Abstract
For centuries, phytochemicals have been used to prevent and cure multiple health ailments. Phytochemicals have been reported to have antioxidant, antidiabetic, antitussive, antiparasitic, anticancer, and antimicrobial properties. Generally, the therapeutic use of phy-tochemicals is based on tradition or word of mouth with few evidence-based studies. Moreo-ver, molecular level interactions or molecular targets for the majority of phytochemicals are unknown. In recent years, antibiotic resistance by microbes has become a major healthcare concern. As such, the use of phytochemicals with antimicrobial properties has become perti-nent. Natural compounds from plants, vegetables, herbs, and spices with strong antimicrobial properties present an excellent opportunity for preventing and combating antibiotic resistant microbial infections. ATP synthase is the fundamental means of cellular energy. Inhibition of ATP synthase may deprive cells of required energy leading to cell death, and a variety of die-tary phytochemicals are known to inhibit ATP synthase. Structural modifications of phyto-chemicals have been shown to increase the inhibitory potency and extent of inhibition. Site-directed mutagenic analysis has elucidated the binding site(s) for some phytochemicals on ATP synthase. Amino acid variations in and around the phytochemical binding sites can re-sult in selective binding and inhibition of microbial ATP synthase. In this review, the therapeu-tic connection between dietary phytochemicals and ATP synthase is summarized based on the inhibition of ATP synthase by dietary phytochemicals. Research suggests selective target-ing of ATP synthase is a valuable alternative molecular level approach to combat antibiotic resistant microbial infections.
Keywords: Microbial and mammalian F1Fo ATP synthase, antimicrobial phytochemicals, polyphenols, enzyme inhibition, molecular drug target
1. Introduction
Antimicrobial resistance is becoming an existential threat to mankind. According to The Review on Antimicrobial Resistance [1] antibiotic resistance will result in about ten million additional deaths per year worldwide by 2050. Currently, more than 700,000 people die from microbial infections every year. Thus, antibiotic-resistant microbes are expected to become the top global killers, surpassing cancer. The impact of this public health crisis on the global economy is projected to be about $100 trillion [2]. Furthermore, bacteria keep evolving so that they can resist the new drugs that are used to combat them. This fast-encroaching antibiotic resistance has become particularly problematic in recent years because the discovery of new antibiotics has not kept pace. Finally, this problem is not limited to bacteria because all microbes that have the potential to mutate can make widely used drugs ineffective [1].
Antibiotic resistance threatens the prevention and treatment of infections caused by bacteria, parasites, viruses, and fungi. Klebsiella pneumonia, Staphylococcus aureus, Streptococcus pneumoniae, and Neisseria gonorrhoeae, in general, and Escherichia coli, in particular, are the main reasons for this alarming situation [3-5]. E. coli, a naturally resistant, gram-negative bacterium with a complex cell wall barrier against several drugs, is at the forefront of drug resistance [6]. Finding new, alternative ways to kill microbes is of paramount importance. In this review, we reiterate the therapeutic link between antimicrobial properties of dietary phytochemicals and energy-generating ATP synthase as a potent molecular drug target.
2. ATP synthase
The enzyme, ATP synthase (EC 3.6.3.14), is important for the normal physiological function of cells because it is the principal energy-generating nanomotor of cells. Structurally, ATP synthase is very similar in almost all organisms from bacteria to man. In its simplest form, bacterial ATP synthase has two sectors, a water-soluble F1 sector and a membrane-embedded Fo sector. The F1 sector is composed of five subunits α3β3γδε, where the catalytic activity occurs; and the Fo sector has three subunits from which protons are pumped (Fig. 1). In the catalytic subunits, the cyclical process known as the binding change mechanism generates adenosine triphosphate (ATP) from adenosine diphosphate (ADP) and inorganic phosphate (Pi) [7].
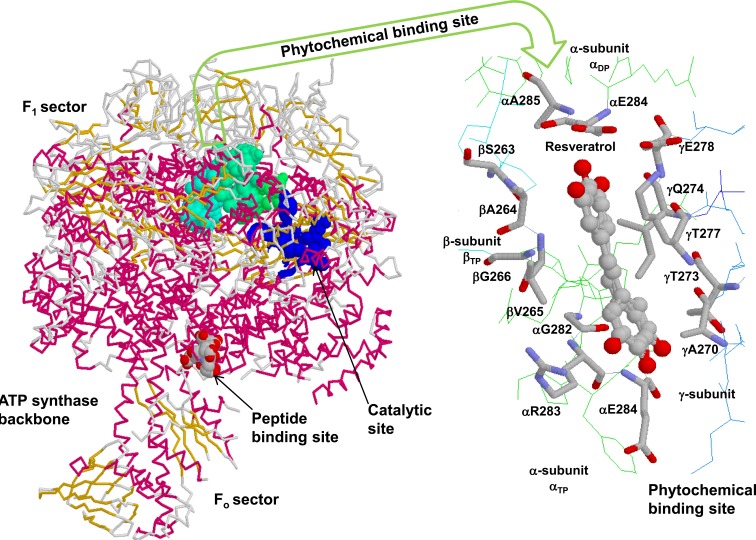
Fig. (1).
Backbone form of F1Fo ATP synthase with phytochemical binding site. The F1 sector of the enzyme shows the catalytic Pi (phosphate) binding subdomain, peptide, and phytochemical binding sites in space fill form. The resveratrol bound phytochemical binding site contributed by α-, β-, and γ-subunit residues is zoomed in wireframe form. Figure was taken from references [11, 28] and was generated by PDB files 1H8E [146] and 2JIZ [10] using Rasmol [147].
Mammalian mitochondrial F1Fo ATP synthase is slightly more complex than the bacterial ATP synthase, with additional subunits and different names. For example, δ subunit of mammalian ATP synthase is homologous to the ε subunit of E. coli. A subunit called oligomycin sensitivity conferral protein of mammalian ATP synthase is homologous to the δ subunit of E. coli ATP synthase. Mammalian ATP synthase also has two additional subunits d and F6 [8, 9]. Additionally, there are multiple variations in amino acid types and positions in all subunits. These variations may play a critical role in selective binding of inhibitors [10-13].
The cytoplasmic concentrations of ATP and Pi in the active cells are in the range of 2-5 mM, whereas that of ADP is at least 10-50-fold lower. Equilibrium binding assays have established that both ADP and ATP bind to catalytic sites of F1Fo ATP synthase with relatively similar binding affinities [14-18]. With such unfavorable low physiological concentration of ADP in the cells, the designated catalytic site amino acids in the α/β interface of ATP synthase bind to Pi and support the ADP and Pi interaction to form ATP [19]. ATP thus formed is the fundamental means of cellular energy. Inhibition of ATP synthase will cease the ATP formation depriving cells of vital energy. Dietary phytochemicals and other inhibitors cause variable degree of ATP synthase inhibition. The extent of inhibition is directly proportional to the molecular level interactions which depend on the interaction between functional groups of inhibitors and binding site amino acids of ATP synthase. The molecular-level interactions between inhibitors and binding-site residues have been resolved for some and are under investigation for many [10-12, 20-25].
3. ATP synthase under disease conditions
ATP synthase is vital to human health. Malfunction of this energy-generating enzyme complex has been associated with a variety of pathological conditions [20, 26], such as hypertension, alcoholism, cardiovascular diseases, cancer, tuberculosis, neuropathy, Alzheimer’s disease, Parkinson’s disease, Down’s syndrome, neuronal diseases, aging, immune responses, uteroplacental insufficiency, albinism and mitochondrial myopathies [20, 26-28]. Multiple studies have linked the alteration of F1Fo ATP synthase to these disease conditions [26]. For instance, alcohol is known to severely damage the mitochondria. With chronic alcoholism, production of ATP by F1Fo ATP synthase declines in the liver and brain [29]. In one study, the cysteine and tyrosine residues of α- and β-subunits of F1Fo ATP synthase were oxidized in rats subjected to prolonged ethanol exposure, resulting in decreased mitochondrial ATP production [30]. In diabetes, F1Fo ATP synthase also plays a significant role. Compromised F1Fo ATP synthase has been shown to result in diminished ATP production, which affects insulin secretion and glucose uptake [31]. Further, the phosphorylation of βTyr-361 and βThr-213 has been shown to be responsible for the down regulation of β-subunit F1Fo ATP synthase, and the ATP synthase β-subunit amino acids from the skeletal muscles of type 2 diabetic and obese patients have shown pronounced phosphorylation at multiple sites [32].
ATP synthase has been linked to high blood pressure. High levels of circulating subunit F6 have been observed with hypertension, suggesting an association between ATP synthase and high blood pressure [33, 34]. The F1Fo enzyme has also been implicated in autoimmune disease conditions, such as systemic lupus erythematosus (SLE). For instance, the ATP depletion in T-cells and defective DNA repair mechanisms may contribute to the autoimmune pathology of SLE. A comparative DNA micro array of healthy and SLE human patients showed down regulation of A6L and a-subunit of ATP synthase [35]. In maternally inherited Leigh syndrome, a neurodegenerative disease and neuropathy, ataxia, the T8993G mutation in the a-subunit causes a severe impairment and dysfunction of ATP synthase [36-39]. Human neuropathological conditions have been documented with mutations in the mitochondrial genome with T8993G or T8993C causing energy depletion or mitochondrial reactive oxygen species production, respectively. These mutations were found to disrupt the ATP synthase coding for aLeu-156 [37]. Abnormal lysosomal ATP synthase c-subunit accumulation has been observed in Batten disease, a neurodegenerative fatal disease of humans and other animals [40].
Aging also affects the activity levels of F1Fo ATP synthase subunits. With age, an increase in the expression of α-, β-, and d-subunits was observed in rat skeletal muscle [41] and an increase in the expression of F-subunit was observed in mouse brain [42]. Age-induced decreases in α- and β-subunits have been documented in mouse heart [43] along with decreased ATPase activity in aging rat kidneys [44]. One of the age-related mitochondrial defects is a 4977 base pair deletion. This deletion negatively affects the biological oxidation of electron transport chain complexes nicotinamide adenine dinucleotide dehydrogenase, cytochrome oxidase, and ATP synthase [45]. In another study, age-related ATP synthase changes in skeletal muscle have been observed, resulting in decreased ATP production per mole of O2 consumed [46]. In Alzheimer’s disease or presenile dementia, a deficiency of mitochondrial ATP synthase was reported [47]. A low expression of β-subunit and buildup of the α-subunit have been noted in Alzheimer’s disease, and ATP synthase α-subunit buildup in the intraneuronal cytosole is associated with the neurodegenerative process [48-50]. Moreover, ectopic ATP synthase on the cell surface of endothelial cells is implicated in the angiogenesis process that is essential for tumor growth [51-54].
4. ATP synthase as a molecular drug target
ATP synthase is also being used and recommended as an effective molecular drug target for multiple disease conditions and for the regulation of energy metabolism ([20, 26, 55, 56] and references therein). Selective and specific inhibition makes ATP synthase an excellent molecular target for the development of new antimicrobial agents. For example, the antituberculosis drug Bedaquiline, approved by the US Food and Drug Administration in 2012, is highly selective in its inhibition of the Fo sector of ATP synthase in mycobacterial species [2, 57-61]. Bz-423 a drug for the autoimmune disorder systemic lupus erythematosus selectively kills pathogenic lymphocytes by inducing apoptosis in lymphoid cells [62]. Bz-423 inhibits the mitochondrial ATP synthase by binding the subunit OSCP the oligomycin sensitivity-conferring protein [63, 64].
Apoptolidin, a selective cytotoxic agent, is an inhibitor of F1Fo ATP synthase. Apoptolidin is a macrolide originally isolated from the Nocardiopsis species and selectively kills E1A and E1A/E1B19K transformed rat glial cells (IC50 = 11 ng/ml) while not killing untransformed glial cells [65]. Apoptolidin is among the most selective cytotoxic agents tested by the National Cancer Institute in human cancer cell lines. The apoptotic mechanism of action of apoptolidin is through selective inhibition of F1Fo ATP synthase [66, 67]. Selective ATP synthase inhibition has been attributed to the type and position of amino acids in and around the inhibitor binding sites [22, 25]. Slight variation in amino acids was shown to cause selective and specific inhibition of ATP synthase from a variety of food sources. For example, tentoxin strongly inhibits F1-ATPase in spinach, potato, and lettuce but causes no inhibition of the same enzyme from species, such as corn and radish, even though they exhibit high sequence and structural similarity [68-70].
5. Ectopic ATP synthase
The inner membrane of the mitochondria was considered the exclusive location of F1Fo-ATP synthase [19]. However, multiple studies have documented the occurrence of ectopic F1Fo-ATP synthase on cell membranes. The actual protein transport mechanism of the ectopic ATP synthase is not clear. The green fluorescent protein-ATP5B fusion introduced into HepG2 cells to study the localization of the ATP synthase suggested that the ectopic expression of ATP synthase occurs from the translocation of mitochondrial ATP synthase [71].
Targeting of ectopic ATP synthase inhibits cytosolic lipid droplet buildup, making ATP synthase a potential molecular target for antiobesity drugs [72]. Ectopic ATP synthase serves as a ligand receptor and participates in numerous cellular processes, such as angiogenesis, lipid metabolism, and the cytolytic pathway of tumor cells. Inhibition of ATP synthase blocks tumor angiogenesis, making it a suitable antiangiogenic therapeutic target [51, 54, 73-77]. Inhibition of ectopic ATP synthase prominently obstructs the migration and proliferation of endothelial cells with little effect on intracellular ATP [75]. On the surface of endothelial cells ectopic ATP synthase β-subunit activates cytotoxic activity by attracting inflammatory cells [78]. Additionally, ectopic ATP synthase was also identified to mediate HIV-1 transfer between antigen-presenting cells and CD4+ target cells [79].
6. ATP synthase inhibitors
More than 300 natural and synthetic molecules are known to bind and inhibit ATP synthase. The interaction between the majority of these inhibitors and ATP synthase residues and the specific sites remains unknown [10, 20, 80]. The two therapeutically important antimicrobial ATP synthase inhibitors are antimicrobial peptides, which mainly bind at the peptide-binding pocket formed by the βDELSEED-motif [13, 81-88], and antimicrobial phytochemicals, which mainly bind at the phytochemical or polyphenol binding pocket contributed by α-, β-, and, γ-subunit residues [10-12, 21-24, 80, 88-103]. Selective inhibition of F1Fo ATP synthase is a promising way to deal with multiple disease conditions, including antibiotic-resistant microbial infections.
7. Phytochemicals as inhibitors of ATP synthase
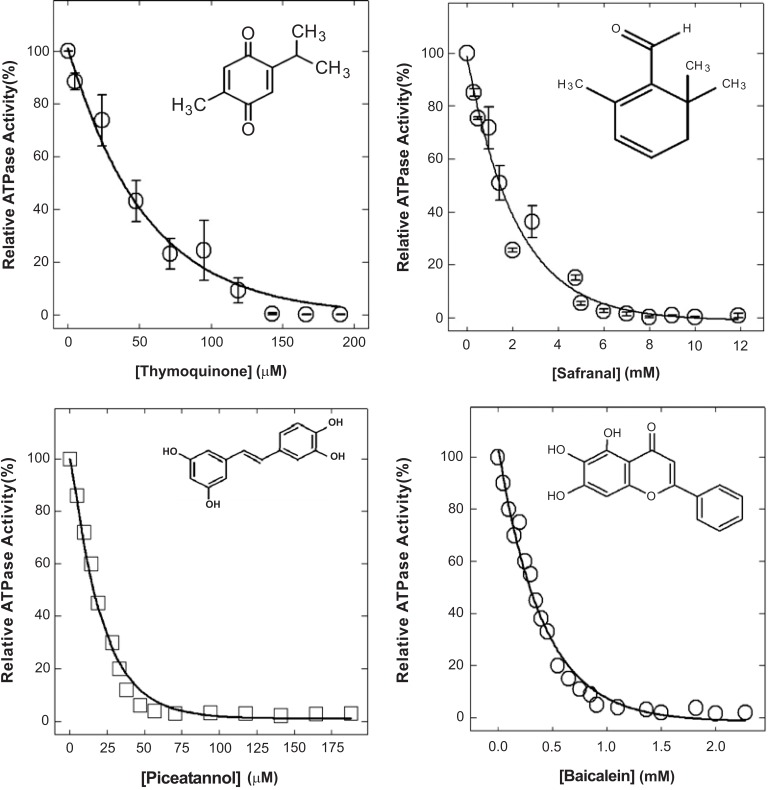
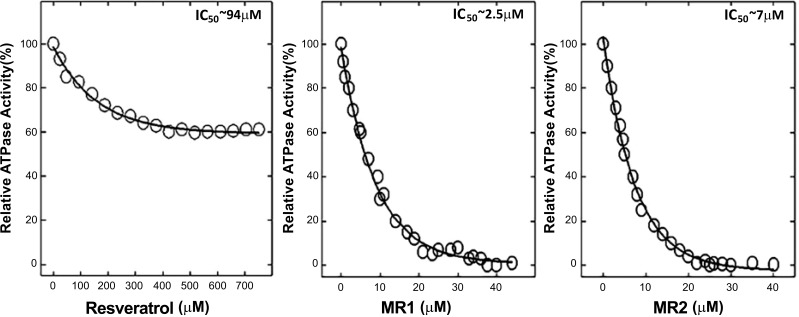
A wide range of phytochemicals are known to have antimicrobial properties [4, 5]. Many of them have been shown to inhibit bacterial ATP synthase (Figs. 2 and 3). Thymoquinone [21], safranal [22], piceatannol [11], and baicalein [12] induced complete inhibition of E. coli wild-type F1Fo ATP synthase as shown in Fig. (2). The Table 1 lists some of the antimicrobial phytochemicals that inhibit bacterial (E. coli) ATP synthase to variable degrees. Potency and the extent of inhibition on a molar scale differ among the various inhibitors. Further, the extent of ATP synthase inhibition depends on the type and positioning of the functional groups of phytochemicals [89]. Thus, addition, deletion, and rearrangement of functional groups can enhance the degree of inhibition. For example, as shown in (Fig. 3), natural resveratrol causes about 40% inhibition of ATP synthase with IC50 at about 94 µM. Structural modulation of resveratrol by removal, addition, or repositioning of its functional groups resulted in 100% inhibition with IC50 values from about 94 µM to about 2.50 µM [11, 89]. Another phytochemical, hydroxytyrosol from olives, caused about 60% inhibition of E. coli membrane-bound F1Fo ATP synthase. Structural modifications with the repositioning of its -OH groups resulted in almost 100% inhibition (Z. Ahmad unpublished data).
Fig. (2).
Phytochemical induced inhibition of F1Fo ATP synthase. Thymoquinone, safranal, piceatannol, and baicalein induced inhibition of E. coli wild-type F1Fo ATP synthase. Figure compiled from references [11, 12, 21, 22].
Fig. (3).
Inhibitory effect of resveratrol and structurally modified resveratrol containing nitro groups hydroxyl-nitrophenyl-imino methylphenol (MR1 & MR2). Structural modification caused 100% inhibition and reduced IC50 from 94 µM to 2.5 µM and 7 µM. Figure taken from reference [89].
Table 1.
Phytochemical induced inhibition of bacterial wild-type F1Fo ATP synthase.
| Phytochemicals |
Maximal inhibition
(~ %) |
Estimated
IC50 values (µM) |
Reference source
(references number) |
|---|---|---|---|
| Resveratrol | 40 | N/A | [11] |
| Piceatannol | 100 | 16 | [11] |
| Quercetin | 80 | N/A | [11] |
| Quercitrin | 40 | N/A | [11] |
| Quercetin-3-β-D glucoside | 50 | N/A | [11] |
| Morin | 100 | 70 | [12] |
| Silymarin | 100 | 110 | [12] |
| Baicalein | 100 | 290 | [12] |
| Amantadine | 100 | 2500 | [12] |
| Rimantadine | 100 | 2000 | [12] |
| Epicatechin | 100 | 4000 | [12] |
| Silibinin | 100 | 340 | [12] |
| Hesperdin | 60 | N/A | [12] |
| Apigenin | 40 | N/A | [12] |
| Diosmin | 50 | N/A | [12] |
| Chrysin | 60 | N/A | [12] |
| Rutin | 40 | N/A | [12] |
| Kaempferol | 58 | N/A | [12] |
| Genistein | 40 | N/A | [12] |
| Galangin | 0 | N/A | [12] |
| Luteolin | 20 | N/A | [12] |
| Daidzein | 10 | N/A | [12] |
| Hydroquinone | 80 | N/A | [89] |
| Dihydrothymoquinone | 40 | N/A | [89] |
| Resorcinol | 20 | N/A | [89] |
| Catechol | 40 | N/A | [89] |
| Modified resveratrol 1 (MR1) | 100 | 2.5 | [89] |
| Modified resveratrol 2 (MR2) | 100 | 7 | [89] |
| Thymoquinone | 100 | 38 | [21] |
| Safranal | 100 | 1600 | [22] |
| Thymol | 87 | N/A | [22] |
| Carvacrol | 100 | 2800 | [22] |
| Damascenone | 85 | N/A | [22] |
| Cuminol | 93 | N/A | [22] |
| 4-Kettoisophorone | 90 | N/A | [22] |
| Curcumin | 60 | N/A | [24] |
| Tyrosol | 100 | 9500 | [25] |
| Hydroxytyrosol | 62 | N/A | [25] |
| Dihydroxyphenylglycol | 35 | N/A | [25] |
| Oleuropein | 40 | N/A | [25] |
| Theaflavin (TF1) | 85 | N/A | [23] |
| Theaflavin-3-gallate (TF2A) | 95 | N/A | [23] |
| Theaflavin-3′-gallate (TF2B) | 95 | N/A | [23] |
| Theaflavin-3,3′-digallate (TF3) | 90 | N/A | [23] |
| Epigallocatechin gallate (EGCG) | 95 | N/A | [23] |
8. Significance and rationale of dietary phytochemicals as inhibitors
Phytochemicals are naturally occurring plant-based compounds that possess antimicrobial, chemopreventive, and antioxidant properties [10, 23, 104-106]. Foods such as apples, berries, cherries, grapes, pears, plums, dates, cantaloupe, ginger, turmeric, garlic, onions, broccoli, olives, and saffron are rich in phytochemicals with antimicrobial properties [107-119]. Phenolic compounds are one of the important classes of phytochemicals shown to block the action of enzymes and other substances that promote the growth of cancer [120-123] and microbial cells [124-128].
Physiological relevance of dietary phytochemicals can be ascribed to their interaction with mitochondrion in eukaryotic cells. Throughout the aging process, many degenerating diseases and neurological disorders are attributed to mitochondrial dysfunction [38]. Thus, the selective inhibition of ATP synthase by natural or structurally modified phytochemicals might play a significant role in the physiology of such conditions [10, 12, 23, 89, 129, 130]. Grape constituent resveratrol, a dietary phenolic phytochemical, is widely known for its chemopreventive actions [121, 122, 131-135] and, in some cases, it has induced apoptosis via mitochondrial pathways [27, 132]. Earlier [136], oligomycin, a highly specific ATP synthase inhibitor, was shown to induce an apoptotic suicide response in cultured human lymphoblastoid and other mammalian cells within 12-18 hours but not in ρo cells that are depleted of a functional mitochondrial respiratory chain. A similar study [137] suggested that inhibition of the components of mitochondrial pathways may lead to the marking of some cells, via CD14, for cell death, whilst allowing differentiation to occur in the surviving population. Thus, there is possibility of targeting tumor cells without affecting the normal cells through selective inhibition of ATP synthase by phenolic phytochemicals [10-12, 23].
For centuries, dietary phytochemicals have been used worldwide as household remedies for multiple ailments, including antimicrobial agents [116, 138-141]. Multiple studies have linked the antimicrobial actions of dietary phytochemicals to the inhibition of bacterial F1Fo ATP synthase [11, 12, 23, 27]. Biological activity against Streptococcus mutans is an excellent example. S. mutans is a primary microbial agent in the pathogenesis of dental caries, and dietary phenolic phytochemicals have been shown to inhibit biofilm formation and acid production by S. mutans. One of the pathways through which phytochemicals are active against traits of S. mutans is the selective inhibition of its proton-translocating ATP synthase activity [130]. Thus, further understanding of the mechanism of inhibition of ATP synthase by natural, synthetic, and structurally modified dietary phytochemicals has a potential to develop better strategies for combating drug-resistant bacteria.
Research suggests that the beneficial effects of dietary phytochemicals are linked to the blocking of ATP synthesis in tumor and bacterial cells [10, 12, 22]. Many dietary phytochemicals, such as safranal, resveratrol, piceatannol, morin, silymarin, baicalein, silibinin, rimantadin, amantidin, epicatechin, and theaflavin, bind and inhibit E. coli ATP synthase [11, 12, 22, 23]. Further, the wild-type (pBWU13.4/DK8) E. coli growth was fully abrogated, and little or no growth inhibition occurred in the null (pUC118/DK8) E. coli strain with the deleted ATPase gene in the presence of dietary phenolic compounds [11, 12, 21, 22, 24]. In the absence of ATP synthase, the little loss of growth among null cells could be the result of action on other possible targets. The damage to the cell membrane by destabilization or permeabilization, inhibition of other microbial enzymes, or blockage of essential substrates, such as iron or zinc, required for microbial growth are possible [26, 96, 142-145]. Likewise, the total loss of growth in wild-type E. coli can be attributed to the inhibition of ATP synthase along with other targets [13].
9. Significance of mutagenic analysis of inhibitor-binding sites and surrounding residues of ATP synthase
To generate structurally and functionally potent antibacterial phytochemicals, it is vital to understand their interaction with binding sites and the surrounding residues. Resveratrol-, piceatannol-, and quercetin-bound x-ray structures suggest that α-, β-, and γ-subunit residues αG282, αR283, αE284, αA285, βS263, βA264, βV265, γA270, γT273, γQ274, γT277, and γE278 may be directly involved in polyphenol binding [10]. Moreover, about 30 plus amino acids closely flank the phytochemical binding pocket, and so far their role has not been deciphered. The side chains of these residues protrude directly into the phytochemical binding pocket. Therefore, the flanking residues may have some role in the binding and orientation of phytochemicals.
A majority of the phytochemical binding and surrounding residues are highly conserved throughout the evolution but do have some species or organismal level variations. There are more than 50 variations in and around the phytochemical binding pocket of E. coli and Homo sapiens (human) ATP synthase [10-12]. Moreover, there is a stretch of 11 amino acids between α311 and α321 omitted in human ATP synthase that encloses the polyphenol binding site of E. coli. These variations may provide the basis for selective inhibition of bacterial ATP synthase. These differences may also help in modifying the functional groups of inhibitors to make them more selective and potent. Charge and mass of the inhibitor binding site and its surrounding residues play a critical role in the binding and interaction with phytochemical functional groups. Therefore, mutagenic analysis of the inhibitor binding site and surrounding residues is pivotal for elucidating the action and selective interaction of phytochemicals with the inhibitor binding site.
Recently mutagenic analysis of phytochemical binding site residues has allowed us to specify the residues required for the binding and inhibition of safranal and tyrosol [22]. For example, αArg-283 plays critical role and is required for the binding of safranal and tyrosol, while residues αGlu-284, βVal-265, and γThr-273A play a less important role in binding and inhibition by safranal and tyrosol [22]. Moreover, both F1 and Fo sector subunit residues have been identified and documented as contributing to binding sites for phytochemicals [10, 20, 26, 28, 55].
Conclusion
Phytochemical induced inhibitory studies of wild-type, mutant, and null E. coli ATP synthase suggest that the antimicrobial properties of dietary phytochemicals can be linked to the inhibition of bacterial ATP synthase. Selective inhibition of ATP synthase provides an alternative way to combat antibiotic-resistant microbial infections. Mutagenic analysis of the phytochemical binding site(s) can help identify selective and potent inhibitors. Functional groups on the dietary phytochemicals are critical for apt and effective inhibition. The potency and degree of inhibition of ATP synthase can be amplified by structural modulations, additions, and subtractions of functional groups on phytochemicals. Moreover, organismal and species level variations in the residues flanking the conserved phytochemical binding site can be utilized to identify the selective and potent microbial inhibitors.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health, grant no. GM085771, to ZA. We are also thankful to Deborah Goggin, scientific writer, Research Support, A.T. Still University for reviewing this article.
List of abbreviations
- ADP
Adenosine diphosphate
- ATP
Adenosine triphosphate
- DNA
Deoxyribonucleic acid
- Pi
Inorganic phosphate
- ROS
Reactive oxygen species
- SLE
Systemic lupus erythematosus
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.O’Neill J. Antimicrobial resistance: Tackling a crisis for health and wealth of nations. https://amr-review.org/sites/default/files/AMR
- 2.Ling L.L., Schneider T., Peoples A.J., Spoering A.L., Engels I., Conlon B.P., Mueller A., Schäberle T.F., Hughes D.E., Epstein S., Jones M., Lazarides L., Steadman V.A., Cohen D.R., Felix C.R., Fetterman K.A., Millett W.P., Nitti A.G., Zullo A.M., Chen C., Lewis K. A new antibiotic kills pathogens without detectable resistance. Nature. 2015;517(7535):455–459. doi: 10.1038/nature14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Antimicrobial Resistance: Global Report on Surveillance. 2014.
- 4.Mahmood H.Y., Jamshidi S., Sutton J.M., Rahman K.M. Current Advances in Developing Inhibitors of Bacterial Multidrug Efflux Pumps. Curr. Med. Chem. 2016;23(10):1062–1081. doi: 10.2174/0929867323666160304150522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borges A., Saavedra M.J., Simões M. Insights on antimicrobial resistance, biofilms and the use of phytochemicals as new antimicrobial agents. Curr. Med. Chem. 2015;22(21):2590–2614. doi: 10.2174/0929867322666150530210522. [DOI] [PubMed] [Google Scholar]
- 6.Blair J.M., Webber M.A., Baylay A.J., Ogbolu D.O., Piddock L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015;13(1):42–51. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- 7.Boyer P.D. A perspective of the binding change mechanism for ATP synthesis. FASEB J. 1989;3(10):2164–2178. doi: 10.1096/fasebj.3.10.2526771. [DOI] [PubMed] [Google Scholar]
- 8.Baker L.A., Watt I.N., Runswick M.J., Walker J.E., Rubinstein J.L. Arrangement of subunits in intact mammalian mitochondrial ATP synthase determined by cryo-EM. Proc. Natl. Acad. Sci. USA. 2012;109(29):11675–11680. doi: 10.1073/pnas.1204935109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu T., Pagadala V., Mueller D.M. Understanding structure, function, and mutations in the mitochondrial ATP synthase. Microb. Cell. 2015;2(4):105–125. doi: 10.15698/mic2015.04.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gledhill J.R., Montgomery M.G., Leslie A.G., Walker J.E. Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proc. Natl. Acad. Sci. USA. 2007;104(34):13632–13637. doi: 10.1073/pnas.0706290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dadi P.K., Ahmad M., Ahmad Z. Inhibition of ATPase activity of Escherichia coli ATP synthase by polyphenols. Int. J. Biol. Macromol. 2009;45(1):72–79. doi: 10.1016/j.ijbiomac.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Chinnam N., Dadi P.K., Sabri S.A., Ahmad M., Kabir M.A., Ahmad Z. Dietary bioflavonoids inhibit Escherichia coli ATP synthase in a differential manner. Int. J. Biol. Macromol. 2010;46(5):478–486. doi: 10.1016/j.ijbiomac.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azim S., McDowell D., Cartagena A., Rodriguez R., Laughlin T.F., Ahmad Z. Venom peptides cathelicidin and lycotoxin cause strong inhibition of Escherichia coli ATP synthase. Int. J. Biol. Macromol. 2016;87:246–251. doi: 10.1016/j.ijbiomac.2016.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmad Z., Senior A.E. Mutagenesis of residue betaArg-246 in the phosphate-binding subdomain of catalytic sites of Escherichia coli F1-ATPase. J. Biol. Chem. 2004;279(30):31505–31513. doi: 10.1074/jbc.M404621200. [DOI] [PubMed] [Google Scholar]
- 15.Weber J., Wilke-Mounts S., Lee R.S., Grell E., Senior A.E. Specific placement of tryptophan in the catalytic sites of Escherichia coli F1-ATPase provides a direct probe of nucleotide binding: maximal ATP hydrolysis occurs with three sites occupied. J. Biol. Chem. 1993;268(27):20126–20133. [PubMed] [Google Scholar]
- 16.Löbau S., Weber J., Senior A.E. Catalytic site nucleotide binding and hydrolysis in F1F0-ATP synthase. Biochemistry. 1998;37(30):10846–10853. doi: 10.1021/bi9807153. [DOI] [PubMed] [Google Scholar]
- 17.Weber J., Hammond S.T., Wilke-Mounts S., Senior A.E. Mg2+ coordination in catalytic sites of F1-ATPase. Biochemistry. 1998;37(2):608–614. doi: 10.1021/bi972370e. [DOI] [PubMed] [Google Scholar]
- 18.Dou C., Fortes P.A., Allison W.S. The alpha 3(beta Y341W)3 gamma subcomplex of the F1-ATPase from the thermophilic Bacillus PS3 fails to dissociate ADP when MgATP is hydrolyzed at a single catalytic site and attains maximal velocity when three catalytic sites are saturated with MgATP. Biochemistry. 1998;37(47):16757–16764. doi: 10.1021/bi981717q. [DOI] [PubMed] [Google Scholar]
- 19.Ahmad Z., Okafor F., Laughlin T.F. Role of charged residues in the catalytic sites of escherichia coli ATP synthase. 2011. [DOI] [PMC free article] [PubMed]
- 20.Hong S., Pedersen P.L. ATP synthase and the actions of inhibitors utilized to study its roles in human health, disease, and other scientific areas. Microbiol. Mol. Biol. Rev. 2008;72(4):590–641. doi: 10.1128/MMBR.00016-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmad Z., Laughlin T.F., Kady I.O. Thymoquinone Inhibits Escherichia coli ATP Synthase and Cell Growth. PLoS One. 2015;10(5):e0127802. doi: 10.1371/journal.pone.0127802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu M., Amini A., Ahmad Z. Safranal and its analogs inhibit Escherichia coli ATP synthase and cell growth. Int. J. Biol. Macromol. 2017;95:145–152. doi: 10.1016/j.ijbiomac.2016.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li B., Vik S.B., Tu Y. Theaflavins inhibit the ATP synthase and the respiratory chain without increasing superoxide production. J. Nutr. Biochem. 2012;23(8):953–960. doi: 10.1016/j.jnutbio.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sekiya M., Chiba E., Satoh M., Yamakoshi H., Iwabuchi Y., Futai M., Nakanishi-Matsui M. Strong inhibitory effects of curcumin and its demethoxy analog on Escherichia coli ATP synthase F1 sector. Int. J. Biol. Macromol. 2014;70:241–245. doi: 10.1016/j.ijbiomac.2014.06.055. [DOI] [PubMed] [Google Scholar]
- 25.Amini A., Liu M., Ahmad Z. Understanding the link between antimicrobial properties of dietary olive phenolics and bacterial ATP synthase. Int. J. Biol. Macromol. 2017;101:153–164. doi: 10.1016/j.ijbiomac.2017.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson J.A., Ogbi M. Targeting the F1Fo ATP synthase: modulation of the body’s powerhouse and its implications for human disease. Curr. Med. Chem. 2011;18(30):4684–4714. doi: 10.2174/092986711797379177. [DOI] [PubMed] [Google Scholar]
- 27.Ahmad Z., Laughlin T.F. Medicinal chemistry of ATP synthase: a potential drug target of dietary polyphenols and amphibian antimicrobial peptides. Curr. Med. Chem. 2010;17(25):2822–2836. doi: 10.2174/092986710791859270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmad Z., Okafor F., Azim S., Laughlin T.F. ATP synthase: a molecular therapeutic drug target for antimicrobial and antitumor peptides. Curr. Med. Chem. 2013;20(15):1956–1973. doi: 10.2174/0929867311320150003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bailey S.M., Cunningham C.C. Contribution of mitochondria to oxidative stress associated with alcoholic liver disease. Free Radic. Biol. Med. 2002;32(1):11–16. doi: 10.1016/s0891-5849(01)00769-9. [DOI] [PubMed] [Google Scholar]
- 30.Moon K.H., Hood B.L., Kim B.J., Hardwick J.P., Conrads T.P., Veenstra T.D., Song B.J. Inactivation of oxidized and S-nitrosylated mitochondrial proteins in alcoholic fatty liver of rats. Hepatology. 2006;44(5):1218–1230. doi: 10.1002/hep.21372. [DOI] [PubMed] [Google Scholar]
- 31.Stump C.S., Short K.R., Bigelow M.L., Schimke J.M., Nair K.S. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc. Natl. Acad. Sci. USA. 2003;100(13):7996–8001. doi: 10.1073/pnas.1332551100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Højlund K., Yi Z., Lefort N., Langlais P., Bowen B., Levin K., Beck-Nielsen H., Mandarino L.J. Human ATP synthase beta is phosphorylated at multiple sites and shows abnormal phosphorylation at specific sites in insulin-resistant muscle. Diabetologia. 2010;53(3):541–551. doi: 10.1007/s00125-009-1624-0. [DOI] [PubMed] [Google Scholar]
- 33.Osanai T., Okada S., Sirato K., Nakano T., Saitoh M., Magota K., Okumura K. Mitochondrial coupling factor 6 is present on the surface of human vascular endothelial cells and is released by shear stress. Circulation. 2001;104(25):3132–3136. doi: 10.1161/hc5001.100832. [DOI] [PubMed] [Google Scholar]
- 34.Osanai T., Magota K., Tanaka M., Shimada M., Murakami R., Sasaki S., Tomita H., Maeda N., Okumura K. Intracellular signaling for vasoconstrictor coupling factor 6: novel function of beta-subunit of ATP synthase as receptor. Hypertension. 2005;46(5):1140–1146. doi: 10.1161/01.HYP.0000186483.86750.85. [DOI] [PubMed] [Google Scholar]
- 35.Lee H.M., Sugino H., Aoki C., Nishimoto N. Underexpression of mitochondrial-DNA encoded ATP synthesis-related genes and DNA repair genes in systemic lupus erythematosus. Arthritis Res. Ther. 2011;13(2):R63. doi: 10.1186/ar3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Vries D.D., van Engelen B.G., Gabreëls F.J., Ruitenbeek W., van Oost B.A. A second missense mutation in the mitochondrial ATPase 6 gene in Leigh’s syndrome. Ann. Neurol. 1993;34(3):410–412. doi: 10.1002/ana.410340319. [DOI] [PubMed] [Google Scholar]
- 37.Baracca A., Sgarbi G., Mattiazzi M., Casalena G., Pagnotta E., Valentino M.L., Moggio M., Lenaz G., Carelli V., Solaini G. Biochemical phenotypes associated with the mitochondrial ATP6 gene mutations at nt8993. Biochim. Biophys. Acta. 2007;1767(7):913–919. doi: 10.1016/j.bbabio.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Wallace D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattiazzi M., Vijayvergiya C., Gajewski C.D., DeVivo D.C., Lenaz G., Wiedmann M., Manfredi G. The mtDNA T8993G (NARP) mutation results in an impairment of oxidative phosphorylation that can be improved by antioxidants. Hum. Mol. Genet. 2004;13(8):869–879. doi: 10.1093/hmg/ddh103. [DOI] [PubMed] [Google Scholar]
- 40.Jolly R.D. Batten disease (ceroid-lipofuscinosis): the enigma of subunit c of mitochondrial ATP synthase accumulation. Neurochem. Res. 1995;20(11):1301–1304. doi: 10.1007/BF00992504. [DOI] [PubMed] [Google Scholar]
- 41.Capitanio D., Vasso M., Fania C., Moriggi M., Viganò A., Procacci P., Magnaghi V., Gelfi C. Comparative proteomic profile of rat sciatic nerve and gastrocnemius muscle tissues in ageing by 2-D DIGE. Proteomics. 2009;9(7):2004–2020. doi: 10.1002/pmic.200701162. [DOI] [PubMed] [Google Scholar]
- 42.Mao L., Zabel C., Wacker M.A., Nebrich G., Sagi D., Schrade P., Bachmann S., Kowald A., Klose J. Estimation of the mtDNA mutation rate in aging mice by proteome analysis and mathematical modeling. Exp. Gerontol. 2006;41(1):11–24. doi: 10.1016/j.exger.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 43.Preston C.C., Oberlin A.S., Holmuhamedov E.L., Gupta A., Sagar S., Syed R.H., Siddiqui S.A., Raghavakaimal S., Terzic A., Jahangir A. Aging-induced alterations in gene transcripts and functional activity of mitochondrial oxidative phosphorylation complexes in the heart. Mech. Ageing Dev. 2008;129(6):304–312. doi: 10.1016/j.mad.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choksi K.B., Nuss J.E., Boylston W.H., Rabek J.P., Papaconstantinou J. Age-related increases in oxidatively damaged proteins of mouse kidney mitochondrial electron transport chain complexes. Free Radic. Biol. Med. 2007;43(10):1423–1438. doi: 10.1016/j.freeradbiomed.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trifunovic A. Mitochondrial DNA and ageing. Biochim. Biophys. Acta. 2006;1757(5-6):611–617. doi: 10.1016/j.bbabio.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Conley K.E., Marcinek D.J., Villarin J. Mitochondrial dysfunction and age. Curr. Opin. Clin. Nutr. Metab. Care. 2007;10(6):688–692. doi: 10.1097/MCO.0b013e3282f0dbfb. [DOI] [PubMed] [Google Scholar]
- 47.Schägger H., Ohm T.G. Human diseases with defects in oxidative phosphorylation. 2. F1F0 ATP-synthase defects in Alzheimer disease revealed by blue native polyacrylamide gel electrophoresis. Eur. J. Biochem. 1995;227(3):916–921. doi: 10.1111/j.1432-1033.1995.tb20219.x. [DOI] [PubMed] [Google Scholar]
- 48.Sergeant N., Wattez A., Galván-valencia M., Ghestem A., David J.P., Lemoine J., Sautiére P.E., Dachary J., Mazat J.P., Michalski J.C., Velours J., Mena-López R., Delacourte A. Association of ATP synthase alpha-chain with neurofibrillary degeneration in Alzheimer’s disease. Neuroscience. 2003;117(2):293–303. doi: 10.1016/s0306-4522(02)00747-9. [DOI] [PubMed] [Google Scholar]
- 49.Kim S.H., Vlkolinsky R., Cairns N., Lubec G. Decreased levels of complex III core protein 1 and complex V beta chain in brains from patients with Alzheimer’s disease and Down syndrome. Cell. Mol. Life Sci. 2000;57(12):1810–1816. doi: 10.1007/PL00000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chandrasekaran K., Hatanpää K., Rapoport S.I., Brady D.R. Decreased expression of nuclear and mitochondrial DNA-encoded genes of oxidative phosphorylation in association neocortex in Alzheimer disease. Brain Res. Mol. Brain Res. 1997;44(1):99–104. doi: 10.1016/s0169-328x(96)00191-x. [DOI] [PubMed] [Google Scholar]
- 51.Moser T.L., Kenan D.J., Ashley T.A., Roy J.A., Goodman M.D., Misra U.K., Cheek D.J., Pizzo S.V. Endothelial cell surface F1-F0 ATP synthase is active in ATP synthesis and is inhibited by angiostatin. Proc. Natl. Acad. Sci. USA. 2001;98(12):6656–6661. doi: 10.1073/pnas.131067798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moser T.L., Stack M.S., Asplin I., Enghild J.J., Højrup P., Everitt L., Hubchak S., Schnaper H.W., Pizzo S.V. Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc. Natl. Acad. Sci. USA. 1999;96(6):2811–2816. doi: 10.1073/pnas.96.6.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moser T.L., Stack M.S., Wahl M.L., Pizzo S.V. The mechanism of action of angiostatin: can you teach an old dog new tricks? Thromb. Haemost. 2002;87(3):394–401. [PubMed] [Google Scholar]
- 54.Wahl M.L., Kenan D.J., Gonzalez-Gronow M., Pizzo S.V. Angiostatin’s molecular mechanism: aspects of specificity and regulation elucidated. J. Cell. Biochem. 2005;96(2):242–261. doi: 10.1002/jcb.20480. [DOI] [PubMed] [Google Scholar]
- 55.Pagliarani A., Nesci S., Ventrella V. Novel drugs targeting the c-ring of the F1FO-ATP synthase. Mini Rev. Med. Chem. 2016;16(10):815–824. doi: 10.2174/1389557516666160211120955. [DOI] [PubMed] [Google Scholar]
- 56.Nesci S., Trombetti F., Ventrella V., Pagliarani A. The c-Ring of the F1FO-ATP synthase: facts and perspectives. J. Membr. Biol. 2016;249(1-2):11–21. doi: 10.1007/s00232-015-9860-3. [DOI] [PubMed] [Google Scholar]
- 57.Andries K., Verhasselt P., Guillemont J., Göhlmann H.W., Neefs J.M., Winkler H., Van Gestel J., Timmerman P., Zhu M., Lee E., Williams P., de Chaffoy D., Huitric E., Hoffner S., Cambau E., Truffot-Pernot C., Lounis N., Jarlier V. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science. 2005;307(5707):223–227. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- 58.Diacon A.H., Pym A., Grobusch M., Patientia R., Rustomjee R., Page-Shipp L., Pistorius C., Krause R., Bogoshi M., Churchyard G., Venter A., Allen J., Palomino J.C., De Marez T., van Heeswijk R.P., Lounis N., Meyvisch P., Verbeeck J., Parys W., de Beule K., Andries K., Mc Neeley D.F. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N. Engl. J. Med. 2009;360(23):2397–2405. doi: 10.1056/NEJMoa0808427. [DOI] [PubMed] [Google Scholar]
- 59.de Jonge M.R., Koymans L.H., Guillemont J.E., Koul A., Andries K. A computational model of the inhibition of Mycobacterium tuberculosis ATPase by a new drug candidate R207910. Proteins. 2007;67(4):971–980. doi: 10.1002/prot.21376. [DOI] [PubMed] [Google Scholar]
- 60.Balemans W., Vranckx L., Lounis N., Pop O., Guillemont J., Vergauwen K., Mol S., Gilissen R., Motte M., Lançois D., De Bolle M., Bonroy K., Lill H., Andries K., Bald D., Koul A. Novel antibiotics targeting respiratory ATP synthesis in Gram-positive pathogenic bacteria. Antimicrob. Agents Chemother. 2012;56(8):4131–4139. doi: 10.1128/AAC.00273-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cole S.T., Alzari P.M. Microbiology. TB--a new target, a new drug. Science. 2005;307(5707):214–215. doi: 10.1126/science.1108379. [DOI] [PubMed] [Google Scholar]
- 62.Blatt N.B., Bednarski J.J., Warner R.E., Leonetti F., Johnson K.M., Boitano A., Yung R., Richardson B.C., Johnson K.J., Ellman J.A., Opipari A.W., Jr, Glick G.D. Benzodiazepine-induced superoxide signals B cell apoptosis: mechanistic insight and potential therapeutic utility. J. Clin. Invest. 2002;110(8):1123–1132. doi: 10.1172/JCI16029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnson K.M., Chen X., Boitano A., Swenson L., Opipari A.W., Jr, Glick G.D. Identification and validation of the mitochondrial F1F0-ATPase as the molecular target of the immunomodulatory benzodiazepine Bz-423. Chem. Biol. 2005;12(4):485–496. doi: 10.1016/j.chembiol.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 64.Johnson K.M., Cleary J., Fierke C.A., Opipari A.W., Jr, Glick G.D. Mechanistic basis for therapeutic targeting of the mitochondrial F1F0-ATPase. ACS Chem. Biol. 2006;1(5):304–308. doi: 10.1021/cb600143j. [DOI] [PubMed] [Google Scholar]
- 65.Salomon A.R., Voehringer D.W., Herzenberg L.A., Khosla C. Apoptolidin, a selective cytotoxic agent, is an inhibitor of F0F1-ATPase. Chem. Biol. 2001;8(1):71–80. doi: 10.1016/s1074-5521(00)00057-0. [DOI] [PubMed] [Google Scholar]
- 66.Salomon A.R., Voehringer D.W., Herzenberg L.A., Khosla C. Understanding and exploiting the mechanistic basis for selectivity of polyketide inhibitors of F(0)F(1)-ATPase. Proc. Natl. Acad. Sci. USA. 2000;97(26):14766–14771. doi: 10.1073/pnas.97.26.14766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim J.W., Adachi H., Shin-ya K., Hayakawa Y., Seto H. Apoptolidin, a new apoptosis inducer in transformed cells from Nocardiopsis sp. J. Antibiot. 1997;50(7):628–630. doi: 10.7164/antibiotics.50.628. [DOI] [PubMed] [Google Scholar]
- 68.Steele J.A., Uchytil T.F., Durbin R.D., Bhatnagar P., Rich D.H. Chloroplast coupling factor 1: A species-specific receptor for tentoxin. Proc. Natl. Acad. Sci. USA. 1976;73(7):2245–2248. doi: 10.1073/pnas.73.7.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Santolini J., Minoletti C., Gomis J.M., Sigalat C., André F., Haraux F. An insight into the mechanism of inhibition and reactivation of the F(1)-ATPases by tentoxin. Biochemistry. 2002;41(19):6008–6018. doi: 10.1021/bi015938z. [DOI] [PubMed] [Google Scholar]
- 70.Pinet E., Cavelier F., Verducci J., Girault G., Dubart L., Haraux F., Sigalat C., André F. Synthesis, structure, and properties of MeSer1-tentoxin, a new cyclic tetrapeptide which interacts specifically with chloroplast F1 H(+)-ATPase differentiation of inhibitory and stimulating effects. Biochemistry. 1996;35(39):12804–12811. doi: 10.1021/bi960955n. [DOI] [PubMed] [Google Scholar]
- 71.Ma Z., Cao M., Liu Y., He Y., Wang Y., Yang C., Wang W., Du Y., Zhou M., Gao F. Mitochondrial F1Fo-ATP synthase translocates to cell surface in hepatocytes and has high activity in tumor-like acidic and hypoxic environment. Acta Biochim. Biophys. Sin. (Shanghai) 2010;42(8):530–537. doi: 10.1093/abbs/gmq063. [DOI] [PubMed] [Google Scholar]
- 72.Arakaki N., Kita T., Shibata H., Higuti T., Cell-surface H. Cell-surface H+-ATP synthase as a potential molecular target for anti-obesity drugs. FEBS Lett. 2007;581(18):3405–3409. doi: 10.1016/j.febslet.2007.06.041. [DOI] [PubMed] [Google Scholar]
- 73.Champagne E., Martinez L.O., Collet X., Barbaras R. Ecto-F1Fo ATP synthase/F1 ATPase: metabolic and immunological functions. Curr. Opin. Lipidol. 2006;17(3):279–284. doi: 10.1097/01.mol.0000226120.27931.76. [DOI] [PubMed] [Google Scholar]
- 74.Kenan D.J., Wahl M.L. Ectopic localization of mitochondrial ATP synthase: a target for anti-angiogenesis intervention? J. Bioenerg. Biomembr. 2005;37(6):461–465. doi: 10.1007/s10863-005-9492-x. [DOI] [PubMed] [Google Scholar]
- 75.Arakaki N., Nagao T., Niki R., Toyofuku A., Tanaka H., Kuramoto Y., Emoto Y., Shibata H., Magota K., Higuti T. Possible role of cell surface H+ -ATP synthase in the extracellular ATP synthesis and proliferation of human umbilical vein endothelial cells. Mol. Cancer Res. 2003;1(13):931–939. [PubMed] [Google Scholar]
- 76.Berger K., Winzell M.S., Mei J., Erlanson-Albertsson C. Enterostatin and its target mechanisms during regulation of fat intake. Physiol. Behav. 2004;83(4):623–630. doi: 10.1016/j.physbeh.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 77.Burwick N.R., Wahl M.L., Fang J., Zhong Z., Moser T.L., Li B., Capaldi R.A., Kenan D.J., Pizzo S.V. An Inhibitor of the F1 subunit of ATP synthase (IF1) modulates the activity of angiostatin on the endothelial cell surface. J. Biol. Chem. 2005;280(3):1740–1745. doi: 10.1074/jbc.M405947200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fu Y., Zhu Y. Ectopic ATP synthase in endothelial cells: a novel cardiovascular therapeutic target. Curr. Pharm. Des. 2010;16(37):4074–4079. doi: 10.2174/138161210794519219. [DOI] [PubMed] [Google Scholar]
- 79.Yavlovich A., Viard M., Zhou M., Veenstra T.D., Wang J.M., Gong W., Heldman E., Blumenthal R., Raviv Y. Ectopic ATP synthase facilitates transfer of HIV-1 from antigen-presenting cells to CD4(+) target cells. Blood. 2012;120(6):1246–1253. doi: 10.1182/blood-2011-12-399063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gledhill J.R., Walker J.E. Inhibition sites in F1-ATPase from bovine heart mitochondria. Biochem. J. 2005;386(Pt 3):591–598. doi: 10.1042/BJ20041513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Laughlin T.F., Ahmad Z. Inhibition of Escherichia coli ATP synthase by amphibian antimicrobial peptides. Int. J. Biol. Macromol. 2010;46(3):367–374. doi: 10.1016/j.ijbiomac.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bullough D.A., Ceccarelli E.A., Roise D., Allison W.S. Inhibition of the bovine-heart mitochondrial F1-ATPase by cationic dyes and amphipathic peptides. Biochim. Biophys. Acta. 1989;975(3):377–383. doi: 10.1016/s0005-2728(89)80346-9. [DOI] [PubMed] [Google Scholar]
- 83.Kato-Yamada Y., Bald D., Koike M., Motohashi K., Hisabori T., Yoshida M. Epsilon subunit, an endogenous inhibitor of bacterial F(1)-ATPase, also inhibits F(0)F(1)-ATPase. J. Biol. Chem. 1999;274(48):33991–33994. doi: 10.1074/jbc.274.48.33991. [DOI] [PubMed] [Google Scholar]
- 84.Ahmad Z., Tayou J., Laughlin T.F. Asp residues of βDELSEED-motif are required for peptide binding in the Escherichia coli ATP synthase. Int. J. Biol. Macromol. 2015;75:37–43. doi: 10.1016/j.ijbiomac.2014.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hara K.Y., Kato-Yamada Y., Kikuchi Y., Hisabori T., Yoshida M. The role of the betaDELSEED motif of F1-ATPase: propagation of the inhibitory effect of the epsilon subunit. J. Biol. Chem. 2001;276(26):23969–23973. doi: 10.1074/jbc.M009303200. [DOI] [PubMed] [Google Scholar]
- 86.Mnatsakanyan N., Krishnakumar A.M., Suzuki T., Weber J. The role of the betaDELSEED-loop of ATP synthase. J. Biol. Chem. 2009;284(17):11336–11345. doi: 10.1074/jbc.M900374200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abrahams J.P., Buchanan S.K., Van Raaij M.J., Fearnley I.M., Leslie A.G., Walker J.E. The structure of bovine F1-ATPase complexed with the peptide antibiotic efrapeptin. Proc. Natl. Acad. Sci. USA. 1996;93(18):9420–9424. doi: 10.1073/pnas.93.18.9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Linnett P.E., Beechey R.B. Inhibitors of the ATP synthethase system. Methods Enzymol. 1979;55:472–518. doi: 10.1016/0076-6879(79)55061-7. [DOI] [PubMed] [Google Scholar]
- 89.Ahmad Z., Ahmad M., Okafor F., Jones J., Abunameh A., Cheniya R.P., Kady I.O. Effect of structural modulation of polyphenolic compounds on the inhibition of Escherichia coli ATP synthase. Int. J. Biol. Macromol. 2012;50(3):476–486. doi: 10.1016/j.ijbiomac.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bernardes C.F., Meyer-Fernandes J.R., Martins O.B., Vercesi A.E. Inhibition of succinic dehydrogenase and F0F1-ATP synthase by 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS). Z. Natforsch. C J. Biosci. 1997;52(11-12):799–806. doi: 10.1515/znc-1997-11-1212. [DOI] [PubMed] [Google Scholar]
- 91.McEnery M.W., Pedersen P.L. Diethylstilbestrol. A novel F0-directed probe of the mitochondrial proton ATPase. J. Biol. Chem. 1986;261(4):1745–1752. [PubMed] [Google Scholar]
- 92.Saishu T., Kagawa Y., Shimizu R. Resistance of thermophilic ATPase (TF1) to specific F1-atpase inhibitors including local anesthetics. Biochem. Biophys. Res. Commun. 1983;112(3):822–826. doi: 10.1016/0006-291x(83)91691-1. [DOI] [PubMed] [Google Scholar]
- 93.Zheng J., Ramirez V.D. Piceatannol, a stilbene phytochemical, inhibits mitochondrial F0F1-ATPase activity by targeting the F1 complex. Biochem. Biophys. Res. Commun. 1999;261(2):499–503. doi: 10.1006/bbrc.1999.1063. [DOI] [PubMed] [Google Scholar]
- 94.Zheng J., Ramirez V.D. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br. J. Pharmacol. 2000;130(5):1115–1123. doi: 10.1038/sj.bjp.0703397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lang D.R., Racker E. Effects of quercetin and F1 inhibitor on mitochondrial ATPase and energy-linked reactions in submitochondrial particles. Biochim. Biophys. Acta. 1974;333(2):180–186. doi: 10.1016/0005-2728(74)90002-4. [DOI] [PubMed] [Google Scholar]
- 96.Caselli A., Cirri P., Santi A., Paoli P. Morin: a promising natural drug. Curr. Med. Chem. 2016;23(8):774–791. doi: 10.2174/0929867323666160106150821. [DOI] [PubMed] [Google Scholar]
- 97.Poór M., Veres B., Jakus P.B., Antus C., Montskó G., Zrínyi Z., Vladimir-Knežević S., Petrik J., Kőszegi T. Flavonoid diosmetin increases ATP levels in kidney cells and relieves ATP depleting effect of ochratoxin A. J. Photochem. Photobiol. B. 2014;132:1–9. doi: 10.1016/j.jphotobiol.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 98.Sekiya M., Nakamoto R.K., Nakanishi-Matsui M., Futai M. Binding of phytopolyphenol piceatannol disrupts β/γ subunit interactions and rate-limiting step of steady-state rotational catalysis in Escherichia coli F1-ATPase. J. Biol. Chem. 2012;287(27):22771–22780. doi: 10.1074/jbc.M112.374868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nakanishi-Matsui M., Sekiya M., Futai M. ATP synthase from Escherichia coli: Mechanism of rotational catalysis, and inhibition with the ε subunit and phytopolyphenols. Biochim. Biophys. Acta. 2016;1857(2):129–140. doi: 10.1016/j.bbabio.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 100.Sandoval-Acuña C., Ferreira J., Speisky H. Polyphenols and mitochondria: an update on their increasingly emerging ROS-scavenging independent actions. Arch. Biochem. Biophys. 2014;559:75–90. doi: 10.1016/j.abb.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 101.Piotto S., Concilio S., Sessa L., Porta A., Calabrese E.C., Zanfardino A., Varcamonti M., Iannelli P. Small azobenzene derivatives active against bacteria and fungi. Eur. J. Med. Chem. 2013;68:178–184. doi: 10.1016/j.ejmech.2013.07.030. [DOI] [PubMed] [Google Scholar]
- 102.Nesci S., Ventrella V., Trombetti F., Pirini M., Pagliarani A. Thiol oxidation of mitochondrial F0-c subunits: a way to switch off antimicrobial drug targets of the mitochondrial ATP synthase. Med. Hypotheses. 2014;83(2):160–165. doi: 10.1016/j.mehy.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 103.Nesci S., Ventrella V., Trombetti F., Pirini M., Pagliarani A. Thiol oxidation is crucial in the desensitization of the mitochondrial F1FO-ATPase to oligomycin and other macrolide antibiotics. Biochim. Biophys. Acta. 2014;1840(6):1882–1891. doi: 10.1016/j.bbagen.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 104.Bárta I., Smerák P., Polívková Z., Sestáková H., Langová M., Turek B., Bártová J. Current trends and perspectives in nutrition and cancer prevention. Neoplasma. 2006;53(1):19–25. [PubMed] [Google Scholar]
- 105.Nishino H., Murakoshi M., Mou X.Y., Wada S., Masuda M., Ohsaka Y., Satomi Y., Jinno K. Cancer prevention by phytochemicals. Oncology. 2005;69(Suppl. 1):38–40. doi: 10.1159/000086631. [DOI] [PubMed] [Google Scholar]
- 106.Cushnie T.P., Lamb A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents. 2011;38(2):99–107. doi: 10.1016/j.ijantimicag.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 107.Jang M., Cai L., Udeani G.O., Slowing K.V., Thomas C.F., Beecher C.W., Fong H.H., Farnsworth N.R., Kinghorn A.D., Mehta R.G., Moon R.C., Pezzuto J.M. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275(5297):218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 108.Ghasemzadeh A., Jaafar H.Z., Rahmat A. Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe). Molecules. 2010;15(6):4324–4333. doi: 10.3390/molecules15064324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Prasad S., Tyagi A.K. Ginger and its constituents: role in prevention and treatment of gastrointestinal cancer. 2015. [DOI] [PMC free article] [PubMed]
- 110.Tyagi A.K., Prasad S., Yuan W., Li S., Aggarwal B.B. Identification of a novel compound (β-sesquiphellandrene) from turmeric (Curcuma longa) with anticancer potential: comparison with curcumin. Invest. New Drugs. 2015;33(6):1175–1186. doi: 10.1007/s10637-015-0296-5. [DOI] [PubMed] [Google Scholar]
- 111.Rahmani A.H., Shabrmi F.M., Aly S.M. Active ingredients of ginger as potential candidates in the prevention and treatment of diseases via modulation of biological activities. Int. J. Physiol. Pathophysiol. Pharmacol. 2014;6(2):125–136. [PMC free article] [PubMed] [Google Scholar]
- 112.Wang C.Z., Qi L.W., Yuan C.S. Cancer chemoprevention effects of ginger and its active constituents: potential for new drug discovery. Am. J. Chin. Med. 2015;43(7):1351–1363. doi: 10.1142/S0192415X15500767. [DOI] [PubMed] [Google Scholar]
- 113.Rahmani A.H., Aly S.M., Ali H., Babiker A.Y., Srikar S., Khan A.A. Therapeutic effects of date fruits (Phoenix dactylifera) in the prevention of diseases via modulation of anti-inflammatory, anti-oxidant and anti-tumour activity. Int. J. Clin. Exp. Med. 2014;7(3):483–491. [PMC free article] [PubMed] [Google Scholar]
- 114.Rosignoli P., Fuccelli R., Sepporta M.V., Fabiani R. In vitro chemo-preventive activities of hydroxytyrosol: the main phenolic compound present in extra-virgin olive oil. Food Funct. 2016;7(1):301–307. doi: 10.1039/c5fo00932d. [DOI] [PubMed] [Google Scholar]
- 115.Sato K., Mihara Y., Kanai K., Yamashita Y., Kimura Y., Itoh N. Relative potency of tyrosol in the treatment of endotoxin-induced uveitis in rats. J. Vet. Med. Sci. 2016;78(10):1631–1634. doi: 10.1292/jvms.16-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hashmi M.A., Khan A., Hanif M., Farooq U., Perveen S. Traditional Uses, Phytochemistry, and Pharmacology of Olea europaea (Olive). Evid. Based Complement Alternat. Med., 2015 doi: 10.1155/2015/541591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lu X., Rasco B.A., Jabal J.M., Aston D.E., Lin M., Konkel M.E. Investigating antibacterial effects of garlic (Allium sativum) concentrate and garlic-derived organosulfur compounds on Campylobacter jejuni by using Fourier transform infrared spectroscopy, Raman spectroscopy, and electron microscopy. Appl. Environ. Microbiol. 2011;77(15):5257–5269. doi: 10.1128/AEM.02845-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Karuppiah P., Rajaram S. Antibacterial effect of Allium sativum cloves and Zingiber officinale rhizomes against multiple-drug resistant clinical pathogens. Asian Pac. J. Trop. Biomed. 2012;2(8):597–601. doi: 10.1016/S2221-1691(12)60104-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Benkeblia N., Dahmouni S., Onodera S., Shiomi N. Antimicrobial Activity of Phenolic Compound Extracts of Various Onions (Allium cepa L.)Cultivars and Garlic (Allium sativum L.). J. Food Technol. 2005;3(1):30–34. [Google Scholar]
- 120.Ahmad I., Muneer K.M., Tamimi I.A., Chang M.E., Ata M.O., Yusuf N. Thymoquinone suppresses metastasis of melanoma cells by inhibition of NLRP3 inflammasome. Toxicol. Appl. Pharmacol. 2013;270(1):70–76. doi: 10.1016/j.taap.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 121.Ahmad K.A., Clement M.V., Hanif I.M., Pervaiz S. Resveratrol inhibits drug-induced apoptosis in human leukemia cells by creating an intracellular milieu nonpermissive for death execution. Cancer Res. 2004;64(4):1452–1459. doi: 10.1158/0008-5472.can-03-2414. [DOI] [PubMed] [Google Scholar]
- 122.Singh C.K., George J., Ahmad N. Resveratrol-based combinatorial strategies for cancer management. Ann. N. Y. Acad. Sci. 2013;1290:113–121. doi: 10.1111/nyas.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chowdhury S.A., Kishino K., Satoh R., Hashimoto K., Kikuchi H., Nishikawa H., Shirataki Y., Sakagami H. Tumor-specificity and apoptosis-inducing activity of stilbenes and flavonoids. Anticancer Res. 2005;25(3B):2055–2063. [PubMed] [Google Scholar]
- 124.Mnayer D., Fabiano-Tixier A.S., Petitcolas E., Hamieh T., Nehme N., Ferrant C., Fernandez X., Chemat F. Chemical composition, antibacterial and antioxidant activities of six essentials oils from the Alliaceae family. Molecules. 2014;19(12):20034–20053. doi: 10.3390/molecules191220034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Puupponen-Pimiä R., Nohynek L., Meier C., Kähkönen M., Heinonen M., Hopia A., Oksman-Caldentey K.M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001;90(4):494–507. doi: 10.1046/j.1365-2672.2001.01271.x. [DOI] [PubMed] [Google Scholar]
- 126.Nohynek L.J., Alakomi H.L., Kähkönen M.P., Heinonen M., Helander I.M., Oksman-Caldentey K.M., Puupponen-Pimiä R.H. Berry phenolics: antimicrobial properties and mechanisms of action against severe human pathogens. Nutr. Cancer. 2006;54(1):18–32. doi: 10.1207/s15327914nc5401_4. [DOI] [PubMed] [Google Scholar]
- 127.Junqueira-Gonçalves M.P., Yáñez L., Morales C., Navarro M.A., Contreras R., Zúñiga G.E. Isolation and characterization of phenolic compounds and anthocyanins from Murta (Ugni molinae Turcz.) fruits. Assessment of antioxidant and antibacterial activity. Molecules. 2015;20(4):5698–5713. doi: 10.3390/molecules20045698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sengul M., Ercisli S., Yildiz H., Gungor N., Kavaz A., Cetin B. Antioxidant, Antimicrobial Activity and Total Phenolic Content within the Aerial Parts of Artemisia absinthum, Artemisia santonicum and Saponaria officinalis. Iran. J. Pharm. Res. 2011;10(1):49–56. [PMC free article] [PubMed] [Google Scholar]
- 129.Hosseinzadeh H., Mehri S., Abolhassani M.M., Ramezani M., Sahebkar A., Abnous K. Affinity-based target deconvolution of safranal. Daru. 2013;21(1):25. doi: 10.1186/2008-2231-21-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Duarte S., Gregoire S., Singh A.P., Vorsa N., Schaich K., Bowen W.H., Koo H. Inhibitory effects of cranberry polyphenols on formation and acidogenicity of Streptococcus mutans biofilms. FEMS Microbiol. Lett. 2006;257(1):50–56. doi: 10.1111/j.1574-6968.2006.00147.x. [DOI] [PubMed] [Google Scholar]
- 131.Aziz M.H., Reagan-Shaw S., Wu J., Longley B.J., Ahmad N. Chemoprevention of skin cancer by grape constituent resveratrol: relevance to human disease? FASEB J. 2005;19(9):1193–1195. doi: 10.1096/fj.04-3582fje. [DOI] [PubMed] [Google Scholar]
- 132.Pervaiz S., Holme A.L. Resveratrol: its biologic targets and functional activity. Antioxid. Redox Signal. 2009;11(11):2851–2897. doi: 10.1089/ars.2008.2412. [DOI] [PubMed] [Google Scholar]
- 133.Pervaiz S. Resveratrol: from grapevines to mammalian biology. FASEB J. 2003;17(14):1975–1985. doi: 10.1096/fj.03-0168rev. [DOI] [PubMed] [Google Scholar]
- 134.Singh C.K., Pitschmann A., Ahmad N. Resveratrol-zinc combination for prostate cancer management. Cell Cycle. 2014;13(12):1867–1874. doi: 10.4161/cc.29334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hsieh T.C., Wu J.M. Resveratrol: Biological and pharmaceutical properties as anticancer molecule. Biofactors. 2010;36(5):360–369. doi: 10.1002/biof.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wolvetang E.J., Johnson K.L., Krauer K., Ralph S.J., Linnane A.W. Mitochondrial respiratory chain inhibitors induce apoptosis. FEBS Lett. 1994;339(1-2):40–44. doi: 10.1016/0014-5793(94)80380-3. [DOI] [PubMed] [Google Scholar]
- 137.Mills K.I., Woodgate L.J., Gilkes A.F., Walsh V., Sweeney M.C., Brown G., Burnett A.K. Inhibition of mitochondrial function in HL60 cells is associated with an increased apoptosis and expression of CD14. Biochem. Biophys. Res. Commun. 1999;263(2):294–300. doi: 10.1006/bbrc.1999.1356. [DOI] [PubMed] [Google Scholar]
- 138.Chusri S., Sinvaraphan N., Chaipak P., Luxsananuwong A., Voravuthikunchai S.P. Evaluation of antibacterial activity, phytochemical constituents, and cytotoxicity effects of Thai household ancient remedies. J. Altern. Complement. Med. 2014;20(12):909–918. doi: 10.1089/acm.2013.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kaur G.J., Arora D.S. Antibacterial and phytochemical screening of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi. BMC Complement. Altern. Med. 2009;9:30. doi: 10.1186/1472-6882-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Muthamilselvan T., Kuo T.F., Wu Y.C., Yang W.C. Herbal Remedies for Coccidiosis Control: A Review of Plants, Compounds, and Anticoccidial Actions. . Evid Based Complement. Alternat. Med., 2016. [DOI] [PMC free article] [PubMed]
- 141.Kato-Noguchi H., Salam M.A., Ohno O., Suenaga K. Nimbolide B and nimbic acid B, phytotoxic substances in neem leaves with allelopathic activity. Molecules. 2014;19(6):6929–6940. doi: 10.3390/molecules19066929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Daglia M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012;23(2):174–181. doi: 10.1016/j.copbio.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 143.Chaieb K., Kouidhi B., Jrah H., Mahdouani K., Bakhrouf A. Antibacterial activity of Thymoquinone, an active principle of Nigella sativa and its potency to prevent bacterial biofilm formation. BMC Complement. Altern. Med. 2011;11:29. doi: 10.1186/1472-6882-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Heinonen M. Antioxidant activity and antimicrobial effect of berry phenolics--a Finnish perspective. Mol. Nutr. Food Res. 2007;51(6):684–691. doi: 10.1002/mnfr.200700006. [DOI] [PubMed] [Google Scholar]
- 145.Dixon R.A., Xie D.Y., Sharma S.B. Proanthocyanidins--a final frontier in flavonoid research? New Phytol. 2005;165(1):9–28. doi: 10.1111/j.1469-8137.2004.01217.x. [DOI] [PubMed] [Google Scholar]
- 146.Menz R.I., Walker J.E., Leslie A.G. Structure of bovine mitochondrial F(1)-ATPase with nucleotide bound to all three catalytic sites: implications for the mechanism of rotary catalysis. Cell. 2001;106(3):331–341. doi: 10.1016/s0092-8674(01)00452-4. [DOI] [PubMed] [Google Scholar]
- 147.Sayle R.A., Milner-White E.J. RASMOL: biomolecular graphics for all. Trends Biochem. Sci. 1995;20(9):374. doi: 10.1016/s0968-0004(00)89080-5. [DOI] [PubMed] [Google Scholar]