Abstract
Background/Aims:
The purpose of this study is to report our results using a computed tomography (CT)-guided fat transversing coaxial biopsy technique for pancreatic lesion biopsy that avoids major organs and vessels. We retrospectively reviewed the records of patients referred to our department for pancreatic mass biopsy.
Patients and Methods:
The records of patients (from June 2008 to August 2014) in whom biopsy was performed under CT guidance with a coaxial needle using a fat transversing technique were reviewed. Patient demographic data and biopsy outcomes were collected. We aimed to compare differences between lesion size and biopsy outcome, the independent two-samples t-test was used.
Results:
A total of 122 patients who underwent 17-G coaxial needle biopsy were included. The mean pancreatic lesion size was 3.2 cm, and in 30 patients it was more than 4 cm. The majority of lesions were located in the head of the pancreas (44.3%). No transorgan biopsies were performed. In most patients, the biopsy was performed via a fat traversing detour route (93.4%), and a successful diagnosis was made based on the biopsy outcome in 96.7% patients. Complications occurred in five patients (4.1%); three of the patients developed a fever, and two developed pancreatitis. All patients recovered with symptomatic treatment.
Conclusion:
CT-guided coaxial core biopsy of pancreatic lesions using a fat detour route appears to be a safe and effective method for obtaining pancreatic lesion biopsies with a high success rate and low complication rate.
Keywords: Biopsy, coaxial needle, computed tomography guidance, computed tomography, pancreas
INTRODUCTION
Biopsy of unresectable malignancies is frequently performed prior to chemotherapy or radiotherapy or if the diagnosis is unclear based on imaging studies.[1] Preoperative biopsy is also appropriate in patients with a pancreatic neoplasm who refuse surgical resection or exploration unless malignancy is confirmed by other means.[2] Both computed tomography (CT)-guided percutaneous biopsy of the pancreas and ultrasound-guided fine-needle aspiration have been found to be acceptable for the diagnosis of pancreatic masses, although some believe core needle biopsy is better as tissue is obtained for histopathological rather than cytological examination.[2,3,4,5,6,7]
Although it is desirable to use the shortest path between the skin and target lesion when performing a percutaneous biopsy, this may not always be possible because of intervening vital structures. This is especially true in cases in which the mass is located in or around the head of the pancreas where bowel, liver, kidney, or major vascular structures often interfere with the access route. Therefore, needle biopsy of this region is considered technically difficult.[8] In such cases, indirect approaches have been used to safely access these lesions.
The most commonly used anterior approach for CT-guided biopsy of lesions in the pancreatic head requires traversing the gastrointestinal tract, the mesenteric vessels, or both, thereby increasing the risk. Alternative routes, including transhepatic, transgastric, posterior transcaval, and transsplenic approaches, have also been used for pancreatic biopsies.[9,10,11] All these approaches have the same disadvantage in that they need to penetrate either organs or vessels to reach the target lesion. Therefore, a safer method for obtaining biopsy tissue from a pancreatic head mass is required.
At our institution, we perform CT-guided pancreatic biopsies with a coaxial needle via a fat transversing route that avoids organs and vessel penetration. The purpose of this report is to describe the technique and report the diagnostic results and complications in a large series of patients.
MATERIALS AND METHODS
Patients
The records of patients referred to our department for pancreatic mass biopsy from June 2008 to August 2014 were retrospectively reviewed. Institutional Review Board approval was obtained for this study, and because of the retrospective nature the requirement of informed patient consent was waived.
Laboratory data, hemodynamic status monitoring, and biopsy tract selection
Laboratory data including complete blood count, prothrombin time, and partial thromboplastin time were obtained within 3 days before biopsy. Blood pressure, PaO2 saturation, and electrocardiogram were monitored during the procedure. Biopsy was performed if the blood coagulation result was 16 seconds or less or the international normalized ratio was 1.5 or less and the platelet count was greater than 50,000/μL.
Based on our prior experience, there were six major detour routes used for pancreatic biopsy [Figure 1]. The selection of the final route was decided according to the preprocedural CT images with the main objectives being to avoid nontarget organ penetration, affect the least number of organs if penetration was required, and use the shortest distance tract for needle insertion.
Figure 1.
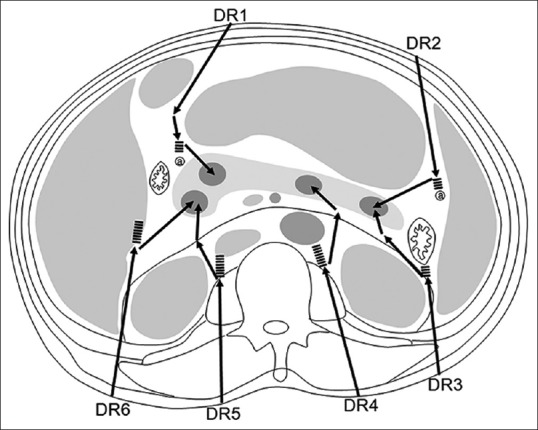
Diagram showing six detour routes (DR1 to DR6) for biopsies of pancreatic lesions located within different portions of the pancreas. Dashed lines indicate that the route may penetrate organs if the biopsy tract continues along a straight line beyond the arrowhead, i.e., continues to go forward. Abbreviations: a, artery; DR, detour route
Computed tomography-guided biopsy technique
Three interventional radiologists performed the CT-guided biopsies, and their years of experience performing were 24, 10, and 3 years. After selection of the biopsy tract, patients received one intramuscular injection of meperidine 50 mg and a subcutaneous injection of 2% xylocaine 2 mL for anesthesia at the needle entry point.
Planning the needle insertion route requires multiple angulations of needle placement, which is in theory the same as single angulation placement,[3] however, the angulation is divided into multiple small steps in which the angle is changed for each small section of placement. It is also important that the angle of insertion through the skin and muscular layer of the abdominal wall be the same as or close to the angle of needle insertion into the lesion. This minimizes the changes that the needle will move from its position in the lesion for collecting the biopsy specimen due to contraction of the abdominal muscles.
The steps in designing the needle insertion route are as follows: (1) Select the direction of needle insertion into the lesion axial images (ventral, dorsal, left, right, caudal, cranial, or oblique) such that it will avoid puncturing adjacent blood vessels, the common bile duct, pancreatic parenchyma, or pancreatic duct. (2) Determine the insertion point in the skin based on the needle insertion direction and the angle of the puncture in the muscular layer of the abdominal wall. (3) When designing the puncture of the tip of the coaxial needle through the muscular layer of the abdominal wall, determine the direction, angle, and depth for multiple angulations (please refer to the previous explanation). (4) Perform a CT scan before puncturing the muscular layer of the abdominal wall with the coaxial needle and confirm the angulation route of the next small section. (5) After the tip of the coaxial needle has passed through the first section, perform another scan to confirm the angulation route of the next section. (6) Repeat the prior steps until the needle has punctured the lesion. (7) Remove the coaxial needle's stylet, replace it with a biopsy needle, and perform another scan to confirm that the biopsy needle has punctured the target lesion. (8) Obtain a tissue specimen and remove the biopsy and coaxial needles.
To perform a biopsy, an outer 17-gauge coaxial needle was inserted under CT guidance via the planned route to reach the target lesion, then an inner 18-gauge biopsy needle was inserted to obtain the tissue specimen [18 G Tru-core needle (15–20 cm); Angiotech, Vancouver, CA]. To perform the biopsy via a fat transversing route, the coaxial needle was inserted along a curved route, with each section of the curve approximating a straight line. Therefore, we focused on steering the tip of the needle along each short straight-line section, and then pulled the tip of the needle back (without rotating it) when we reached each inflection point, so that the needle continued to advance along the next short section after the inflection point. Because the coaxial needle must be pulled back from outside the patient's body, a 17-G coaxial needle makes it easier to turn the tip than a finer needle (such as 20 G or 21 G).
One to four pancreatic lesion core specimens were considered an adequate biopsy. If no tissue was obtained, a second CT-guided biopsy was performed or the tissue sample was obtained during surgery. All specimens were sent for histopathological evaluation.
After completion of the biopsy, an abdominal CT was performed to identify immediate procedure-related complications such as retroperitoneal hematoma or erroneous penetration of a bowel loop.
Follow-up and confirmation of biopsy results
All patients were followed-up 1 month after the procedure for possible delayed complications such as retroperitoneal hematoma. Biopsy results were confirmed according to the patient's treatment course, i.e., if a patient was treated for an infection or malignancy according to the biopsy results, the response to therapy was used to determine if the biopsy results were correct or not.
Statistical analysis
Continuous data (age and lesion size) were expressed as mean and standard deviation (SD); categorical data were expressed as number and percentage. To compare differences in age and lesion size between biopsy outcomes (i.e., successful diagnosis or negative tissue), independent two-samples t-test was used. Fisher's exact test was performed to examine association between biopsy outcomes and gender and pancreas lesion location. A two-sided P value < 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics 22.0 software (IBM Corporation, Armonk, New York).
RESULTS
A total of 114 patients who underwent 17-G coaxial needle biopsy were included in the analysis. Patient demographic and clinical data are summarized in Table 1.
Table 1.
Patient demographic and clinical characteristics (N=114)
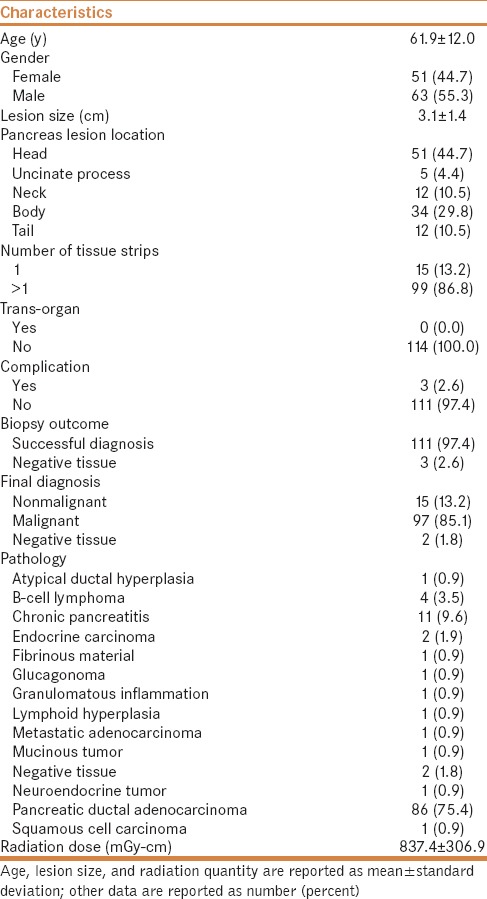
The mean pancreatic lesion size was 3.1 cm, and in 24 patients, it was more than 4 cm. The majority of lesions were located in the head of the pancreas (44.7%), followed by the body of the pancreas (29.8%). No patient had a biopsy via a transorgan route. The biopsy was performed via a fat traversing the detour route in all patients [Figures 2 and 3]. Two or more tissue strips were obtained during the procedure in 86.8% of patients, no complications occurred in 97.4%, a successful diagnosis was made based on biopsy outcome in 97.4%, a final diagnosis of malignancy was made in 85.1%, and pancreatic ductal adenocarcinoma was noted in 75.4%.
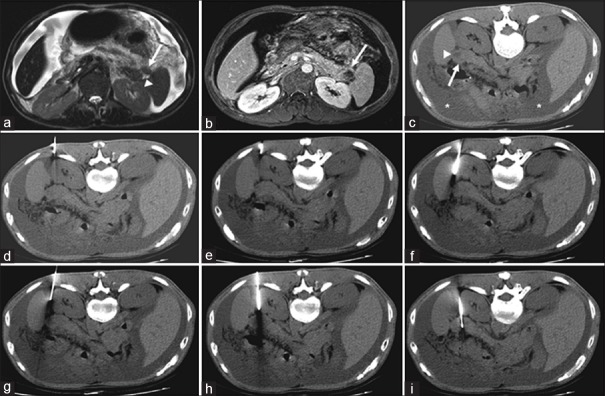
Figure 2.
(a-i) Computed tomography (CT)-guided biopsy images via detour route in a 54-year-old male with a pancreatic tail mass. Detour route 3, as shown in Figure 1, was used. The needle insertion angle/path was calculated to avoid going through any organs, and the insertion was one-way. (a) T2-weighted axial image revealed an isointense mass (arrow) within the pancreatic tail with a cystic component (arrowhead). (b) Contrast-enhanced T1-weighted axial image revealed an enhancing mass within the pancreatic tail (arrow), with a nonenhancing cystic component. (c) Prone nonenhanced axial CT image showed an isodense mass (arrow) with a cystic component (arrowhead) and ascites (asterisk). (d-h) Nonenhanced axial CT images showed successful insertion of the coaxial guiding needle just to the level of the pancreatic mass by slightly altering its course to avoid non-target organ penetration (i.e., the spleen). (i) The biopsy gun was then fired into the pancreatic tail mass on the axial CT image
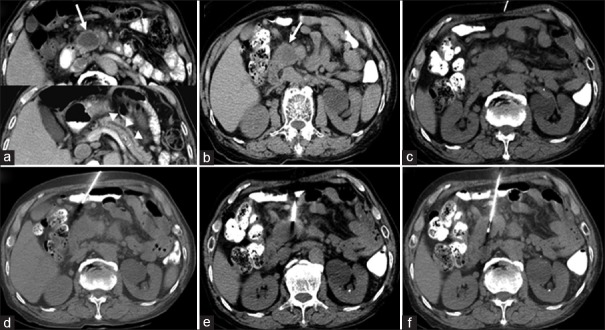
Figure 3.
(a-f) Computed tomography (CT)-guided biopsy images via a fat detour route in an 80-year-old male with a pancreatic head and neck mass. Detour route 1, as shown in Figure 1, was used. The needle insertion angle/path was calculated to avoid going through any organs, and the insertion was one-way. (a) Contrast-enhanced axial CT image revealed a poorly enhancing mass (arrow) within the pancreatic head/neck with a dilated pancreatic duct (arrowheads). (b) Preprocedural CT image showed a hypodense mass within the pancreatic head/neck (arrow). (c) Axial CT image with subcutaneous anesthetic needle inserted. (d-e) Nonenhanced axial CT images showed insertion of the coaxial guiding needle to the level of the pancreatic mass by changing its course to avoid non-target organ penetration (i.e. small intestine) to reach the mass.* (f) The biopsy gun was fired into the pancreatic head/neck mass on the axial CT image. * The needle appears to be traversing the bowel loop because of the partial volume effect of CT images. Briefly, the partial volume effect is the loss of apparent activity in small objects or regions because of the limited resolution of the imaging system. The needle indeed avoided penetrating the bowel because of the detour route, and the route used made it appear as if it traversed the bowel loop
The mean radiation dose administered to the patients was 837.4 ± 306.9 mGy-cm [Table 1]. To determine if there was a learning curve with earlier patients receiving a greater dose than later patients in the series, mean radiation dose was examined with different patient grouping, and regardless of the grouping the mean dose was similar [Supplemental Table 1 (285.1KB, tif) ].
Comparisons of radiation dose between patients with various groupings
Complications occurred in three patients [Supplemental Table 2 (186.9KB, tif) ]. One of the patients developed a fever and two developed pancreatitis. The age of the patients ranged from 50 to 78 years; a transorgan route puncture was not performed; two tissue strips were obtained; and no re-biopsy procedures were required. The lesion was in the head of the pancreas in all patients. The pathologic results were pancreatic ductal adenocarcinoma.
Details of patients with complications
Association of lesion size vs. biopsy outcome
There was no difference in age, sex, lesion size, and lesion location between patients with a successful and unsuccessful biopsy outcome [Table 2]. The average age of patients with a successful diagnosis was 61.8 ± 12.1 years, and that of patients in which tissue was not obtained was 66.7 ± 10.6 years. The mean lesion size was 3.1 ± 1.4 cm for patients with a successful diagnosis and 3.0 ± 1.9 cm for patients in which tissue was not obtained [Table 2].
Table 2.
Association between lesion size and biopsy outcome (N=114)

DISCUSSION
The results of this study showed that coaxial biopsy was associated with a high diagnostic rate of 97.4% and a complication rate of approximately 2–3%, with most complications being minor. No delayed complications were noted as all patients were followed-up for at least 1 month. To our knowledge, the detour route has not been previously reported. Our inspiration for the development of the detour route was based on prior work in which angulated needle placement or triangulation of needle placement was examined.[3,4]
A coaxial system involves the insertion of a coaxial needle, followed by the use of a biopsy needle to remove samples. Because a coaxial needle is relatively light, it can be inserted in stages in alternation with CT scans and a detour route can be employed. Because CT images were used in a step-by-step manner, we did not continuously hold the core biopsy needle by hand. Therefore, we prefer to use a semi-automatic biopsy needle. Its relative light weight allows the operator to stand above the tissue or organ, and the risk of the needle tip advancing unexpectedly is reduced. Using a coaxial needle, our standard has been to determine whether the total length of the tissue stripes exceeds 2 cm using the naked eye. Because the coaxial needle can be used to perform multiple biopsies, two or more tissue stripes can be obtained from many patients.
Noncoaxial systems involve the straightforward insertion of a biopsy needle and removal of samples. While there are many different types of core biopsy needles, with some less bulky and easier to control and held in position during scanning, only one biopsy can be performed using a noncoaxial needle. A second core can only be obtained when the needle is completely free of solid tissue. Noncoaxial systems are also heavier, making it more difficult to maneuver the needle, insert it to the correct depth, and use a detour route.
Other study has reported similar diagnostic accuracies with the coaxial needle technique. Tyng et al.[8] reported a diagnostic accuracy of 98.1% for CT-guided percutaneous core needle biopsies of pancreatic lesions. Coaxial biopsy is expected to improve diagnostic rates because it allows repeat sampling, allowing possible immunochemical evaluation.[12] The ability to rescan the patient with the needle in place to confirm location, contributes to both the higher diagnostic rate and the lower complication rate. Furthermore, the ability to use a detour route through fat afforded by coaxial needle such that organ and vessel penetration is avoided reduces the risk of injury, and may be one of the reasons why the amount of tissue obtained using the coaxial needle and pathology diagnostic rate are both relatively high.
The transgastric route is universally accepted as a safe route for pancreatic biopsy, and is intuitive, easily understood, and is relatively free of operator-dependent problems. However, the primary disadvantage of the transgastric route is the risk of bleeding and peritonitis.[4,5] In comparison with liver, spleen, kidney, colon, and inferior vena cava routes, from a theoretical perspective not penetrating unnecessary organs should be at least as safe as, if not safer than, penetrating unnecessary organs. Therefore, we believe that a detour route should be superior to the transgastric route.
Alternatives to avoid traversing vital organs during biopsy include hydrodissection and pneumodissection.[13,14] With these techniques, either a 0.9% saline solution (in hydrodissection) or air (in pneumodissection) is injected to displace the parietal pleura and expand the posterior paravertebral space while simultaneously advancing the coaxial needle carefully under CT control. The location of the lesion can also be confirmed with intravenous contrast administration to improve the diagnostic accuracy of the procedure.[8,15]
While ultrasound can be used to identify the needle path, it is limited by its ability to penetrate tissue (i.e., deep tissues often cannot be seen) and is blocked by air in the stomach and intestines. Consequently, ultrasound may be more suitable for patients of small stature and when there is little air in the gastrointestinal tract.[16] On the other hand, CT-guided biopsy is not limited by the patient's physique and air in the gastrointestinal tract.
Major hemorrhage after pancreatic biopsy, although uncommon, has been reported in the literature.[10] These hemorrhages are likely caused by inadvertent puncture of mesenteric or pancreatic vessels. To decrease such risks, we used a technique that traversed fat. This technique allowed multiple sampling of different areas of the pancreas through the same needle guide without puncturing other organs. Compared to biopsy without the use of a coaxial needle, no patient in the coaxial needle biopsy group had a transorgan route puncture, and the coaxial group had significantly less complications than the noncoaxial group.
The complication rate of 4.1% in the current study is similar to that reported by other authors.[17,18] In their retrospective review of 211 CT-guided and 58 ultrasound-guided biopsies of pancreatic lesions, Brandt et al.[17] reported a total complication rate of 3.8%. However, in contrast to our findings, where no organ was traversed, Brandt et al. reported a transorgan route in 24% of the ultrasound-guided and 40% of CT-guided biopsy procedures. In that study, the needle passed through the liver in 27 cases and through other organs including the stomach (41 passes), small bowel (18 passes), colon (seven passes), and spleen (one pass). Another study has shown only mild/moderate complications in 8.7% of patients who had pancreatic biopsies using a coaxial system, and complications were more commonly associated with lesions located in the head/uncinate process than lesions in the tail.[8]
Acute pancreatitis is a potentially fatal complication of imaging-guided pancreas biopsy.[7,10,11] One possible mechanism for the development of post-biopsy pancreatitis is the release of pancreatic enzymes into the surrounding tissue each time the needle passes through pancreatic tissue. To avoid post-biopsy pancreatitis, the needle should pass only through the tumor and not enter normal pancreatic tissue or pancreatic ducts.[19] The detour route decreased the chance of entering normal pancreatic tissue, thereby decreasing the possibility of post-biopsy pancreatitis.
There are, however, potential problems of using a fat detour route. They include possible fracture of the coaxial needle, and the problem of frictional forces rectifying the path of the guiding needle when inserting the cutting needle causing imprecision and complications. In addition, frictional forces within the tissue can potentially cause disruption of tissue and vessels. Finally, vessels running through fat in the region of the pancreas are vulnerable to injury, possibly resulting in hemorrhage.
The current study has several limitations including its retrospective nature. To obtain optimal needle positioning to reach the target and avoid puncturing the intervening vessel and organs frequent windowing was required, thereby increasing the scan time, procedural time, and radiation dose. There was no control group involving a different technique for comparison to the coaxial technique with detour route.
In conclusion, CT-guided coaxial core biopsy of pancreatic lesions using a fat detour route appears to be a safe and effective method for obtaining pancreatic lesion biopsies. The low complication rate is likely due to the ability to use a detour route through fat afforded by coaxial needle use.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e142S–65S. doi: 10.1378/chest.12-2353. [DOI] [PubMed] [Google Scholar]
- 2.Fogel EL, Shahda S, Sandrasegaran K, DeWitt J, Easler JJ, Agarwal DM, et al. A multidisciplinary approach to pancreas cancer in 2016: A review. Am J Gastroenterol. 2017;112:537–54. doi: 10.1038/ajg.2016.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caliskan KC, Ozcelik G, Cakmakci E, Ulusay SM, Celebi AS, Turk S, et al. Real time ultrasound guided pediatric percutaneous renal biopsy: The traditional method versus angled tangential approach. JBR-BTR. 2014;97:206–10. doi: 10.5334/jbr-btr.96. [DOI] [PubMed] [Google Scholar]
- 4.Tyng CJ, Almeida MF, Barbosa PN, Bitencourt AG, Berg JA, Maciel MS, et al. Computed tomography-guided percutaneous core needle biopsy in pancreatic tumor diagnosis. World J Gastroenterol. 2015;21:3579–86. doi: 10.3748/wjg.v21.i12.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tseng HS, Chen CY, Chan WP, Chiang JH. Percutaneous transgastric computed tomography-guided biopsy of the pancreas using large needles. World J Gastroenterol. 2009;15:5972–5. doi: 10.3748/wjg.15.5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Onofrio M, De Robertis R, Barbi E, Martone E, Manfrin E, Gobbo S, et al. Ultrasound-guided percutaneous fine-needle aspiration of solid pancreatic neoplasms: 10-year experience with more than 2,000 cases and a review of the literature. Eur Radiol. 2016;26:1801–7. doi: 10.1007/s00330-015-4003-x. [DOI] [PubMed] [Google Scholar]
- 7.Strobl FF, Schwarz JB, Haeussler SM, Paprottka PM, Rist C, Thierfelder KM, et al. Percutaneous CT fluoroscopy-guided core biopsy of pancreatic lesions: Technical and clinical outcome of 104 procedures during a 10-year period. Acta Radiol. 2017;58:906–13. doi: 10.1177/0284185116678274. [DOI] [PubMed] [Google Scholar]
- 8.Tyng CJ, Almeida MF, Barbosa PN, Bitencourt AG, Berg JA, Maciel MS, et al. Computed tomography-guided percutaneous core needle biopsy in pancreatic tumor diagnosis. World J Gastroenterol. 2015;21:3579–86. doi: 10.3748/wjg.v21.i12.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tseng HS, Chen CY, Chan WP, Chiang JH. Percutaneous transgastric computed tomography-guided biopsy of the pancreas using large needles. World J Gastroenterol. 2009;15:5972–5. doi: 10.3748/wjg.15.5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta S, Ahrar K, Morello FA, Jr, Wallace MJ, Hicks ME. Masses in or around the pancreatic head: CT-guided coaxial fine-needle aspiration biopsy with a posterior transcaval approach. Radiology. 2002;222:63–9. doi: 10.1148/radiol.2221010437. [DOI] [PubMed] [Google Scholar]
- 11.Hsu MY, Pan KT, Chen CM, Lui KW, Chu SY, Lin YY, et al. CT-guided percutaneous core-needle biopsy of pancreatic masses: Comparison of the standard mesenteric/retroperitoneal versus the trans-organ approaches. Clin Radiol. 2016;71:507–12. doi: 10.1016/j.crad.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu I, Okazaki Y, Takeda W, Kirihara T, Sato K, Fujikawa Y, et al. Use of percutaneous image-guided coaxial core-needle biopsy for diagnosis of intraabdominal lymphoma. Cancer Med. 2014;3:1336–41. doi: 10.1002/cam4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tyng CJ, Bitencourt AG, Martins EB, Pinto PN, Chojniak R. Technical note: CT-guided paravertebral adrenal biopsy using hydrodissection-a safe and technically easy approach. Br J Radiol. 2012;85:e339–42. doi: 10.1259/bjr/16118679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tyng CJ, Bitencourt AG, Almeida MF, Barbosa PN, Martins EB, Matushita JP, et al. Computed tomography-guided percutaneous biopsy of pancreatic masses using pneumodissection. Radiol Bras. 2013;46:139–42. [Google Scholar]
- 15.Meier-Meitinger M, Anders K, Alibek S, Uder M, Baum U. CT-guided biopsies of pancreatic lesions: Impact of contrast application prior to versus following needle placement. Acad Radiol. 2009;16:1386–92. doi: 10.1016/j.acra.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Wei Y, Yu XL, Liang P, Cheng ZG, Han ZY, Liu FY, et al. Guiding and controlling percutaneous pancreas biopsies with contrast-enhanced ultrasound: Target lesions are not localized on B-mode ultrasound. Ultrasound Med Biol. 2015;41:1561–9. doi: 10.1016/j.ultrasmedbio.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Brandt KR, Charboneau JW, Stephens DH, Welch TJ, Goellner JR. CT- and US-guided biopsy of the pancreas. Radiology. 1993;187:99–104. doi: 10.1148/radiology.187.1.8451443. [DOI] [PubMed] [Google Scholar]
- 18.Paulsen SD, Nghiem HV, Negussie E, Higgins EJ, Caoili EM, Francis IR. Evaluation of imaging-guided core biopsy of pancreatic masses. AJR Am J Roentgenol. 2006;187:769–72. doi: 10.2214/AJR.05.0366. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Liu LZ, Wu QL, Mo YX, Liu XW, Cui CY, et al. CT-guided core needle biopsy in the diagnosis of pancreatic diseases with an automated biopsy gun. J Vasc Interv Radiol. 2008;9:89–94. doi: 10.1016/j.jvir.2007.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparisons of radiation dose between patients with various groupings
Details of patients with complications