Abstract
Background:
Varicella zoster virus is the etiologic agent of primary varicella (chickenpox) during childhood, and varicella vaccination has not been introduced in Iran. The aim of this study is to estimate cost-effectiveness of one- and two-dose Varicella Vaccination Program in Iran.
Methods:
A decision-tree model was conducted to evaluate the cost-effectiveness of the Varicella Vaccination Program in a cohort of 12 months children in Iran. Epidemiologic parameters of varicella were extracted from local and international sources, and cost of disease was estimated based on societal prospective in 2015 US$. Incremental cost per disability-adjusted life years (DALY) averted calculated as final outcome. Sensitivity analysis was also performed for lower and upper estimate of incidence, DALY, and vaccine efficacy.
Results:
Considering the vaccine efficacy of 95%, for the two-dose and 85% for the one-dose vaccination, incremental cost-effectiveness ratio (ICER) per DALYs averted were US$41,531 and US$17,280, respectively. ICER has changed between (US$ 6,177–US$167,047) in lower and upper base estimate of epidemiological burden parameters in sensitivity analysis.
Conclusions:
Varicella vaccination is not cost-effective in Iran in one-dose and two-dose scenario under the assumptions of this study in base case scenario according to the threshold of incremental cost per DALY averted less than three time of GDP per capita in Iran = US$ 14,292. One-dose vaccination program might be cost-effective in upper scenario of epidemiological burden of varicella in sensitivity analysis.
Keywords: Chicken pox, children, cost-effectiveness, Iran, varicella vaccination, varicella zoster virus
Introduction
Varicella zoster virus (VZV) is a DNA virus which can cause varicella (chickenpox) in young adulthood. This virus could establish a latent infection in dorsal roots which can cause herpes zoster (HZ) in future. Usually, VZV infections are benign but can cause major and significant infection which could be occasionally fatal.[1,2,3] About 90% of the population could become infected with VZV by the age of 15 years.[4,5,6] Although VZV seroprevalence increases with aging, a significant proportion of adolescences are still susceptible to varicella infection in Iran. Among adults, the disease is more severe and prolonged and has 15–25 times higher mortality than pediatric patients.[7] The United States added Varicella Vaccination Program in the mid1990s. However, many other countries did not introduce varicella prevention programs due to this believe that infant vaccination may increase the occurrence of adult varicella or because the vaccination may not be cost-effective for their health-care system.[8,9]
Most of the epidemiologic data about varicella are from developed countries’ databases. The incidence of varicella is seasonal and rallies in spring and summer. The epidemic outbreak appears every 2–5 years.[10] Case fatality rate of varicella is much less than other infectious diseases, including smallpox, polio, rotavirus, pertussis, and measles.[11] Based on literature, burden created by chicken pox is 4.2 million hospitalizations as a result of severe complications and 4200 deaths.[12] Recent study in Iran determined that 64.8% of children had no detectable antibody levels for varicella zoster. The overall varicella seroprevalence rate is 35.2%.[13]
Literature highly supports suitability of establishing mass Varicella Vaccination Programs in Iran[7,13] by highlighting the importance of reaching high levels of virus coverage. However, there is not any suitable research on cost-effectiveness of varicella vaccination in Iran.[7] The varicella seroprevalence in Iran is in accordance with average of tropical and temperate areas. Seroprevalence of VZV did not change from 2003 to 2011 in Iran.[14] The aim of the current study was to determine the cost-effectiveness of varicella vaccination in Iran.
Methods
Decision-tree-based analysis model
The cost-effectiveness analysis is based on a deterministic decision-tree model to compare alternative scenario against chickenpox in children 12 months old including no vaccination, one-dose of varicella vaccination at 12 months, and two-dose varicella vaccination at 12 and 15 month of age from societal prospective in 2014 in Iran [Figure 1]. In this analysis, we followed the pathway from the original decision node (vaccination or no vaccination) through to each of the terminal node. A baseline assumption that 85% and 95% of each birth cohort receive vaccination in one-dose and two-dose, respectively, based on vaccination coverage rate. There are two distinct pathways including with chickenpox and without chickenpox for children who were not vaccinated as well as for whom the vaccination was not effective; based on vaccine efficacy rate We assumed probability of inpatient admission, outpatient visit, and no treatment for children with chickenpox throughout the decision tree model which was obtained from expert panel opinion.
Figure 1.
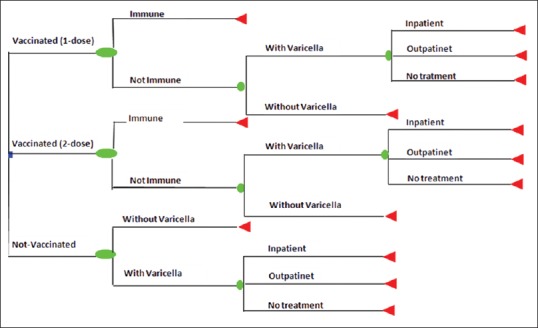
Decision tree analysis model to calculate incremental cost per disability-adjusted life year averted of varicella vaccination in Iran
Outcomes in decision model are incorporated within a common framework and it includes the consequence of alternative actions (incremental analysis). In this study, effectiveness was estimated as a total number of varicella cases averted, as well as disability-adjusted life years (DALYs) averted due to vaccination in 2014. Herd immunity was not considered in this analysis. Parameters of the model are shown in Table 1.
Table 1.
Parameters of decision tree model
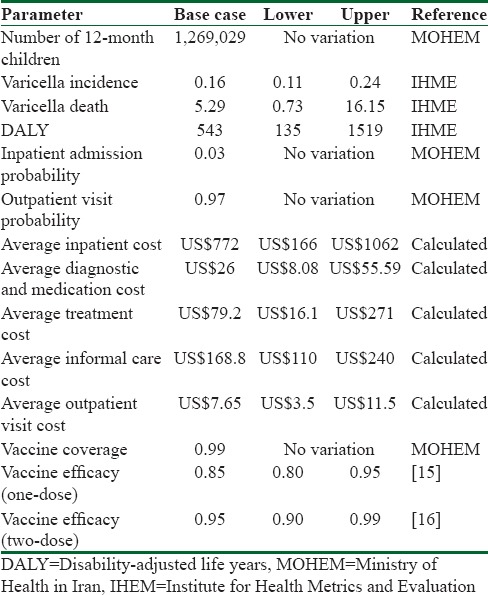
Epidemiological and disease burden estimates
To estimate the burden of disease information, due to lack of epidemiological Iranian data, we used the Global Burden of Disease Study (GBD 2013) conducted by the Institute for Health Metrics and Evaluation to extract incidence, death, and DALYs due to varicella in Iran. Furthermore, we used information at the center of control and prevention of communicable diseases at the Ministry of Health in Iran for vaccination cost program, also we used literature and expert panel for other parameters and assumptions.
Vaccine efficacy
We assumed efficacy of 1-dose and 2-dose of varicella vaccination was 85% and 95%, respectively, for all varicella cases since the start of vaccination.[15,16] Vaccination coverage was assumed to be 99% in 2014.
Cost of disease management
To estimate direct treatment cost, we investigated the medical records of 30 patients (age range 1-month to 5 years) who were hospitalized at Mofid Hospital in 2014–2015 for chickenpox. Because the tariff of medical services in public sector is identical across Iran, the results from this public hospital can be generalized to the public sector across country. Since we did not have access to information about burden of service in private sector and their tariff, we assumed that all of patients receive treatment from public sector. Total direct medical cost included hospitalization cost and medication; diagnostic, nursing care, and consultation cost were calculated. To calculate the outpatient services costs, we extracted pharmaceutical items, diagnostic tests, and outpatient visits after consultation with expert's panel including health professionals, general practitioners, pediatric assistants, and pediatricians in public hospital and estimated average cost per outpatient visit based on it. Furthermore, we estimated the percentage of children diagnosed with chickenpox who receive inpatient and outpatient visit based on expert panel via questionnaires which was 0.97 and 0.03, respectively, and also we assumed that all of varicella cases receive treatment.
To estimate indirect cost of treatment, we calculated informal care cost with cost opportunity method (human capital approach) based on duration of disease of our sample (5.7 days) and minimum daily wage in Iran (US$7.9) based on the Iranian Ministry of Cooperation, Labor and Social Welfare in 2015. All the costs were converted to US Dollars (US$) using the average annual 2015 exchange rate in market (US$1 = Rial 30,000).
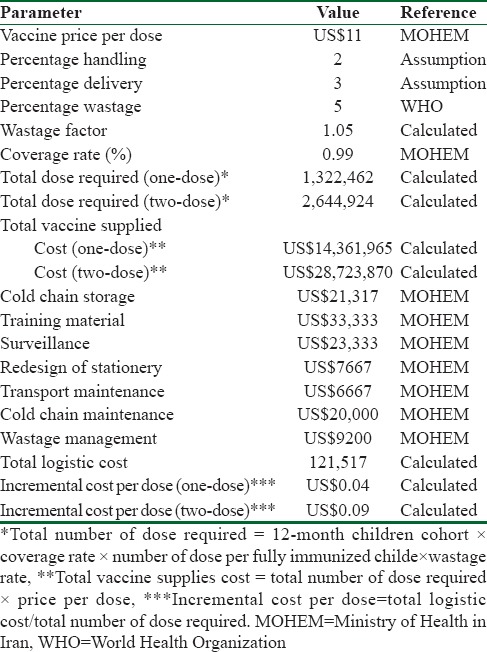
Vaccination cost
Vaccine price per dose was taken from the local representative of the vaccine's manufacturer (Behestan Darou) that would be US$11 per dose, we added to the price of the vaccine 3% charge of purchasing from the manufacturer in addition 2% for delivery including the insurance, shipping, and delivery to the airport. Furthermore, 5% wastage rate was assumed on the basis of expert opinion for the vaccine. To estimate the vaccine supplies, cost was calculated using the following formula:
C = P * I × B × D × (1/[1−w])
P = Vaccine Price per dose
I = Immunization coverage rate
B = Birth cohort
D = Number of doses per fully immunized child
W = Wastage rate.
The number of doses per children fully immunized for varicella vaccine was considered as one-dose and two-dose scenario. We assumed 1,269,029 as 12-month-old children cohort in Iran and 99% coverage rate of vaccination. As a result, total vaccine supplies would be US$28,723,870 for one-dose scenario and US$14,361,935 for two-dose scenario. We used World Health Organization (WHO) guideline to calculate incremental system cost of adding a new vaccine to national immunization system in Iran. We estimated distribution system's cost, cold chain, surveillance monitoring, training, maintenance, and other facilities required by the Ministry of Health that are not currently available. The total annualized capital cost was calculated according to equipment prices and useful life and an annualizing factor [Table 2].
Table 2.
Vaccination cost parameters
Cost-effectiveness analysis
Cost-effectiveness analysis for varicella vaccination was calculated in comparison with the case of no immunization program. Based on the following formula, the difference between the costs in each scenario which was divided by the difference between the DALY averted in each scenario considered as ratio of incremental cost per DALY averted.
ICER = total vaccination cost − cost saved/DALY without vaccine − DALY with vaccine.
We used the threshold recommended by the WHO to interpret cost-effectiveness result. The intervention was considered highly cost-effective if cost per (DALY) averted was below the per capita gross domestic product (GDP).[17] Per capita GDP of Iran was US$4763 in 2013.
Sensitivity analysis
A one-way sensitivity analysis has been conducted on each scenario (1-dose and 2-dose varicella vaccination) to detect those parameters which had the most impact on the incremental cost-effectiveness ratio (ICER). We were considered upper and lower estimate of incidence, disability –adjusted life years, and vaccine efficacy in this regard.
Results
In the unvaccinated cohort, 203,045 varicella cases among 1 year of age children, 6091 inpatient admission, 196,953 outpatient visit, and 5 deaths would happen in Iran 2014.
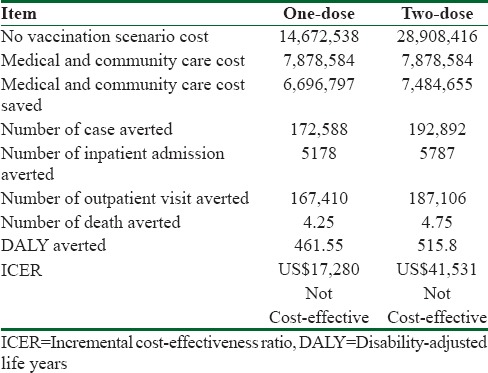
The average cost of outpatient visit per patient is estimated at US $ 7.6, and the average cost per inpatient admissions is US $1046 in societal prospective. Total cost of varicella disease management would be US $ 7,878,584 in base case scenario for base year. Using one-dose vaccination of varicella scenario, deaths averted would amount to 4.25; the number of hospitalizations and outpatient visits avoided would be 5,178 and 167,410, respectively, and total cost of varicella management saved would be US $ 6,696,797 during base year. Total cost of one-dose vaccination scenario would account to US $14,672,539. Incremental cost per DALY averted would amount to $17,271for one-dose vaccination scenario compared to no vaccination in the base-case analysis.
In the two-dose vaccination strategy, 4.75 deaths, 5,878 hospitalizations, and 187,106 consultations would be avoided in the 1-year children cohort in base year. Total cost of 2- dose varicella vaccination scenario would cost US $28,908,416 in base year. Incremental cost per DALY averted of 2-dose varicella vaccination compared to no vaccination would be US $41,508. Result of sensitivity analysis showed that in lower estimate of incidence and DALY, ICER for two-dose vaccination program has changed between US$45,975–US$167,047 and this value has changed between ranges of US$21,587–US$69,505 for one- dose vaccination while in upper estimate of incidence and DALY, result of ICER changes in range of US$14,856–US$34,410 for two-dose program and US$6,177–US$10,532 in one-dose program [Figure 2 and Table 3].
Figure 2.
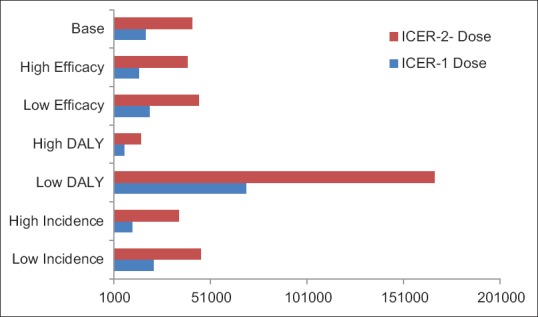
Scenario analysis of incremental cost-effectiveness ratio in Varicella Vaccination Program in Iran
Table 3.
Cost-effectiveness result
Discussion
According to the WHO threshold to compare the incremental cost per DALY prevented, with the Iran's GDP per capita of US$4763 in 2013 and the results of this study, it can be concluded that the implementation of the Varicella Vaccination Program in both one-dose and two-dose scenario given that the ICER is more than Iran's three time of GDP per capita, so this intervention will not be cost effective. Assuming upper scenario estimate for incidence (0.24) and DALY (1519) showed that ICER for one-dose vaccination program is less that 3 × GDP per capita which means might be cost effective intervention.
Scuffham et al. supporting our results concluded that the Varicella Vaccination Program for infants could prevented 4.4 million cases in Australia. Authors compared three different strategy with no vaccination programs, including, varicella vaccination for infant, adolescents without a history of varicella, and catch-up strategy.[18] Cost -effectiveness analysis conducted by Valentim J et al. shows, Varicella Vaccination Program as cost-effective vaccination in Brazil. They announce that this immunization program could be saved R$ 15,000 per life-year gained under the societal and the healthcare system's perspective. Vaccine price and the number of doses parameters had most impact on the result of cost-effectiveness in this study.[9]
Despite the existence of varicella vaccine, many developed countries have not introduced it into their national schedules, partly because of concerns about whether HZ (shingles) will increase due to a lack of exogenous boosting. Van Hoek AJ et al. conducted the cost-effectiveness of combined varicella and zoster vaccination and compare to alternative scenario. Their finding determined that, combined vaccination is cost-effective. However, incremental cost-effectiveness ratio is influenced by projected outcome that happen in next decade after the start of vaccination and this program would be unlikely to be cost-effective in short time horizon, that may result in projected rise in the incidence of HZ in long-run[8] USA is one of the developed countries which have introduced vaccination of varicella into the national schedule. Frequent varicella outbreaks with sizable impact on the US public health system and has continued to occur despite the success of the country's 1-dose Varicella Vaccination Program.[19] Zhou et al. evaluated the economic impact of the projected 2-dose Varicella Vaccination Program as well as the existing 1-dose program. they showed both mentioned programs are cost saving from the social prospective (societal benefit-cost ratio was 4.37 and 2.73 for 1-dose and 2-dose respectively). Authors also estimated the second dose's vaccination program could save $109,000 per QALY gained.[19]
Important limitation of the current study was the lack of local epidemiological information for burden of chickenpox in Iran. We used deterministic decision tree model in this study, but author suggested using probabilistic model to estimate cost-effectiveness of vaccination program over life time horizon by having adequate cost and effectiveness parameters in the future.
Conclusions
According to the results obtained in this study, adding varicella vaccination into the national immunization program of Iran is not cost-effective under the assumption mentioned about epidemiological burden of varicella in Iran.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Nikkels AF, Delvenne P, Sadzot-Delvaux C, Debrus S, Piette J, Rentier B, et al. Distribution of varicella zoster virus and herpes simplex virus in disseminated fatal infections. J Clin Pathol. 1996;49:243–8. doi: 10.1136/jcp.49.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holland P, Isaacs D, Moxon ER. Fatal neonatal varicella infection. Lancet. 1986;2:1156. doi: 10.1016/s0140-6736(86)90556-8. [DOI] [PubMed] [Google Scholar]
- 3.Arvin AM, Gershon AA. Live attenuated varicella vaccine. Annu Rev Microbiol. 1996;50:59–100. doi: 10.1146/annurev.micro.50.1.59. [DOI] [PubMed] [Google Scholar]
- 4.Preblud S. Varicella: Complications and costs. Pediatrics. 1986;78:728–35. [PubMed] [Google Scholar]
- 5.Finger R, Hughes JP, Meade BJ, Pelletier AR, Palmer CT. Age-specific incidence of chickenpox. Public Health Rep. 1994;109:750–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC). Prevention of Varicella: Recommendations of the Advisory Committee on Immunization Practices (ACIP) Atlanta: GA: US Department of Health and Human Services; 1996. [Google Scholar]
- 7.Sharifi Z, Emadi Ghanjin S. The seroepidemiology of varicella zoster virus (VZV) in different age groups in Tehran, Iran. Iran J Allergy Asthma Immunol. 2005;4:95–8. [PubMed] [Google Scholar]
- 8.van Hoek AJ, Melegaro A, Gay N, Bilcke J, Edmunds WJ. The cost-effectiveness of varicella and combined varicella and herpes zoster vaccination programmes in the United Kingdom. Vaccine. 2012;30:1225–34. doi: 10.1016/j.vaccine.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 9.Valentim J, Sartori AM, de Soárez PC, Amaku M, Azevedo RS, Novaes HM. Cost-effectiveness analysis of universal childhood vaccination against varicella in Brazil. Vaccine. 2008;26:6281–91. doi: 10.1016/j.vaccine.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Background Paper on Varicella Vaccines. SAGE Working Group. [Last accessed on 2014. Apr 15]. Available from: http://www.who.int/immunization/sage/meetings/2014/april/presentations_backgrounddocs/en/
- 11.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varicella Disease Burden and Varicella Vaccine. [Last accessed on 2014 May 21]. Available from: http://www.who.int/immunization/sage/meetings/2014/april/2_SAGE_April_VZV_Seward_Varicella.pdf?ua=1 .
- 13.Motamedifar M, Handjani F, Hadi N, Shahkarami MK, Mehrabani D. Seroprevalence of varicella-zoster virus in children from Shiraz-Iran. Iran J Immunol. 2006;3:43–6. [Google Scholar]
- 14.Allami A, Mohammadi N. Varicella immunity in Iran: An age-stratified systematic review and meta-analysis. Iran J Microbiol. 2014;6:372–81. [PMC free article] [PubMed] [Google Scholar]
- 15.Seward JF, Marin M, Vázquez M. Varicella vaccine effectiveness in the US vaccination program: A review. J Infect Dis. 2008;197(Suppl 2):S82–9. doi: 10.1086/522145. [DOI] [PubMed] [Google Scholar]
- 16.Kuter B, Matthews H, Shinefield H, Black S, Dennehy P, Watson B, et al. Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatr Infect Dis J. 2004;23:132–7. doi: 10.1097/01.inf.0000109287.97518.67. [DOI] [PubMed] [Google Scholar]
- 17.Waitzkin H. Report of the WHO Commission on Macroeconomics and Health: a summary and critique. Lancet. 2003 Feb 8;361:523–6. doi: 10.1016/S0140-6736(03)12491-9. [DOI] [PubMed] [Google Scholar]
- 18.Scuffham PA, Lowin AV, Burgess MA. The cost-effectiveness of varicella vaccine programs for Australia. Vaccine. 2000;18:407–15. doi: 10.1016/s0264-410x(99)00261-3. [DOI] [PubMed] [Google Scholar]
- 19.Zhou F, Ortega-Sanchez IR, Guris D, Shefer A, Lieu T, Seward JF. An economic analysis of the universal varicella vaccination program in the United States. J Infect Dis. 2008;197(Suppl 2):S156–64. doi: 10.1086/522135. [DOI] [PubMed] [Google Scholar]


