Abstract
Background\Aim:
Consanguinity influences the phenotypic variations of some hereditary and immune-mediated disorders, including inflammatory bowel disease. This study estimated the prevalence of consanguinity among the ancestors of patients with inflammatory bowel disease and examined the effect of various consanguinity levels on inflammatory bowel disease onset.
Patients and Methods:
Patients with inflammatory bowel disease who were seen at two gastroenterology outpatient clinics were consecutively recruited and surveyed for demographics, disease onset, and presence of ancestral consanguinity within three generations. The prevalence of different consanguinity levels was calculated. The association between age at inflammatory bowel disease onset and consanguinity was examined.
Results:
Two hundred seventeen patients were recruited. The mean age, mean age at diagnosis, and mean illness duration were 32.9 ± 13.4, 18.6 ± 11.5, and 8.6 ± 7.7 years, respectively. Of the cohort, 53.5% were women, and 74.2% were native Saudis. Cigarette smoking was reported in 17.1%; 51% had Crohn's disease, while the remaining patients had ulcerative colitis. A family history of inflammatory bowel disease was reported in 29.5% of patients; consanguinity within three generations was reported in 57.6%. Consanguinity in more than one generation was reported in 38.7%; 17.5% had consanguinity in three consecutive generations. There was no association between inflammatory bowel disease onset and multi-generation consanguinity, but there was an association with disease subtype in favor of ulcerative colitis (b coefficient = 7.1 [95% confidence interval = 4.1, 10]).
Conclusions:
Consanguinity is extremely common among Saudi patients with inflammatory bowel disease but does not seem to influence age at disease onset. Genetic studies are needed to further clarify the effect of consanguinity on disease behavior.
Keywords: Consanguinity, Crohn's disease, Disease onset, Inflammatory bowel disease, Ulcerative colitis
INTRODUCTION
Inflammatory bowel disease (IBD) involves chronic inflammation of the entire gut or a part of the gut. IBD primarily includes ulcerative colitis (UC) and Crohn's disease (CD). Both diseases can lead to severe diarrhea, fatigue, and weight loss when left untreated. IBD can be debilitating and sometimes leads to life-threatening complications. UC primarily affects the large intestine and rectum, while CD can affect any part of the gut.[1] IBD can present at any age, but the incidence peaks in the third decade of life.[1] The factors that predispose patients to early onset remain unclear.
It is hypothesized that both genetic and environmental factors play an etiological role in CD. The hypothesis of genetic susceptibility is largely based on the high frequency of familial clustering that is seen with IBD (6–33%), and relatively high risk of IBD is seen among first-degree relatives of patients with IBD (up to 10-fold).[2] The incidence of IBD has been noted to increase over the past decade in the Kingdom of Saudi Arabia (KSA).[3,4,5] Consanguinity is very common in Saudi culture[6] and it has been linked to many hereditary and congenital disorders,[7] which is why studying the impact of consanguinity on IBD epidemiology is important. A recent survey of 600 Saudi women revealed a parental consanguinity prevalence of 30%.[6] In comparison, the rate of consanguineous marriages in the United States is estimated to be < 1%.[7] In 1987, after studying multiple families with IBD, Purrmann et al.[8] suggested that the occurrence of IBD among blood relatives of patients with either CD or UC was more frequent than that expected by chance alone. A recent study by Elmouzan et al. reported that there was no significant association between IBD and parental consanguinity, especially in the absence of a family history of IBD.[9] Conversely, another study by AlSaleem et al.[10] reported that there was a significant association between the increasing incidence of IBD and history of positive consanguinity between parents.
Consanguinity has been linked to monogenic immune-mediated diseases, such as primary immunodeficiency syndromes,[11,12] and to the occurrence of simultaneous early-onset IBD through newly recognized genetic mutations.[13] Moreover, a recent study suggested that consanguinity had a significant impact on the emergence of early-onset UC in those with high rates of familial aggregation of IBD.[14] However, whether consanguinity truly influences early IBD onset and phenotype remains unknown.
Given the increasing rate of IBD incidence in the KSA, we aimed to estimate the prevalence of ancestral consanguinity among IBD patients and evaluated the impact of multi-generation consanguinity on age at onset of IBD.
PATIENTS AND METHODS
We performed a cross-sectional study involving all consecutive patients diagnosed with IBD, based on typical clinical, endoscopic, and histologic criteria, who were seen at two outpatient gastroenterology clinics in the KSA between August and October 2016. The research Ethics Committee of our institution provided approval for this study to be conducted. Prospectively, patients of all ages who agreed to provide written informed consent to participate were recruited. Data on demographics, disease onset, and a detailed family and consanguinity history were collected through a standard data collection sheet. Additionally, retrospective clinical data were collected from hospital medical records to confirm the diagnosis.
Outcomes
Consanguinity, defined as marriage between first- and second-degree blood relatives, was examined in parents, grandparents, and great grandparents of the patients in this study. The age at onset of IBD, a history or no history of ancestral consanguinity, and first, second, and third-generation consanguinity were compared.
Statistical analysis
We compared baseline characteristics using descriptive statistics. Data for continuous variables are reported as means ± standard deviations, while frequencies are reported for categorical variables. Standard t-tests (or one-way analysis of variance for multiple groups) and Chi-squared testing were performed when comparing means and frequencies, respectively, where appropriate. Simple and multiple linear regression analyses were used to identify predictors of continuous outcomes. For dependent binary categorical outcomes, simple logistic regression testing was used to examine their association with independent variables. Regression b coefficients and odds ratios with 95% confidence intervals (CI) were estimated for linear and logistic regression, respectively. STATA 12.1 (Stata Corp., College Station, Texas, USA) was used, and the statistical significance level was set at 5%.
Ethical considerations
The research and ethical approval was granted by the Research Ethics Committee at King Abdulaziz University, Saudi Arabia, prior to initiating this study. The study was conducted in compliance with all of the principles stated in the Declaration of Helsinki. None of the authors have financial conflicts to declare in relation to this study.
RESULTS
Of 250 patients who were approached at either of the medical centers that participated in this study, 217 patients were recruited (response rate: 87%). The baseline demographics are summarized in Table 1. The mean age was 32.9 ± 13.4 years, the average age at the time of diagnosis was 18.6 ± 11.5 years, and the average duration of illness was 8.6 ± 7.7 years. Of the patients who were included, 53.5% were women, and 74.2% were native Saudi Arabians. Cigarette smoking was reported in 17.1% of patients. Of the 217 patients, 51% had CD, while the remaining patients had UC. A family history of IBD was reported in 29.5% of patients, and any form of consanguinity within three generations was reported in 57.6%. Consanguinity in more than one generation was reported in 38.7%, and 17.5% of patients had consanguinity in three consecutive generations.
Table 1.
Baseline demographics for the 217 patients who were evaluated in this study
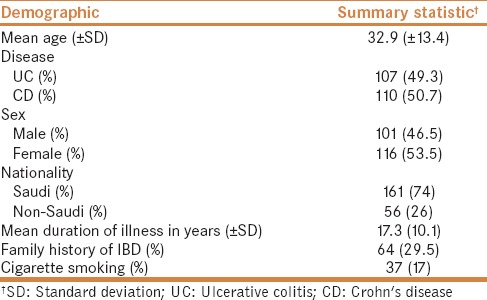
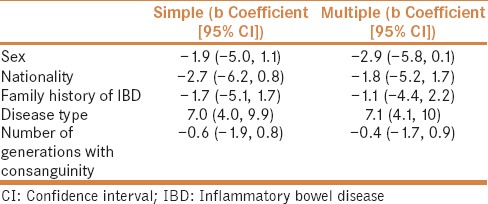
On comparing various levels of consanguinity, a significant difference was observed with regards to family history of IBD (P = 0.007). Although no statistical significance was seen, trends were observed for the average patient age (P = 0.088) and disease subset, i.e., UC versus CD (P = 0.052) [Table 2]. In the multiple linear regression analysis, we did not find that there was any association between IBD onset and multi-generation consanguinity; however, an association was observed for disease type in favor of UC (b coefficient = 7.1 [95% CI = 4.1, 10]) [Table 3].
Table 2.
Comparison between various levels of ancestral consanguinity
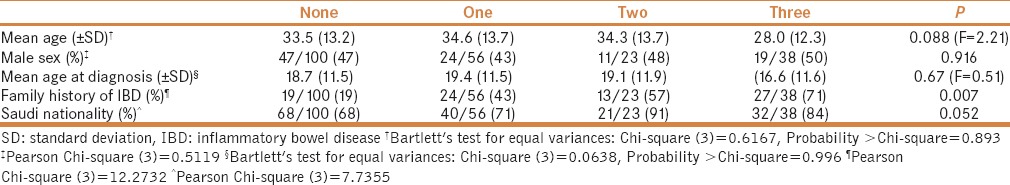
Table 3.
Simple and multiple linear regression analysis of the association between inflammatory bowel disease onset and demographic characteristics
DISCUSSION
In this study, we aimed to estimate the prevalence of ancestral consanguinity among IBD patients and evaluated the impact of multi-generation consanguinity on age at onset of IBD in a cohort of Saudi Arabian patients.
The recent emergence of IBD in the Arabic peninsula has been constantly attributed to the westernization of diet.[3] Variations in disease phenotype compared to those described in other countries have been reported in Arabian countries; however, genetic profiles similar to those described in Western countries have been identified.[15] Little attention has been given to the influence that consanguinity might have on this phenomenon. Based on cross-sectional surveys, 56% of the general Saudi population are reported to have consanguineous marital relationships, which have been linked with many inherited diseases.[16] A report on European countries have implicated consanguinity as a cause of oligo- and monogenic diseases such as immunodeficiencies, and has directly linked consanguinity with the early onset of IBD.[13] Conversely, a Saudi cohort study involving 138 pediatric patients with IBD suggested no relationship between early-onset CD and consanguinity.[9] Similarly, our data could not provide any evidence that simple and complex forms of consanguinity have any effect on age at onset of IBD.
Familial clustering of autoimmune diseases is a well-described phenomenon that has provided researchers with an opportunity to study the genetic background of such illnesses.[17] Our data suggest that there is an association between consanguinity and familial clustering of IBD; however, dedicated studies that examine the genetic profiles of patients with both consanguineous ancestral trees and a family history of IBD must be performed before this observation can be validated.
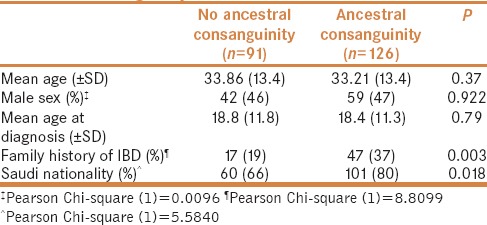
Confirming an association between phenotypic disease characteristics and consanguinity could greatly affect patient risk profiling. Additionally, some implications related to pre-marital counseling might surface. Based on our descriptive survey, there seems to be an association between ancestral consanguinity and IBD subtype [Table 4]; however, more data including disease phenotype, site of involvement, disease behavior, and response to treatment are required before a firm conclusion can be drawn.
Table 4.
Comparison between patients with and without ancestral consanguinity
Our study has many limitations, including the potential for recall bias and information bias. Additionally, genetic material to confirm our observations and prospective collection of clinical data are lacking.
CONCLUSION
Based on this cohort study, ancestral consanguinity does not seem to influence patients' age at the time of IBD onset. However, there seems to be an association with disease subtype. Further research is needed to examine the effect of consanguinity on disease behavior and outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We would like to acknowledge and thank Rayan Muneer Malibari for his efforts as a data collector for this research project. We would also like to thank Editage (www.editage.com) for English language editing.
REFERENCES
- 1.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–78. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nunes T, Fiorino G, Danese S, San M. Familial aggregation in inflammatory bowel disease: Is it genes or environment? World J Gastroenterol. 2011;17:2715–22. doi: 10.3748/wjg.v17.i22.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Mofleh IA, Azzam NA. Crohn's disease. Increasing trend in Saudi Arabia. Saudi Med J. 2013;34:1105–13. [PubMed] [Google Scholar]
- 4.El Mouzan MI, Saadah O, Al-Saleem K, Alanazi A, Asery A, Wali S, et al. Incidence of pediatric inflammatory bowel disease in Saudi Arabia: A multicenter national study. Inflamm Bowel Dis. 2014;20:1085–90. doi: 10.1097/MIB.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 5.Al-Mofarreh MA, Al-Mofleh IA. Emerging inflammatory bowel disease in Saudi outpatients: A report of 693 cases. Saudi J Gastroenterol. 2013;19:16–22. doi: 10.4103/1319-3767.105915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warsy AS, Al-Jaser MH, Albdass A, Al-Daihan S, Alanazi M. Is consanguinity prevalence decreasing in Saudis.: A study in two generations? Afr Health Sci. 2014;14:314–21. doi: 10.4314/ahs.v14i2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bittles AH, Black ML. Evolution in health and medicine Sackler colloquium: Consanguinity, human evolution, and complex diseases. Proc Natl Acad Sci USA. 2010;107:1779–86. doi: 10.1073/pnas.0906079106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Purrmann J, Miller B. Therapeutic management and genetic counseling of pregnant patients with chronic inflammatory intestinal diseases. Internist (Berl) 1987;28:770–6. [PubMed] [Google Scholar]
- 9.El Mouzan M, Al-Mofarreh M, Assiri A, Hamid Y, Saeed A. Consanguinity and inflammatory bowel diseases: Is there a relation? J Pediatr Gastroenterol Nutr. 2013;56:182–5. doi: 10.1097/MPG.0b013e31826d9987. [DOI] [PubMed] [Google Scholar]
- 10.AlSaleem K, El Mouzan MI, Saadah OI, Ali M, AlSaleem B, Al-Hussaini A, et al. Characteristics of pediatric ulcerative colitis in Saudi Arabia: A multicenter national study. Ann Saudi Med. 2015;35:19–22. doi: 10.5144/0256-4947.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kammermeier J, Dziubak R, Pescarin M, Dury S, Goodwin H, Shah N, et al. Phenotypic and genotypic characterisation of inflammatory bowel disease presenting before the age of 2 years. J Crohn's Colitis. 2017;11:60–9. doi: 10.1093/ecco-jcc/jjw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Pierro V, Zuntini R, Cancrini C, Finocchi A, Angelino G, Rossi P, et al. Consanguinity and polygenic diseases: A model for antibody deficiencies. Hum Hered. 2014;77:144–9. doi: 10.1159/000362364. [DOI] [PubMed] [Google Scholar]
- 13.Salzer E, Kansu A, Sic H, Majek P, Dogu FE, Pickl WF, et al. Early-onset inflammatory bowel disease and common variable immunodeficiency-like disease caused by IL-21 deficiency. J Allergy Clin Immunol. 2014;133:1651–9. doi: 10.1016/j.jaci.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 14.Al-Hussaini A, El Mouzan M, Hasosah M, Al-Mehaidib A, Al Saleem K, Saadah OI, et al. Clinical pattern of early-onset inflammatory bowel disease in Saudi Arabia: A multicenter national study. Inflamm Bowel Dis. 2016;22:1961–70. doi: 10.1097/MIB.0000000000000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azzam N, Nounou H, Alharbi O, Aljebreen A, Shalaby M. CARD15/NOD2, CD14 and toll-like 4 receptor gene polymorphisms in Saudi patients with Crohn's disease. Int J Mol Sci. 2012;13:4268–80. doi: 10.3390/ijms13044268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Mouzan MI, Al Salloum AA, Al Herbish AS, Qurachi MM, Al Omar AA. Consanguinity and major genetic disorders in Saudi children: A community-based cross-sectional study. Ann Saudi Med. 2008;28:169–73. doi: 10.5144/0256-4947.2008.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anwar WA, Khyatti M, Hemminki K. Consanguinity and genetic diseases in North Africa and immigrants to Europe. Eur J Public Health. 2014;24:57–63. doi: 10.1093/eurpub/cku104. [DOI] [PubMed] [Google Scholar]


