Abstract
BACKGROUND
Studies in breast cancer-related lymphedema (BRCL) have exclusively examined total arm volume, but not the specific tissue composition that contribute to total volume. We evaluated baseline differences in arm tissue composition (fat mass, lean mass, bone mineral content [BMC] and bone mineral density [BMD]) between the affected and unaffected arms in women with BRCL. We compared changes in arm tissue composition and self-reported lymphedema symptoms after one-year of weight-lifting vs. control.
METHODS
We utilized data from Physical Activity and Lymphedema (PAL) trial that included 141 women with BRCL. Arm tissue composition was quantified using dual-energy x-ray absorptiometry. The severity of lymphedema was quantified using self-report survey. Weight-lifting was performed at community fitness facilities.
RESULTS
At baseline, the affected arm had more fat (Δ=89.7g; P<0.001) and lean mass (Δ=149.1g; P<0.001), but less BMC (Δ=−3.2g; P<0.001) and less BMD (Δ=−5.5mg/cm2; P=0.04) than the unaffected arm. After 12-months of weight-lifting, composition of the affected arm was improved: lean mass (71.2g; P=0.01) and BMD (14.0mg/cm2; P=0.02) increased, arm fat percentage decreased (−1.5%; P=0.003). Composition of the unaffected arm was only improved in lean mass (65.2g; P=0 04). Increases in lean mass were associated with less severe BCRL symptoms.
CONCLUSIONS
Among women with BRCL, slowly progressive weight-lifting could improve arm tissue composition. Changes in arm tissue composition predict changes in symptom burden. Investigating the combined effects of exercise and weight-loss on arm tissue composition and BCRL symptoms may provide additional insight into the benefits of lifestyle modification on lymphedema biology.
Keywords: lymphedema, tissue composition, physical activity, survivorship
Introduction
Breast cancer (BrCa) is the most common cancer in women, with more than 3.1 million survivors alive in the United States(1). A frequent side-effect of BrCa treatment is breast cancer-related lymphedema (BCRL) (2). Women with BCRL may experience pain, impaired upper-extremity mobility and function, decreased physical activity, fatigue, distress, and poorer quality of life (3–6). BCRL results from lymph node removal, radiation therapy, and postoperative infections (7). The reported prevalence of BCRL varies from 5–54%, depending on the method of measurement used, threshold for diagnosis, length of follow-up, and population studied (8).
BCRL changes the tissue composition of the affected limb (9). Initially, swelling is caused by the accumulation of watery lymphatic fluid. As lymphedema progresses, an increased deposition of fibrosclerotic tissue and fat occurs (10). Aspirates from liposuction of lymphedematous arms consists of 68–93% fat and 7–32% interstitial fluid(11, 12). Persistent lymphedema in the affected arm may result in altered fat and lean (muscle: non-fat, non-bone) tissues when compared to the unaffected limb(13).
Prior clinical trials have suggested that slowly-progressive weight-lifting among women with BCRL can increase upper-and lower-extremity muscular strength, reduce the number and severity of BCRL symptoms, and reduce the incidence of BCRL exacerbations(14–17). Many of these trials have demonstrated that limb volume does not significantly change with slowly-progressive weight-lifting(15). However, studies to date have exclusively examined total arm volume, and not the specific tissue compartments that contribute to total volume. Improvements in muscular strength, BCRL symptoms, and BCRL exacerbations are consistent with the hypothesis that the tissue compartments of the affected limb (e.g. fat, lean, and bone) may be favorably altered in response to slowly-progressive weight-lifting.
To explore this hypothesis, we conducted a post hoc analysis of a randomized trial of slowly-progressive weight-lifting among women with BCRL. We explored whether: 1) baseline arm tissue composition in the affected limb differed from the unaffected limb; 2) arm tissue composition in the affected and unaffected limb changed after 12-months of slowly-progressive weight-lifting; 3) baseline body mass index (BMI) or grade of BCRL modify the magnitude of 12-months weight-lifting effect on the changes in the arm tissue composition; and 4) changes in arm tissue composition correlate with changes in arm volume, patient-reported BCRL symptom number or severity.
Methods
Participants
The primary aim of the PAL trial was to assess the safety of slowly progressive weight-lifting among breast cancer survivors with lymphedema (n=141) (16). A detailed description of the PAL trial methods are described elsewhere(18). Breast cancer survivors were recruited from the metropolitan Philadelphia region. The presence of lymphedema was defined as one of the following conditions: 1) ≥10% interlimb discrepancy in volume or circumference at the point of greatest visible difference; 2) meeting any of the Common Toxicity Criteria Adverse Event version 3.0 for BRCL (swelling, obscuration, or pitting); 3) prior clinical diagnosis of BCRL that was confirmed by study measurements or by a clinician (16, 18). Participants were eligible for the study if they were a female breast cancer survivor, 1–15 years post-diagnosis, free from cancer at study entry, with ≥1 lymph node removed, and with no medical conditions or contraindicated medications that would prohibit participation in an exercise program. Additional eligibility criteria included a BMI ≤50 kg/m2, no plans for surgery during the study, no history of bilateral lymph node removal, no weight-lifting in the previous year, stable body weight (<10% change in the past year), and not attempting to lose weight. Participants were randomized to one of the two study groups described below. This trial was approved by the University of Pennsylvania Institutional Review Board. All participants provided written informed consent and provided written clearance from their physician prior to participating in any study-related activities.
Intervention
Briefly, study participants randomized to the weight-lifting group were provided with a 12-months community fitness center membership. For the first 13-weeks, participants were instructed on safe completion of weight-lifting exercises in groups of 2–6 participants. Certified exercise professionals employed by the fitness centers led the twice-weekly 90-minutes exercise sessions. Each session included stretching, cardiovascular warm up, abdominal and lower back strengthening exercises, and weight-lifting exercises. Weight-lifting exercises for the upper-body included the dumbbell press, seated row, lateral or front raise, bicep curls, and triceps extension. Weight-lifting exercises for the lower-body included the leg press, back extension, leg extension, and leg curl. For each exercise session, three-sets of each weight-lifting exercise were performed, 10-repetitions per set. Weight was progressed by the smallest possible increment after two sessions at which three-sets of 10-repetitions could be performed without symptom changes. No maximal upper limit was placed on the weight progression.
After 13-weeks, participants continued with unsupervised weight-lifting for 39-weeks. Participants continued to adhere to the same exercise prescription. Attendance logs completed by study participants were verified for completion by the exercise professionals. Adherence was defined as attendance to weight-lifting sessions. The exercise professionals contacted study participants if they missed more than one exercise session each week throughout the year. Participants in the control group were asked to maintain their baseline level of physical activity. Upon study completion, control group participants were offered a 12-months fitness center membership and 13-weeks of supervised exercise instruction, similar to that of the weight-lifting group.
At baseline, study participants received a custom-fitted compression garment (Jobst, BSN Medical) to wear during exercise sessions. A second garment was provided at six-months. At night, participants were allowed to follow their usual lymphedema care routine and were not required to wear garments (19).
Measurement
Measurements were obtained from all participants at baseline and 12-months by trained staff that followed a standardized protocol and were blinded to study group. Demographic characteristics including age, and race were self-reported at baseline. Clinical characteristics including time since cancer diagnosis, cancer stage, and cancer treatment were collected from the state cancer registry, surgical pathology report, or self-report. Body mass (kg) and height (m) were used to calculate BMI (kg/m2). BCRL burden was quantified by self-report on a validated survey assessing the presence and severity of 14 common BCRL symptoms (16, 18).
Arm volume was quantified by water displacement volumetry (ml). DXA was used to quantify arm tissue composition including fat mass (g), lean mass (non-fat and non-bone, g), bone mineral density (BMD, mg/cm2), and bone mineral content (BMC, g) using Hologic APEX v. 13.4 software. Arm fat percentage was calculated as the percent of arm fat mass to arm limb mass. DXA presents high reliability and validity to quantify soft-tissue mass across upper and lower extremity and has been used to quantify arm tissue composition, such as fat mass, lean mass among older adults, postmenopausal and women with BCRL (20–22).
Statistical Analysis
All statistical analyses were completed using Stata MP Version 14.0 (StataCorp, College Station, TX). Chi-square and t-test were used to compare baseline characteristics. All inferential analyses were conducted on an intention-to-treat basis. Comparisons of baseline arm tissue composition differences between the affected and unaffected limbs were made using a t-test. In addition, multivariable models used linear regression to adjust for covariates that may explain differences in arm volume (i.e., hand dominance may influence lymphedema outcomes (23)). Between-group changes in arm volume (ml), fat mass (g), arm fat percentage, lean mass (g), BMD (mg/m2), and BMC (g), as well as the self-reported lymphedema symptoms were evaluated from baseline to 12-months using repeated-measures linear mixed-effects regression models. This statistical approach includes all available data and accounts for the correlation between repeated measures. The baseline value of the dependent variable was included as a covariate in the regression models to reduce variance (24) and a group-by-time interaction term was included as a fixed effect to assess the between-group change over time. To determine if the magnitude of benefit from weight-lifting on arm tissue composition varied by baseline BMI category (normal weight, overweight, obese) and baseline lymphedema grade (grade 1, 2, 3), we examined interaction effects using the 3-way interaction of group-by-time-by-BMI category (or group-by-time-by-lymphedema grade), and tests for trend were calculated using linear contrasts in the regression model. Correlation and linear regression were used to examine the relationship between changes in arm tissue composition over 12-months and changes in lymphedema symptoms (arm volume and self-reported survey). In sensitivity analyses, baseline values were carried forward for the 11 women for whom DXA data were not available at 12-months. Additionally, per-protocol analyses were conducted that excluded the participants who did not have a 12-months follow-up measurement. Results of the sensitivity analyses did not differ from those presented herein.
Results
Between October 2005 and February 2007, 141 breast cancer survivor with BCRL were recruited and randomly assigned into the weight-lifting (n=71) and control groups (n=70) (Figure 1). Median attendance to exercise sessions was 88% in weight-lifting group, including the five women who were lost to follow-up (16, 18). Table 1 depicts demographic and clinical characteristics. At baseline, there were no differences in interlimb volume difference between the two groups. Within-person, compared to unaffected arm, the affected arm had significantly higher absolute volume (+414.8 mL, P<0.001), fat mass (+89.7 g, P<0.001), lean mass (+149.1g, P<0.001), but less BMD (−5.5 mg/cm2, P=0.04) and BMC (−3.2 g, P<0.001) (Table 2.).
Fig 1. CONSORT diagram of enrollment, random assignment, and follow-up of women with lymphedema in PAL study (141/295, 47.8% of total sample size).
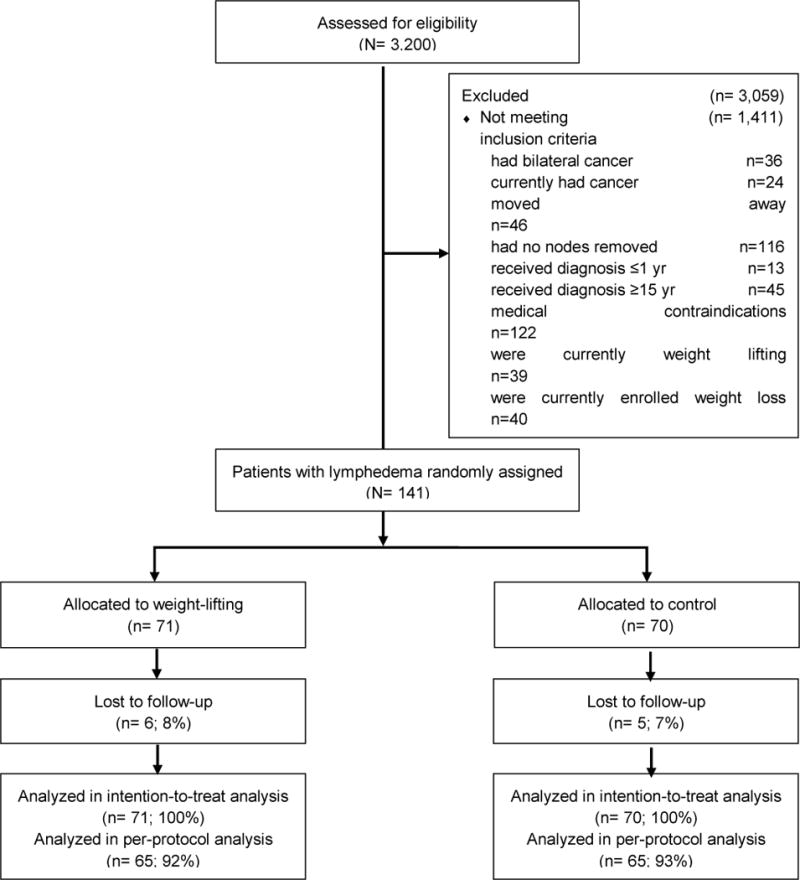
Other reasons for exclusion: never called back to finish screening, lived too far from participating fitness centers, were eligible for phone screening, but never provided consent, consented but did not undergo randomization, had life circumstances that interfered, were determined to be ineligible after providing consent, did not respond to attempts by study staff to make contact
Table 1.
Demographic and clinical characteristics (N=141)
| Overall n=141 |
Control n=70 |
Weight-lifting n=71 |
Pa | |
|---|---|---|---|---|
| Age, Yr, Mean±SD | 57±10 | 58±10 | 56±9 | 0.56 |
| Race, n(%) | 0.87 | |||
| Black | 54 (38%) | 26 (37%) | 28 (39%) | |
| White | 82 (58%) | 42 (60%) | 40 (56%) | |
| Other | 5 (4%) | 2 (3%) | 3 (4%) | |
| Education, n(%) | 0.56 | |||
| High school or less | 29 (21%) | 16 (23%) | 13 (18%) | |
| Some college | 50 (35%) | 24 (34%) | 26 (37%) | |
| College degree or more | 62 (44%) | 30 (43%) | 32 (45%) | |
| BMI, kg/m2, Mean±SD | 30.5±6.4 | 29.9±6.6 | 31.0±6.2 | 0.33 |
| BMI category, n(%) | 0.59 | |||
| Normal weight (<25kg/m2) | 30 (21%) | 17 (24%) | 13 (18%) | |
| Overweight (25–30kg/m2) | 41 (29%) | 21 (30%) | 20 (28%) | |
| Obese (>30kg/m2) | 70 (50%) | 32 (46%) | 38 (54%) | |
| Month since diagnosis, Mean±SD | 83±45 | 88±45 | 79±45 | 0.23 |
| Month since lymphedema diagnosis, Mean±SD | 61±45 | 61±45 | 61±45 | 0.95 |
| Month since last lymphedema treatment, Mean±SD | 36±36 | 36±37 | 36±35 | 0.92 |
| Interlimb volume % difference, Mean±SD | 16.1±15.2 | 17.3±16.6 | 15.0±14.7 | 0.49 |
| Interlimb volume % difference stage, n(%) | 0.48 | |||
| Stage 0 (<5%) | 30 (21%) | 16 (23%) | 14 (20%) | |
| Stage I (5-<10%) | 22 (16%) | 10 (14%) | 12 (17%) | |
| Stage II (10-<30%) | 63 (45%) | 28 (40%) | 35 (49%) | |
| Stage III (>30%) | 26 (18%) | 16 (23%) | 10 (14%) | |
| Common toxicity criteria lymphedema grade, n(%) | 0.25 | |||
| 0 | 12 (8%) | 7 (10%) | 5 (7%) | |
| 1 | 30 (21%) | 12 (17%) | 18 (25%) | |
| 2 | 58 (41%) | 26 (37%) | 32 (45%) | |
| 3 | 41 (29%) | 25 (36%) | 16 (22%) | |
| Breast cancer stage, n(%) | 0.19 | |||
| 1 | 57 (40%) | 24 (34%) | 33 (4 6%) | |
| 2 | 1 (1.0%) | 0 | 1 (1%) | |
| 3 | 44 (31%) | 22 (31%) | 22 (31%) | |
| Unknown | 39 (28%) | 24 (34%) | 15 (21%) | |
| Treatment, n(%) | ||||
| Radiation | 112 (79%) | 53 (76%) | 59 (83%) | 0.30 |
| Chemotherapy | 115 (82%) | 56 (80%) | 59 (83%) | 0.67 |
| Tamoxifen | 17 (12.2) | 3 (4%) | 14 (20%) | <0.01 |
| Aromatase Inhibitor | 1 (1%) | 1 (1%) | 0 (0%) | 0.31 |
| Breast cancer in dominant side, n (%) | 72 (51%) | 35 (50%) | 37 (52%) | 0.87 |
two-sided t-test or chi-square test
Table 2.
Baseline Tissue Composition in Breast Cancer Women with Lymphedema (N=141)
| Affected Arm | Unaffected Arm | Absolute Interlimb Differencea |
Pb | |
|---|---|---|---|---|
| Mean (95%CI) | Mean (95%CI) | Mean (95%CI) | ||
| Arm Volume, ml | 3017.3 (2882.7, 3151.9) | 2602.5 (2498.9, 2706.2) | +414.8 (346.4, 483.1) | <0.001 |
| Fat, g | 2059.3 (1938.3, 2180.2) | 1969.6 (1844.8, 2094.3) | +89.7 (48.2, 131,2) | <0.001 |
| Arm Fat Percentage, Fat Mass/Limb Mass, % |
46.5 (45.4, 47.6) | 46.5 (45.4, 47.7) | −0.03 (−0.6, 0.5) | 0.90 |
| Lean, g | 2551.5 (2457.4, 2646.7) | 2402.5 (2320.5, 2484.4) | +149.1 (99.0, 199.2) | <0.001 |
| BMD, mg/cm2 | 684.4 (673.8, 694.9) | 689.9 (678.9, 700.9) | −5.5 (−10.7,−0.4) | 0.04 |
| BMC, g | 133.9 (130.2, 137.7) | 137.2 (133.3, 141.0) | −3.2 (−4.9,−1.5) | <0.001 |
95%CI: 95% Confidence Interval
Subtracting unaffected from affected limb
t-test
Adjusted for dominance, no effect changes
At 12-months, compared to the control group, women in the weight-lifting group demonstrated improvements in arm tissue composition in their affected limb, with no changes observed in arm volume as assessed by water volumetry (Table 3). In the affected limb, lean mass (71.2 g, P=0.01) and BMD (14.0 mg/cm2, P=0.02) increased significantly among women in the weight-lifting group when compared to the control group. Arm fat percentage decreased (−1.5%, P=0.003). Fat mass reduced without meeting statistical significant (−57.9 g, P=0.13). In the unaffected arm, lean mass also significantly increased (65.2 g, P=0.04) (Table 3.). Stratified analyses for arm dominance and time since lymphedema diagnosis did not modify our conclusions (data not shown).
Table 3.
Effect of Weight-lifting on Arm Tissue Composition (N=141)
| Baselinec | Δ Baseline to Month 12 | Δ Between Group over Time | ||
|---|---|---|---|---|
| LS Mean (95% CI) | LS Mean (95% CI) | LS Mean (95% CI) | P | |
| Affected Arm | ||||
| Arm Volume, ml | 0.60 | |||
| Control | 2956.3 (2922.3, 2989.7) | −29.0±2 (−77.3, 19.3) | ||
| Weight-lifting | 2966.0 (2932.8, 2999.8) | −10.9 (−59.1, 37.3) | 18.3 (−50.1, 86.3) | |
| Fat, ga | 0.13 | |||
| Control | 2018.1 (1981.2, 2055.1) | −28.7 (81.9, 24.6) | ||
| Weight-lifting | 2024.7 (1988.3, 2061.3) | −86.6 (−139.0, −34.2) | −57.9 (−132.6, 16.7) | |
| Arm Fat Percentage, %b | 0.003 | |||
| Control | 46.5 (46.0, 47.0) | −0.2 (−0.9, 0.5) | ||
| Weight-lifting | 46.0 (45.9, 46.9) | −1.7 (−2.4, −1.0) | −1.5 −2.5,−0.5) | |
| Lean,ga | 0.01 | |||
| Control | 2509.2 (2482.2, 2536.3) | −9.6 (−48.6, 29.4) | ||
| Weight-lifting | 2518.8 (2492.1, 2545.5) | 61.6 (23.2, 100.0) | 71.2 (16.5, 125.9) | |
| BMD, mg/cm2a | 0.02 | |||
| Control | 680.1 (674.4, 685.7) | −1.2 (−9.2, 6.9) | ||
| Weight-lifting | 680.6 (675.0, 686.1) | 12.8 (4.8, 20.8) | 14.0 (2.6, 25.3) | |
| BMC, ga | 0.46 | |||
| Control | 132.0 (130. 3, 133.7) | −1.3 (−3.7, 1.1) | ||
| Weight-lifting | 132.4 (130.8, 134.1) | −0.02 (−2.4, 2.4) | 1.3 (−2.1, 4.7) | |
| Unaffected Arm | ||||
| Arm Volume, ml | 0.58 | |||
| Control | 2556.9 (2531.4, 2582.4) | −4.2±18.7 (− 40.7, 32.4) | ||
| Weight-lifting | 2566.9 (2541.6, 2592.3) | 10.5 (−25.9, 47.0) | 14.7 (−37.0, 66.3) | |
| Fat, ga | 0.80 | |||
| Control | 1929.8 (1893.9, 1965.6) | −42.8 (−9 4.5, 8.9) | ||
| Weight-lifting | 1928.3 (1892.9, 1963.7) | −52.2±25.9 (−103.1, −1.4) | −9.4 (82.0, 63.1) | |
| Arm Fat Percentage, %b | 0.10 | |||
| Control | 46.4 (45.9, 46.9) | −0.7 (−1.4, 0.1) | ||
| Weight-lifting | 46.3 (45.8, 46.8) | −1.6 −2.3,−0.8) | −0.9 (−1.9, 0.2) | |
| Lean, ga | 0.04 | |||
| Control | 2368.3 (2336.8, 2399.7) | 12.5 (−32.7, 57.8) | ||
| Weight-lifting | 2377.4 (2346.4, 2408.5) | 77.7 (33.1, 122.2) | 65.2 (1.7, 128.7) | 0.36 |
| BMD, mg/cm2a | ||||
| Control | 684.9 (680.2, 689.6) | 8.1 (1.3, 14.9) | ||
| Weight-lifting | 684.9 (680.3, 689.6) | 3.6 (−3.1, 10.3) | −4.5 (−14.0, 5.0) | |
| BMC, ga | 0.62 | |||
| Control | 135.5 (134. 3, 136.8) | −2.3 (−4.1, −0.4) | ||
| Weight-lifting | 135.6 (134.4, 136.9) | −1.6 (−3.4, 0.2) | −0.6 (−1. 9, 3.2) | |
| Norman Lymphedema Surveyb | ||||
| Number of Symptoms | 0.06 | |||
| Control | 5.4±0.2 (5.1, 5.8) | −1.2 (−1.7, −0.7) | ||
| Weight-lifting | 5.5 (5.2, 5.8) | −1.9 −2.3,−1.4) | −0.65 (−1.3, 0.04) | |
| Severity of Symptoms | 0.08 | |||
| Control | 2.0 (1.8, 2.1) | −0.2 (−0.4, −0.1) | ||
| Weight-lifting | 2.0 (1.9, 2.1) | −0.4 −0.6,−0.3) | −0.20 (−0.4, 0.02) | |
LS Mean: least-squares mean, 95%CI: 95% Confidence Interval
adjusted for baseline measurements, arm volume, race, lymphedema grade, cancer stage, months since diagnosis, radiation. No 3-way (group-time-arm) interactions observed for fat, muscle, BMC
adjusted for baseline measurements, race, lymphedema grade, cancer stage, months since diagnosis, radiation.
baseline values were estimated from the linear mixed models adjusted for covariates
An interaction was observed between BMI category and changes of affected arm tissue composition after 12-month weight-lifting (Pinteraction’s<0.04, Table 4). Compared with normal BMI, women with higher BMI had a smaller magnitude of loss in affected arm fat mass percentage (P linear trend=0.002), and a smaller magnitude of improvement in lean mass (P linear trend =0.03) as a result of weight-lifting. Another interaction was observed between BCRL grade and changes of affected arm tissue composition after 12-month weight-lifting (Pinteraction’s<0.05, Table 4). Compared to BCRL grade 1, women with higher lymphedema grade had a smaller magnitude of loss in affected arm fat mass percentage (P linear trend =0.04) in response to weight-lifting (Table 4.). In the unaffected limb, BMI and BCRL grade did not modify the effects of weight-lifting (results not shown). Supplementary Table presents weight-lifting effects on absolute differences of arm tissue compositions.
Table 4.
Interaction of BMI / Lymphedema Grade in Affected Arm (N=141)
| A between group over time LS Mean (95% CI) |
A between group over time LS Mean (95% CI) |
||||
|---|---|---|---|---|---|
| Arm volume, mla | Arm volume, mlb | ||||
| BMI | Normal | 51.1 (−74.1, 176.4) | Lymph grade | 1 | 71.4 (−33.1, 175.8) |
| Overweight | 56.8 (−52.7, 166.4) | 2 | 33.2 (−58.9, 128.3) | ||
| Obese | −10.5 (−102.3, 81.4) | 3 | −28.4 (−149.5, 92.7) | ||
| Plinear trend | 0.94 | Plinear trend | 0.91 | ||
| Fat, ga* | Fat, gb* | ||||
| BMI | Normal | −37.9 (−173.6, 97.6) | Lymph grade | 1 | −48.8 (−163.6, 66.0) |
| Overweight | −26.4 (−145.7, 92.9) | 2 | −33.0 (−133.9, 67.8) | ||
| Obese | −77.0 (−176.7, 22.7) | 3 | −77.9 (−210.3, 54.4) | ||
| Plinear trend | 0.15 | Plinear trend | 0.24 | ||
| Arm Fat Percentage, %a* | Arm Fat Percentage, %b | ||||
| BMI | Normal | −2.0 (−3.8, −0.3) | Lymph grade | 1 | −1.8 (−3.3, −0.3) |
| Overweight | −1.6 −3.2, −0.01) | 2 | −1.5 (−2.9, −0.2) | ||
| Obese | −1.4 (−2.7, −0.1) | 3 | −1.1 (−2.9, −0.7) | ||
| Plinear trend | 0.002 | Plinear trend | 0.04 | ||
| Lean, ga* | Lean, gb | ||||
| BMI | Normal | 100.5 (−0.7, 201.8) | Lymph grade | 1 | 94.9 (9.3, 180.5) |
| Overweight | 87.8 (−1.3, 176.9) | 2 | 100.3 (25.1, 175.5) | ||
| Obese | 76.0 (1.5, 150.5) | 3 | 28.5 (−70.2, 127.1) | ||
| P linear trend | 0.03 | Plinear trend | 0.07 | ||
| BMD, mg/cm2b* | BMD, mg/cm2b* | ||||
| BMI | Normal | 15.5 (−19.8, 50.1) | Lymph grade | 1 | 1.3 (−42.8, 17.8) |
| Overweight | −21.9 (−52.9, 9.1) | 2 | 13.8 (−40.5, 12.9) | ||
| Obese | −18.5 (−44.5, 7.5) | 3 | 11.1 (−43.6, 26.5) | ||
| P linear trend | 0.10 | Plinear trend | 0.36 | ||
| BMC, ga | BMC, gb | ||||
| BMI | Normal | 3.2 (−2.9, 9.3) | Lymph grade | 1 | −0.2 (−5.3, 5.0) |
| Overweight | 0.6 (−4.7, 6.0) | 2 | 1.4 (−3.2, 5.9) | ||
| Obese | 2.3 (−2.2, 6.8) | 3 | 5.2 (−0.8, 11.1) | ||
| Plinear trend | 0.35 | Plinear trend | 0.13 | ||
LS Mean: least-squares mean, 95%CI: 95% Confidence Interval
P interaction <0.05. Interaction of group by time by BMI category (or lymphedema grade)
adjusted lymphedema grade, number of lymph nodes removed, and months since diagnosis.
adjusted bmi, number of lymph nodes removed, and months since diagnosis
We also assessed the correlation between change in arm tissue composition after 12-months weight-lifting and severity and frequency of lymphedema symptoms. Arm volume, arm fat mass, arm fat percentage, and BMD and BMC were not associated with changes in lymphedema symptom severity or frequency. However, we found that improvement of lean mass in affected limb after 12-months weight-lifting was correlated with reduced the severity of lymphedema symptoms. For each 1kg increase in lean mass, the severity of lymphedema symptoms decreased 0.86 on the scale of 0–3 (P=0.04). The improvement of lean mass was not associated with changes in the frequency of lymphedema symptoms (data not shown). In addition, with every 1% decrease in arm fat percentage, affected limb volume reduced 13.81ml (P=0.03).
Discussion
Our findings demonstrate that weight-lifting results in significant improvements in tissue composition in the affected limbs of women with BCRL. Weight-lifting increased lean mass and BMD, and reduced arm fat percentage, despite no changes in overall arm volume (previously reported in our main results paper for the PAL Trial (18)). These effects were not observed in the unaffected limb, in which a significant increase in lean mass was noted. The beneficial effect of weight-lifting may be attenuated among women with a high BMI or who had higher lymphedema grade at baseline. In addition, our findings suggest that improvement in lean mass from 12-months weight-lifting are associated with reduced severity of lymphedema symptoms, and the decrease in arm fat percentage was associated with a reduction in affected limb volume. To our knowledge, this is the first report of the effects of any intervention to improve arm tissue composition among women with BCRL.
In the unaffected arm, the only improvement observed as a result of 12-months weight-lifting was in the lean mass. It appears a lymphedematous environment responds more favorably to a weight-lifting stimulus, perhaps in an attempt to reestablish homeostasis within the arm. Lymphedematous tissue differs from non-lymphedematous tissue in a number of ways. For example, lymphedematous tissue has been shown to have vascular insufficiency, demonstrating higher adipogenic gene expression and the enhanced ability to undergo adipogenic differentiation, coupled with a lower vasculogenic gene expression and diminished capability to form tubules (25). In the early stages of BCRL, swelling is caused by a watery lymphatic edema. As BCRL progresses, the tissue consistency begins to harden and is characterized by fibrosclerotisis and fat deposition. The dominant structural elements in chronic lymphedema are collagen fibers and fibroblast migration. However, there have been no systematic studies of BCRL-related changes in the extracellular matrix components (10). Our finding is consistent with prior cross-sectional studies that the affected limb includes more fat and lean mass compared with unaffected limb (13).
Women with BCRL report functional limitations due to reduced strength, increased pain and edema (26–28). BCRL also leads to psychological distress and lower quality of life (3, 5, 26, 29). Weight training exercise can provide benefits by challenging skeletal muscles with controlled physiological stress to the onset of muscle fatigue (30, 31). Specifically, weight training exercise significantly improves lean body mass and muscular strength compared with usual care, and even aerobic exercise (16, 32, 33). Weight-lifting could also attenuate the decline of appendicular skeletal muscle mass(14). Upper body function and strength are also improved with weight-lifting, and these outcomes might be particularly important for breast cancer survivors (15). In addition, a revised weight-lifting intervention (Strength After Breast Cancer; SABC) has been translated and delivered in the outpatient rehabilitation clinic setting using similar intervention elements from the PAL trial (37). This revised intervention included four small group physical therapy sessions and an expectation that participants would be able to complete twice weekly weight-lifting training at home. Participants in the SABC programs also demonstrated improvements in lymphedema symptoms, muscular strength and body image among women with breast cancer, which suggested that breast cancer survivors could benefit from clinicians’ referral to such programs.
Therefore, previous evidence, together with the findings from this post hoc analysis, suggest that women with BCRL could improve arm tissue composition and arm strength from weight training exercise, to reduce lymphedema symptoms, prevent functional impairment and improve the quality of life after breast cancer surgery. Future studies to identify morphological changes in lymphedema progressions and to quantify the association of improvements of lean mass and arm strength are needed to better understand the pathophysiology and clinical relevance of compositional changes in BCRL.
Strengths and limitations
There are several limitations of this trial. We observed modifications of the effect of weight-lifting by BMI category and lymphedema grade. However, sample size precluded examination of the interaction effect of lymphedema grade and BMI category on changes in arm tissue composition. Given the long-standing evidence that obesity is associated with worse clinical course of lymphedema (34, 35), it will also be important for future studies to examine the effect of weight loss alone and together with weight-lifting. Understanding how weight loss and weight-lifting training may work synergistically for tissue composition improvement among women with BCRL is important to finding ways to control this chronic condition to garner other health and quality of life benefits for breast cancer survivors. Change in arm tissue composition after 12-months weight-lifting was not a primary outcome of the PAL trial. Therefore, participants were not enrolled into this trial on the basis of improving arm tissue composition measured by DXA. Assessment of the validity of measuring changes in arm tissue composition by DXA data to present the morphological changes in lymphedematous tissue requires further attention. Although the self-reported survey we used to quantify the severity of lymphedema symptoms is valid, a better clinical assessment tool, perhaps lymphoscintigraphy, should be developed to measure the severity of lymphedema symptoms objectively. This analysis was not pre-specified in the PAL trial protocol and therefore should be interpreted as exploratory and hypothesis generating.
There are several strengths to this analysis. To our knowledge, this is the first study to examine the effect of slowly-progressive weight-lifting on the change in arm tissue composition. In addition, we used DXA to quantify segmental arm tissue compartments (fat, lean and bone), which is a well-validated measure of body composition, including fat, lean, and bone (20–22, 36). Another strength of this study is, PAL trial included participants with a wide range of arm volume (1630–5890 mL) and BMI (range 17.7–48 kg/m2), which provided the opportunity to examine the benefit of weight-lifting intervention among women with BCRL in different body shapes. We also included a racially diverse population (42% minority women) with a wide range of time since diagnosis (1–15 years) and the high rate of follow-up. This suggests that the arm tissue benefits of a slowly progressive weight-lifting program might be broadly generalizable to women with BCRL.
Conclusion
In conclusion, despite no change in overall arm volume, a twice-weekly slowly-progressive weight-lifting program resulted in a significant improvement in the fat and lean tissue composition, of the lymphedematous limb. Observed changes are consistent with the hypothesis that the changes are in lymphedematous tissue. Moreover, improvement of lean mass from weight-lifting intervention was associated with reduced severity of lymphedema symptoms, and the decrease in arm fat percentage was associated with a reduction of arm volume. Clinicians treating BRCA survivors with lymphedema may wish to prescribe such program (i.e. SABC, http://klosetraining.com) with the goal of improving this chronic condition.
Supplementary Material
Acknowledgments
Funding Source: This work was upported by R01-CA106851 from the National Cancer Institute
Footnotes
Disclosures: The authors report there are no conflicts of interest exist.
Clinicaltrials.gov Identifier: NCT00194363
References
- 1.American Cancer S. Breast Cancer Facts and Figures. 2015 [Google Scholar]
- 2.Armer JM, Stewart BR. Post-breast cancer lymphedema: incidence increases from 12 to 30 to 60 months. Lymphology. 2010;43(3):118. [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed RL, Prizment A, Lazovich D, et al. Lymphedema and quality of life in breast cancer survivors: the Iowa Women’s Health Study. J Clin Oncol. 2008;26(35):5689. doi: 10.1200/JCO.2008.16.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heiney SP, McWayne J, Cunningham JE, et al. Quality of life and lymphedema following breast cancer. Lymphology. 2007;40(4):177. [PubMed] [Google Scholar]
- 5.Paskett ED, Dean JA, Oliveri JM, et al. Cancer-related lymphedema risk factors, diagnosis, treatment, and impact: a review. J Clin Oncol. 2012;30(30):3726. doi: 10.1200/JCO.2012.41.8574. [DOI] [PubMed] [Google Scholar]
- 6.Shih YC, Xu Y, Cormier JN, et al. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2-year follow-up study. J Clin Oncol. 2009;27(12):2007. doi: 10.1200/JCO.2008.18.3517. [DOI] [PubMed] [Google Scholar]
- 7.Coward DD. Lymphedema prevention and management knowledge in women treated for breast cancer. Oncol Nurs Forum. 1999;26(6):1047. [PubMed] [Google Scholar]
- 8.Goker M, Devoogdt N, Van de Putte G, et al. Systematic review of breast cancer related lymphoedema: making a balanced decision to perform an axillary clearance. Facts Views Vis Obgyn. 2013;5(2):106. [PMC free article] [PubMed] [Google Scholar]
- 9.Rockson SG. Update on the biology and treatment of lymphedema. Current treatment options in cardiovascular medicine. 2012;14(2):184. doi: 10.1007/s11936-012-0170-0. [DOI] [PubMed] [Google Scholar]
- 10.Rusznyák I, Földi M, Szabó G. Lymphatics and lymph circulation: Physiology and pathology. Elsevier; 2013. [Google Scholar]
- 11.Brorson H, Ohlin K, Olsson G, et al. Adipose tissue dominates chronic arm lymphedema following breast cancer: an analysis using volume rendered CT images. Lymphatic research and biology. 2006;4(4):199. doi: 10.1089/lrb.2006.4404. [DOI] [PubMed] [Google Scholar]
- 12.Brorson H, Svensson H. Complete reduction of lymphoedema of the arm by liposuction after breast cancer. Scandinavian journal of plastic and reconstructive surgery and hand surgery. 1997;31(2):137. doi: 10.3109/02844319709085480. [DOI] [PubMed] [Google Scholar]
- 13.Brorson H, Ohlin K, Olsson G, et al. Breast cancer-related chronic arm lymphedema is associated with excess adipose and muscle tissue. Lymphatic research and biology. 2009;7(1):3. doi: 10.1089/lrb.2008.1022. [DOI] [PubMed] [Google Scholar]
- 14.Brown JC, Schmitz KH. Weight lifting and appendicular skeletal muscle mass among breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2015;151(2):385. doi: 10.1007/s10549-015-3409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheema B, Gaul CA, Lane K, et al. Progressive resistance training in breast cancer: a systematic review of clinical trials. Breast Cancer Res Treat. 2008;109(1):9. doi: 10.1007/s10549-007-9638-0. [DOI] [PubMed] [Google Scholar]
- 16.Schmitz KH, Ahmed RL, Troxel A, et al. Weight lifting in women with breast-cancer—related lymphedema. N Engl J Med. 2009;361(7):664. doi: 10.1056/NEJMoa0810118. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz KH, Ahmed RL, Troxel AB, et al. Weight lifting for women at risk for breast cancer—related lymphedema: a randomized trial. Jama. 2010;304(24):2699. doi: 10.1001/jama.2010.1837. [DOI] [PubMed] [Google Scholar]
- 18.Schmitz KH, Troxel AB, Cheville A, et al. Physical Activity and Lymphedema (the PAL trial): assessing the safety of progressive strength training in breast cancer survivors. Contemporary clinical trials. 2009;30(3):233. doi: 10.1016/j.cct.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown JC, Cheville AL, Tchou JC, et al. Prescription and adherence to lymphedema self-care modalities among women with breast cancer-related lymphedema. Supportive Care in Cancer. 2014;22(1):135. doi: 10.1007/s00520-013-1962-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Czerniec SA, Ward LC, Meerkin JD, et al. Assessment of segmental arm soft tissue composition in breast cancer-related lymphedema: A pilot study using dual energy X-ray absorptiometry and bioimpedance spectroscopy. Lymphatic research and biology. 2015;13(1):33. doi: 10.1089/lrb.2014.0033. [DOI] [PubMed] [Google Scholar]
- 21.Gianoudis J, Bailey CA, Daly RM. Associations between sedentary behaviour and body composition, muscle function and sarcopenia in community-dwelling older adults. Osteoporosis Int. 2015;26(2):571. doi: 10.1007/s00198-014-2895-y. [DOI] [PubMed] [Google Scholar]
- 22.Reid IR, Evans MC, Ames R. Relationships between upper-arm anthropometry and soft-tissue composition in postmenopausal women. Am J Clin Nutr. 1992;56(3):463. doi: 10.1093/ajcn/56.3.463. [DOI] [PubMed] [Google Scholar]
- 23.Cornish BH, Chapman M, Hirst C, et al. Early diagnosis of lymphedema using multiple frequency bioimpedance. Lymphology. 2001;34(1):2. [PubMed] [Google Scholar]
- 24.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. John Wiley & Sons; 2012. [Google Scholar]
- 25.Levi B, Glotzbach JP, Sorkin M, et al. Molecular analysis and differentiation capacity of adipose-derived stem cells from lymphedema tissue. Plast Reconstr Surg. 2013;132(3):580. doi: 10.1097/PRS.0b013e31829ace13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawes DJ, Meterissian S, Goldberg M, et al. Impact of lymphoedema on arm function and health-related quality of life in women following breast cancer surgery. J Rehabil Med. 2008;40(8):651. doi: 10.2340/16501977-0232. [DOI] [PubMed] [Google Scholar]
- 27.Rietman JS, Dijkstra PU, Debreczeni R, et al. Impairments, disabilities and health related quality of life after treatment for breast cancer: a follow-up study 2.7 years after surgery. Disabil Rehabil. 2004;26(2):78. doi: 10.1080/09638280310001629642. [DOI] [PubMed] [Google Scholar]
- 28.Rietman JS, Dijkstra PU, Geertzen JHB, et al. Treatment-related upper limb morbidity 1 year after sentinel lymph node biopsy or axillary lymph node dissection for stage I or II breast cancer. Annals of surgical oncology. 2004;11(11):1018. doi: 10.1245/ASO.2004.03.512. [DOI] [PubMed] [Google Scholar]
- 29.Passik SD, McDonald MV. Psychosocial aspects of upper extremity lymphedema in women treated for breast carcinoma. Cancer. 1998;83(S12B):2817. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2817::aid-cncr32>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 30.Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 31.Thompson WR, Gordon NF, Pescatello LS. American College of Sport Medicine. ACSM’s Guidelines for exercise testing and prescription. 8. painos. 2009. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed RL, Thomas W, Yee D, et al. Randomized controlled trial of weight training and lymphedema in breast cancer survivors. J Clin Oncol. 2006;24(18):2765. doi: 10.1200/JCO.2005.03.6749. [DOI] [PubMed] [Google Scholar]
- 33.Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25(28):4396. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 34.Soran A, D’ Angelo G, Begovic M, et al. Breast Cancer — Related Lymphedema—What Are the Significant Predictors and How They Affect the Severity of Lymphedema? Breast J. 2006;12(6):536. doi: 10.1111/j.1524-4741.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 35.Vignes S, Arrault M, Dupuy A. Factors associated with increased breast cancer-related lymphedema volume. Acta Oncol. 2007;46(8):1138. doi: 10.1080/02841860701403020. [DOI] [PubMed] [Google Scholar]
- 36.Bredella MA, Ghomi RH, Thomas BJ, et al. Comparison of DXA and CT in the assessment of body composition in premenopausal women with obesity and anorexia nervosa. Obesity. 2010;18(11):2227. doi: 10.1038/oby.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beidas RS, Paciotti B, Barg F, et al. A hybrid effectiveness-implementation trial of an evidence-based exercise intervention for breast cancer survivors. J Natl Cancer Inst Monogr. 2014;2014(50):338. doi: 10.1093/jncimonographs/lgu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


