Research Center, College of Veterinary Medicine, Auburn University. TS and FB thank Dr. Prather and his group (Division of Animal Sciences, National Swine Resource and Research Center, University of Missouri, Columbia, MO) for help with in vitro fertilization studies.
LIST OF ABBREVIATIONS
- APC
Antigen presenting cell
- CpG
Cytosine-phosphate-guanosine
- Ct
Chlamydia trachomatis
- CTL
Cytotoxic T lymphocyte
- DC
Dendritic cell
- DTH
Delayed type hypersensitivity
- FA-1
Fertilization antigen 1
- Fab
Antibody fragment
- FSH
Follicle-stimulating hormone
- FSHR
Follicle-stimulating hormone receptor
- GnRH
Gonadotropin-releasing hormone
- GnRHR
Gonadotropin-releasing hormone receptor
- hCG
Human chorionic gonadotropin
- HCMV
Human cytomegalovirus
- HIV
Human immunodeficiency virus
- IVF
In vitro fertilization
- LH
Luteinizing hormone
- LHR
Luteinizing hormone receptor
- MAGE
Melanoma antigen gene
- MyD88
Myeloid differentiation primary response gene
- NGS
Next generation sequencing
- RGD
Arginine-glycine-aspartic acid peptide
- scFv
Single-chain variable fragment
- Tc
T cytotoxic
- Th
T helper
- TLR
Toll like receptor
- VLP
Virus like particle
- ZP
Zona pellucida
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Gupta S.K., Srinivasan V.A., Suman P., Rajan S., Nagendrakumar S.B., Gupta N., Shrestha A., Joshi P., Panda A.K. Contraceptive vaccines based on the zona pellucida glycoproteins for dogs and other wildlife population management. Am. J. Reprod. Immunol. 2011;66(1):51–62. doi: 10.1111/j.1600-0897.2011.01004.x. [DOI] [PubMed] [Google Scholar]
- 2.Kirkpatrick J.F., Lyda R.O., Frank K.M. Contraceptive vaccines for wildlife: a review. Am. J. Reprod. Immunol. 2011;66(1):40–50. doi: 10.1111/j.1600-0897.2011.01003.x. [DOI] [PubMed] [Google Scholar]
- 3.Levy J.K. Contraceptive vaccines for the humane control of community cat populations. Am. J. Reprod. Immunol. 2011;66(1):63–70. doi: 10.1111/j.1600-0897.2011.01005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swegen A., Aitken R.J. Prospects for immunocontraception in feral horse population control: exploring novel targets for an equine fertility vaccine. Reprod. Fertil. Dev. 2016;28:853–863. doi: 10.1071/RD14280. [DOI] [PubMed] [Google Scholar]
- 5.Ackermann H-W. Bacteriophages classification. In: Kutter E., Sulakvelidze A., editors. Bacteriophages: Biology and application. CRC Press; 2005. pp. 67–89. [Google Scholar]
- 6.van Houten N.E., Zwick M.B., Menendez A., Scott J.K. Filamentous phage as an immunogenic carrier to elicit focused antibody responses against a synthetic peptide. Vaccine. 2006;24(19):4188–4200. doi: 10.1016/j.vaccine.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grieco S.H., Lee S., Dunbar W.S., MacGillivray R.T., Curtis S.B. Maximizing filamentous phage yield during computer-controlled fermentation. Bioprocess Biosyst. Eng. 2009;32(6):773–779. doi: 10.1007/s00449-009-0303-3. [DOI] [PubMed] [Google Scholar]
- 8.Brigati J.R., Petrenko V.A. Thermostability of landscape phage probes. Anal. Bioanal. Chem. 2005;382(6):1346–1350. doi: 10.1007/s00216-005-3289-y. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson F., Tsagozis P., Lundberg K., Parsa R., Mangsbo S.M., Persson M.A., Harris R.A., Pisa P. Tumor-specific bacteriophages induce tumor destruction through activation of tumor-associated macrophages. J. Immunol. 2009;182(5):3105–3111. doi: 10.4049/jimmunol.0800224. [DOI] [PubMed] [Google Scholar]
- 10.Sartorius R., Pisu P., D’Apice L., Pizzella L., Romano C., Cortese G., Giorgini A., Santoni A., Velotti F., De Berardinis P. The use of filamentous bacteriophage fd to deliver MAGE-A10 or MAGE-A3 HLA-A2-restricted peptides and to induce strong antitumor CTL responses. J. Immunol. 2008;180(6):3719–3728. doi: 10.4049/jimmunol.180.6.3719. [DOI] [PubMed] [Google Scholar]
- 11.De Berardinis P., Sartorius R., Caivano A., Mascolo D., Domingo G.J., Del Pozzo G., Gaubin M., Perham R.N., Piatier-Tonneau D., Guardiola J. Use of fusion proteins and procaryotic display systems for delivery of HIV-1 antigens: development of novel vaccines for HIV-1 infection. Curr. HIV Res. 2003;1(4):441–446. doi: 10.2174/1570162033485168. [DOI] [PubMed] [Google Scholar]
- 12.Hassapis K.A., Stylianou D.C., Kostrikis L.G. Architectural insight into inovirus-associated vectors(IAVs) and development of IAV-based vaccines inducing humoral and cellular responses: implications in HIV-1 vaccines. Viruses. 2014;6(12):5047–5076. doi: 10.3390/v6125047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solomon B. Filamentous bacteriophage as a novel therapeutic tool for Alzheimer’s disease treatment. J. Alzheimers Dis. 2008;15(2):193–198. doi: 10.3233/jad-2008-15205. [DOI] [PubMed] [Google Scholar]
- 14.Wang G., Sun M., Fang J., Yang Q., Tong H., Wang L. Protective immune responses against systemic candidiasis mediated by phage-displayed specific epitope of Candida albicans heat shock protein 90 in C57BL/6J mice. Vaccine. 2006;24(35-36):6065–6073. doi: 10.1016/j.vaccine.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 15.Houimel M., Dellagi K. Peptide mimotopes of rabies virus glycoprotein with immunogenic activity. Vaccine. 2009;27(34):4648–4655. doi: 10.1016/j.vaccine.2009.05.055. [DOI] [PubMed] [Google Scholar]
- 16.Deng L., Ibanez L.I., Van den Bossche V., Roose K., Youssef S.A., de Bruin A., Fiers W., Saelens X. Protection against Influenza A Virus Challenge with M2e-Displaying Filamentous Escherichia coli Phages. PLoS One. 2015;10(5):e0126650. doi: 10.1371/journal.pone.0126650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrenko V.A., Smith G.P., Gong X., Quinn T. A library of organic landscapes on filamentous phage. Protein Eng. 1996;9(9):797–801. doi: 10.1093/protein/9.9.797. [DOI] [PubMed] [Google Scholar]
- 18.Webster R. Filamentous phage biology. In: Barbas C. III, Burton D., Scott J.K., Silverman G., editors. Phage Display: A laboratory manual. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; 2001. pp. 1.1–1.36. [Google Scholar]
- 19.Malik P., Terry T.D., Gowda L.R., Langara A., Petukhov S.A., Symmons M.F., Welsh L.C., Marvin D.A., Perham R.N. Role of capsid structure and membrane protein processing in determining the size and copy number of peptides displayed on the major coat protein of filamentous bacteriophage. J. Mol. Biol. 1996;260(1):9–21. doi: 10.1006/jmbi.1996.0378. [DOI] [PubMed] [Google Scholar]
- 20.Prisco A., De Berardinis P. Filamentous bacteriophage fd as an antigen delivery system in vaccination. Int. J. Mol. Sci. 2012;13(4):5179–5194. doi: 10.3390/ijms13045179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrenko V. Smith GP, Vectors and Modes of Display. In: Sidhu S.S., editor. Phage Display In Biotechnology and Drug Discovery. CRC Press; 2005. pp. 63–110. [Google Scholar]
- 22.Kouzmitcheva G.A., Petrenko V.A., Smith G.P. Identifying diagnostic peptides for lyme disease through epitope discovery. Clin. Diagn. Lab. Immunol. 2001;8(1):150–160. doi: 10.1128/CDLI.8.1.150-160.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthews L.J., Davis R., Smith G.P. Immunogenically fit subunit vaccine components via epitope discovery from natural peptide libraries. J. Immunol. 2002;169(2):837–846. doi: 10.4049/jimmunol.169.2.837. [DOI] [PubMed] [Google Scholar]
- 24.Blancafort P., Steinberg S.V., Paquin B., Klinck R., Scott J.K., Cedergren R. The recognition of a noncanonical RNA base pair by a zinc finger protein. Chem. Biol. 1999;6(8):585–597. doi: 10.1016/s1074-5521(99)80091-x. [DOI] [PubMed] [Google Scholar]
- 25.Iannolo G., Minenkova O., Petruzzelli R., Cesareni G. Modifying filamentous phage capsid: limits in the size of the major capsid protein. J. Mol. Biol. 1995;248(4):835–844. doi: 10.1006/jmbi.1995.0264. [DOI] [PubMed] [Google Scholar]
- 26.Jegerlehner A., Storni T., Lipowsky G., Schmid M., Pumpens P., Bachmann M.F. Regulation of IgG antibody responses by epitope density and CD21-mediated costimulation. Eur. J. Immunol. 2002;32(11):3305–3314. doi: 10.1002/1521-4141(200211)32:11<3305::AID-IMMU3305>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 27.Scott J., Barbas C., III . Phage Display Vectors. In: Barbas C. III, Burton D., Scott J., Silverman G., editors. Phage Display: A Laboratory Manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 2001. pp. 2.1–2.19. [Google Scholar]
- 28.Brigati J.R., Samoylova T.I., Jayanna P.K., Petrenko V.A. 2008. Phage display for generating peptide reagents.In Current Protocols in Protein Science, [DOI] [PubMed] [Google Scholar]
- 29.Cao L., Ge X., Gao Y., Zarlenga D.S., Wang K., Li X., Qin Z., Yin X., Liu J., Ren X., Li G. Putative phage-display epitopes of the porcine epidemic diarrhea virus S1 protein and their anti-viral activity. Virus Genes. 2015;51(2):217–224. doi: 10.1007/s11262-015-1234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang L., Cen J., Xue Q., Li J., Bi Y., Sun L., Liu W. Identification of rabies virus mimotopes screened from a phage display peptide library with purified dog anti-rabies virus serum IgG. Virus Res. 2013;174(1-2):47–51. doi: 10.1016/j.virusres.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 31.t Hoen P. A., Jirka S. M., Ten Broeke B. R., Schultes E. A., Aguilera B., Pang K. H., Heemskerk H., Aartsma-Rus A., van Ommen G. J., den Dunnen J. T. Phage display screening without repetitious selection rounds. Anal. Biochem. 2012;421(2):622–631. doi: 10.1016/j.ab.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Matochko W.L., Cory Li S., Tang S.K., Derda R. Prospective identification of parasitic sequences in phage display screens. Nucleic Acids Res. 2014;42(3):1784–1798. doi: 10.1093/nar/gkt1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matochko W.L., Derda R. Next-generation sequencing of phage-displayed peptide libraries. Methods Mol. Biol. 2015;1248:249–266. doi: 10.1007/978-1-4939-2020-4_17. [DOI] [PubMed] [Google Scholar]
- 34.Bernard J.M., Francis M.B. Chemical strategies for the covalent modification of filamentous phage. Front. Microbiol. 2014;5:734. doi: 10.3389/fmicb.2014.00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaks L., Benhar I. In vivo characteristics of targeted drug-carrying filamentous bacteriophage nanomedicines. J. Nanobiotechnology. 2011;9:58. doi: 10.1186/1477-3155-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karimi M., Mirshekari H., Moosavi Basri S. M., Bahrami S., Moghoofei M., Hamblin M. R. Bacteriophages and phage-inspired nanocarriers for targeted delivery of therapeutic cargos. . Adv. Drug Del. Rev. 2016;45:62. doi: 10.1016/j.addr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roehnisch T., Then C., Nagel W., Blumenthal C., Braciak T., Donzeau M., Bohm T., Bourquin C., Oduncu F. Chemically linked phage idiotype vaccination in the murine B cell lymphoma 1 model. J. Transl. Med. 2013;11:267. doi: 10.1186/1479-5876-11-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roehnisch T., Then C., Nagel W., Blumenthal C., Braciak T., Donzeau M., Bohm T., Flaig M., Bourquin C., Oduncu F.S. Phage idiotype vaccination: first phase I/II clinical trial in patients with multiple myeloma. J. Transl. Med. 2014;12:119. doi: 10.1186/1479-5876-12-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith G.P. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228(4705):1315–1317. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 40.de la Cruz V.F., Lal A.A., McCutchan T.F. Immunogenicity and epitope mapping of foreign sequences via genetically engineered filamentous phage. J. Biol. Chem. 1988;263(9):4318–4322. [PubMed] [Google Scholar]
- 41.De Berardinis P., Sartorius R., Fanutti C., Perham R.N., Del Pozzo G., Guardiola J. Phage display of peptide epitopes from HIV-1 elicits strong cytolytic responses. Nat. Biotechnol. 2000;18(8):873–876. doi: 10.1038/78490. [DOI] [PubMed] [Google Scholar]
- 42.Willis A.E., Perham R.N., Wraith D. Immunological properties of foreign peptides in multiple display on a filamentous bacteriophage. Gene. 1993;128(1):79–83. doi: 10.1016/0378-1119(93)90156-w. [DOI] [PubMed] [Google Scholar]
- 43.Costa L.E., Chavez-Fumagalli M.A., Martins V.T., Duarte M.C., Lage D.P., Lima M.I., Pereira N.C., Soto M., Tavares C.A., Goulart L.R., Coelho E.A. Phage-fused epitopes from Leishmania infantum used as immunogenic vaccines confer partial protection against Leishmania amazonensis infection. Parasitology. 2015;142(10):1335–1347. doi: 10.1017/S0031182015000724. [DOI] [PubMed] [Google Scholar]
- 44.Chen X., Scala G., Quinto I., Liu W., Chun T.W., Justement J.S., Cohen O.J., vanCott T.C., Iwanicki M., Lewis M.G., Greenhouse J., Barry T., Venzon D., Fauci A.S. Protection of rhesus macaques against disease progression from pathogenic SHIV-89.6PD by vaccination with phage-displayed HIV-1 epitopes. Nat. Med. 2001;7(11):1225–1231. doi: 10.1038/nm1101-1225. [DOI] [PubMed] [Google Scholar]
- 45.Villa-Mancera A., Quiroz-Romero H., Correa D., Ibarra F., Reyes-Perez M., Reyes-Vivas H., Lopez-Velazquez G., Gazarian K., Gazarian T., Alonso R.A. Induction of immunity in sheep to Fasciola hepatica with mimotopes of cathepsin L selected from a phage display library. Parasitology. 2008;135(12):1437–1445. doi: 10.1017/S003118200800471X. [DOI] [PubMed] [Google Scholar]
- 46.Morales J., Martinez J.J., Manoutcharian K., Hernandez M., Fleury A., Gevorkian G., Acero G., Blancas A., Toledo A., Cervantes J., Maza V., Quet F., Bonnabau H., de Aluja A.S., Fragoso G., Larralde C., Sciutto E. Inexpensive anti-cysticercosis vaccine: S3Pvac expressed in heat inactivated M13 filamentous phage proves effective against naturally acquired Taenia solium porcine cysticercosis. Vaccine. 2008;26(23):2899–2905. doi: 10.1016/j.vaccine.2008.03.042. [DOI] [PubMed] [Google Scholar]
- 47.Samoylova T.I., Norris M.D., Samoylov A.M., Cochran A.M., Wolfe K.G., Petrenko V.A., Cox N.R. Infective and inactivated filamentous phage as carriers for immunogenic peptides. J. Virol. Methods. 2012;183(1):63–68. doi: 10.1016/j.jviromet.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 48.Delmastro P., Meola A., Monaci P., Cortese R., Galfre G. Immunogenicity of filamentous phage displaying peptide mimotopes after oral administration. Vaccine. 1997;15(11):1276–1285. doi: 10.1016/s0264-410x(97)00072-8. [DOI] [PubMed] [Google Scholar]
- 49.Manoutcharian K., Diaz-Orea A., Gevorkian G., Fragoso G., Acero G., Gonzalez E., De Aluja A., Villalobos N., Gomez-Conde E., Sciutto E. Recombinant bacteriophage-based multiepitope vaccine against Taenia solium pig cysticercosis. Vet. Immunol. Immunopathol. 2004;99(1-2):11–24. doi: 10.1016/j.vetimm.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 50.Zuercher A.W., Miescher S.M., Vogel M., Rudolf M.P., Stadler M.B., Stadler B.M. Oral anti-IgE immunization with epitope-displaying phage. Eur. J. Immunol. 2000;30(1):128–135. doi: 10.1002/1521-4141(200001)30:1<128::AID-IMMU128>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 51.Hart S.L., Knight A.M., Harbottle R.P., Mistry A., Hunger H.D., Cutler D.F., Williamson R., Coutelle C. Cell binding and internalization by filamentous phage displaying a cyclic Arg-Gly-Asp-containing peptide. J. Biol. Chem. 1994;269(17):12468–12474. [PubMed] [Google Scholar]
- 52.Ivanenkov V., Felici F., Menon A.G. Uptake and intracellular fate of phage display vectors in mammalian cells. Biochim. Biophys. Acta. 1999;1448(3):450–462. doi: 10.1016/s0167-4889(98)00162-1. [DOI] [PubMed] [Google Scholar]
- 53.Bhattarai S.R., Yoo S.Y., Lee S.W., Dean D. Engineered phage-based therapeutic materials inhibit Chlamydia trachomatis intracellular infection. Biomaterials. 2012;33(20):5166–5174. doi: 10.1016/j.biomaterials.2012.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okada N., Tsukada Y., Nakagawa S., Mizuguchi H., Mori K., Saito T., Fujita T., Yamamoto A., Hayakawa T., Mayumi T. Efficient gene delivery into dendritic cells by fiber-mutant adenovirus vectors. Biochem. Biophys. Res. Commun. 2001;282(1):173–179. doi: 10.1006/bbrc.2001.4527. [DOI] [PubMed] [Google Scholar]
- 55.Curiel T.J., Morris C., Brumlik M., Landry S.J., Finstad K., Nelson A., Joshi V., Hawkins C., Alarez X., Lackner A., Mohamadzadeh M. Peptides identified through phage display direct immunogenic antigen to dendritic cells. J. Immunol. 2004;172(12):7425–7431. doi: 10.4049/jimmunol.172.12.7425. [DOI] [PubMed] [Google Scholar]
- 56.Kim A., Shin T.H., Shin S.M., Pham C.D., Choi D.K., Kwon M.H., Kim Y.S. Cellular internalization mechanism and intracellular trafficking of filamentous M13 phages displaying a cell-penetrating transbody and TAT peptide. PLoS One. 2012;7(12):e51813. doi: 10.1371/journal.pone.0051813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gaubin M., Fanutti C., Mishal Z., Durrbach A., De Berardinis P., Sartorius R., Del Pozzo G., Guardiola J., Perham R.N., Piatier-Tonneau D. Processing of filamentous bacteriophage virions in antigen-presenting cells targets both HLA class I and class II peptide loading compartments. DNA Cell Biol. 2003;22(1):11–18. doi: 10.1089/104454903321112451. [DOI] [PubMed] [Google Scholar]
- 58.Wan Y., Wu Y., Zhou J., Zou L., Liang Y., Zhao J., Jia Z., Engberg J., Bian J., Zhou W. Cross-presentation of phage particle antigen in MHC class II and endoplasmic reticulum marker-positive compartments. Eur. J. Immunol. 2005;35(7):2041–2050. doi: 10.1002/eji.200425322. [DOI] [PubMed] [Google Scholar]
- 59.Hashiguchi S., Yamaguchi Y., Takeuchi O., Akira S., Sugimura K. Immunological basis of M13 phage vaccine: Regulation under MyD88 and TLR9 signaling. Biochem. Biophys. Res. Commun. 2010;402(1):19–22. doi: 10.1016/j.bbrc.2010.09.094. [DOI] [PubMed] [Google Scholar]
- 60.Mascolo D., Barba P., De Berardinis P., Di Rosa F., Del Pozzo G. Phage display of a CTL epitope elicits a long-term in vivo cytotoxic response. FEMS Immunol. Med. Microbiol. 2007;50(1):59–66. doi: 10.1111/j.1574-695X.2007.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ulivieri C., Citro A., Ivaldi F., Mascolo D., Ghittoni R., Fanigliulo D., Manca F., Baldari C.T., Li Pira G., Del Pozzo G. Antigenic properties of HCMV peptides displayed by filamentous bacteriophages vs. synthetic peptides. Immunol. Lett. 2008;119(1-2):62–70. doi: 10.1016/j.imlet.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 62.Del Pozzo G., Mascolo D., Sartorius R., Citro A., Barba P., D'Apice L., De Berardinis P. Triggering DTH and CTL activity by fd filamentous bacteriophages: role of CD4+ T cells in memory responses. J. Biomed. Biotechnol. 2010. [DOI] [PMC free article] [PubMed]
- 63.Dor-On E., Solomon B. Targeting glioblastoma via intranasal administration of Ff bacteriophages. Front. Microbiol. 2015;6:530. doi: 10.3389/fmicb.2015.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sartorius R., D’Apice L., Trovato M., Cuccaro F., Costa V., De Leo M.G., Marzullo V.M., Biondo C., D’Auria S., De Matteis M.A., Ciccodicola A., De Berardinis P. Antigen delivery by filamentous bacteriophage fd displaying an anti-DEC-205 single-chain variable fragment confers adjuvanticity by triggering a TLR9-mediated immune response. EMBO Mol. Med. 2015;7(7):973–988. doi: 10.15252/emmm.201404525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Segura-Velazquez R., Cervantes J., Acosta E., Sanchez-Betancourt I., Villalobos N., Rodarte L.F., Restelli M., Fragoso G., Sciutto E. Influenza vaccine: development of a novel intranasal and subcutaneous recombinant adjuvant. Vaccine. 2013;31(37):4009–4016. doi: 10.1016/j.vaccine.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 66.Samoylov A., Cochran A., Schemera B., Kutzler M., Donovan C., Petrenko V., Bartol F., Samoylova T. Humoral immune responses against gonadotropin releasing hormone elicited by immunization with phage-peptide constructs obtained via phage display. J. Biotechnol. 2015;216:20–28. doi: 10.1016/j.jbiotec.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 67.Trepel M., Arap W., Pasqualini R. Modulation of the immune response by systemic targeting of antigens to lymph nodes. Cancer Res. 2001;61(22):8110–8112. [PubMed] [Google Scholar]
- 68.Sartorius R., Bettua C., D’Apice L., Caivano A., Trovato M., Russo D., Zanoni I., Granucci F., Mascolo D., Barba P., Del Pozzo G., De Berardinis P. Vaccination with filamentous bacteriophages targeting DEC-205 induces DC maturation and potent anti-tumor T-cell responses in the absence of adjuvants. Eur. J. Immunol. 2011;41(9):2573–2584. doi: 10.1002/eji.201141526. [DOI] [PubMed] [Google Scholar]
- 69.Prudencio C.R., Marra A.O., Cardoso R., Goulart L.R. Recombinant peptides as new immunogens for the control of the bovine tick, Rhipicephalus(Boophilus) microplus. Vet. Parasitol. 2010;172(1-2):122–131. doi: 10.1016/j.vetpar.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 70.Wu H.W., Hu X.M., Wang Y., Kurtis J.D., Zeng F.J., McGarvey S.T., Wu G.L., Zhang Z.S., Hua Z.C. Protective immunity induced by phage displayed mitochondrial related peptides of Schistosoma japonicum. Acta Trop. 2006;99(2-3):200–207. doi: 10.1016/j.actatropica.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 71.van Houten N.E., Henry K.A., Smith G.P., Scott J.K. Engineering filamentous phage carriers to improve focusing of antibody responses against peptides. Vaccine. 2010;28(10):2174–2185. doi: 10.1016/j.vaccine.2009.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Henry K.A., Murira A., van Houten N.E., Scott J.K. Developing strategies to enhance and focus humoral immune responses using filamentous phage as a model antigen. Bioeng. Bugs. 2011;2(5):275–283. doi: 10.4161/bbug.2.5.16559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Naz R.K. Contraceptive vaccines: success, status, and future perspective. Am. J. Reprod. Immunol. 2011;66(1):2–4. doi: 10.1111/j.1600-0897.2011.00999.x. [DOI] [PubMed] [Google Scholar]
- 74.Yu M., Zeng W., Pagnon J., Walker J., Ghosh S., Wang L.F., Jackson D.C. Identification of dominant epitopes of synthetic immunocontraceptive vaccines that induce antibodies in dogs. Vaccine. 2005;23(37):4589–4597. doi: 10.1016/j.vaccine.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 75.Zeng W., Pagnon J., Jackson D.C. The C-terminal pentapeptide of LHRH is a dominant B cell epitope with antigenic and biological function. Mol. Immunol. 2007;44(15):3724–3731. doi: 10.1016/j.molimm.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 76.Remy J.J., Couture L., Rabesona H., Haertle T., Salesse R. Immunization against exon 1 decapeptides from the lutropin/choriogonadotropin receptor or the follitropin receptor as potential male contraceptive. J. Reprod. Immunol. 1996;32(1):37–54. doi: 10.1016/s0165-0378(96)00991-6. [DOI] [PubMed] [Google Scholar]
- 77.Abdennebi L., Couture L., Grebert D., Pajot E., Salesse R., Remy J.J. Generating FSH antagonists and agonists through immunization against FSH receptor N-terminal decapeptides. J. Mol. Endocrinol. 1999;22(2):151–159. doi: 10.1677/jme.0.0220151. [DOI] [PubMed] [Google Scholar]
- 78.Abdennebi L., Chun E.Y., Jammes H., Wei D., Remy J.J. Maintenance of sexual immaturity in male mice and bucks by immunization against N-terminal peptides of the follicle-stimulating hormone receptor. Biol. Reprod. 2003;68(1):323–327. doi: 10.1095/biolreprod.102.003699. [DOI] [PubMed] [Google Scholar]
- 79.Rao A.J., Ramachandra S.G., Ramesh V., Couture L., Abdennebi L., Salesse R., Remy J.J. Induction of infertility in adult male bonnet monkeys by immunization with phage-expressed peptides of the extracellular domain of FSH receptor. Reprod. Biomed. Online. 2004;8(4):385–391. doi: 10.1016/s1472-6483(10)60921-2. [DOI] [PubMed] [Google Scholar]
- 80.Sambroni E., Abdennebi-Najar L., Remy J.J., Le Gac F. Delayed sexual maturation through gonadotropin receptor vaccination in the rainbow trout Oncorhynchus mykiss. Gen. Comp. Endocrinol. 2009;164(2-3):107–116. doi: 10.1016/j.ygcen.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 81.Clayton R., Cooke I.D., Partridge L.J., Moore H.D. A combinatorial phage display library for the generation of specific Fab fragments recognizing human spermatozoa and inhibiting fertilizing capacity in vitro. Biol. Reprod. 1998;59(5):1180–1186. doi: 10.1095/biolreprod59.5.1180. [DOI] [PubMed] [Google Scholar]
- 82.Samuel A.S., Naz R.K. Isolation of human single chain variable fragment antibodies against specific sperm antigens for immunocontraceptive development. Hum. Reprod. 2008;23(6):1324–1337. doi: 10.1093/humrep/den088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fiszer D., Pupecka M., Schmidt K., Rozwadowska N., Kamieniczna M., Grygielska B., Kurpisz M. Specific Fab fragments recovered by phage display technique recognizing human spermatozoa. Int. J. Androl. 2009;32(5):442–452. doi: 10.1111/j.1365-2605.2008.00876.x. [DOI] [PubMed] [Google Scholar]
- 84.Eidne K.A., Henery C.C., Aitken R.J. Selection of peptides targeting the human sperm surface using random peptide phage display identify ligands homologous to ZP3. Biol. Reprod. 2000;63(5):1396–1402. doi: 10.1095/biolreprod63.5.1396. [DOI] [PubMed] [Google Scholar]
- 85.Samoylova T.I., Cox N.R., Cochran A.M., Samoylov A.M., Griffin B., Baker H.J. ZP-binding peptides identified via phage display stimulate production of sperm antibodies in dogs. Anim. Reprod. Sci. 2010;120(1-4):151–157. doi: 10.1016/j.anireprosci.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 86.Bartol F., Spate L., Samoylov A., Petrenko V., Prather R., Samoylova T. In Phage-peptide constructs for immunocontraception of wild pigs: effects on events associated with fertilization in vitro., Society for the Study of Reproduction 48th Annual Meeting, San Juan, Puerto Rico, USA, June 18-22,2015; San Juan, Puerto Rico, USA.; 2015. [Google Scholar]
- 87.Samoylova T.I., Cochran A.M., Samoylov A.M., Schemera B., Breiteneicher A.H., Ditchkoff S.S., Petrenko V.A., Cox N.R. Phage display allows identification of zona pellucida-binding peptides with species-specific properties: novel approach for development of contraceptive vaccines for wildlife. J. Biotechnol. 2012;162(2-3):311–318. doi: 10.1016/j.jbiotec.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 88.Tissot A.C., Renhofa R., Schmitz N., Cielens I., Meijerink E., Ose V., Jennings G.T., Saudan P., Pumpens P., Bachmann M.F. Versatile virus-like particle carrier for epitope based vaccines. PLoS One. 2010;5(3):e9809. doi: 10.1371/journal.pone.0009809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Caldeira J., Bustos J., Peabody J., Chackerian B., Peabody D.S. Epitope-Specific Anti-hCG Vaccines on a Virus Like Particle Platform. PLoS One. 2015;10(10):e0141407. doi: 10.1371/journal.pone.0141407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lua L.H., Connors N.K., Sainsbury F., Chuan Y.P., Wibowo N., Middelberg A.P. Bioengineering virus-like particles as vaccines. Biotechnol. Bioeng. 2014;111(3):425–440. doi: 10.1002/bit.25159. [DOI] [PubMed] [Google Scholar]
- 91.Jeong H., Seong B.L. Exploiting virus-like particles as innovative vaccines against emerging viral infections. J. Microbiol. 2017;55(3):220–230. doi: 10.1007/s12275-017-7058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]