Abstract
Chemotherapy-induced peripheral neuropathy (CIPN), a consequence of peripheral nerve fiber dysfunction or degeneration, continues to be a dose-limiting and debilitating side effect during and/or after cancer chemotherapy. Paclitaxel, a taxane commonly used to treat breast, lung, and ovarian cancers, causes CIPN in 59–78% of cancer patients. Novel interventions are needed due to the current lack of effective CIPN treatments. Our studies were designed to investigate whether nicotine can prevent and/or reverse paclitaxel-induced peripheral neuropathy in a mouse model of CIPN, while ensuring that nicotine will not stimulate lung tumor cell proliferation or interfere with the antitumor properties of paclitaxel. Male C57BL/6J mice received paclitaxel every other day for a total of four injections (8 mg/kg, i.p.). Acute (0.3–0.9 mg/kg, i.p.) and chronic (24 mg/kg per day, s.c.) administration of nicotine respectively reversed and prevented paclitaxel-induced mechanical allodynia. Blockade of the antinociceptive effect of nicotine with mecamylamine and methyllycaconitine suggests that the reversal of paclitaxel-induced mechanical allodynia is primarily mediated by the α7 nicotinic acetylcholine receptor subtype. Chronic nicotine treatment also prevented paclitaxel-induced intraepidermal nerve fiber loss. Notably, nicotine neither promoted proliferation of A549 and H460 non–small cell lung cancer cells nor interfered with paclitaxel-induced antitumor effects, including apoptosis. Most importantly, chronic nicotine administration did not enhance Lewis lung carcinoma tumor growth in C57BL/6J mice. These data suggest that the nicotinic acetylcholine receptor–mediated pathways may be promising drug targets for the prevention and treatment of CIPN.
Introduction
Chemotherapy continues to play a significant role in the treatment and survival of cancer patients. However, a number of cancer chemotherapeutic drugs can promote either transient or prolonged tissue and organ toxicities, including chemotherapy-induced peripheral neuropathy (CIPN). CIPN, a result of peripheral nerve fiber dysfunction or degeneration, is characterized by sensory symptoms, including numbness, tingling, burning, hyperalgesia, allodynia, and, in some cases, neuropathic pain. Approximately 68% of cancer patients experience CIPN within a month after the completion of their treatment, whereas 30% suffer from symptoms of CIPN for 6 months or longer after chemotherapy (Seretny et al., 2014). When CIPN manifests during the administration of chemotherapy, it can become dose limiting and/or delay treatment, thereby interfering with the full course of treatment that may be required for a positive clinical outcome (Hama and Takamatsu, 2016).
Cancer chemotherapeutic drugs and drug classes associated with peripheral neuropathy include the taxanes (paclitaxel), platinum-based compounds (cisplatin, oxaliplatin), vinca alkaloids (vincristine), and bortezomib. Paclitaxel, a taxane commonly used to treat breast, lung, and ovarian cancers, increases both progression-free and overall survival time in cancer patients (Dranitsaris et al., 2015). Unfortunately, paclitaxel has been found to cause CIPN both acutely and chronically in 59–78% and 30% of cancer patients, respectively (Beijers et al., 2012).
There are currently no effective preventative or therapeutic treatments for CIPN. Opioids, anticonvulsants, antidepressants, anesthetics, and muscle relaxants perform modestly in relieving CIPN pain, do not show consistent efficacy in the majority of patients, and/or produce intolerable side effects (Hershman et al., 2014; Kim et al., 2015; Majithia et al., 2016).
Nicotine and nicotine analogs have demonstrated potential utility as analgesic and/or antinociceptive drugs, and as anti-inflammatory agents in both human and experimental pain studies (Alsharari et al., 2013; Umana et al., 2013; Flood and Damaj, 2014). For example, nicotine elicits analgesic effects in nonsmokers suffering from spinal cord injury in a randomized, placebo-controlled, crossover design experiment (Richardson et al., 2012). Additionally, a recent preclinical study demonstrated that i.p. administration of nicotine at a dose of 1.5 mg/kg reverses allodynia induced by oxaliplatin, a chemotherapeutic agent used to induce peripheral neuropathy in rats (Di Cesare Mannelli et al., 2013).
The studies described in this report characterize the antinociceptive and/or neuroprotective effects of nicotine in a CIPN mouse model while further evaluating the influence of nicotine on lung tumor cell proliferation and sensitivity to the antitumor properties of paclitaxel.
Materials and Methods
Animals.
Adult male C57BL/6J mice (8 weeks old at the beginning of experiments; weight, 20–30 g) were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were housed in groups of four in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (Frederick, MD); the mice in each cage were randomly allocated to different treatment groups. Food and water were available ad libitum. Experiments were performed during the light cycle (7:00 AM to 7:00 PM) and were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University (VCU) and followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Animals were euthanized via CO2 asphyxiation followed by cervical dislocation. Any subjects that showed behavioral disturbances unrelated to chemotherapy-induced pain were excluded from further behavioral testing.
Drugs.
Paclitaxel was purchased from Tocris Bioscience (catalog #1097; Bristol, UK) and dissolved in a mixture of 1:1:18 [1 volume ethanol/1 volume Emulphor-620 (Rhone-Poulenc Inc., Princeton, NJ)/18 volumes distilled water]. Paclitaxel injections were administered i.p. every other day for a total of four injections to induce neuropathy, as previously described by Toma et al. (2017). (−)-Nicotine hydrogen tartrate salt and mecamylamine HCl were purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in 0.9% saline. For acute administration, nicotine was injected i.p. at doses of 0.3, 0.6, or 0.9 mg/kg (Di Cesare Mannelli et al., 2013; Bagdas et al., 2017). Nicotine at doses of 6, 12, or 24 mg/kg per day was also administered chronically via 7-day s.c. osmotic minipumps (model 1007D; Alzet, Cupertino, CA), which were implanted 2 days prior to paclitaxel treatment (Alsharari et al., 2015). Mecamylamine was administered at a dose of 2 mg/kg s.c. Fifteen minutes before administration of nicotine or saline (Bagdas et al., 2014). Methyllycaconitine (MLA) was purchased from RBI (Natick, MA) and administered at a dose of 10 mg/kg s.c. 10 minutes before administration of nicotine (Freitas et al., 2013). All doses were chosen based on previous work that demonstrated which dose, time of exposure, and route of administration for each drug effectively acted upon the appropriate receptor and was not toxic to the animal. All i.p. or s.c. injections were given in a volume of 1 ml/100 g b.wt., whereas the osmotic minipumps released 0.5 μl/h
Immunohistochemistry and Quantification of Intraepidermal Nerve Fibers.
The hind paw epidermis was collected from the following groups of mice: vehicle-saline, vehicle-nicotine (24 mg/kg per day), paclitaxel (8 mg/kg)-saline, and paclitaxel (8 mg/kg)-nicotine (24 mg/kg per day). The staining procedure was performed as previously described (Toma et al., 2017). Briefly, the glabrous skin of the hind paw was excised, placed in freshly prepared 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS) (pH 7.4), and stored overnight at 4°C in the same fixative. The samples were embedded in paraffin, sectioned at 25 μm, and stained with PGP9.5 (catalog #70R-30722; Fitzgerald, Acton, MA) and goat anti-rabbit IgG (heavy and light chains) secondary antibody conjugated with Alexa Fluor 594 (A11037; Life Technologies, Eugene, OR). Sections were examined using a Zeiss Axio Imager A1–Fluorescence microscope (Carl Zeiss, AG, Oberkochen, Germany) in a blinded fashion under 63× magnification, but imaged under 40× magnification; the density of fibers is expressed as fibers per millimeter.
Mechanical Allodynia Evaluation (von Frey Test).
Mechanical allodynia thresholds were determined using von Frey filaments according to the method suggested by Chaplan et al. (1994) and as described previously (Bagdas et al., 2015). The mechanical threshold is expressed as log10 (10 £ force in milligrams). For the nicotine-mediated reversal of the CIPN experiment, paclitaxel-treated mice were tested for mechanical allodynia after acute nicotine administration on days 7–14 after the initial paclitaxel injection. All behavioral testing on animals was performed in a blinded manner.
Minipump Implantation.
The procedure was performed as previously described by Alsharari et al. (2013) with minor modifications. Mice were anesthetized with 2.5% isoflurane/ 97.5% oxygen. The anesthetized mice were prepared by shaving of the back and swabbing with betadine, followed by 70% ethanol pads. Sharp, sterile scissors was used to make a 1-cm incision in the skin of the upper back/neck. The sterile, preloaded minipump (model 1007D; Alzet) with different doses of nicotine or saline was inserted with sterile forceps by a technician wearing sterile gloves. The wound was closed with sterile 9-mm stainless steel wound clips. The mice were allowed to recover on heated pads and were monitored before returning to their home cages.
Cell Culture.
All lung cancer cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS) (catalog #FB22-500HI; Serum Source International, Charlotte, NC) and 1% (v/v) combination of 10,000 U/ml penicillin and 10,000 μg/ml streptomycin (15140-122; Thermo Fisher Scientific, Carlsbad, CA), unless stated otherwise. Cells were incubated at 37°C under a humidified, 5% CO2 atmosphere. The H460 non–small cell lung cancer (NSCLC) cell line was provided by the laboratory of Dr. Richard Moran at VCU, the A549 NSCLC cell line was a gift from the laboratory of Dr. Charles Chalfant at VCU, and the Lewis lung carcinoma (LLC) cells were provided by Dr. Andrew Larner at VCU. To establish the T1 primary lung cancer cell line, tissues were obtained from adenocarcinoma tumors in accordance with the VCU institutional review board protocol. Tissues were minced well and washed multiple times by centrifugation in sterile PBS. Thereafter, the tissues were resuspended in DMEM. Tissue homogenates were layered on plates coated with collagen (catalog #C3867; Sigma-Aldrich). Cell colonies started to appear after 2–3 weeks. Upon confluence, the cells were trypsinized and passaged. The ovarian cancer cell lines SKOV-3/DDP and OVCAR-3 were generously provided by the laboratory of Dr. Xianjun Fang at VCU and were cultured in RPMI 1640 supplemented with 10% (v/v) FBS and 1% (v/v) 10,000 U/ml penicillin and 10,000 μg/ml streptomycin.
Paclitaxel was dissolved in dimethylsulfoxide (DMSO), diluted with sterile PBS, and added to the medium to obtain the desired concentration. Staurosporine (catalog #S6942; Sigma-Aldrich) was purchased as 1 mM in DMSO. Cells were not exposed to greater than 0.1% DMSO in any experiment. (−)-Nicotine hydrogen tartrate salt was dissolved in sterile PBS. All experiments involving these light-sensitive drugs were performed in the dark.
Assessment of Cell Viability.
Cell viability was measured by either the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)/3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) colorimetric assay or trypan blue exclusion. For the MTT/MTS assay, cells were seeded in 96-well plates and treated with various concentrations of nicotine for 24 hours, at which time the drug was removed and replaced with fresh medium. Depending on the replication rate of the cell line, the cells were allowed 24 or 48 hours to proliferate after drug exposure. For the serum deprivation study, cells were seeded in DMEM (10% FBS) for 24 hours, then the medium was removed and replaced with DMEM supplemented with various concentrations of FBS (0–10%) with or without nicotine (1 μM); cell viability was assessed at either 48 or 96 hours post-treatment without drug removal. At the time of testing, the medium was removed, then the cells were washed with PBS and stained with MTT (2 mg/ml; catalog #M2128; Sigma-Aldrich) in PBS for 3 hours. The MTT solution was aspirated and replaced with DMSO. The color change was measured by a spectrophotometer (ELx800UV; BioTek Instruments, Winooski, VT) at 490 nm. To avoid potentially aspirating cells, the CellTiter 96 Aqueous One Solution Cell Proliferation Assay (MTS; G358C; Promega, Madison, WI) was used for less adherent cell lines (A549, LLC, and T1); the use of MTS rather than MTT eliminates washing steps before and after staining.
For trypan blue exclusion, cells were incubated with trypsin (0.25% trypsin-EDTA) for 3 minutes, stained with trypan blue (catalog #15250; Invitrogen, Carlsbad, CA), and the viable, unstained cells were counted using a hemocytometer with bright-field microscopy.
Assessment of Colony Formation.
Cells were seeded at a low density in DMEM (10% FBS). After 24 hours, the paclitaxel and paclitaxel + nicotine samples were exposed to paclitaxel (50 nM) for 24 hours, after which the medium was replaced with fresh, drug-free medium. After 24 hours, the nicotine and paclitaxel + nicotine samples were exposed to nicotine (1 μM) for 24 hours, after which the medium was replaced with fresh, drug-free medium. Once the control colonies reached a size of 50 cells per colony (approximately 8–10 days after seeding), the samples were fixed with methanol, stained with crystal violet, and quantified (ColCount software; Discovery Technology International, Inc., Sarasota, FL).
Assessment of Apoptosis and DNA Content.
Flow cytometry analyses were performed using BD FACSCanto II (BD Biosciences, San Jose, CA) and BD FACSDiva software (BD Biosciences) at the VCU Flow Cytometry Core facility. For all studies, 10,000 cells per replicate within the gated region were analyzed. When collecting samples, both adherent and floating cells were harvested with 0.1% trypsin-EDTA and neutralized with medium after 48 hours of drug exposure. For quantification of apoptosis, cells were centrifuged and washed with PBS, then resuspended in 100 μl of 1× binding buffer with 5 μl of Annexin V and 5 μl of propidium iodide (PI) (FITC Annexin V Apoptosis Detection Kit, catalog #556547; BD Biosciences). The samples were then incubated at room temperature while being protected from light for 15 minutes. The suspension solution was then brought up to 500 μl using the 1× binding buffer and analyzed by flow cytometry. For quantification of DNA content, the cells were resuspended in 500 μl of a PI solution (50 μg/ml PI, 4 mM sodium citrate, 0.2 mg/ml DNase-free RNase A, and 0.1% Triton X-100) for 1 hour at room temperature while being protected from light (Tate et al., 1983). Before flow cytometry analysis, NaCl was added to the cell suspensions to achieve a final concentration of 0.20 M.
Assessment of Tumor Growth In Vivo.
Male C57BL/6J adult mice were injected s.c. with 1.5 × 106 LLC cells in both flanks. The LLC cells were collected via trypsinization then neutralized with medium, centrifuged, and washed with PBS. Pellets of 1.5 × 106 LLC cells were then resuspended in 30 μl of 80% basement membrane extract (catalog #3632-010-02; Trevigen, Gaithersburg, MD)/20% PBS. Mice were anesthetized with isoflurane via inhalation during tumor cell injection. Palpable tumors formed at approximately 7 days post–tumor cell injection, and on day 11 tumor volumes (length × width × height) were sufficient to be assessed with calipers; subsequent tumor volume measurements were collected every other day. Osmotic minipumps (model 1007D; Alzet) were implanted s.c. as previously described at 13 days post–tumor cell injection to release 24 mg/kg nicotine daily for a total of 7 days. Body weight and tumor volume were observed until humane endpoints were reached, at which time mice were euthanized via CO2 asphyxiation followed by cervical dislocation.
Statistical Analysis.
A power analysis calculation was performed with the Lamorte Power Calculator (Boston University Research Compliance, Boston, MA) to determine the sample size of animals for each group (Charan and Kantharia, 2013). For assessing nociceptive behavior and tumor volume, the calculations showed that an n of 5 was required to achieve a power of 90% with an α error of 0.05; we used eight mice per group for the nociceptive assay and four to six mice per group for the in vivo cancer study. The data were analyzed with GraphPad Prism software, version 6 (GraphPad Software, Inc., La Jolla, CA) and SPSS, version 24 (IBM, Armonk, NY) and are expressed as the mean ± S.E.M. One-way and two-way analysis of variance (ANOVA) tests were conducted and followed by the Bonferroni post hoc test, three-way mixed factor ANOVAs were performed and followed by the Sidak post hoc test, and linear mixed models were conducted to account for the loss of tumor-bearing mice (Little and Rubin, 1987); repeated measures were considered for all in vivo studies. Differences were determined to be significant at P < 0.05.
Results
Nicotine Reverses and Prevents Paclitaxel-Induced Mechanical Allodynia.
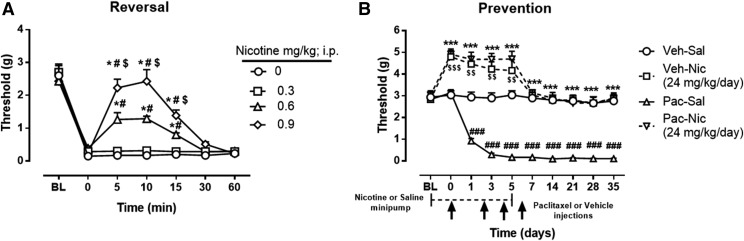
Initial experiments were designed to determine whether acute administration of nicotine reverses paclitaxel-induced mechanical allodynia. Figure 1A demonstrates that nicotine reversed mechanical allodynia in paclitaxel-treated mice in a time- and dose-dependent manner [Ftime × dose(18,126) = 17.10, P < 0.0001], with full reversal (mechanical threshold values restored to baseline levels) after administration of 0.9 mg/kg and partial reversal with 0.6 mg/kg. Nicotine did not alter mechanical thresholds in vehicle-treated mice [Ftime × dose(18,126) = 0.6122, P = 0.88] (Supplemental Fig. 1).
Fig. 1.
Antinociceptive and preventative effect of nicotine in a mouse model of paclitaxel-induced peripheral neuropathy. (A) Reversal of mechanical allodynia by acute administration of nicotine at doses of 0.3, 0.6, and 0.9 mg/kg i.p. in paclitaxel-treated mice at days 7–14 after initial paclitaxel injection. *P < 0.0001 versus saline (0 mg/kg); #P < 0.0001 versus nicotine (0.3 mg/kg); $P < 0.0001 versus nicotine (0.6 mg/kg). (B) Prevention of mechanical allodynia by chronic administration of nicotine at a dose of 24 mg/kg per day. Arrows indicate vehicle/paclitaxel injections on days 0, 2, 4, and 6. Minipumps with nicotine were implanted s.c. in the mouse, starting 2 days before the vehicle/paclitaxel treatment cycle and ending on day 5. Baseline measurements were taken at baseline before saline/nicotine minipump implantation and on day 0 before paclitaxel/vehicle administration. ***P < 0.001 Pac-Nic versus Pac-Sal; ###P < 0.001 Pac-Sal versus Veh-Sal; $$$P < 0.001, $$P < 0.01 Veh-Nic versus Veh-Sal. BL, baseline; Nic, nicotine; Pac, paclitaxel; Sal, saline; Veh, vehicle. n = 8 per group; data are expressed as mean ± S.E.M. Statistical analysis: for mice treated with 24 mg/kg nicotine, a 2 × 2 × 10 mixed-factor ANOVA of chemotherapy drug (paclitaxel or vehicle) in nicotine- or saline-treated mice by day showed a significant three-way interaction [F(9,252) = 7.851, P < 0.001]. A subsequent 2 × 10 mixed-factor ANOVA of paclitaxel or vehicle treatment by day was calculated for each level of treatment (nicotine or saline). Saline-treated mice demonstrated a significant interaction of chemotherapy drug (paclitaxel or vehicle) by day [F(9,252) = 15.054, P < 0.001], where a Sidak post hoc test revealed a lower threshold responding in paclitaxel-treated mice compared with vehicle-treated mice on days 0–35 (P < 0.001). A separate 2 × 10 mixed-factor ANOVA calculated where nicotine or saline treatment by day differed at each level of chemotherapy drug (paclitaxel or vehicle). Paclitaxel-treated mice demonstrated a significant interaction of drug pretreatment (nicotine or saline) by day [F(9,252) = 6.703, P < 0.001], where a Sidak post hoc test revealed a higher threshold responding in nicotine-treated mice compared with saline-treated mice on days 0–35 (P < 0.001). Vehicle-treated mice also demonstrated a significant interaction of drug pretreatment (nicotine or saline) by day [F(9,252) = 37.064, P < 0.001], where a Sidak post hoc test revealed a higher threshold responding in nicotine-treated mice compared with saline-treated mice, but only on days 0, 1, and 3 (P < 0.001).
Having demonstrated that nicotine reversed the allodynic effect of paclitaxel, the next series of experiments was designed to investigate whether nicotine also prevents the development of paclitaxel-induced nociceptive (allodynic) responses. Seven days of nicotine (24 mg/kg per day) administration prevented the development of mechanical allodynia throughout the entire duration of the experiment, up to 35 days post–paclitaxel injection [F(9,252) = 6.703, P < 0.001] (Fig. 1B). As shown in Supplemental Fig. 2, 6 and 12 mg/kg per day nicotine did not prevent the development of paclitaxel-induced mechanical allodynia.
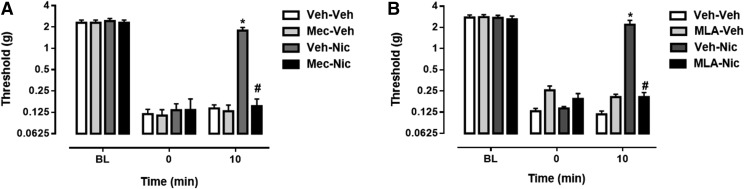
To examine the possibility that nicotinic acetylcholine receptors (nAChRs) mediate the antinociceptive effect of nicotine, mecamylamine, a nonselective nAChR antagonist, was administered prior to nicotine. Mecamylamine effectively blocked the antinociceptive effect of nicotine in paclitaxel-treated mice [Ftime × dose(6,42) = 10.38, P < 0.0001] (Fig. 2A). To begin determining which nAChR subtypes are involved in the reversal of paclitaxel-induced mechanical allodynia, we administered MLA, an α7 nAChR antagonist, before nicotine treatment, which effectively blocked the antinociceptive effect of nicotine in paclitaxel-treated mice [Ftime × dose(6,42) = 15.58, P < 0.0001] (Fig. 2B).
Fig. 2.
The antinociceptive effect of nicotine is mediated by nAChRs. (A) Mecamylamine, a nonselective nAChR antagonist, was injected at a dose of 2 mg/kg, s.c., 15 minutes prior to nicotine treatment to block the nicotine-mediated (0.9 mg/kg, i.p.) reversal of mechanical allodynia in paclitaxel-treated mice.(B) MLA, an α7 nAChR antagonist, was administered at a dose of 10 mg/kg, s.c., 10 minutes before nicotine treatment (0.9 mg/kg, i.p.) in paclitaxel-treated mice. *P < 0.0001 Veh-Nic at 10 versus 0 minutes; #P < 0.0001 Mec-Nic or MLA-Nic versus Veh-Nic at 10 minutes. Mec, mecamylamine; MLA, methyllycaconitine; Nic, nicotine; Veh, vehicle. n = 8 per group; data are expressed as the mean ± S.E.M.
Nicotine Prevents Paclitaxel-Induced Reduction of Intraepidermal Nerve Fibers.
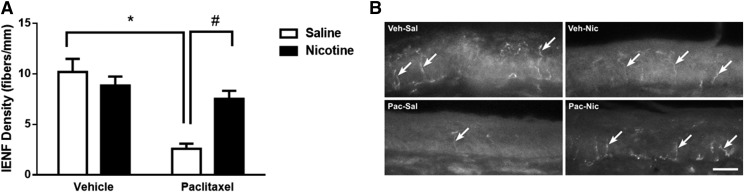
A decrease in the intraepidermal nerve fiber (IENF) density in the paw epidermis is a common marker for evaluating CIPN in rodent models (Bennett et al., 2011). To determine whether nicotine also protects the IENFs from the toxic effect of paclitaxel, mice were treated with vehicle or paclitaxel (8 mg/kg, i.p.) and implanted with minipumps releasing saline or nicotine (24 mg/kg per day), and sacrificed 35 days after the first paclitaxel injection, when their hind paw epidermis was collected for immunohistochemical analysis. Quantification of IENFs revealed a significant overall interaction between paclitaxel and nicotine treatment [Fpaclitaxel × nicotine(1,28) = 11.58, P < 0.01] (Fig. 3A). As illustrated in Fig. 3B, mice treated with paclitaxel-saline demonstrated a significant decrease in IENF density when compared with vehicle-saline–treated mice (P < 0.0001). In contrast, paclitaxel-nicotine–treated mice showed a significant increase in IENF density when compared with paclitaxel-saline–treated mice (P < 0.01). Paclitaxel-nicotine–treated mice did not show a change in the IENF density when compared with vehicle-nicotine–treated mice (P = 0.54), and vehicle-nicotine–treated mice did not exhibit an alteration in IENF density when compared with the vehicle-saline group (P = 0.53).
Fig. 3.
Paclitaxel induces a decrease in IENF density at 35 days post–paclitaxel injection, which is prevented by nicotine administration at a dose of 24 mg/kg per day, s.c. (A) Paclitaxel at a dose of 8 mg/kg, i.p., significantly decreased IENF density compared with vehicle-saline and paclitaxel-nicotine groups. *P < 0.05 paclitaxel-saline versus vehicle-saline; #P < 0.05 paclitaxel-nicotine versus paclitaxel-saline. (B) Immunostained sections of hind paw epidermis represent the reduction of IENF density by paclitaxel and protection by nicotine. Scale bar, 20 μm. Original magnification, 40×. Nic, nicotine; Pac, paclitaxel; Sal, saline; Veh, vehicle. n = 8 per group; data are expressed as the mean ± S.E.M.
Collectively, the behavioral and immunohistochemical studies presented in Figs. 1–3 indicate that nicotine reverses paclitaxel-induced mechanical allodynia, via the α7 nAChR, but also protects against paclitaxel-induced mechanical allodynia and IENF loss. However, multiple reports have argued that nicotine can stimulate tumor growth or interfere with cancer chemotherapeutic drug–induced apoptosis, which would severely limit the potential utility of nicotine in the clinic (Dasgupta et al., 2006; Zhang et al., 2009; Pillai et al., 2011; Wu et al., 2013; Liu et al., 2015). As a review of the relevant literature revealed a number of inconsistencies (see Discussion), we re-evaluated the effect of nicotine on tumor cell proliferation and paclitaxel-induced apoptosis.
Nicotine Fails to Stimulate Lung Cancer Cell Proliferation or Interfere with Paclitaxel-Induced Cytotoxicity.
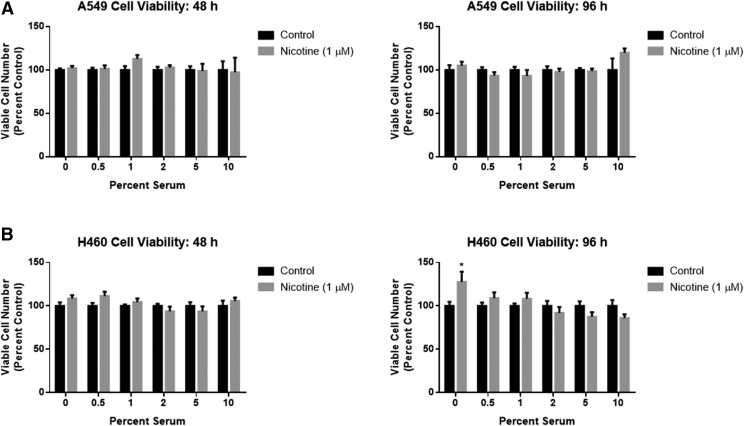
Initial experiments were performed by utilizing the MTT/MTS colorimetric assay with both A549 and H460 cells, two commonly used experimental models of NSCLC that express multiple nAChRs (Tsurutani et al., 2005; Dasgupta et al., 2006; Yoo et al., 2014). Figure 4 indicates that 48 and 96 hours of exposure to nicotine (1 μM) did not induce a significant increase in viable cell number when compared with untreated cells under both normal (10% FBS) and serum deprivation (0–5% FBS) conditions in A549 (Fig. 4A) and H460 (Fig. 4B) cells. The only observed effect was a significant increase in viable cell numbers under serum starvation (0% FBS) conditions in one cell line at a single time point (Fig. 4B).
Fig. 4.
Nicotine fails to enhance NSCLC viable cell numbers under normal and serum-deprivation conditions. (A) A549 and (B) H460 cells were treated with nicotine (1 μM) for 48 or 96 hours in DMEM supplemented with various concentrations of FBS. Viability was determined with an MTT or MTS colorimetric assay. *P < 0.05 vs. control with 0% serum. Data are expressed as the mean ± S.E.M of three independent experiments.
The influence of various concentrations of nicotine on tumor cell proliferation was further investigated under full serum (10% FBS) conditions that are the standard for cancer cell studies. Again, exposure to a range of nicotine concentrations (0.1–10 μM) for 24 hours under full serum conditions did not significantly increase the numbers of viable A549, H460, LLC, or T1 (primary lung cancer) cells (Supplemental Fig. 3).
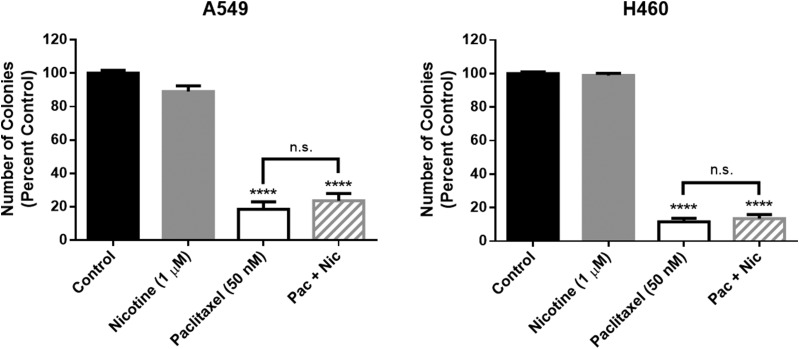
Additional studies were designed to more closely mimic the potential use of nicotine after chemotherapy treatment in the clinic. NSCLC cells were first exposed to paclitaxel (50 nM) for 24 hours, followed by a 24-hour drug-free period and subsequent treatment with nicotine (1 μM) for 24 hours. Paclitaxel significantly decreased the number of A549 and H460 colonies, and the impact of paclitaxel was not altered by nicotine; nicotine alone did not stimulate colony formation (Fig. 5).
Fig. 5.
Nicotine fails to stimulate NSCLC colony formation alone or after paclitaxel treatment. For the single-drug treatment conditions, A549 cells (left) and H460 cells (right) were exposed to nicotine (1 μM) or paclitaxel (50 nM) for 24 hours. For the combination treatment, cells were first exposed to paclitaxel for 24 hours, followed by a 24-hour drug-free period, then treatment with nicotine for 24 hours. Colony number was determined by crystal violet staining. ****P < 0.0001 versus control. n.s., not significant. Data are expressed as the mean ± S.E.M. of three independent experiments.
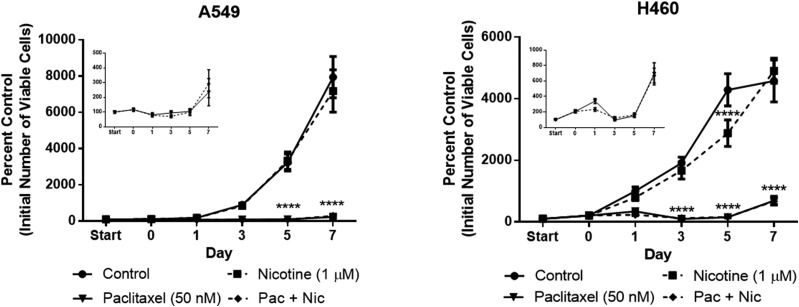
In previous work (Jones et al., 2005; Roberson et al., 2005; Efimova et al., 2010; Emery et al., 2014; Webster et al., 2015; Alotaibi et al., 2016), we and others have reported that growth arrest induced by cancer therapy is transient and that tumor cells recover proliferative capacity within 7–10 days post-treatment. To determine whether prior exposure to nicotine stimulates proliferation and/or promotes early tumor cell recovery from paclitaxel-induced growth arrest, NSCLC cell proliferation was monitored for a period of 7 days after treatment with nicotine (1 μM, 48 hours), paclitaxel (50 nM, 24 hours), or a combination of the two drugs, which consisted of a 24-hour nicotine pretreatment period preceding 24-hour cotreatment. Nicotine did not stimulate the proliferation of either the A549 or H460 cells throughout the duration of the assay and even induced a slight but significant decrease in H460 cell number on day 5 (Fig. 6). Most importantly, nicotine did not interfere with the paclitaxel-induced decrease in viable cell number at any time point (Fig. 6). Furthermore, nicotine did not promote an early proliferative recovery in either cell line (insets of Fig. 6).
Fig. 6.
Nicotine fails to stimulate NSCLC cell proliferation alone or interfere with paclitaxel-induced growth inhibition of NSCLC cells. The ‘start’ time point represents the initial number of A549 cells (left) or H460 cells (right) after seeding. A 24-hour nicotine pretreatment period occurred from start to day 0 for the nicotine and Pac + Nic conditions, then all subsequent treatments lasted 24 hours; no drugs were present after day 1. The number of cells was determined via trypan blue exclusion. ****P < 0.0001 versus control. Data are expressed as the mean ± S.E.M. of three independent experiments.
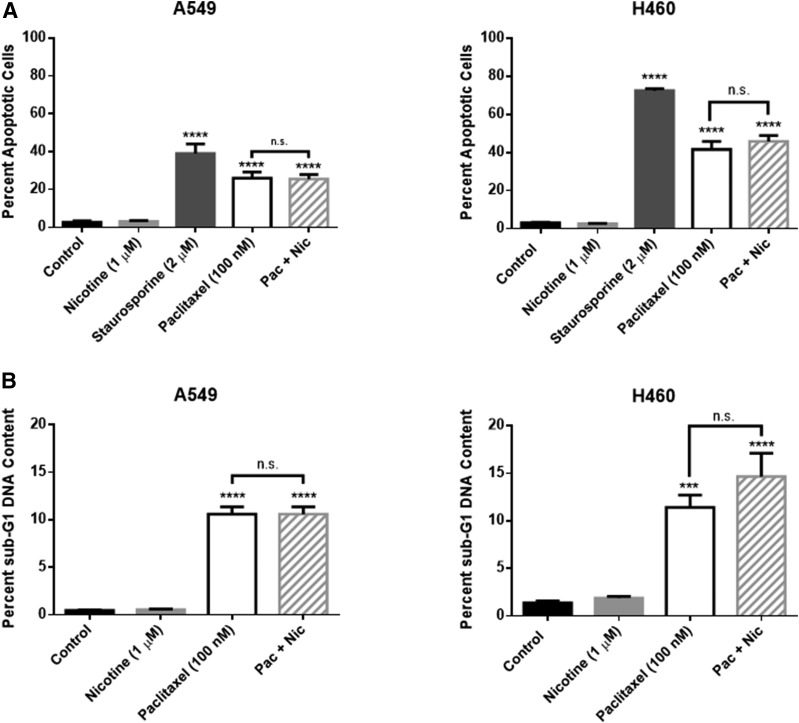
Although we failed to detect any interference with the antitumor activity of paclitaxel in two different assays, other studies have argued that nicotine suppresses paclitaxel-induced apoptosis (Tsurutani et al., 2005; Dasgupta et al., 2006). Consequently, additional experiments were performed to evaluate the effects of nicotine on paclitaxel-induced apoptosis. Paclitaxel (100 nM) induced significant apoptosis in both the A549 and H460 cells after 48 hours of treatment (Fig. 7A). Most importantly, nicotine (1 μM) did not interfere with the promotion of paclitaxel-induced apoptosis in either NSCLC cell line after cotreatment (Fig. 7A); staurosporine (2 µM), a nonselective protein kinase inhibitor, induced significant apoptosis and was used as a positive control. Similarly, cell cycle analysis revealed that paclitaxel (100 nM) induces significant sub-G1 fragmented DNA content, an indicator of late-stage cell death, in both A549 and H460 cells, and that nicotine does not attenuate this effect (Fig. 7B).
Fig. 7.
Nicotine fails to interfere with paclitaxel-induced apoptosis (A) and sub-G1 DNA content (B) of NSCLC cells. A549 and H460 cells were treated with nicotine (1 μM), staurosporine (2 μM), paclitaxel (100 nM), or the combination of paclitaxel and nicotine for 48 hours. Quantification of apoptotic cells and sub-G1 DNA content was determined by the Annexin V/PI assay and PI staining, respectively, followed by flow cytometry analysis. ***P < 0.001, ****P < 0.0001 versus control. n.s., not significant. Data are expressed as the mean ± S.E.M. of three (A) or two (B) independent experiments.
To ensure that our observations applied to other cancer types commonly treated with paclitaxel, we also evaluated the effects of nicotine on cancer cell proliferation in two human ovarian cancer cell lines. As was the case with the lung cancer cells, nicotine did not stimulate ovarian cancer cell proliferation in SKOV-3/DDP and OVCAR-3 cells (Supplemental Fig. 4).
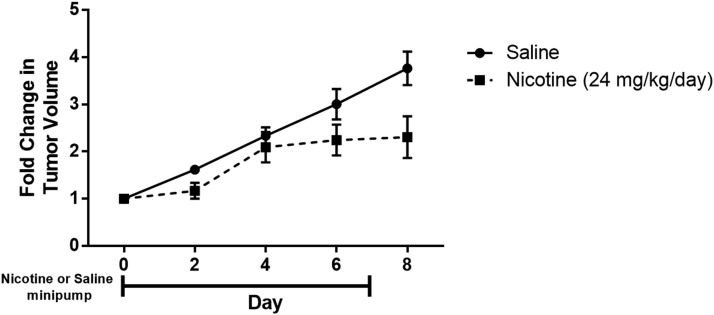
To investigate whether the in vitro findings are indicative of tumor cell responses in vivo, immunocompetent C57BL/6J mice were injected s.c. with LLC cells in the flank, a commonly used syngeneic model of lung cancer (Kellar et al., 2015). Once the tumors formed, the mice were treated with nicotine at a dose of 24 mg/kg per day for 7 days via an s.c. osmotic minipump to mimic the nicotine treatment regimen administered in the peripheral neuropathy studies. In accordance with the in vitro findings, chronic administration of nicotine failed to enhance LLC tumor growth (Fig. 8).
Fig. 8.
Nicotine fails to enhance LLC tumor growth in vivo. C57BL/6J mice were injected s.c. with 1.5 × 106 LLC cells in both flanks. Once tumors formed, s.c. osmotic minipumps were implanted on day 0 to release 24 mg/kg nicotine daily for a total of 7 days. The left and right flank tumor volumes (length × width × height) were compared with the respective baseline tumor volumes to calculate fold change; the fold change values were averaged for each mouse. A linear mixed-model analysis revealed a significant effect of time [F(4,39) = 25.747, P < 0.001] and treatment [F(1,39) = 15.683, P < 0.001] but no interaction between time and treatment [F(4,39) = 2.560, P = 0.054]. n = 5–6/group. Data are expressed as the mean ± S.E.M.
Discussion
Effects of Nicotine on Chemotherapy-Induced Peripheral Neuropathy.
To the best of our knowledge, this is the first study to report that nicotine reverses paclitaxel-induced mechanical allodynia as well as prevents paclitaxel-induced peripheral neuropathy when administered prior to and during paclitaxel treatment in the mouse. Our work also indicates that nAChRs mediate the antinociceptive effects of nicotine based on interference by mecamylamine, a nonselective nAChR antagonist, and MLA, an α7 nAChR antagonist. Our studies further demonstrate that chronic nicotine infusion prevents the loss of IENFs in the epidermis of the hind paw after paclitaxel treatment. Taken together, these findings suggest that nicotine could have potential utility for the prevention and/or treatment of CIPN.
Mice treated with 8 mg/kg paclitaxel developed significant mechanical allodynia, which is consistent with our recent report (Toma et al., 2017) and previous studies (Deng et al., 2015; Neelakantan et al., 2016; Slivicki et al., 2016). Acute administration of nicotine reverses the mechanical allodynia induced by paclitaxel, which is consistent with studies by Di Cesare Mannelli et al. (2013) in a rat model of oxaliplatin-induced peripheral neuropathy. As nicotine has a very short half-life of approximately 15 minutes in mice (Damaj et al., 2007), it was also administered chronically via 7-day osmotic minipumps to achieve and maintain steady-state levels. We have previously reported that s.c. minipump administration of 12 and 25 mg/kg per day nicotine leads to plasma nicotine concentrations of approximately 56 and 97 ng/ml or 0.121 and 0.210 μM, respectively (Alsharari et al., 2013, 2015). Chronic administration of nicotine (24 mg/kg per day) prior to and during paclitaxel injections significantly prevents both the development of mechanical allodynia and the reduction of IENFs induced by paclitaxel, as previously described by our group (Toma et al., 2017); others have also shown protection from paclitaxel-induced IENF loss with pifithrin-μ (Krukowski et al., 2015).
The antinociceptive and antiallodynic properties of nicotine have been demonstrated in numerous animal and human studies (Flood and Damaj, 2014), including neuropathic pain in humans (Rowbotham et al., 2009; Richardson et al., 2012). Randomized, double-blind, placebo-controlled trials have reported that intranasal or transdermal administration of nicotine preoperatively or postoperatively results in significantly decreased pain scores and lower morphine consumption, respectively (Flood and Daniel, 2004; Habib et al., 2008; Yagoubian et al., 2011). Similarly, laboratory animal studies have revealed that nicotine acts as an antinociceptive drug in a variety of acute and chronic pain models in rodents (Alsharari et al., 2012, 2015). More specifically, the α7 nAChR subtype has been reported to mediate the antinociceptive effects of nicotine in a mouse model of postoperative pain (Rowley et al., 2008).
Others have also investigated targeting nAChRs for the treatment of CIPN. For example, a recent study (Romero et al., 2017) indicated that pharmacological and genetic blockade of the α9α10 nAChR subtype prevents the development of neuropathic pain induced by oxaliplatin in mice, suggesting that nAChRs play a significant role in the development and, potentially, the treatment of CIPN. Furthermore, nicotine reduces the ratio of proinflammatory monocytes compared with anti-inflammatory monocytes in murine bone marrow via the α7 nAChR subtype, thus significantly decreasing the level of proinflammatory cytokines, including tumor necrosis factor-α and interleukin-1β, and enhancing the release of anti-inflammatory cytokines, such as interleukin-12 (St-Pierre et al., 2016). Moreover, nicotine exhibits a neuroprotective effect in animal models of neurodegenerative diseases, such as Alzheimer’s disease, an action that is predominantly mediated through the α7 nAChR subtype (Ferrea and Winterer, 2009). Overall, it appears that the α7 nAChR may be one of the predominant nAChR subtypes involved in the neuroprotective actions of nicotine.
Although the current work clearly demonstrates the potential for nicotine to both prevent and reverse paclitaxel-induced peripheral neuropathy, there is an extensive body of literature suggesting that nicotine may stimulate tumor growth and/or interfere with the effectiveness of chemotherapy. This untoward effect is thought to occur via the binding of nicotine to an nAChR on the plasma membrane of the tumor cell, thereby promoting proliferative and antiapoptotic signaling via the extracellular signal-regulated kinase and phosphoinositide 3-kinase/Akt (protein kinase B) pathways, respectively (Grando, 2014; Schaal and Chellappan, 2014). However, the only experimental condition under which we identified an effect of nicotine was on tumor cell proliferation in serum-free media, in which cells are deprived of nutrients, cytokines, and other growth factors, which is a nonphysiological environment. Under standard cell growth conditions, nicotine did not enhance viability, colony formation, or proliferation of a number of experimental tumor cell lines or interfere with apoptosis induced by paclitaxel.
It is somewhat difficult to make direct comparisons between our studies and those in the literature focusing on human NSCLC cell lines because the concentrations of paclitaxel and nicotine vary widely in these experiments, with paclitaxel concentrations ranging between 0.1 and 20 µM, and nicotine concentrations ranging from 0.1 to 10 μM. The human steady-state plasma concentration of paclitaxel falls between 5 and 200 nM, whereas the nicotine concentration in cigarette smokers ranges from 20 to 60 ng/ml, or 0.1 to 0.4 μM (Blagosklonny and Fojo, 1999; Benowitz et al., 2009). In addition to the lack of consistency in the concentrations of paclitaxel and nicotine, the duration of drug exposure (18 hours to 7 days) and the serum concentration (0–10%) also cover a wide range. In our work, we used 1 µM nicotine for 24–96 hours, a treatment regimen that involves both acute and chronic exposure to a nicotine concentration that is slightly higher than peak human plasma levels to use a clinically relevant dose of nicotine. Similarly, we used paclitaxel concentrations of 50 and 100 nM because the former is appropriate for experiments involving low cell numbers, such as the clonogenic assay, and the latter induces substantial apoptosis; yet, most importantly, both concentrations are within the range of human plasma levels.
Given the various experimental conditions, it is perhaps not surprising that the reported effects of nicotine vary widely as well. For example, some studies have shown increases of 23–200% in NSCLC cell proliferation (Zhang et al., 2009; Pillai et al., 2011; Wu et al., 2013; Liu et al., 2015), whereas others demonstrate modest increases of 7–18% (Chen et al., 2002; Jarzynka et al., 2006; Puliyappadamba et al., 2010) and in one case decreases of 40–72% (Gao et al., 2016). Nicotine has been reported to reduce paclitaxel-induced apoptosis by a significant 50% (Dasgupta et al., 2006) or only by a modest 8% (Tsurutani et al., 2005). These studies used sub-G1 DNA content, the terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling assay, and poly-ADP ribose polymerase cleavage to assess the impact of nicotine on paclitaxel-induced apoptosis, whereas we included quantification of early and late apoptotic populations with the Annexin V/PI assay. In part, this may provide a rationale for the inconsistencies in outcomes since sub-G1 DNA content alone does not distinguish between apoptotic and necrotic cell death (Mattes, 2007). The observation of substantially more apoptosis than sub-G1 DNA content after 48 hours of paclitaxel treatment likely reflects the possibility that early apoptotic cells had not yet become fragmented.
With regard to in vivo studies, although nicotine exposure has been reported to significantly increase lung tumor incidence, volume, weight, and Ki-67+ populations (Heeschen et al., 2001; Jarzynka et al., 2006; Improgo et al., 2013; Iskandar et al., 2013; Liu et al., 2015), other reports have shown that chronic nicotine treatment does not significantly stimulate lung tumor growth in mice (Maier et al., 2011; Murphy et al., 2011; Warren et al., 2012). Similarly, we found that chronic nicotine administration did not enhance LLC tumor growth in immunocompetent mice, suggesting that nicotine may be a potential therapy for CIPN prior to or after chemotherapy with the aim of preventing and reversing peripheral neuropathy, respectively.
In summary, our results provide a proof of concept that nicotine is efficacious in preventing and reversing CIPN, actions that may enhance the quality of life of cancer patients and survivors. In addition, our findings suggest that nicotine does not significantly promote tumor cell proliferation or interfere with chemotherapy in lung cancer cell lines. In this context, we are encouraged by the report that nicotine replacement therapy is not a significant predictor of cancer in humans (Murray et al., 2009).
Abbreviations
- ANOVA
analysis of variance
- CIPN
chemotherapy-induced peripheral neuropathy
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
dimethylsulfoxide
- FBS
fetal bovine serum
- IENF
intraepidermal nerve fiber
- LLC
Lewis lung carcinoma
- MLA
methyllycaconitine
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- nAChR
nicotinic acetylcholine receptor
- NSCLC
non–small cell lung cancer
- PBS
phosphate-buffered saline
- PI
propidium iodide
- VCU
Virginia Commonwealth University
Authorship Contributions
Participated in research design: Kyte, Toma, Bagdas, Fang, Damaj, and Gewirtz.
Conducted experiments: Kyte, Toma, Bagdas, Meade, and Fang.
Contributed new reagents or analytic tools: Bigbee.
Performed data analysis: Kyte, Toma, Bagdas, and Schurman.
Wrote or contributed to the writing of the manuscript: Kyte, Toma, Schurman, Lichtman, Chen, Del Fabbro, Damaj, and Gewirtz.
Footnotes
The research was supported by the National Institutes of Health (NIH) [Grant 1R01-CA-206028-01] (to M.I.D. and D.A.G.), [Grant T32-DA-007027-41] (to S.L.K.), [Grant 1F31-NS-095628-01A1] (to L.D.S.) and, in part, by a Massey Cancer Center Pilot Project Grant (to D.A.G. and M.I.D.). Microscopy was performed at the VCU Microscopy Facility, and flow cytometry analysis was conducted at the VCU Massey Cancer Center Flow Cytometry Shared Resource, which were supported, in part, with funding by NIH-National Cancer Institute Cancer Center Support Grant P30-CA-016059. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Alotaibi M, Sharma K, Saleh T, Povirk LF, Hendrickson EA, Gewirtz DA. (2016) Radiosensitization by PARP inhibition in DNA repair proficient and deficient tumor cells: proliferative recovery in senescent cells. Radiat Res 185:229–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlSharari SD, Akbarali HI, Abdullah RA, Shahab O, Auttachoat W, Ferreira GA, White KL, Lichtman AH, Cabral GA, Damaj MI. (2013) Novel insights on the effect of nicotine in a murine colitis model. J Pharmacol Exp Ther 344:207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlSharari SD, Carroll FI, McIntosh JM, Damaj MI. (2012) The antinociceptive effects of nicotinic partial agonists varenicline and sazetidine-A in murine acute and tonic pain models. J Pharmacol Exp Ther 342:742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsharari SD, King JR, Nordman JC, Muldoon PP, Jackson A, Zhu AZX, Tyndale RF, Kabbani N, Damaj MI. (2015) Effects of menthol on nicotine pharmacokinetic, pharmacology and dependence in mice. PLoS One 10:e0137070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, AlSharari SD, Freitas K, Tracy M, Damaj MI. (2015) The role of alpha5 nicotinic acetylcholine receptors in mouse models of chronic inflammatory and neuropathic pain. Biochem Pharmacol 97:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, Ergun D, Jackson A, Toma W, Schulte MK, Damaj MI. (2017) Allosteric modulation of α4β2* nicotinic acetylcholine receptors: desformylflustrabromine potentiates antiallodynic response of nicotine in a mouse model of neuropathic pain. Eur J Pain [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, Muldoon PP, Zhu AZX, Tyndale RF, Damaj MI. (2014) Effects of methoxsalen, a CYP2A5/6 inhibitor, on nicotine dependence behaviors in mice. Neuropharmacology 85:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beijers AJM, Jongen JLM, Vreugdenhil G. (2012) Chemotherapy-induced neurotoxicity: the value of neuroprotective strategies. Neth J Med 70:18–25. [PubMed] [Google Scholar]
- Bennett GJ, Liu GK, Xiao WH, Jin HW, Siau C. (2011) Terminal arbor degeneration--a novel lesion produced by the antineoplastic agent paclitaxel. Eur J Neurosci 33:1667–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Hukkanen J, Jacob P., III (2009) Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol 192:29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV, Fojo T. (1999) Molecular effects of paclitaxel: myths and reality (a critical review). Int J Cancer 83:151–156. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63. [DOI] [PubMed] [Google Scholar]
- Charan J, Kantharia ND. (2013) How to calculate sample size in animal studies? J Pharmacol Pharmacother 4:303–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GQ, Lin B, Dawson MI, Zhang XK. (2002) Nicotine modulates the effects of retinoids on growth inhibition and RAR β expression in lung cancer cells. Int J Cancer 99:171–178. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Siu ECK, Sellers EM, Tyndale RF, Martin BR. (2007) Inhibition of nicotine metabolism by methoxysalen: pharmacokinetic and pharmacological studies in mice. J Pharmacol Exp Ther 320:250–257. [DOI] [PubMed] [Google Scholar]
- Dasgupta P, Kinkade R, Joshi B, Decook C, Haura E, Chellappan S. (2006) Nicotine inhibits apoptosis induced by chemotherapeutic drugs by up-regulating XIAP and survivin. Proc Natl Acad Sci USA 103:6332–6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Guindon J, Cornett BL, Makriyannis A, Mackie K, Hohmann AG. (2015) Chronic cannabinoid receptor 2 activation reverses paclitaxel neuropathy without tolerance or cannabinoid receptor 1-dependent withdrawal. Biol Psychiatry 77:475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cesare Mannelli L, Zanardelli M, Ghelardini C. (2013) Nicotine is a pain reliever in trauma- and chemotherapy-induced neuropathy models. Eur J Pharmacol 711:87–94. [DOI] [PubMed] [Google Scholar]
- Dranitsaris G, Yu B, King J, Kaura S, Zhang A. (2015) Nab-paclitaxel, docetaxel, or solvent-based paclitaxel in metastatic breast cancer: a cost-utility analysis from a Chinese health care perspective. Clinicoecon Outcomes Res 7:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimova EV, Mauceri HJ, Golden DW, Labay E, Bindokas VP, Darga TE, Chakraborty C, Barreto-Andrade JC, Crawley C, Sutton HG, et al. (2010) Poly(ADP-ribose) polymerase inhibitor induces accelerated senescence in irradiated breast cancer cells and tumors. Cancer Res 70:6277–6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery SM, Alotaibi MR, Tao Q, Selley DE, Lichtman AH, Gewirtz DA. (2014) Combined antiproliferative effects of the aminoalkylindole WIN55,212-2 and radiation in breast cancer cells. J Pharmacol Exp Ther 348:293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrea S, Winterer G. (2009) Neuroprotective and neurotoxic effects of nicotine. Pharmacopsychiatry 42:255–265. [DOI] [PubMed] [Google Scholar]
- Flood P, Daniel D. (2004) Intranasal nicotine for postoperative pain treatment. Anesthesiology 101:1417–1421. [DOI] [PubMed] [Google Scholar]
- Flood P, Damaj MI. (2014) Nicotine is out: nicotinic agonists may have utility as analgesics. Anesth Analg 119:232–233. [DOI] [PubMed] [Google Scholar]
- Freitas K, Ghosh S, Ivy Carroll F, Lichtman AH, Imad Damaj M. (2013) Effects of α7 positive allosteric modulators in murine inflammatory and chronic neuropathic pain models. Neuropharmacology 65:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T, Zhou X-L, Liu S, Rao C-X, Shi W, Liu J-C. (2016) In vitro effects of nicotine on the non-small-cell lung cancer line A549. J Pak Med Assoc 66:368–72. [PubMed] [Google Scholar]
- Grando SA. (2014) Connections of nicotine to cancer. Nat Rev Cancer 14:419–429. [DOI] [PubMed] [Google Scholar]
- Habib AS, White WD, El Gasim MA, Saleh G, Polascik TJ, Moul JW, Gan TJ. (2008) Transdermal nicotine for analgesia after radical retropubic prostatectomy. Anesth Analg 107:999–1004. [DOI] [PubMed] [Google Scholar]
- Hama A, Takamatsu H. (2016) Chemotherapy-induced peripheral neuropathic pain and rodent models. CNS Neurol Disord Drug Targets 15:7–19. [DOI] [PubMed] [Google Scholar]
- Heeschen C, Jang JJ, Weis M, Pathak A, Kaji S, Hu RS, Tsao PS, Johnson FL, Cooke JP. (2001) Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med 7:833–839. [DOI] [PubMed] [Google Scholar]
- Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, Chauhan C, Gavin P, Lavino A, Lustberg MB, et al. American Society of Clinical Oncology (2014) Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 32:1941–1967. [DOI] [PubMed] [Google Scholar]
- Improgo MR, Soll LG, Tapper AR, Gardner PD. (2013) Nicotinic acetylcholine receptors mediate lung cancer growth. Front Physiol 4:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iskandar AR, Liu C, Smith DE, Hu KQ, Choi SW, Ausman LM, Wang XD. (2013) β-cryptoxanthin restores nicotine-reduced lung SIRT1 to normal levels and inhibits nicotine-promoted lung tumorigenesis and emphysema in A/J mice. Cancer Prev Res (Phila) 6:309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarzynka MJ, Guo P, Bar-Joseph I, Hu B, Cheng SY. (2006) Estradiol and nicotine exposure enhances A549 bronchioloalveolar carcinoma xenograft growth in mice through the stimulation of angiogenesis. Int J Oncol 28:337–344. [PMC free article] [PubMed] [Google Scholar]
- Jones KR, Elmore LW, Jackson-Cook C, Demasters G, Povirk LF, Holt SE, Gewirtz DA. (2005) p53-Dependent accelerated senescence induced by ionizing radiation in breast tumour cells. Int J Radiat Biol 81:445–458. [DOI] [PubMed] [Google Scholar]
- Kim JH, Dougherty PM, Abdi S. (2015) Basic science and clinical management of painful and non-painful chemotherapy-related neuropathy. Gynecol Oncol 136:453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellar A, Egan C, Morris D. (2015) Preclinical murine models for lung cancer: clinical trail applications. BioMed Res Int 2015:621324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krukowski K, Nijboer CH, Huo X, Kavelaars A, Heijnen CJ. (2015) Prevention of chemotherapy-induced peripheral neuropathy by the small-molecule inhibitor pifithrin-μ. Pain 156:2184–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. (1987) Statistical Analysis with Missing Data, Wiley, New York. [Google Scholar]
- Liu W, Yi DD, Guo JL, Xiang ZX, Deng LF, He L. (2015) Nuciferine, extracted from Nelumbo nucifera Gaertn, inhibits tumor-promoting effect of nicotine involving Wnt/β-catenin signaling in non-small cell lung cancer. J Ethnopharmacol 165:83–93. [DOI] [PubMed] [Google Scholar]
- Maier CR, Hollander MC, Hobbs EA, Dogan I, Linnoila RI, Dennis PA. (2011) Nicotine does not enhance tumorigenesis in mutant K-ras-driven mouse models of lung cancer. Cancer Prev Res (Phila) 4:1743–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majithia N, Temkin SM, Ruddy KJ, Beutler AS, Hershman DL, Loprinzi CL. (2016) National Cancer Institute-supported chemotherapy-induced peripheral neuropathy trials: outcomes and lessons. Support Care Cancer 24:1439–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes MJ. (2007) Apoptosis assays with lymphoma cell lines: problems and pitfalls. Br J Cancer 96:928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SE, von Weymarn LB, Schutten MM, Kassie F, Modiano JF. (2011) Chronic nicotine consumption does not influence 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis. Cancer Prev Res (Phila) 4:1752–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RP, Connett JE, Zapawa LM. (2009) Does nicotine replacement therapy cause cancer? Evidence from the Lung Health Study. Nicotine Tob Res 11:1076–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelakantan H, Ward SJ, Walker EA. (2016) Effects of paclitaxel on mechanical sensitivity and morphine reward in male and female C57Bl6 mice. Exp Clin Psychopharmacol 24:485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S, Rizwani W, Li X, Rawal B, Nair S, Schell MJ, Bepler G, Haura E, Coppola D, Chellappan S. (2011) ID1 facilitates the growth and metastasis of non-small cell lung cancer in response to nicotinic acetylcholine receptor and epidermal growth factor receptor signaling. Mol Cell Biol 31:3052–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puliyappadamba VT, Cheriyan VT, Thulasidasan AKT, Bava SV, Vinod BS, Prabhu PR, Varghese R, Bevin A, Venugopal S, Anto RJ. (2010) Nicotine-induced survival signaling in lung cancer cells is dependent on their p53 status while its down-regulation by curcumin is independent. Mol Cancer 9:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson EJ, Ness TJ, Redden DT, Stewart CC, Richards JS. (2012) Effects of nicotine on spinal cord injury pain vary among subtypes of pain and smoking status: results from a randomized, controlled experiment. J Pain 13:1206–1214. [DOI] [PubMed] [Google Scholar]
- Roberson RS, Kussick SJ, Vallieres E, Chen SY, Wu DY. (2005) Escape from therapy-induced accelerated cellular senescence in p53-null lung cancer cells and in human lung cancers. Cancer Res 65:2795–2803. [DOI] [PubMed] [Google Scholar]
- Romero HK, Christensen SB, Di Cesare Mannelli L, Gajewiak J, Ramachandra R, Elmslie KS, Vetter DE, Ghelardini C, Iadonato SP, Mercado JL, et al. (2017) Inhibition of α9α10 nicotinic acetylcholine receptors prevents chemotherapy-induced neuropathic pain. Proc Natl Acad Sci USA 114:E1825–E1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbotham MC, Duan WR, Thomas J, Nothaft W, Backonja MM. (2009) A randomized, double-blind, placebo-controlled trial evaluating the efficacy and safety of ABT-594 in patients with diabetic peripheral neuropathic pain. Pain 146:245–252. [DOI] [PubMed] [Google Scholar]
- Rowley TJ, Payappilly J, Lu J, Flood P. (2008) The antinociceptive response to nicotinic agonists in a mouse model of postoperative pain. Anesth Analg 107:1052–1057. [DOI] [PubMed] [Google Scholar]
- Schaal C, Chellappan SP. (2014) Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol Cancer Res 12:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M. (2014) Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain 155:2461–2470. [DOI] [PubMed] [Google Scholar]
- Slivicki RA, Ali YO, Lu HC, Hohmann AG. (2016) Impact of genetic reduction of NMNAT2 on chemotherapy-induced losses in cell viability in vitro and peripheral neuropathy in vivo. PLoS One 11:e0147620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre S, Jiang W, Roy P, Champigny C, LeBlanc É, Morley BJ, Hao J, Simard AR. (2016) Nicotinic acetylcholine receptors modulate bone marrow-derived pro-inflammatory monocyte production and survival. PLoS One 11:e0150230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate EH, Wilder ME, Cram LS, Wharton W. (1983) A method for staining 3T3 cell nuclei with propidium iodide in hypotonic solution. Cytometry 4:211–215. [DOI] [PubMed] [Google Scholar]
- Toma W, Kyte SL, Bagdas D, Alkhlaif Y, Alsharari SD, Lichtman AH, Chen ZJ, Del Fabbro E, Bigbee JW, Gewirtz DA, et al. (2017) Effects of paclitaxel on the development of neuropathy and affective behaviors in the mouse. Neuropharmacology 117:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurutani J, Castillo SS, Brognard J, Granville CA, Zhang C, Gills JJ, Sayyah J, Dennis PA. (2005) Tobacco components stimulate Akt-dependent proliferation and NFkappaB-dependent survival in lung cancer cells. Carcinogenesis 26:1182–1195. [DOI] [PubMed] [Google Scholar]
- Umana IC, Daniele CA, McGehee DS. (2013) Neuronal nicotinic receptors as analgesic targets: It’s a winding road. Biochem Pharmacol 86:1208–1214, Elsevier Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren GW, Romano MA, Kudrimoti MR, Randall ME, McGarry RC, Singh AK, Rangnekar VM. (2012) Nicotinic modulation of therapeutic response in vitro and in vivo. Int J Cancer 131:2519–2527. [DOI] [PubMed] [Google Scholar]
- Webster MR, Xu M, Kinzler KA, Kaur A, Appleton J, O’Connell MP, Marchbank K, Valiga A, Dang VM, Perego M, et al. (2015) Wnt5A promotes an adaptive, senescent-like stress response, while continuing to drive invasion in melanoma cells. Pigment Cell Melanoma Res 28:184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SQ, Lv YE, Lin BH, Luo LM, Lv SL, Bi AH, Jia YS. (2013) Silencing of periostin inhibits nicotine-mediated tumor cell growth and epithelial-mesenchymal transition in lung cancer cells. Mol Med Rep 7:875–880. [DOI] [PubMed] [Google Scholar]
- Yagoubian B, Akkara J, Afzali P, Alfi DM, Olson L, Conell-Price J, Yeh J, Eisig SB, Flood P. (2011) Nicotine nasal spray as an adjuvant analgesic for third molar surgery. J Oral Maxillofac Surg 69:1316–1319. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Lee SM, Do SK, Lee WK, Kim DS, Park JY. (2014) Unmethylation of the CHRNB4 gene is an unfavorable prognostic factor in non-small cell lung cancer. Lung Cancer 86:85–90. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kamdar O, Le W, Rosen GD, Upadhyay D. (2009) Nicotine induces resistance to chemotherapy by modulating mitochondrial signaling in lung cancer. Am J Respir Cell Mol Biol 40:135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]